Corrosion Behaviour of High-Pressure Die-Cast AZ91 Alloy in NaCl Solution: Effects of Friction Stir Process at High Rotational Speed
Abstract
:1. Introduction
2. Materials and Methods
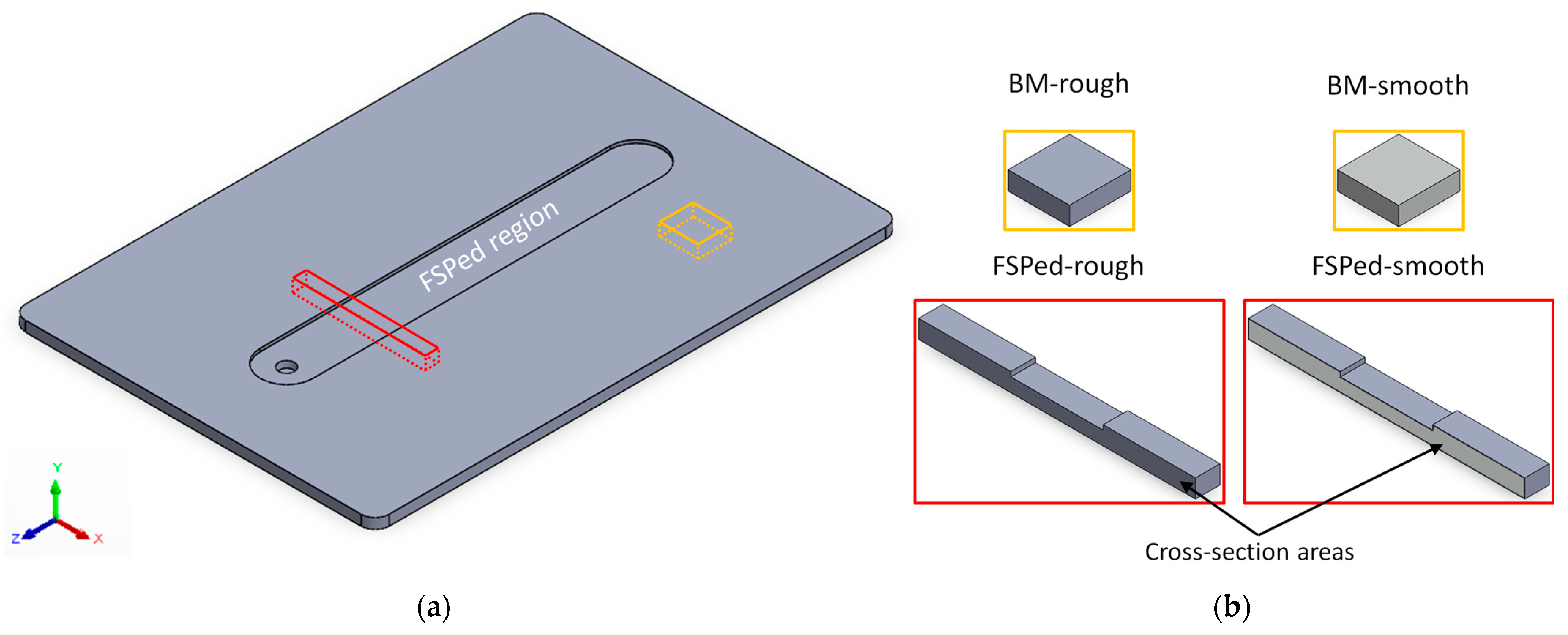
2.1. Sample Preparation
