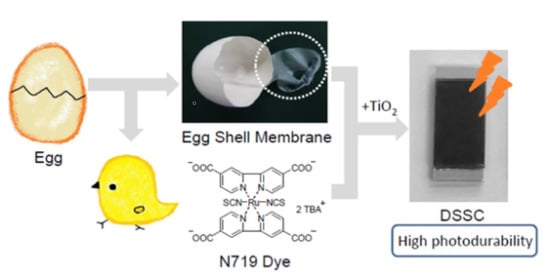
Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes
Abstract
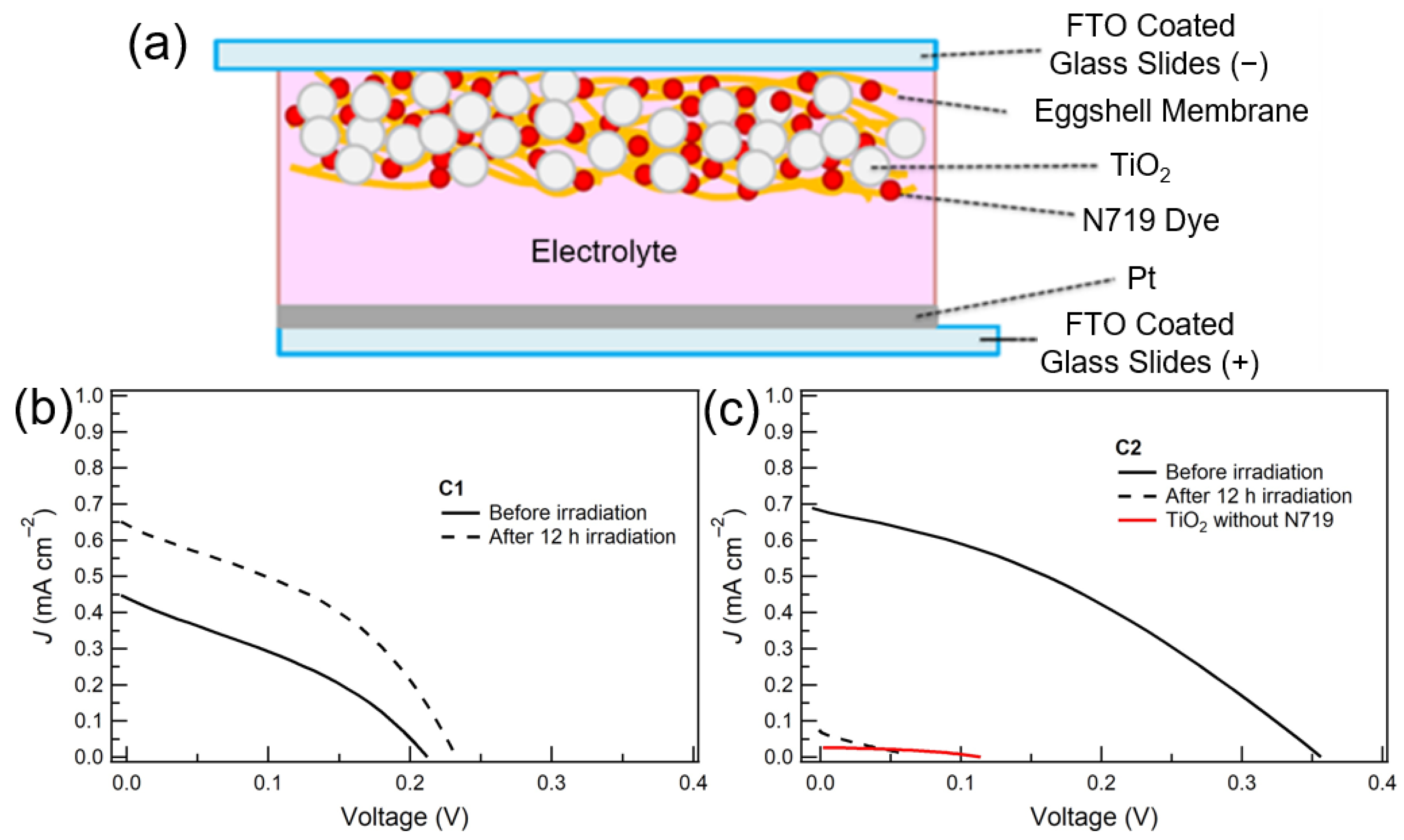
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
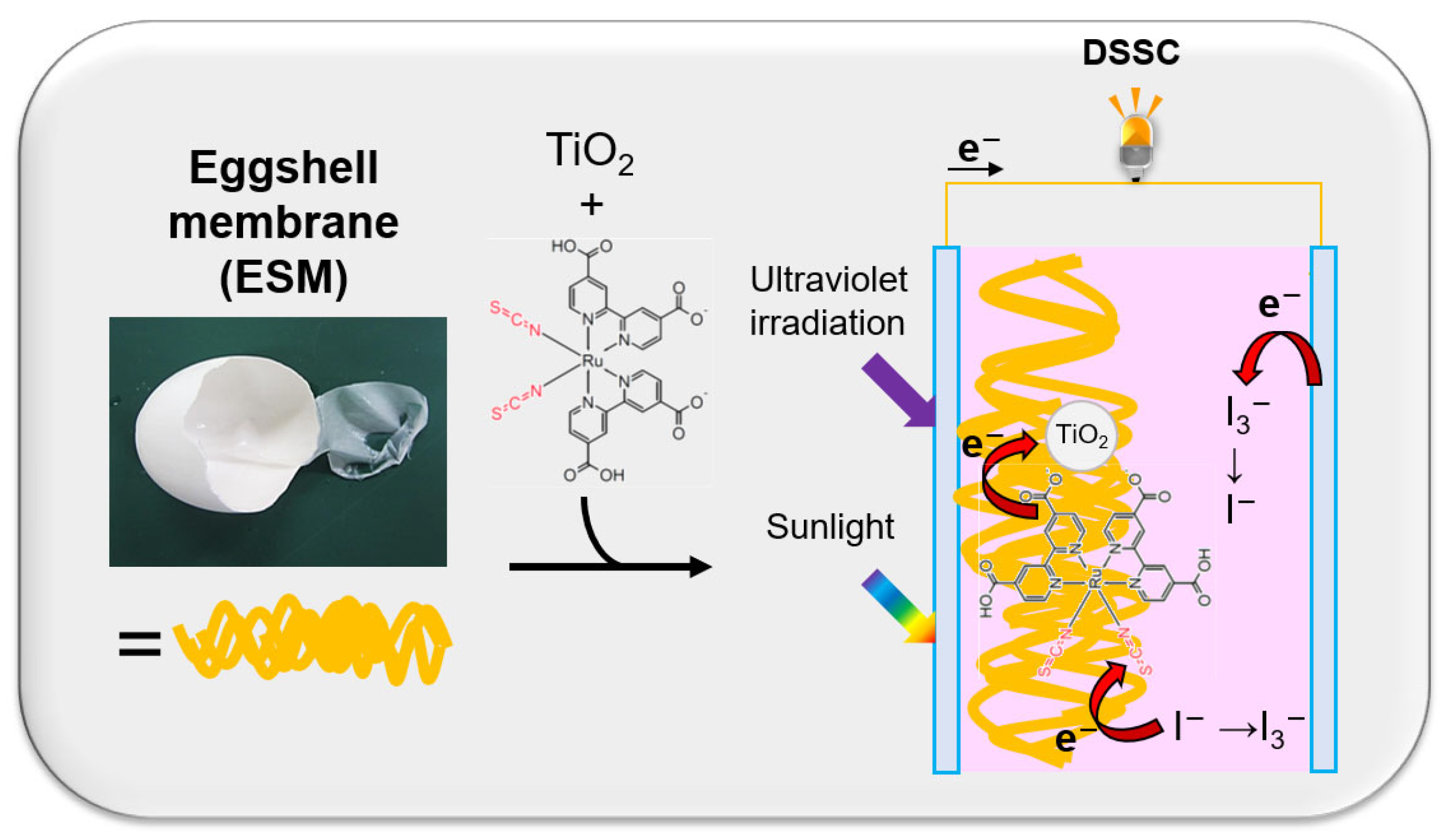
2.2.1. EMS
2.2.2. N719-Adsorbed ESM-TiO2 Composite
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM) with Energy Dispersion X-ray Spectroscopy (EDX)
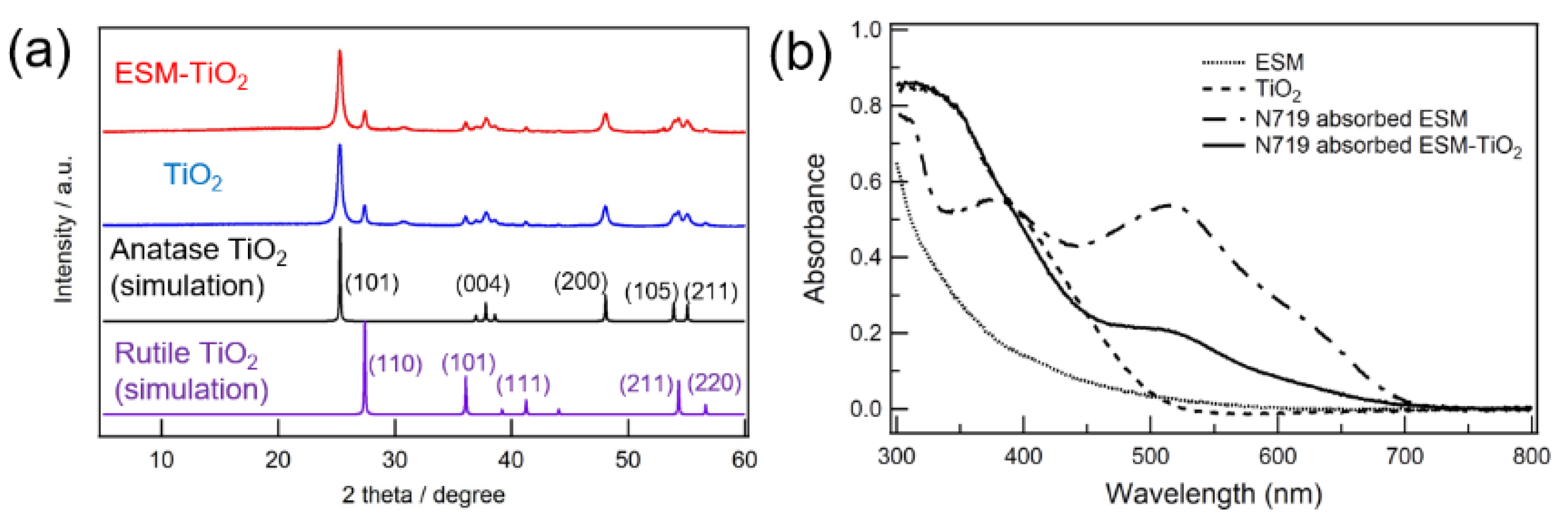
2.3.2. X-ray Diffraction (XRD)
2.3.3. UV-Vis Spectroscopy
2.4. Fabrication of DSSCs
2.5. The Photovoltaic Performance Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 354, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research progress on photosensitizers for DSSC. Front Chem. 2018, 6, 481. [Google Scholar] [CrossRef]
- Song, C.E.; Ryu, K.Y.; Hong, S.J.; Bathula, C.; Lee, S.K.; Shin, W.S.; Lee, J.C.; Choi, S.K.; Kim, J.H.; Moon, S.J. Enhanced Performance in Inverted Polymer Solar Cells with D-π-A-Type Molecular Dye Incorporated on ZnO Buffer Layer. ChemSusChem 2013, 6, 1445–1454. [Google Scholar] [CrossRef]
- Khadtare, S.; Bansode, A.S.; Mathe, V.L.; Shrestha, N.K.; Bathula, C.; Han, S.H.; Pathan, H.M. Effect of Oxygen Plasma Treatment on Performance of ZnO Based Dye Sensitized Solar Cells. J. Alloys Compd. 2017, 724, 348–352. [Google Scholar] [CrossRef]
- Khadtare, S.; Ansari, A.S.; Pathan, H.M.; Han, S.H.; Mahadevan, K.M.; Mane, S.D.; Bathula, C. Silver Nanoparticles Loaded ZnO Photoelectrode with Rose Bengal as a Sensitizer for Dye Sensitized Solar Cells. Inorg. Chem. Commun. 2019, 104, 155–159. