The Ablation Performance of Silicon Nitride/Boron Nitride Fibrous Monolithic Ceramics under an Oxyacetylene Combustion Torch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedure
2.3. Characterization
3. Results and Discussions
3.1. Ablation Resistance
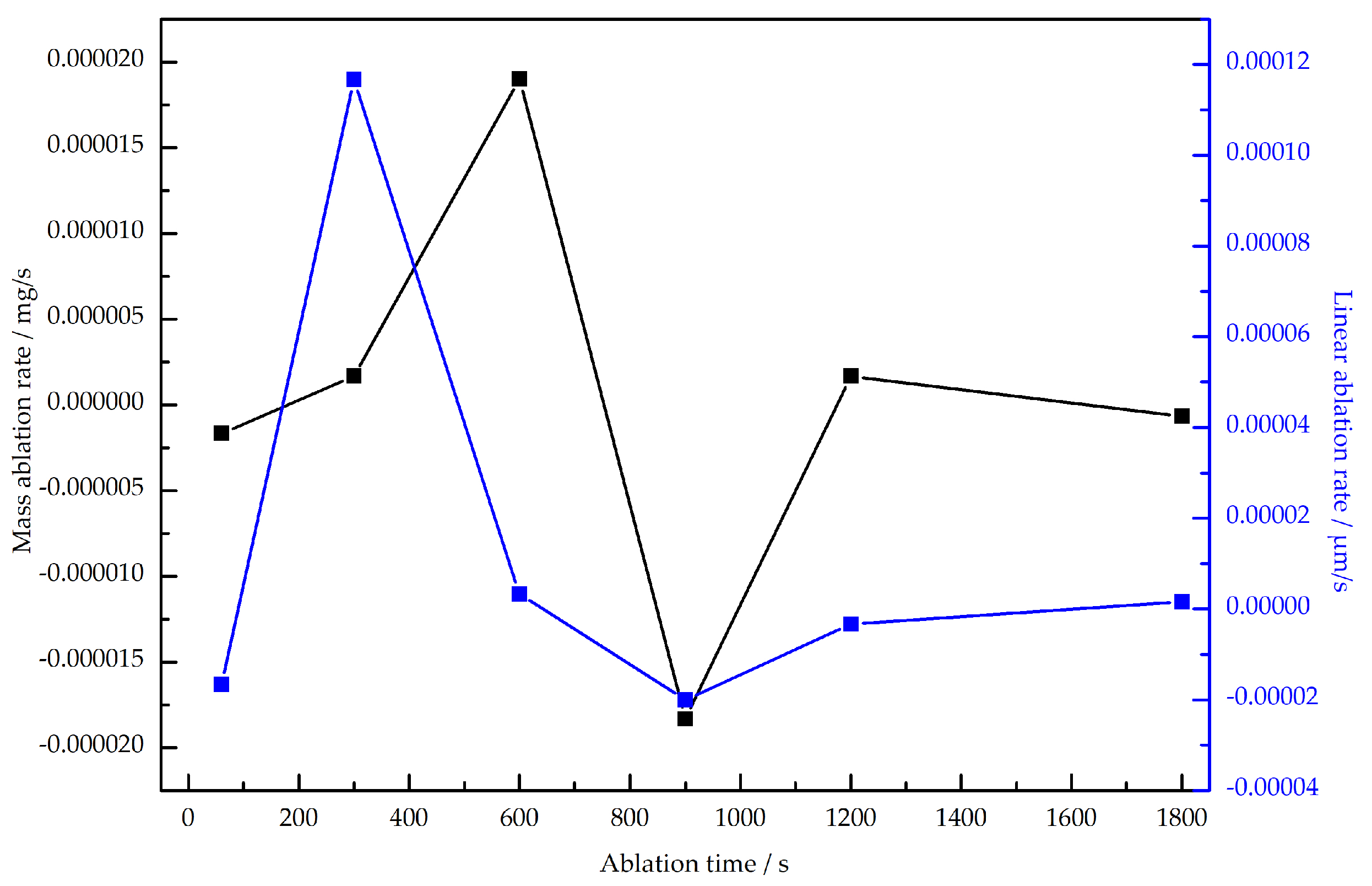
3.1.1. Ablation Rate
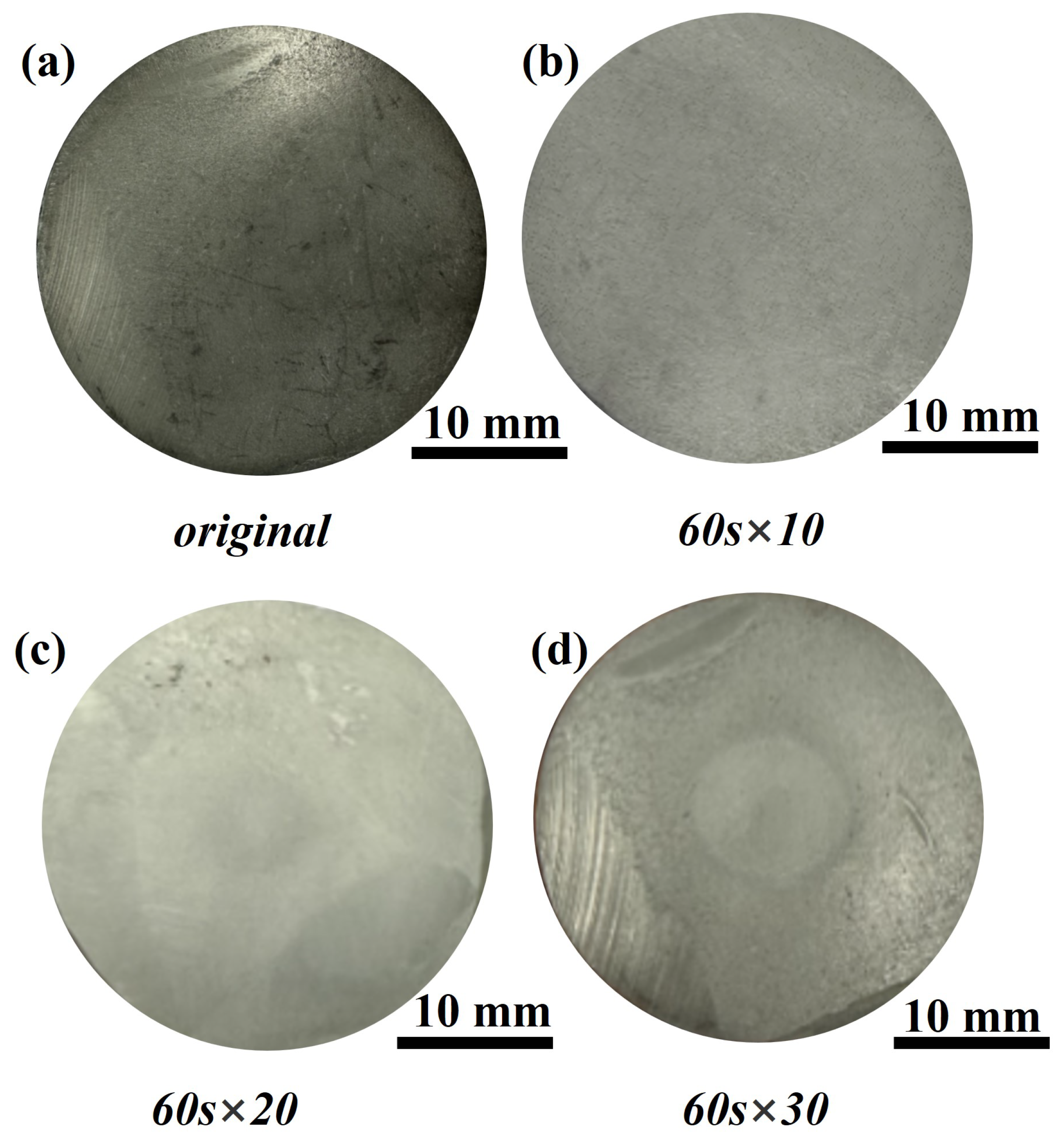
3.1.2. Macroscopic Morphology
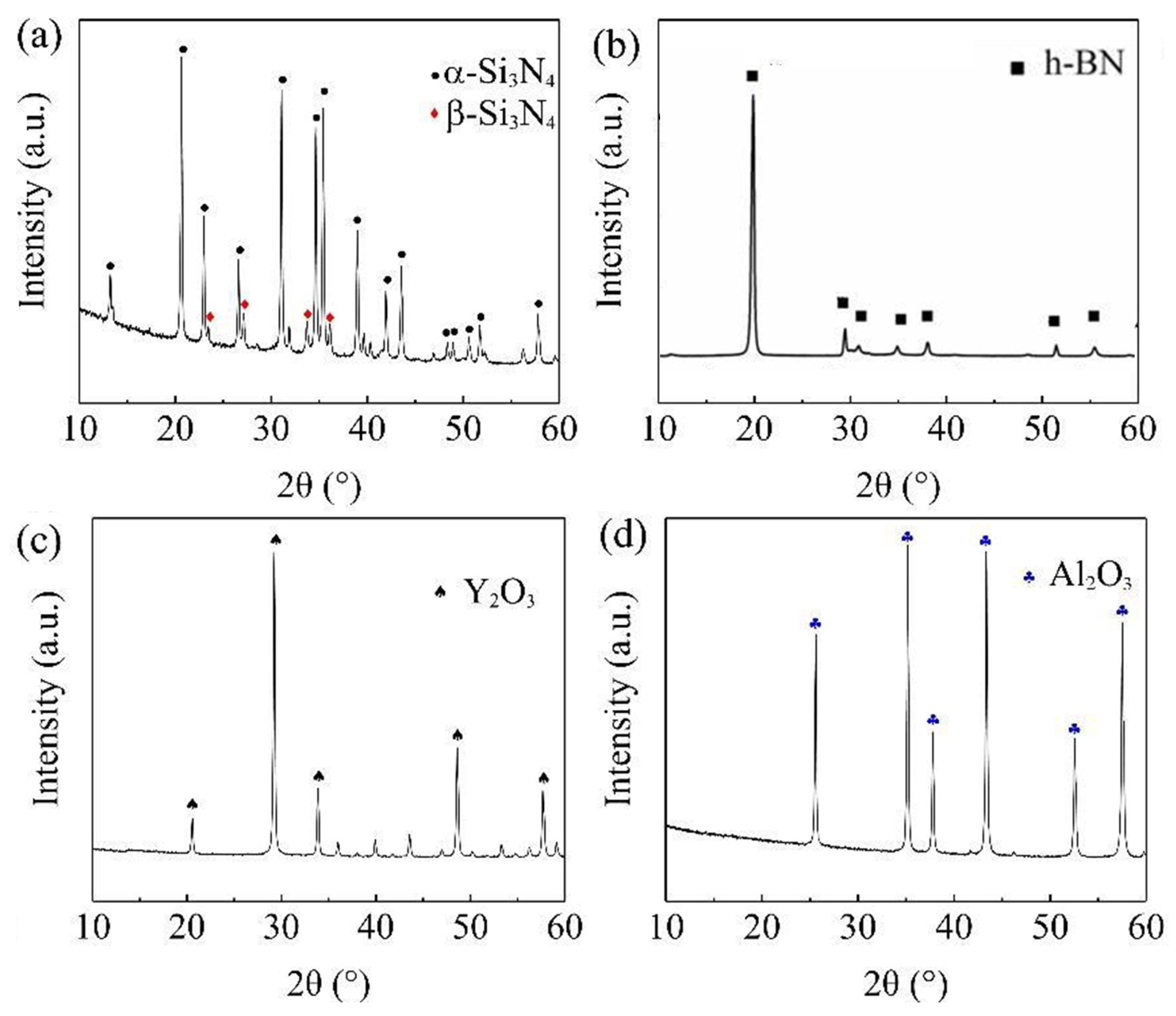
3.2. Surface Phase Composition
3.3. Surface Micrographs
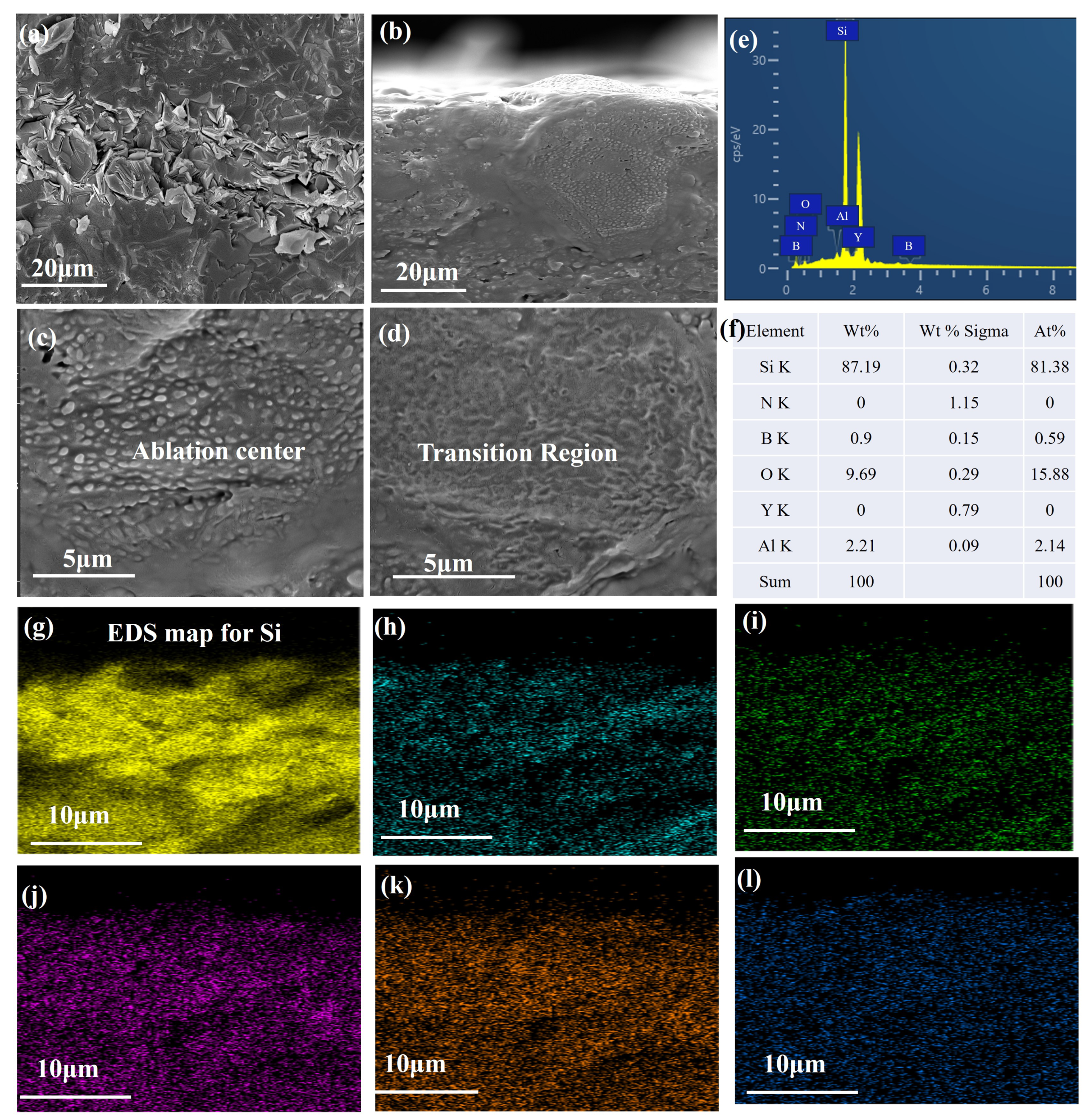
3.4. Cross-Sectional Micrographs
3.5. Ablation Behavior
4. Conclusions
- 1.
- The mass and linear ablation rates of SiN/BN fibrous monolithic ceramics initially increase with prolonged ablation time, then decrease, followed by a subsequent rise. Specifically, for ablation durations of 60 s 10, 60 s 20, and 60 s 30, the corresponding mass ablation rates are mg/s, mg/s, mg/s, respectively; the linear ablation rates are m/s,m/s and m/s. The ablation resistance of SiN/BN fibrous monolithic ceramics is notably superior to other SiN-based ceramics.;
- 2.