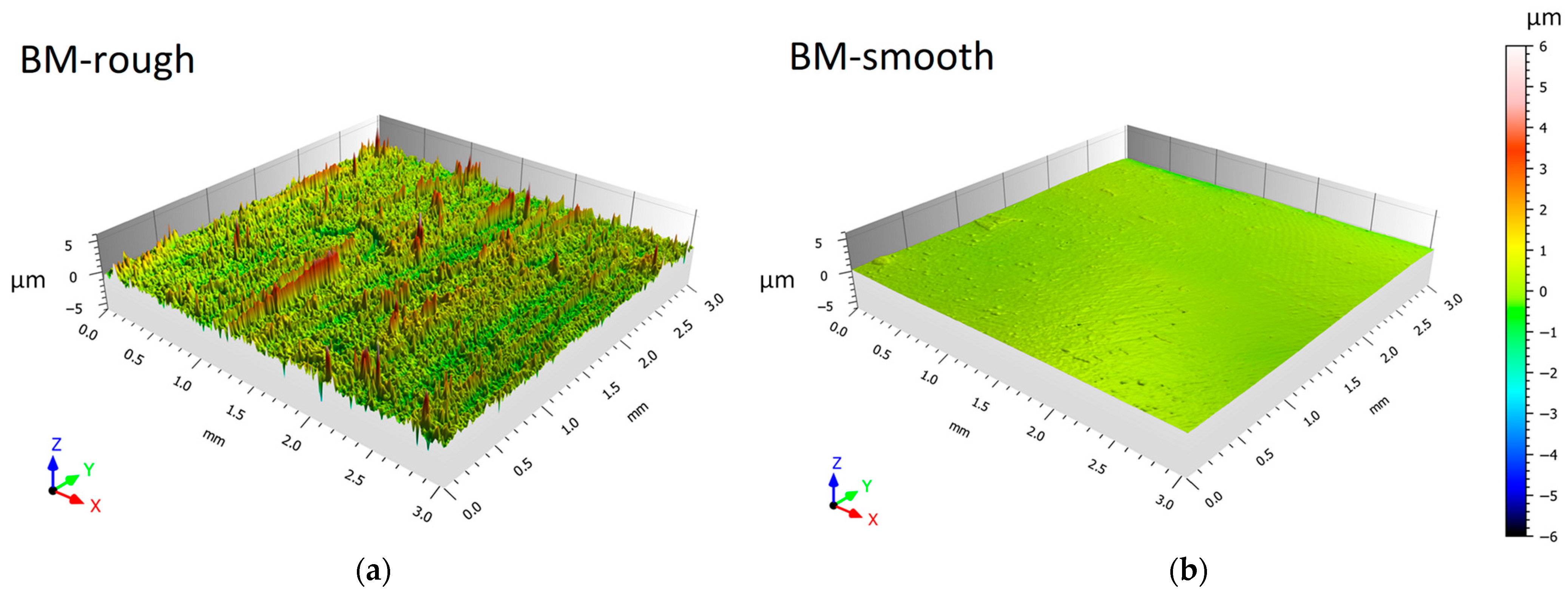
2.2. Surface and Microstructural Characterizations
2.3. Corrosion Behaviour: Immersion Test
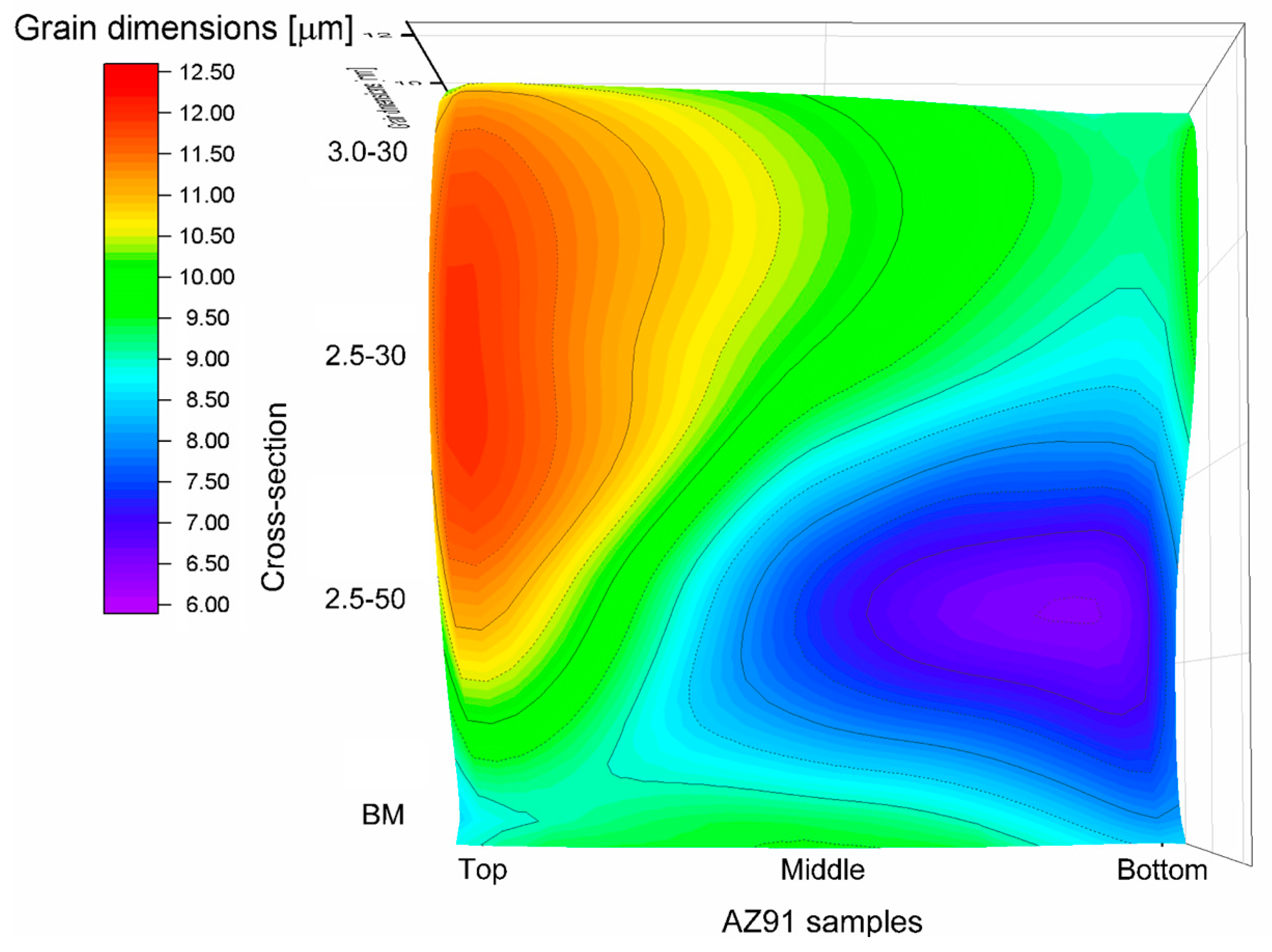
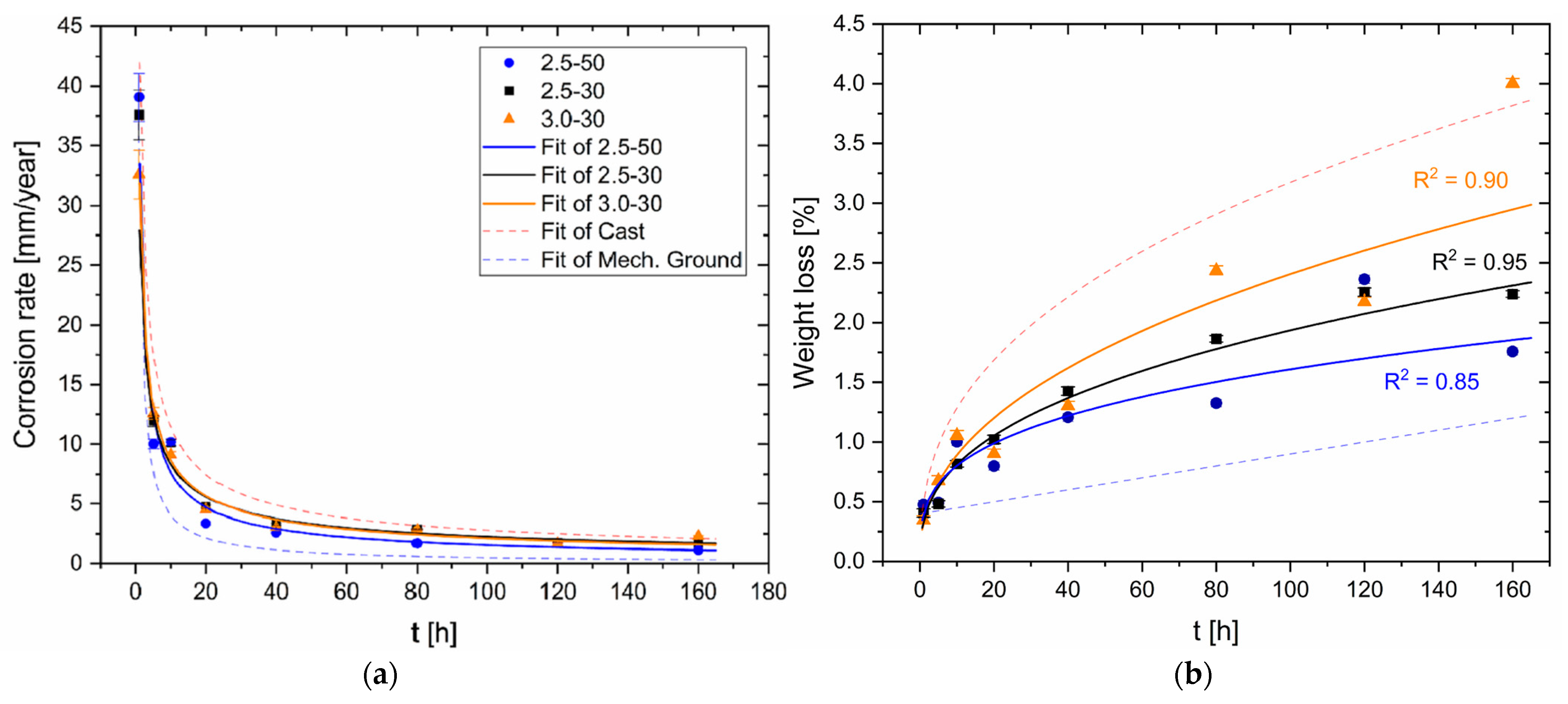
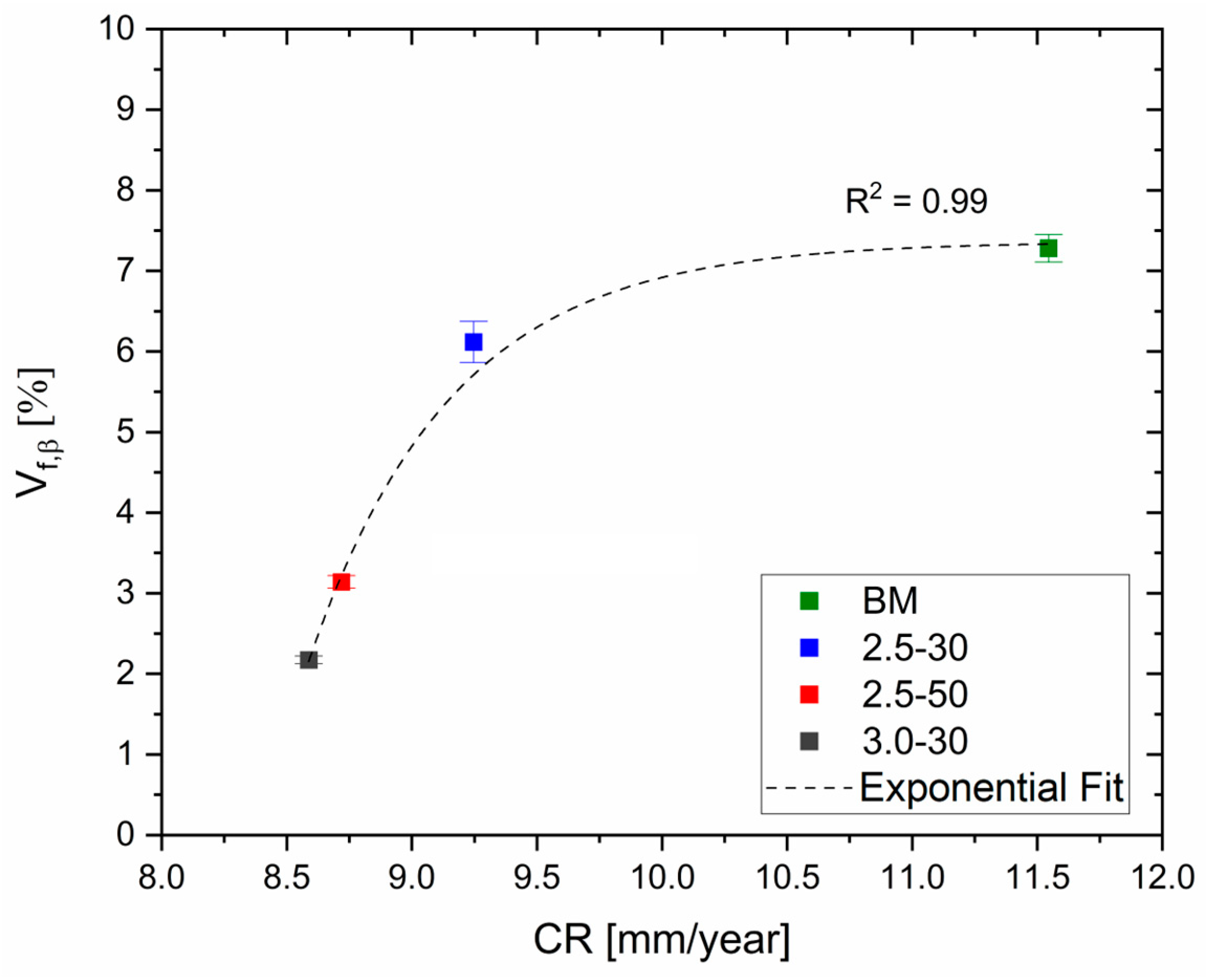