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Andersen, R.A.; Skou, E.M.; Lund, T. Dye stability and performances of dye-sensitized solar cells with different nitrogen additives at elevated temperatures can sterically hindered pyridines prevent dye degradation? Sol. Energy Mater. Sol. Cells 2010, 94, 1582–1590. [Google Scholar] [CrossRef]
- Kashif, M.K.; Nippe, M.; Duffy, N.W.; Forsyth, C.M.; Chang, C.J.; Long, J.R.; Spiccia, L.; Bach, U. Stable dye-sensitized solar cell electrolytes based on cobalt(ii)/(iii) complexes of a hexadentate pyridyl ligand. Angew. Chem. Int. Ed. 2013, 52, 5527–5531. [Google Scholar] [CrossRef]
- Mori, S.; Fukuda, S.; Sumikura, S.; Takeda, Y.; Tamaki, Y.; Suzuki, E.; Abe, T. Charge-transfer processes in dye-sensitized NiO solar cells. J. Phys. Chem. C 2008, 112, 16134–16139. [Google Scholar] [CrossRef]
- Fan, J.; Liu, S.; Yu, J. Enhanced photovoltaic performance of dye-sensitized solar cells based on TiO2 nanosheets/graphene composite films. J. Mater. Chem. 2012, 22, 17027–17036. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, Y.M.; Sarker, S.; Lee, J.J.; Lee, Y.; Lee, K.; Oh, J.J.; Joo, S.W. Temperature-dependent infrared spectrum of (Bu4N)2[Ru(dcbpyH)2-(NCS)2] on nanocrystalline TiO2 surfaces. Sol. Energy Mater. Sol. Cells 2010, 94, 857–864. [Google Scholar]
- Filippo, D.A.; Simona, F.; Edoardo, M.; Mohammad, K.N.; Michael, G. Absorption Spectra and Excited State Energy Levels of the N719 Dye on TiO2 in Dye-Sensitized Solar Cell Models. J. Phys. Chem. C 2011, 115, 8825–8831. [Google Scholar]
- Johansson, E.M.J.; Hedlund, M.; Siegbahn, H.; Rensmo, H. Electronic and molecular surface structure of Ru(tcterpy)(NCS)3 and Ru(dcbpy)2(NCS)2 adsorbed from solution onto nanostructured TiO2: A photoelectron spectroscopy study. J. Phys. Chem. B 2005, 109, 22256–22263. [Google Scholar] [CrossRef]