- With the prolongation of ablation time, a dense oxidation layer forms on the surface of SiN/BN fibrous monolithic ceramics. However, after 60 s 30 of ablation, this oxidation layer is eroded by the oxyacetylene flame, revealing a porous, coral-like structure with numerous cracks. A glass phase, primarily formed from sintering aids and covering the SiN ceramic surface, is observed on the ablated surface of the sample, effectively preventing further ablation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Xu, J.; Yang, R.; Chen, K.; Yan, H.; Gao, F. Effect of composition on the dielectric properties and thermal conductivity of α-SiAlON ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 22480–22491. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Zhu, D. Fabrication and properties of porous Si3N4-SiO2 ceramics with dense surface and gradient pore distribution. Ceram. Int. 2014, 40, 5079–5084. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, D.; Wang, J.; Zhang, J.; Zhou, J.; Zhang, Y. Synthesis of self-toughness porous Si3N4 ceramics with three-dimensional cage structures. Mater. Lett. 2020, 270, 127651. [Google Scholar] [CrossRef]
- Cao, M.S.; Hou, Z.L.; Yuan, J.; Xiong, L.T.; Shi, X.L. Low dielectric loss and non-Debye relaxation of γ-Y2Si2O7 ceramic at elevated temperature in X-band. J. Appl. Phys. 2009, 105, 106102-3. [Google Scholar] [CrossRef]
- Wang, K.; Bao, C.; Zhang, C.; Li, Y.; Liu, R.; Xu, H.; Ma, H.; Man, J.; Song, S. Preparation of high-strength Si3N4 antenna window using selective laser sintering. Ceram. Int. 2021, 47, 31277–31285. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Zhu, D. The effect of the crystallization of oxidation-derived SiO2 on the properties of porous Si3N4-SiO2 ceramics synthesized by oxidation. Ceram. Int. 2014, 40, 4897–4902. [Google Scholar] [CrossRef]
- Wei, S.; Liu, Y.; Liu, X.; Zhao, H. Investigation on edge chipping evaluation of Si3N4 ceramics milling surface. Measurement 2019, 133, 241–250. [Google Scholar] [CrossRef]
- Wei, Z.H.; Cheng, L.J.; Ma, Y.X.; Chen, A.N.; Guo, X.F.; Wu, J.M.; Shi, Y.S. Direct fabrication mechanism of pre-sintered Si3N4 ceramic with ultra-high porosity by laser additive manufacturing. Scr. Mater. 2019, 173, 91–95. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Y.; Zhang, D.; Zhang, J.; Zhou, J.; Zhang, J.; Liu, X.; Zhang, J. Effect of surface nano-modification on the antioxidation properties of Si3N4 ceramics. J. Alloys Compd. 2018, 766, 678–685. [Google Scholar] [CrossRef]
- Yin, S.; Pan, L.; Liu, Y.; Wang, Y.; Qiu, T.; Yang, J. Effect of β-Si3N4 seeds on microstructure and properties of porous Si3N4 ceramics prepared by gelcasting using DMAA system. Ceram. Int. 2020, 46, 4924–4932. [Google Scholar] [CrossRef]
- Liang, H.; Zeng, Y.; Zuo, K.; Xia, X.; Yao, D.; Yin, J. The effect of oxidation on the mechanical properties and dielectric properties of porous Si3N4 ceramics. Ceram. Int. 2017, 43, 5517–5523. [Google Scholar] [CrossRef]
- Liu, J.X.; Yuan, B.; Zhang, G.J.; Kan, Y.M.; Wang, P.L. Properties of porous Si3N4/BN composites fabricated by RBSN technique. Int. J. Appl. Ceram. Technol. 2010, 7, 536–545. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.; Qiao, R.; Zhang, F.; Wang, Z.; Chen, L. Effect of sintering temperature on microstructures and tribological characteristics of dense α-Si3N4-based ceramic coating on porous Si3N4 ceramics. J. Alloys Compd. 2019, 776, 927–933. [Google Scholar] [CrossRef]
- Wang, B.; Shangguan, D.; Qiao, R.; Zhang, F.; Bai, Y.; Wang, Z.; Wang, C.; Lu, X. Fabrication, mechanical properties and thermal shock resistance of a dense SiC NWs/α-Si3N4 composite coating for protecting porous Si3N4 ceramics. Ceram. Int. 2019, 45, 23241–23247. [Google Scholar] [CrossRef]
- Brazel, J.; Fenton, R.; Roetling, J.A.; Tanzilli, R.A. Millimeter wave hardened antenna window materials development. In Final Report; General Electric Co.: Philadelphia, PA, USA, 1979. [Google Scholar]
- Xia, Y.; Zeng, Y.P.; Jiang, D. Dielectric and mechanical properties of porous Si3N4 ceramics prepared via low temperature sintering. Ceram. Int. 2009, 35, 1699–1703. [Google Scholar] [CrossRef]