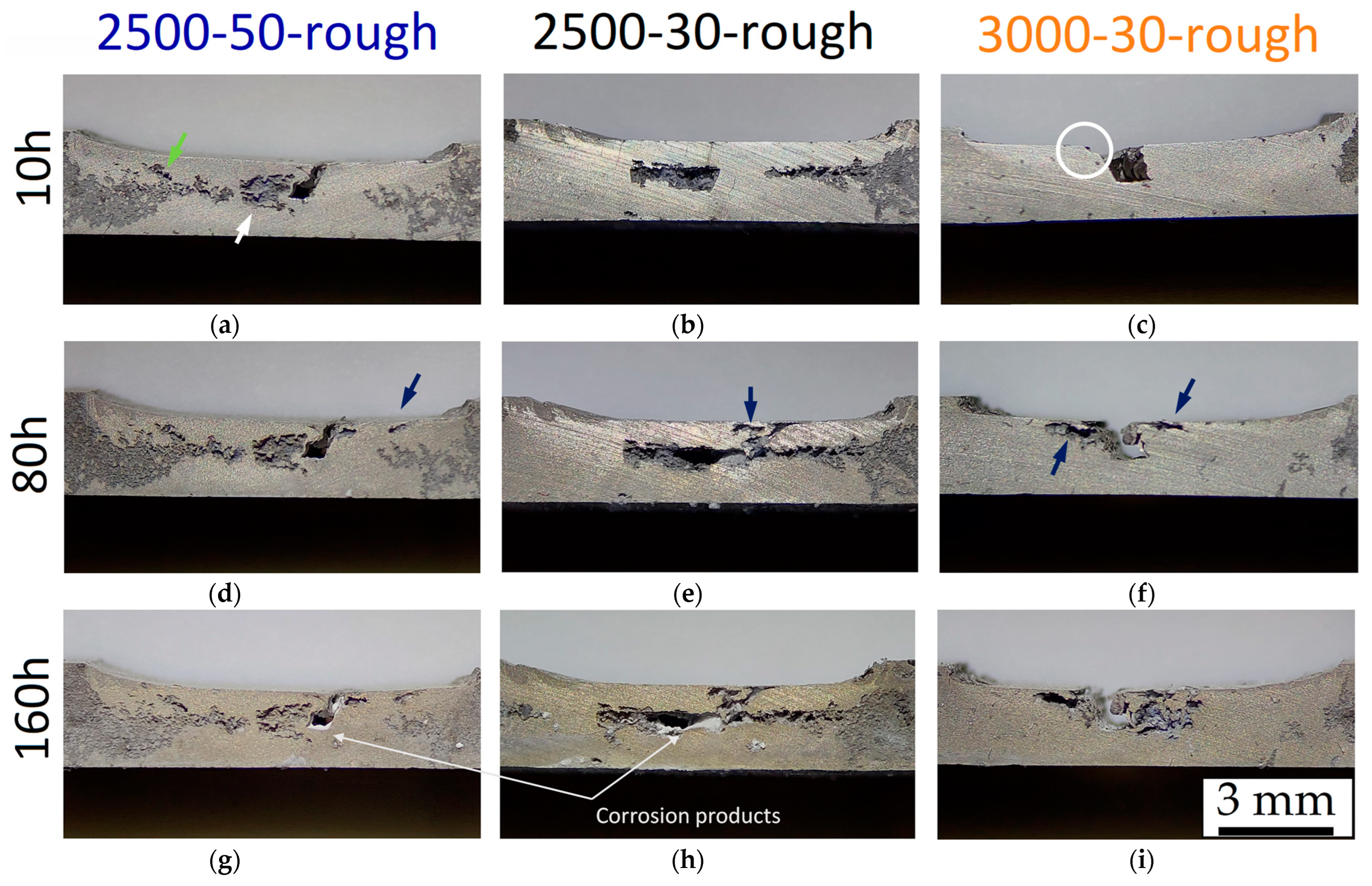
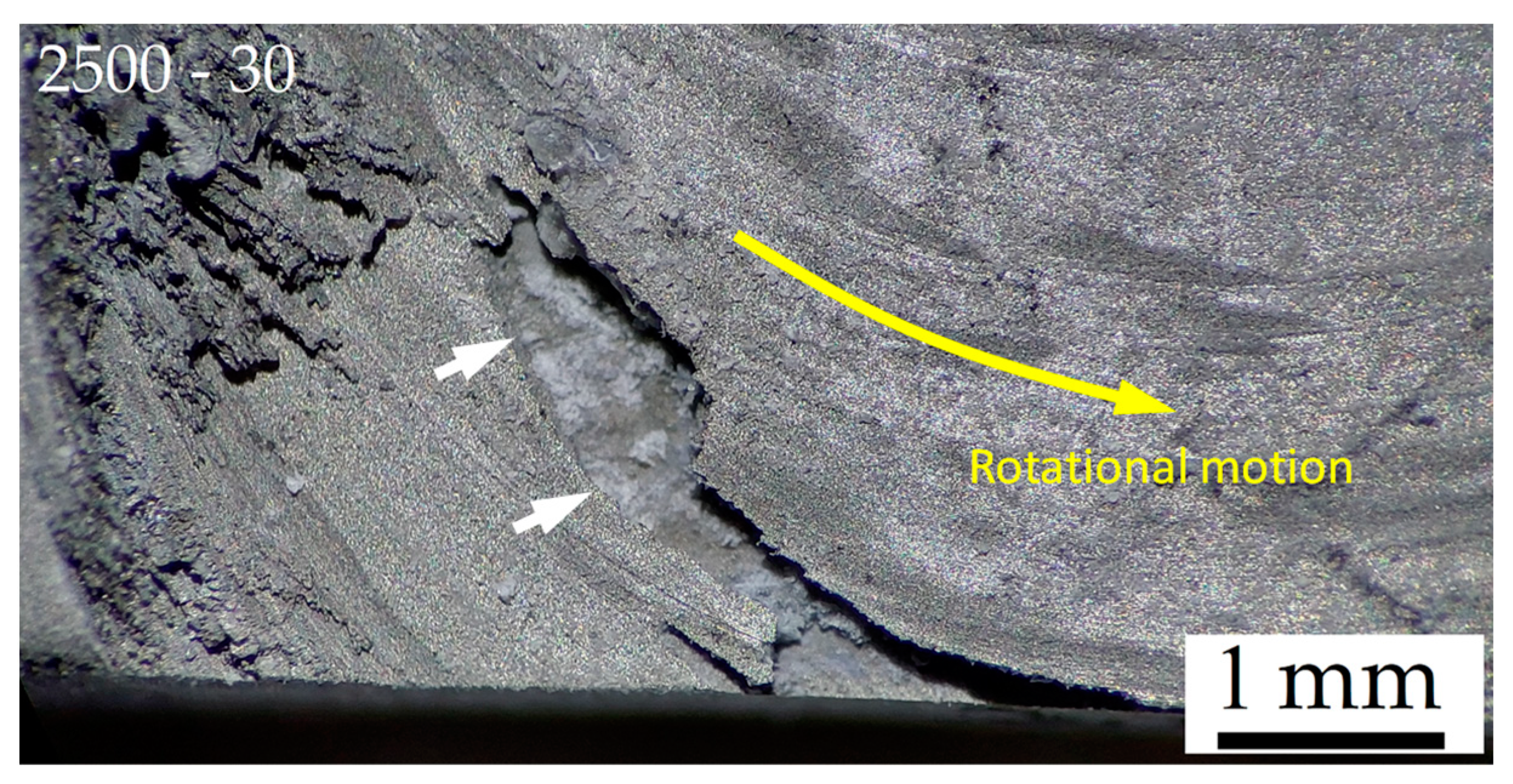
3. Results and Discussion
4. Conclusions
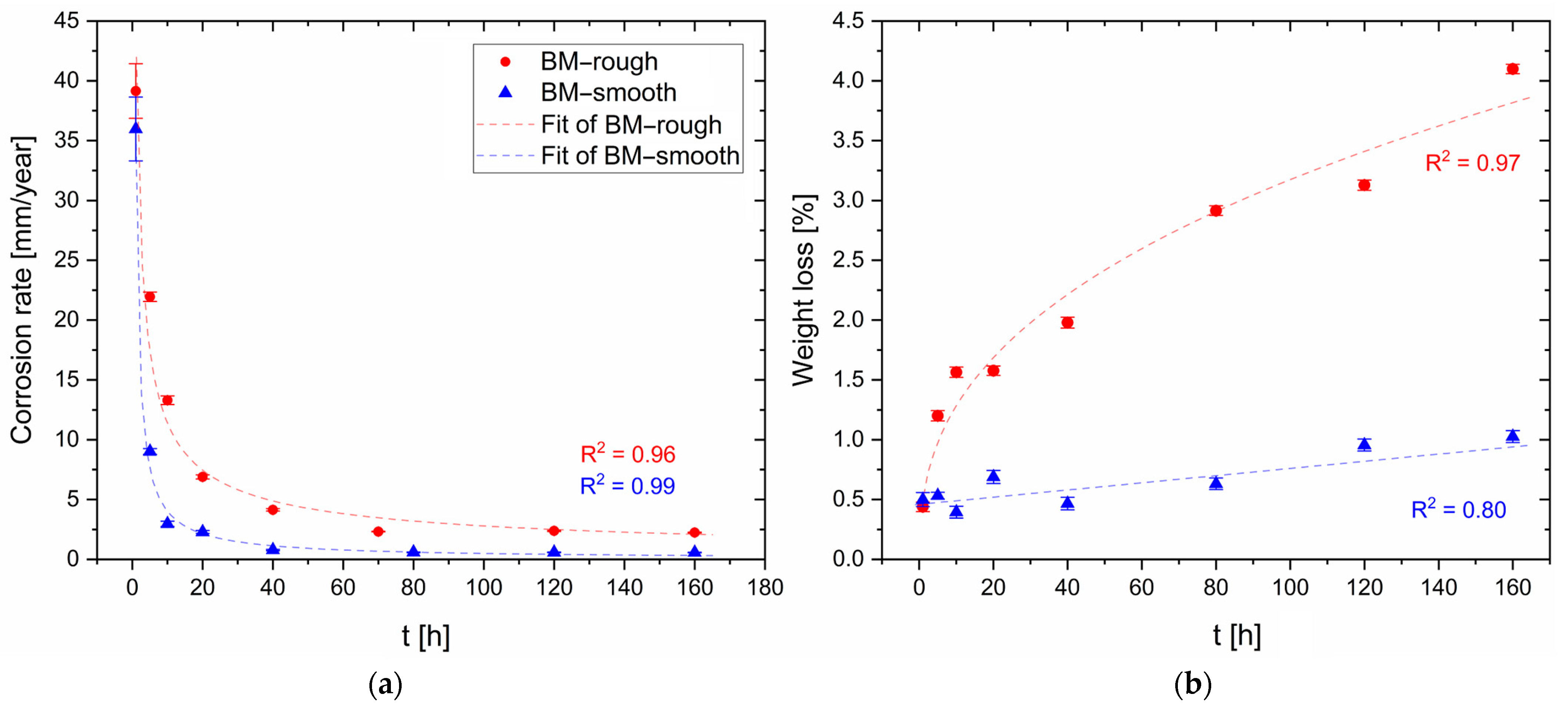
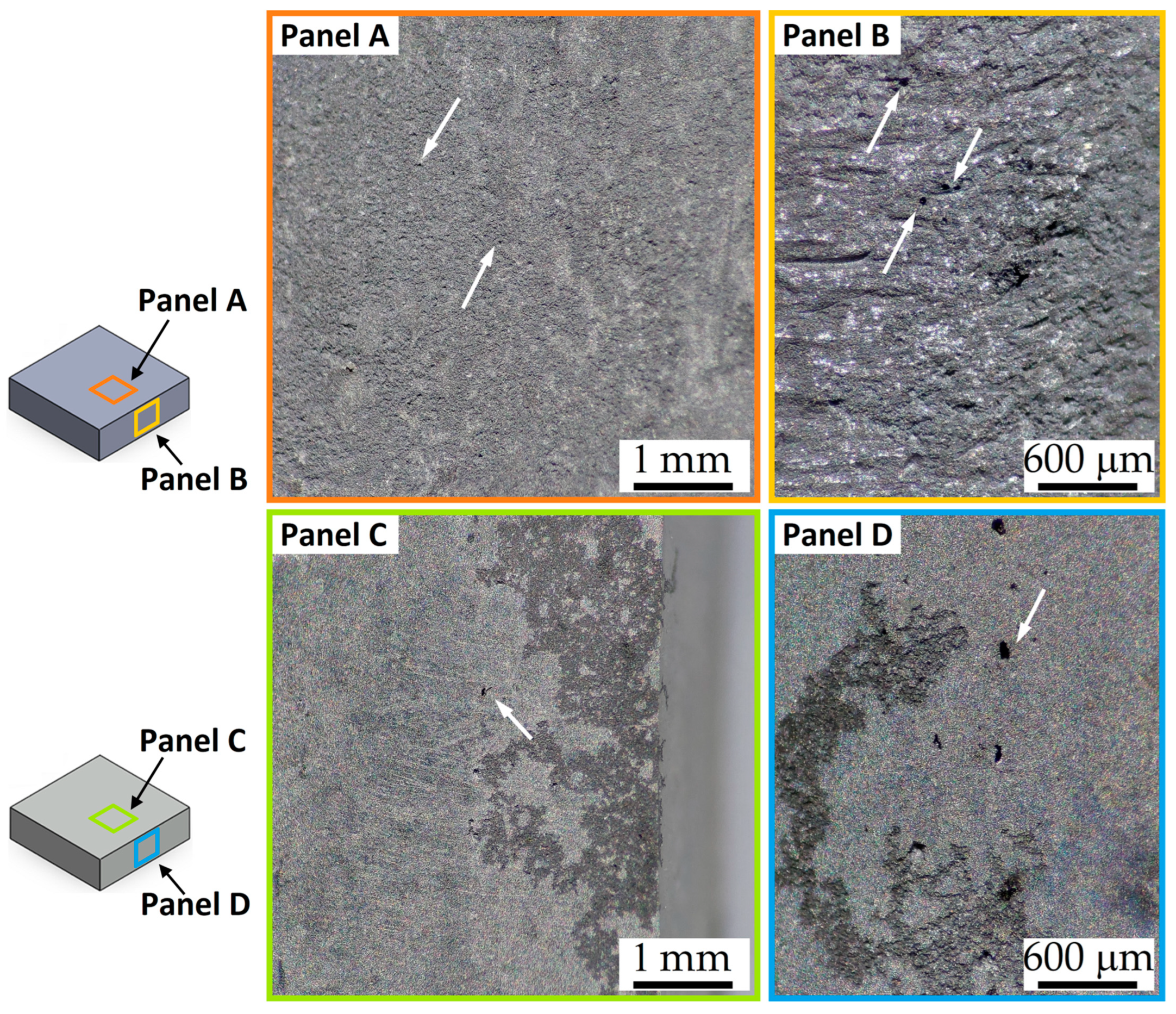
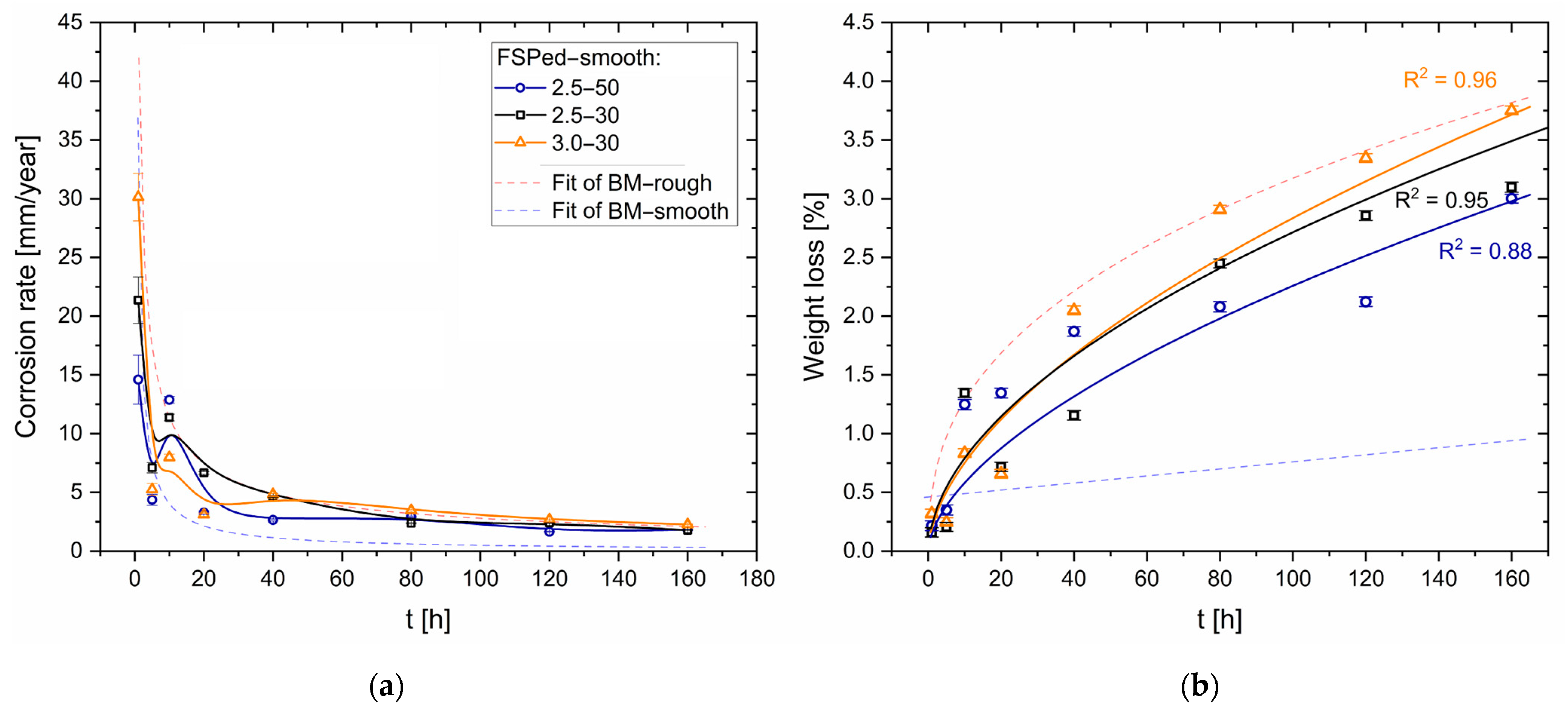
- The corrosion rate of the HPDC AZ91 alloy decreases in samples with lower surface roughness. The smooth samples (Sa = ~0.07 μm) are indeed characterized by slower corrosion rates (<1 mm/year) than rougher samples (Sa = ~0.50 μm), which indeed show a higher number of pits.