- Yusuke, O.; Keitaro, S.; Liyuan, H.; Yoshitaka, T. Possibility of NCS Group Anchor for Ru Dye Adsorption to Anatase TiO2(101) Surface: A Density Functional Theory Investigation. J. Phys. Chem. C 2015, 119, 234–241. [Google Scholar]
- Junghänel, M.; Tributsch, H. Role of nanochemical environments in porous TiO2 in photocurrent efficiency and degradation in dye sensitized solar cells. J. Phys. Chem. B 2005, 109, 22876–22883. [Google Scholar] [CrossRef]
- Nour-Mohhamadi, F.; Nguyen, S.D.; Boschloo, G.; Hagfeldt, A.; Lund, T. Determination of the light-induced degradation rate of the solar cell sensitizer N719 on TiO2 nanocrystalline particles. J. Phys. Chem. B 2005, 109, 22413–22419. [Google Scholar] [CrossRef]
- Agrell, H.G.; Lindgren, J.; Hagfeldt, A. Degradation mechanisms in a dye-sensitized solar cell studied by UV-VIS and IR spectroscopy. Sol. Energy 2003, 75, 169–180. [Google Scholar] [CrossRef]
- Yoshida, C.; Nakajima, S.; Shoji, Y.; Itoh, E.; Momiyama, K.; Kanomata, K.; Hirose, F. In Situ Observation of Structural Change in N719 Dye Molecule in Dye Sensitized Solar Cells with a Light Exposure and a Heat Treatment. J. Electrochem. Soc. 2012, 159, H881–H884. [Google Scholar] [CrossRef]
- Tanifuji, N.; Shimizu, T.; Yoshikawa, H.; Tanaka, M.; Nishio, K.; Ida, K.; Shimizu, A.; Hasebe, Y. Assessment of Eggshell Membrane as a New Type of Proton-Conductive Membrane in Fuel Cells. ACS Oemga 2022, 7, 12637–12642. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Koumanova, B. Decolourisation of Water/Wastewater Using Adsorption (Review). J. Univ. Chem. Technol. Metall. 2005, 40, 175–192. [Google Scholar]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Zhang, W. Flower-like hydroxyapatite modified carbon paste electrodes applicable for highly sensitive detection of heavy metal ions. J. Mater. Chem. 2011, 21, 7552–7554. [Google Scholar] [CrossRef]
- Su, H.; Dong, Q.; Han, J.; Zhang, D.; Guo, Q. Biogenic synthesis and photocatalysis of Pd-PdO nanoclusters reinforced hierarchical TiO2 films with interwoven and tubular conformations. Biomacromolecules 2008, 9, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sabu, U.; Tripathi, N.; Logesh, G.; Rashad, M.; Joy, A.; Balasubramanian, M. Development of Biomorphic C-ZnO with in Situ Formation of ZnS Using Eggshell Membrane as Bio-Template. Ceram. Int. 2020, 46, 22869–22875. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Ab Aziz, M.A. Spherical CeO2 Nanoparticles Prepared Using an Egg-Shell Membrane as a Bio-Template for High CO2 Adsorption. Chem. Phys. Lett. 2021, 779, 138842. [Google Scholar] [CrossRef]
- Garay-Rodríguez, L.F.; Luévano-Hipólito, E.; Torres-Martínez, L.M. Renewable formic acid production from CO2 reduction using green ZnO nanoarchitectures. Mater. Sci. Semicond. Process. 2023, 161, 107458. [Google Scholar] [CrossRef]
- Salman, D.D.; Ulaiwi, W.S.; Tariq, N.M. Determination the optimal conditions of Methylene blue adsorption by the chicken egg shell membrane. Int. J. Poult. Sci. 2012, 11, 391–396. [Google Scholar] [CrossRef]
- Jalu, R.G.; Chamada, T.A.; Kasirajan, D.R. Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution. Environ. Chall. 2021, 4, 100193. [Google Scholar] [CrossRef]