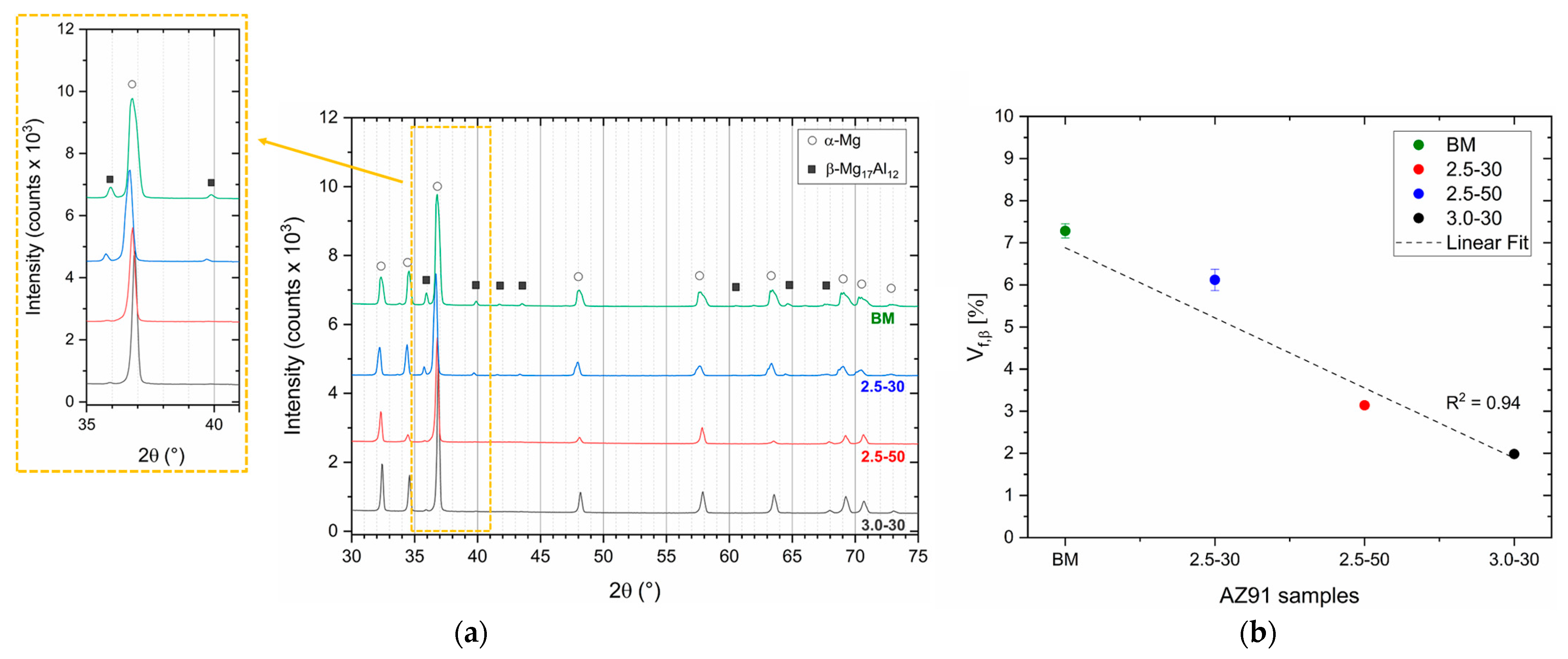
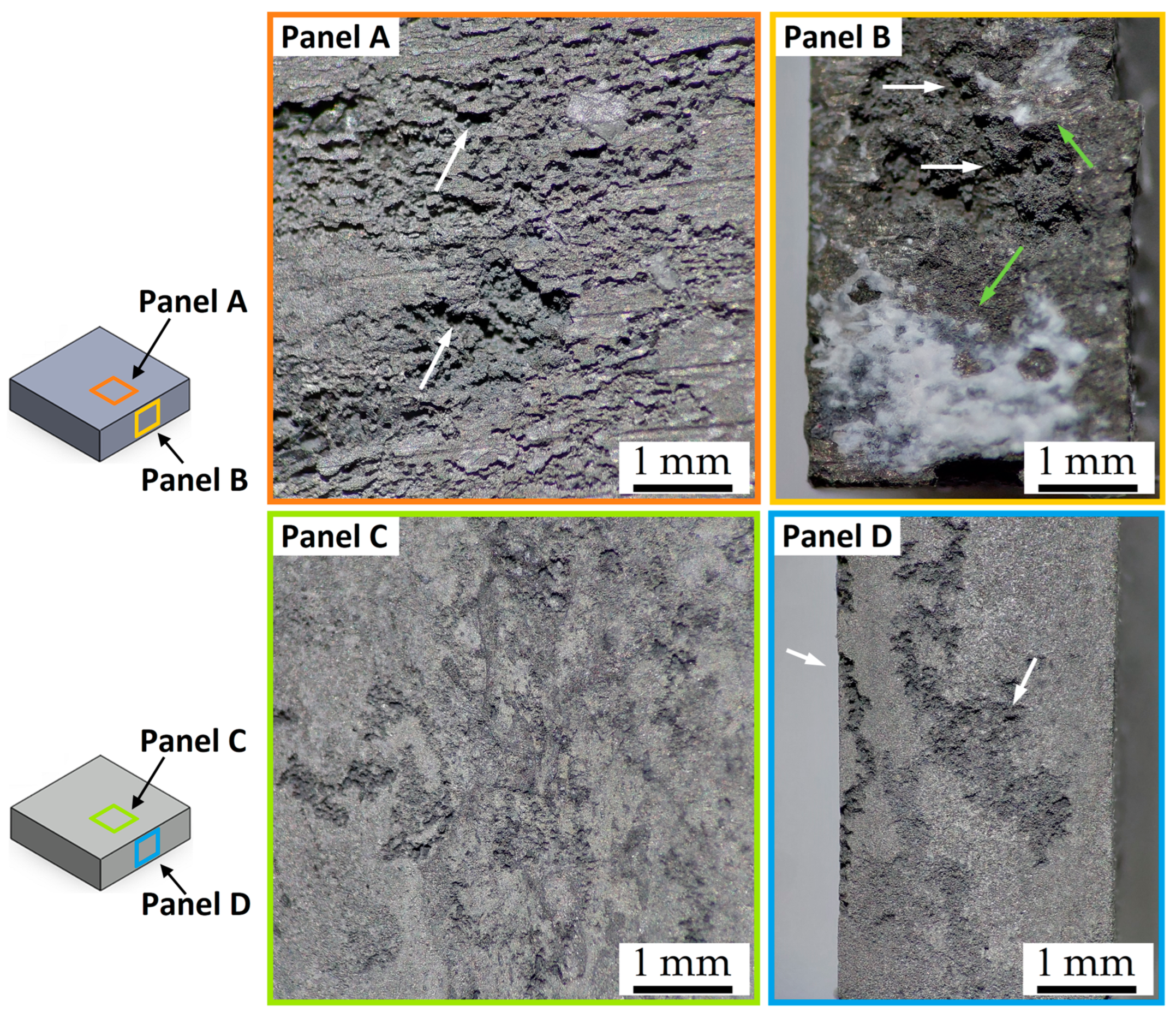
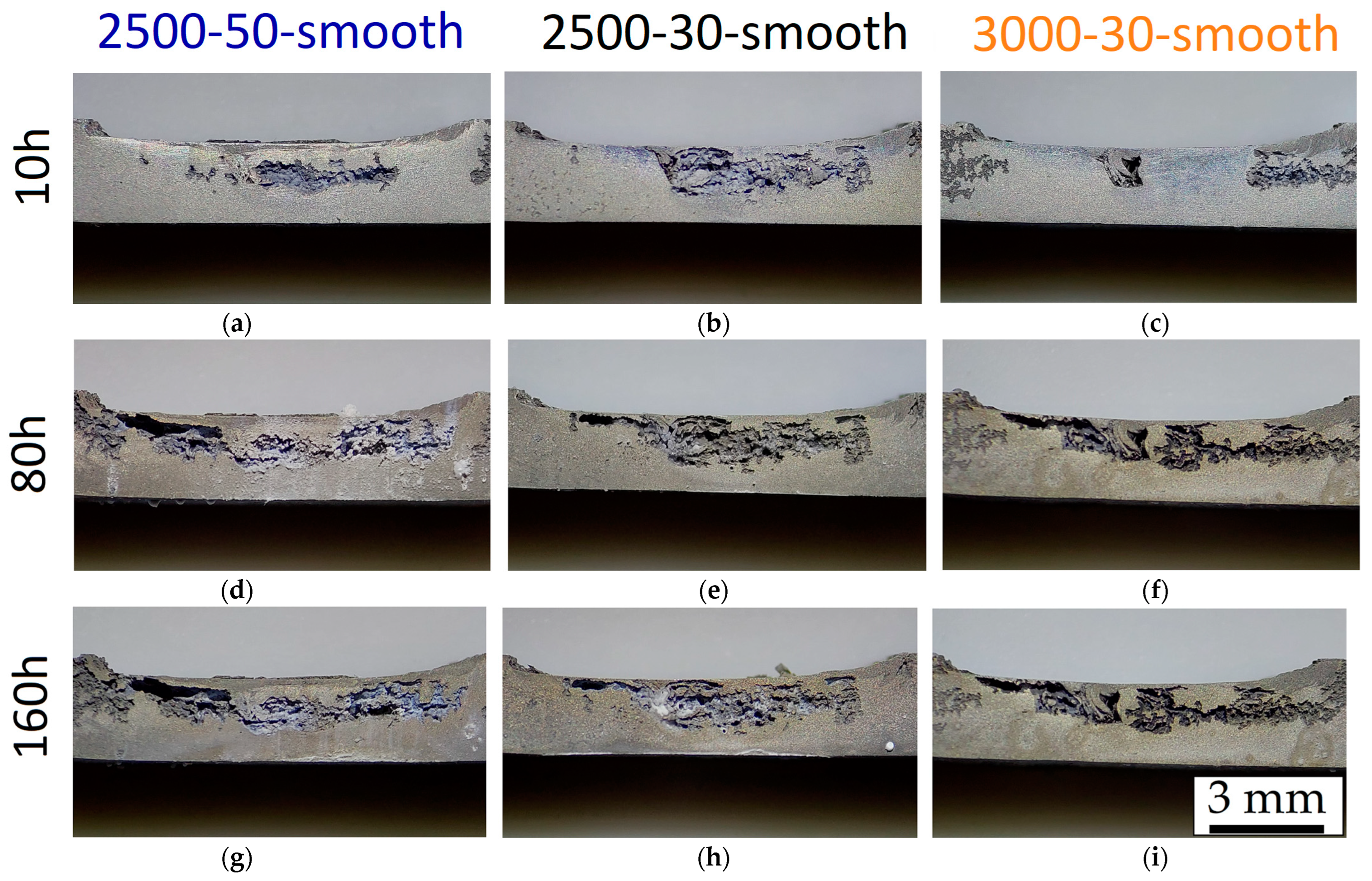
- FSP improves the corrosion resistance of HPDC samples due to the fracture and dissolution of β-Mg17Al12 eutectic and the resulting increase in wt.% Al in the α-Mg matrix. These phenomena play a predominant role in corrosion behaviour with respect to the grain size. On the other hand, FSPed-smooth samples show better corrosion resistance than the FSPed-rough thanks to the greatest stability of the passive layer (t < 10 h). After 10 h of immersion time, the corrosion attack becomes strongly localized in the SZ regions (t > 10 h) because a macro-galvanic couple between the passive layer and the SZ may be formed.
- The HPDC samples friction stir processed at 2500 rpm and 50 mm/min show better corrosion resistance than the other conditions.
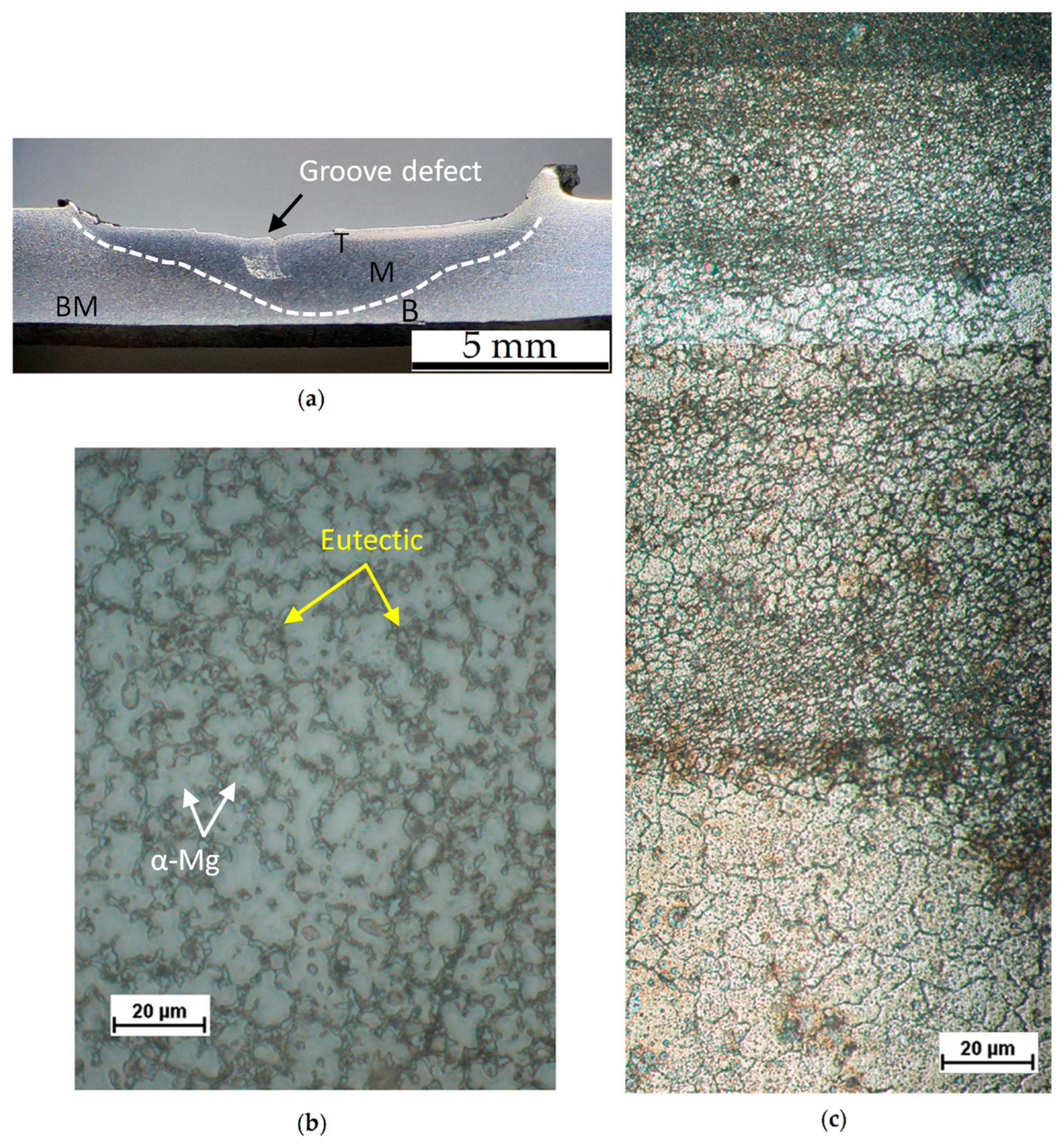
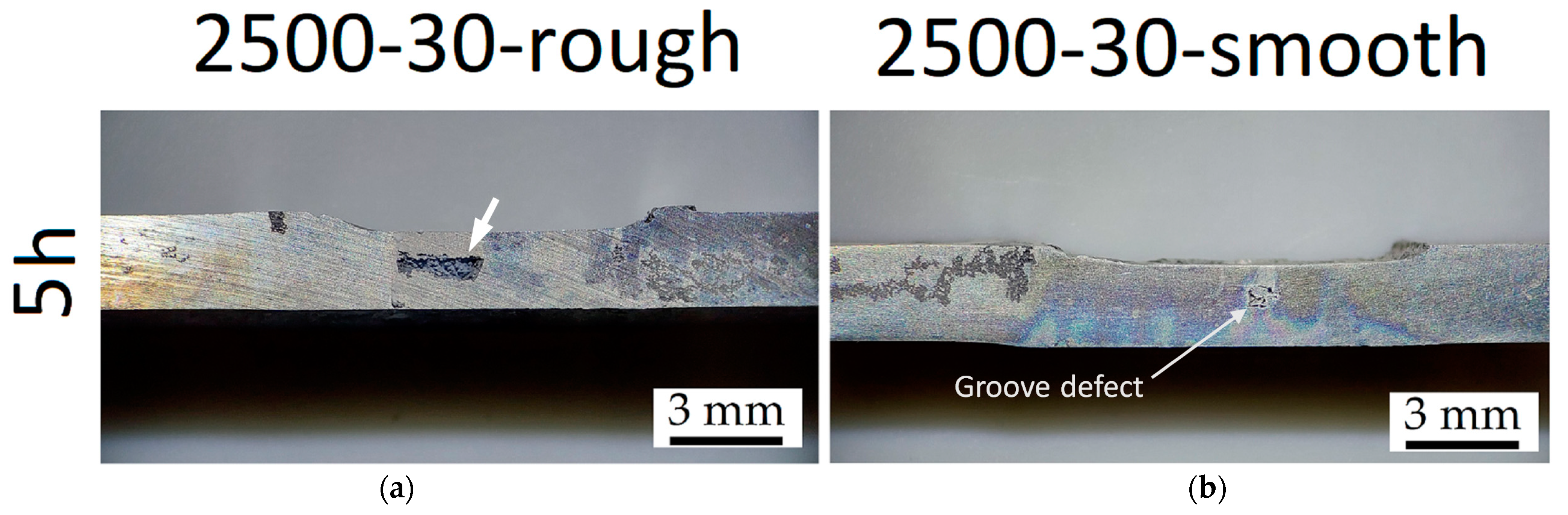
- The corrosion phenomena are not influenced by the groove defects, but the typical onion ring structure seems to promote a localized corrosion attack in the nugget zone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BM | Base Material |
| CR | Corrosion Rate |
| HPDC | High-Pressure Die Cast |
| FSP | Friction Stir Process |
| SZ | Stirred Zone |
| TMAZ | Thermomechanically Affected Zone |
| XRD | X-ray Diffraction |
References
- Mordike, B.L.; Ebert, T. Magnesium. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Dong, J.; Lin, T.; Shao, H.; Wang, H.; Wang, X.; Song, K.; Li, Q. Advances in Degradation Behavior of Biomedical Magnesium Alloys: A Review. J. Alloys Compd. 2022, 908, 164600. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kund, N.K.; Mohapatra, P. Experimental Investigations on Corrosion Performance of Heat-Treated AZ91 Mg Alloy. Mater. Today Proc. 2023, 74, 1012–1016. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; del Valle, J.A.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion Behaviour of AZ31 Magnesium Alloy with Different Grain Sizes in Simulated Biological Fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of Microstructural Effects on Corrosion Behaviour of AZ91D Magnesium Alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- Merino, M.C.; Pardo, A.; Arrabal, R.; Merino, S.; Casajús, P.; Mohedano, M. Influence of Chloride Ion Concentration and Temperature on the Corrosion of Mg–Al Alloys in Salt Fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Grgur, B.N.; Jugović, B.Z.; Gvozdenović, M.M. Influence of Chloride Ion Concentration on Initial Corrosion of AZ63 Magnesium Alloy. Trans. Nonferrous Met. Soc. China 2022, 32, 1133–1143. [Google Scholar] [CrossRef]
- Warner, T.J.; Thorne, N.A.; Nussbaum, G.; Stobbs, W.M. A Cross-Sectional TEM Study of Corrosion Initiation in Rapidly Solidified Mg-Based Ribbons. Surf. Interface Anal. 1992, 19, 386–392. [Google Scholar] [CrossRef]