- Pramanpol, N.; Nitayapat, N. Adsorption of reactive dye by eggshell and its membrane. Nat. Sci. 2006, 40, 192–197. [Google Scholar]
- Arami, M.; Yousefi Limaee, N.; Mahmoodi, N.M. Investigation on the adsorption capability of egg shell membrane towards model textile dyes. Chemosphere 2006, 65, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Nugroho, A.; Floresyona, D.; Lau, S.K.; Manap, A.; Chia, H.C.; Afandi, N. Bio and non-bio materials-based quasi-solid state electrolytes in DSSC: A review. Int. J. Energy Res. 2022, 46, 5399–5422. [Google Scholar] [CrossRef]
- Perera, A.S.; Subbaiyan, N.K.; Kalita, M.; Wendel, S.O.; Samarakoon, T.N.; D’Souza, F.; Bossmann, S.H. A hybrid 254 soft solar cell based on the mycobacterial porin MspA linked to a sensitizer-viologen diad. J. Am. Chem. Soc. 2013, 255, 6842–6845. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.S.; Chen, C.Y.; Lin, S.H.; Lu, W.H.; Wu, P. Preparation of TiO2/bamboo-charcoal-powder composite particles and their applications in dye-sensitized solar cells. Adv. Powder Technol. 2015, 26, 711–717. [Google Scholar] [CrossRef]
- Chawla, P.; Srivastava, A.; Tripathi, M. Performance of chitosan based polymer electrolyte for natural dye sensitized solar cell. Environ. Prog. Sustain. Energy 2019, 38, 630–634. [Google Scholar] [CrossRef]
- Koli, P. Characterization, stability, and feasibility of long-term use of light-absorbing components of aqueous spinach extract-based photogalvanic electrolyte. Sci. Rep. 2022, 12, 13518. [Google Scholar] [CrossRef] [PubMed]
- Kinsinger, N.M.; Wong, A.; Li, D.; Villalobos, F.; Kisailus, D. Nucleation and crystal growth of nanocrystalline anatase and rutile phase TiO2 from a water-soluble precursor. Cryst. Growth Des. 2010, 10, 5254–5261. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadizadeh, M.R. Superhydrophilicity and photocatalytic activity of sol-gel deposited nanosized titania thin films. Thin Solid Films 2011, 519, 6432–6437. [Google Scholar] [CrossRef]
- Chena, L.; Pang, X.; Yu, G.; Zhang, J. In-situ coating of MWNTs with sol-gel TiO2 nanoparticles. Adv. Mat. Lett. 2010, 1, 75–78. [Google Scholar] [CrossRef]
- Gao, M.; Shen, Z.; Liu, X.; Yue, G.; Huo, J.; Dong, C.; Tan, F. Tungsten Phosphide Microsheets In-Situ Grown on Carbon Fiber as Counter Electrode Catalyst for Efficient Dye-Sensitized Solar Cells. Adv. Mater. Interfaces 2023, 10, 2201494. [Google Scholar] [CrossRef]
- Saritha, F.; Aiswarya, N.; Aswath Kumar, R.; Dileep, K.V. Structural analysis and ensemble docking revealed the binding modes of selected progesterone receptor modulators. J. Biomol. Struct. Dyn. 2023, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.S.; Bergfors, T.; Jones, T.A.; Högbom, M. Structural mechanics of the pH-dependent activity of β-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Crombie, G.; Snider, R.; Faris, B.; Franzblau, C. Lysine-Drived Cross-Links in The Egg Shell Membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 1981, 640, 365–367. [Google Scholar] [CrossRef]

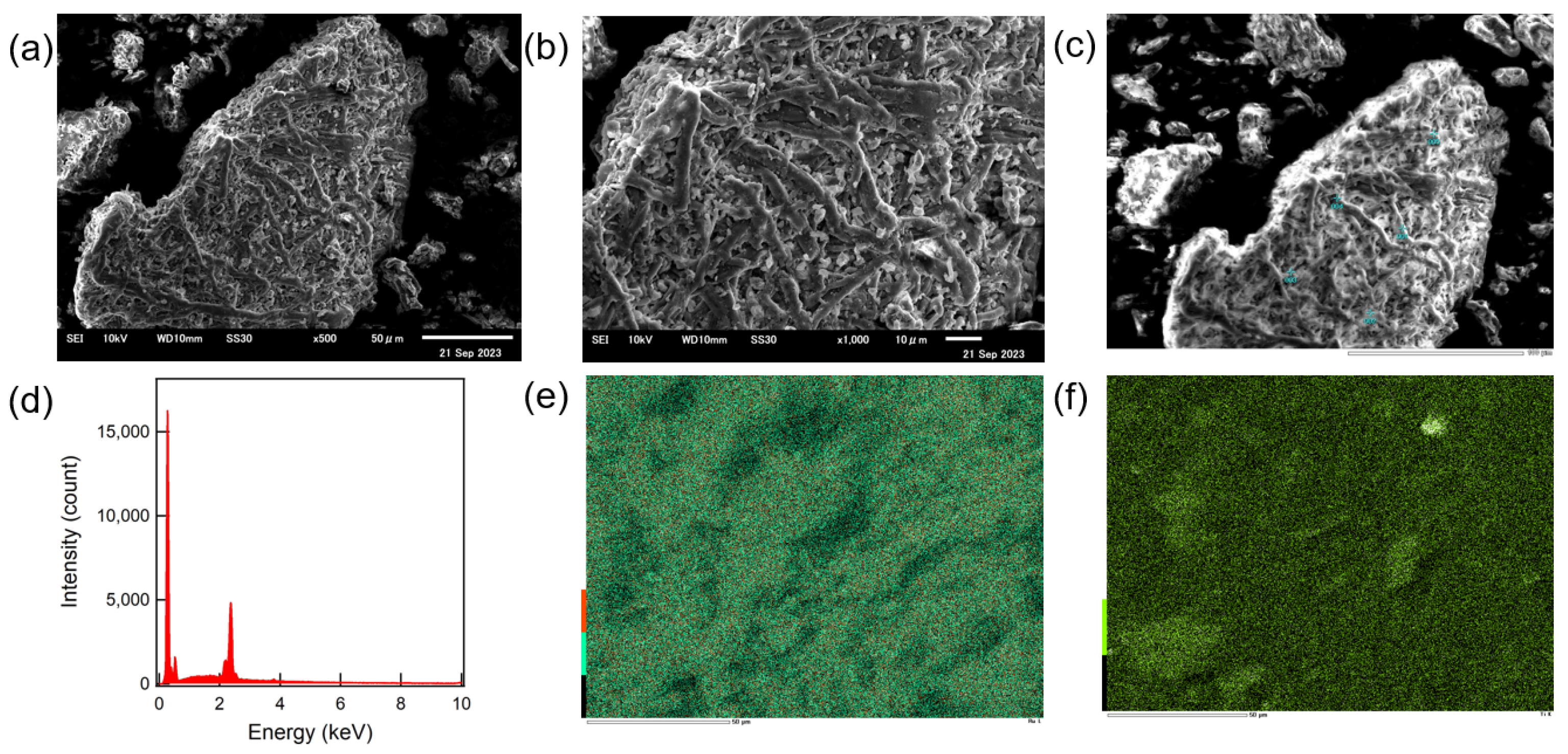

| Element | Energy [keV] | Atomic Ratio [%] |
|---|---|---|
| C | 0.277 | 72.87 |
| N | 0.392 | 13.88 |
| O | 0.525 | 9.18 |
| S | 2.307 | 3.10 |