- Froes, F.H.; Kim, Y.-W.; Hehmann, F. Rapid Solidification of Al, Mg and Ti. JOM 1987, 39, 14–21. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Liu, J.; Xu, J.; Guo, D.; Zhang, H.; Zhou, X.; Yang, P.; Zhang, S. Comparison of Corrosion Behavior of WE43 and AZ80 Alloys in NaCl and Na2SO4 Solutions. Crystals 2023, 13, 506. [Google Scholar] [CrossRef]
- Singh, I.B.; Singh, M.; Das, S. A Comparative Corrosion Behavior of Mg, AZ31 and AZ91 Alloys in 3.5% NaCl Solution. J. Magnes. Alloys 2015, 3, 142–148. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion Behaviour of Magnesium/Aluminium Alloys in 3.5wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.; Atrens, A. Influence of the β-Phase Morphology on the Corrosion of the Mg Alloy AZ91. Corros. Sci. 2008, 50, 1939–1953. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Dietzel, W.; Zettler, R. In Vitro Degradation Behaviour of a Friction Stir Processed Magnesium Alloy. J. Mater. Sci. Mater. Med. 2011, 22, 2397–2401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, Q.; Chen, G.; Cao, X.; Zhang, S.; Pan, J.; Zhang, G.; Shi, Q. Enhanced Corrosion Resistance of AZ91 Magnesium Alloy through Refinement and Homogenization of Surface Microstructure by Friction Stir Processing. Corros. Sci. 2018, 138, 284–296. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Yang, S. De-Alloying Behavior of Mg–Al Alloy in Sulphuric Acid and Acetic Acid Aqueous Solutions. Materials 2019, 12, 2046. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005–075013. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain Size Distribution Effects on the Corrosion Behaviour of Materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Hamu, G.B.; Eliezer, D.; Wagner, L. The Relation between Severe Plastic Deformation Microstructure and Corrosion Behavior of AZ31 Magnesium Alloy. J. Alloys Compd. 2009, 468, 222–229. [Google Scholar] [CrossRef]
- Wang, H.; Estrin, Y.; Fu, H.; Song, G.; Zúberová, Z. The Effect of Pre-Processing and Grain Structure on the Bio-Corrosion and Fatigue Resistance of Magnesium Alloy AZ31. Adv. Eng. Mater. 2007, 9, 967–972. [Google Scholar] [CrossRef]
- Cerri, E.; Knez, D.; Rimoldi, T.; Di Giovanni, M.T. Microstructure Evolution and Mechanical Properties of Hot Deformed Mg9Al1Zn Samples Containing a Friction Stir Processed Zone. J. Magnes. Alloys 2017, 5, 388–403. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Wu, R.; Hou, L.; Zhang, M. Recent Developments in High-Strength Mg-RE-Based Alloys: Focusing on Mg-Gd and Mg-Y Systems. J. Magnes. Alloys 2018, 6, 277–291. [Google Scholar] [CrossRef]
- Hütter, A.; Huemer, W.; Ramskogler, C.; Warchomicka, F.; Lachehab, A.; Vallant, R.; Sommitsch, C. Surface Modification of Pure Magnesium and Magnesium Alloy AZ91 by Friction Stir Processing. Key Eng. Mater. 2015, 651–653, 796–801. [Google Scholar] [CrossRef]