| Mo | 2.293 | 0.60 |
| Au | 2.121 | 0.38 |
| DSSC | Short-Circuit Current Density JSC [mA cm−2] | Open Circuit Voltage VOC [V] | Max Power Density Pmax [mW cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|
| C1 | 0.441 | 0.212 | 0.032 | 0.337 | 0.005 |
| C2 | 0.682 | 0.356 | 0.085 | 0.401 | 0.100 |
| DSSC | Short-Circuit Current Density JSC [mA cm−2] | Open Circuit Voltage VOC [V] | Max Power Density Pmax [mW cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|
| C1 | 0.643 | 0.232 | 0.060 | 0.347 | 0.008 |
| C2 | 0.071 | 0.063 | 0.001 | 0.223 | 0.001 |
| TiO2 (Comparison) | 0.057 | --- | 0.008 | --- | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanifuji, N.; Shimizu, T.; Shimizu, A.; Shimizu, K.; Abe, K.; Tanaka, M.; Wang, H.; Yoshikawa, H. Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes. Materials 2023, 16, 6654. https://doi.org/10.3390/ma16206654
Tanifuji N, Shimizu T, Shimizu A, Shimizu K, Abe K, Tanaka M, Wang H, Yoshikawa H. Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes. Materials. 2023; 16(20):6654. https://doi.org/10.3390/ma16206654
Chicago/Turabian StyleTanifuji, Naoki, Takeshi Shimizu, Akihiro Shimizu, Kaho Shimizu, Kizuna Abe, Miki Tanaka, Heng Wang, and Hirofumi Yoshikawa. 2023. "Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes" Materials 16, no. 20: 6654. https://doi.org/10.3390/ma16206654
APA StyleTanifuji, N., Shimizu, T., Shimizu, A., Shimizu, K., Abe, K., Tanaka, M., Wang, H., & Yoshikawa, H. (2023). Stability Modification of Dye-sensitized Solar Cells by Ruthenium Dyes Embedded on Eggshell Membranes. Materials, 16(20), 6654. https://doi.org/10.3390/ma16206654