- Mehdipour, M.; Jamshidi Aval, H. Effect of Friction Stir Back Extrusion Rotational Speed on Microstructure, Mechanical Properties, and Corrosion Behaviour of AZ91-Ca Alloy. J. Mater. Res. Technol. 2023, 25, 4441–4456. [Google Scholar] [CrossRef]
- Saikrishna, N.; Pradeep Kumar Reddy, G.; Munirathinam, B.; Ratna Sunil, B. Influence of Bimodal Grain Size Distribution on the Corrosion Behavior of Friction Stir Processed Biodegradable AZ31 Magnesium Alloy. J. Magnes. Alloys 2016, 4, 68–76. [Google Scholar] [CrossRef]
- Singh Sidhu, H.; Singh, B.; Kumar, P. To Study the Corrosion Behavior of Friction Stir Processed Magnesium Alloy AZ91. Mater. Today Proc. 2021, 44, 4633–4639. [Google Scholar] [CrossRef]
- Hasani, B.M.; Hedaiatmofidi, H.; Zarebidaki, A. Effect of Friction Stir Process on the Microstructure and Corrosion Behavior of AZ91 Mg Alloy. Mater. Chem. Phys. 2021, 267, 124672. [Google Scholar] [CrossRef]
- Patle, H.; Sunil, B.R.; Dumpala, R. Machining Characteristics, Wear and Corrosion Behavior of AZ91 Magnesium Alloy—Fly Ash Composites Produced by Friction Stir Processing. Materwiss Werksttech 2021, 52, 88–99. [Google Scholar] [CrossRef]
- Dani, M.S.; Dave, I.B.; Parmar, B. Corrosion Behavior of Die-Cast and Friction Stir-Processed AZ91 Magnesium Alloys in 5% NaCl. J. Inst. Eng. (India) Ser. D 2019, 100, 21–27. [Google Scholar] [CrossRef]
- Seifiyan, H.; Heydarzadeh Sohi, M.; Ansari, M.; Ahmadkhaniha, D.; Saremi, M. Influence of Friction Stir Processing Conditions on Corrosion Behavior of AZ31B Magnesium Alloy. J. Magnes. Alloys 2019, 7, 605–616. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent Developments in High-Pressure Die-Cast Magnesium Alloys for Automotive and Future Applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tabary, S.A.A.B.; Rahmatabadi, D.; Ebrahimi, M.S.; Abrinia, K.; Hashemi, R. Review of Selective Laser Melting of Magnesium Alloys: Advantages, Microstructure and Mechanical Characterizations, Defects, Challenges, and Applications. J. Mater. Res. Technol. 2022, 19, 1537–1562. [Google Scholar] [CrossRef]
- Hamza, H.M.; Deen, K.M.; Haider, W. Microstructural Examination and Corrosion Behavior of Selective Laser Melted and Conventionally Manufactured Ti6Al4V for Dental Applications. Mater. Sci. Eng. C 2020, 113, 110980. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. Influence of Surface Roughness on the Corrosion Behaviour of Magnesium Alloy. Mater. Des. 2011, 32, 2350–2354. [Google Scholar] [CrossRef]
- Cabeza, S.; Pérez Zubiaur, P.; Garcés, G.; Andrade, C.; Adeva, P. Corrosion Behaviour of Mg98.5Nd1Zn0.5 (at. %) Alloy in Phosphate Buffered Saline Solution. Metals 2020, 10, 148. [Google Scholar] [CrossRef]
- ASTM G1:03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2017.
- Nwaogu, U.C.; Blawert, C.; Scharnagl, N.; Dietzel, W.; Kainer, K.U. Effects of Organic Acid Pickling on the Corrosion Resistance of Magnesium Alloy AZ31 Sheet. Corros. Sci. 2010, 52, 2143–2154. [Google Scholar] [CrossRef]
- Agha Amini Fashami, H.; Bani Mostafa Arab, N.; Hoseinpour Gollo, M.; Nami, B. Numerical and Experimental Investigation of Defects Formation during Friction Stir Processing on AZ91. SN Appl. Sci. 2021, 3, 108. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N. Discontinuous and Continuous Precipitation in Magnesium–Aluminium Type Alloys. J. Alloys Compd. 2009, 477, 870–876. [Google Scholar] [CrossRef]
- Duly, D.; Simon, J.P.; Brechet, Y. On the Competition between Continuous and Discontinuous Precipitations in Binary Mg-Al Alloys. Acta Metall. Mater. 1995, 43, 101–106. [Google Scholar] [CrossRef]
- Cerri, E.; Ghio, E. Effect of Friction Stir Processing at High Rotational Speed on Aging of a HPDC Mg9Al1Zn. Metals 2020, 10, 1014. [Google Scholar] [CrossRef]
- Cerri, E.; Renna, G.; Cabibbo, M.; Simoncini, M.; Forcellese, A. Friction Stir Processing at High Rotation Rates of a Magnesium Alloy: Mechanical Properties at High Temperatures and Microstructure. Mater. Sci. Forum 2016, 879, 295–300. [Google Scholar] [CrossRef]
- Mishra, R.S.; Ma, Z.Y. Friction Stir Welding and Processing. Mater. Sci. Eng. R Rep. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Marodkar, A.S.; Patil, H.; Chavhan, J.; Borkar, H. Effect of Gravity Die Casting, Squeeze Casting and Extrusion on Microstructure, Mechanical Properties and Corrosion Behaviour of AZ91 Magnesium Alloy. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yousefpour, F.; Jamaati, R.; Aval, H.J. Effect of Traverse and Rotational Speeds on Microstructure, Texture, and Mechanical Properties of Friction Stir Processed AZ91 Alloy. Mater. Charact. 2021, 178, 111235. [Google Scholar] [CrossRef]
- Asadi, P.; Besharati Givi, M.K.; Faraji, G. Producing Ultrafine-Grained AZ91 from As-Cast AZ91 by FSP. Mater. Manuf. Process. 2010, 25, 1219–1226. [Google Scholar] [CrossRef]
- Mitchell, J.; Crow, N.; Nieto, A. Effect of Surface Roughness on Pitting Corrosion of AZ31 Mg Alloy. Metals 2020, 10, 651. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.R.; Hwang, D.Y.; Lee, D.H.; Shin, D.H. Effect of Surface Roughness on Leakage Current and Corrosion Resistance of Oxide Layer on AZ91 Mg Alloy Prepared by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]
- Kim, W.-C.; Han, K.-H.; Kim, J.-G.; Yang, S.-J.; Seok, H.-K.; Han, H.-S.; Kim, Y.-Y. Effect of Surface Area on Corrosion Properties of Magnesium for Biomaterials. Met. Mater. Int. 2013, 19, 1131–1137. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Gupta, R.K.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Ghandvar, H. Investigation of Corrosion Protection Performance of Multiphase PEO (Mg2SiO4, MgO, MgAl2O4) Coatings on Mg Alloy Formed in Aluminate-Silicate- Based Mixture Electrolyte. Prot. Met. Phys. Chem. Surf. 2018, 54, 425–441. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, G.; Atrens, A.; Ding, W. Influence of Trace as Content on the Microstructure and Corrosion Behavior of the AZ91 Alloy in Different Metallurgical Conditions. J. Magnes. Alloys 2020, 8, 301–317. [Google Scholar] [CrossRef]
- Han, L.; Li, X.; Bai, J.; Xue, F.; Zheng, Y.; Chu, C. Effects of Flow Velocity and Different Corrosion Media on the in Vitro Bio-Corrosion Behaviors of AZ31 Magnesium Alloy. Mater. Chem. Phys. 2018, 217, 300–307. [Google Scholar] [CrossRef]
- Cerri, E.; Leo, P. Effect of Friction Stir Processing on Microstructure and Mechanical Properties of a HPDC Magnesium Alloy. Mater. Sci. Forum 2014, 783–786, 1735–1740. [Google Scholar] [CrossRef]
- Zeng, R.; Kainer, K.U.; Blawert, C.; Dietzel, W. Corrosion of an Extruded Magnesium Alloy ZK60 Component—The Role of Microstructural Features. J. Alloys Compd. 2011, 509, 4462–4469. [Google Scholar] [CrossRef]
- Iwaszko, J.; Strzelecka, M. Microstructure and Corrosion Resistance of AZ91 Magnesium Alloy after Surface Remelting Treatment. Materials 2022, 15, 8980. [Google Scholar] [CrossRef]
- Cerri, E.; Cabibbo, M.; Leo, P. Nanoindentation and Microstructure of Friction Stir Processed Die Cast Mg-Al-Zn Alloy. La Metall. Ital. 2014, 3–10. [Google Scholar]


| Mg | Al | Zn | Mn | Si | Cu | Ni | Fe | Be |
|---|---|---|---|---|---|---|---|---|
| Bal. | 8.90 | 0.79 | 0.28 | 0.0093 | 0.0013 | 0.001 | 0.0017 | 3.6 ppm |
| Designation | [rpm]-[mm/min] | w/v [r/mm] | Temperature [°C] |
|---|---|---|---|
| 2.5-50 | 2500-50 | 50 | 198 |
| 2.5-30 | 2500-30 | 83.3 | 220 |
| 3.0-30 | 3000-30 | 100 | 265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghio, E.; Cerri, E. Corrosion Behaviour of High-Pressure Die-Cast AZ91 Alloy in NaCl Solution: Effects of Friction Stir Process at High Rotational Speed. Materials 2023, 16, 6620. https://doi.org/10.3390/ma16206620
Ghio E, Cerri E. Corrosion Behaviour of High-Pressure Die-Cast AZ91 Alloy in NaCl Solution: Effects of Friction Stir Process at High Rotational Speed. Materials. 2023; 16(20):6620. https://doi.org/10.3390/ma16206620
Chicago/Turabian StyleGhio, Emanuele, and Emanuela Cerri. 2023. "Corrosion Behaviour of High-Pressure Die-Cast AZ91 Alloy in NaCl Solution: Effects of Friction Stir Process at High Rotational Speed" Materials 16, no. 20: 6620. https://doi.org/10.3390/ma16206620
APA StyleGhio, E., & Cerri, E. (2023). Corrosion Behaviour of High-Pressure Die-Cast AZ91 Alloy in NaCl Solution: Effects of Friction Stir Process at High Rotational Speed. Materials, 16(20), 6620. https://doi.org/10.3390/ma16206620