- Hou, Z.L.; Cao, M.S.; Yuan, J.; Fang, X.Y.; Shi, X.L. High-temperature conductance loss dominated defect level in h-BN: Experiments and first principles calculations. J. Appl. Phys. 2009, 105, 076103-3. [Google Scholar] [CrossRef]
- Li, B.; Liu, K.; Zhang, C.R.; Wang, S.Q. Fabrication and properties of borazine derived boron nitride bonded porous silicon aluminum oxynitride wave-transparent composite. J. Eur. Ceram. Soc. 2014, 34, 3591–3595. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.; Xie, X.; Feng, W.; Wu, K. Calculation of the ionic conductivity of h-BN and its effect on the dielectric loss. J. Phys. D Appl. Phys. 2007, 40, 846. [Google Scholar] [CrossRef]
- Cao, F.; Fang, Z.; Zhang, C. High-temperature properties and associated structure evolution of continuous SiNO fiber-reinforced BN composites for wave transparency. Mater. Des. 2013, 43, 258–263. [Google Scholar] [CrossRef]
- Paquette, D.G. Method of Making a Radar Transparent Window Material Operable above 2000 °C. U.S. Patent 06/812420, 6 May 1997. [Google Scholar]
- Zou, C.; Li, B.; Meng, X.; Liu, K.; Yang, X.; Li, D.; Wang, S. Ablation behavior and mechanism of SiO2f/SiO2, SiO2f/BN and Si3N4f/BN radar wave transparent composites. Corros. Sci. 2018, 139, 243–254. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, B.; Xiao, G.; Qiao, R.; Zhang, F.; Bai, Y.; Li, Y.; Wu, Y.; Wang, Z.; et al. Enhancement of thermal shock resistance in β-Si3N4 coating with in situ synthesized β-Si3N4 nanowires/nanobelts on porous Si3N4 ceramics. Ceram. Int. 2021, 47, 25449–25457. [Google Scholar] [CrossRef]
- GJB323A-96; Test Method for Abaltion of Ablators. Commission of Science, Industry and Technology for National Defence: Beijing, China, 1997.
- Fang, D.; Chen, Z.; Song, Y.; Sun, Z. Morphology and microstructure of 2.5 dimension C/SiC composites ablated by oxyacetylene torch. Ceram. Int. 2009, 35, 1249–1253. [Google Scholar] [CrossRef]
- Wang, C.; Chen, M.; Wang, H.; Fan, X.; Xia, H. Fabrication and thermal shock resistance of multilayer γ-Y2Si2O7 environmental barrier coating on porous Si3N4/BN ceramic. J. Eur. Ceram. Soc. 2016, 36, 689–695. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Cai, D.; Wang, B.; Jia, D. Thermal shock and ablation behavior of α-cordierite glass-ceramic coating on porous BN/Si2N2O ceramic. Ceram. Int. 2019, 45, 24793–24801. [Google Scholar] [CrossRef]
- Park, D.S.; Choi, H.J.; Han, B.D.; Kim, H.D.; Lim, D.S. Effect of Si2N2O content on the microstructure, properties, and erosion of silicon nitride-Si2N2O in situ composites. J. Mater. Res. 2002, 17, 2275–2280. [Google Scholar] [CrossRef]





| Raw Material | Purity (%) | Particle Size (μm) | Manufacturer |
|---|---|---|---|
| -SiN | 99 | 0.5 | Hefei Aijia New Material Technology Co., Ltd., Hefei, China |
| BN | 99 | 1.31 | Dandong Chemical Research Institute Co., Ltd., Dandong, China |
| YO | 99.99 | 2 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| AlO | 98 | 0.73 | Showa Denko, Yokohama, Japan |
| -SiN (wt.%) | YO (wt.%) | AlO (wt.%) | Deionized Water (wt.%) | (CHNO) (wt.%) | BN |
|---|---|---|---|---|---|
| 47.5 | 1.78 | 0.72 | 48.75 | 1.25 | 3.0 |
| Parameters | Unit | Numerical Value |
|---|---|---|
| Heat flux | Kw/m | 4168.8 ± 418.7 |
| Oxygen flow | L/h | 1443 |
| Acetylene flow | L/h | 927 |
| Oxygen pressure | MPa | 0.4 |
| Acetylene pressure | MPa | 0.095 |
| Distance from initial specimen surface to flame nozzle | mm | 10 |
| Flame nozzle diameter | mm | 2 |
| Flame ablation Angle | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhang, Y.; Zhou, Y.; Li, D.; Ying, G. The Ablation Performance of Silicon Nitride/Boron Nitride Fibrous Monolithic Ceramics under an Oxyacetylene Combustion Torch. Materials 2023, 16, 6703. https://doi.org/10.3390/ma16206703
Chen Q, Zhang Y, Zhou Y, Li D, Ying G. The Ablation Performance of Silicon Nitride/Boron Nitride Fibrous Monolithic Ceramics under an Oxyacetylene Combustion Torch. Materials. 2023; 16(20):6703. https://doi.org/10.3390/ma16206703
Chicago/Turabian StyleChen, Qingqing, Yuan Zhang, Yu Zhou, Daxin Li, and Guobing Ying. 2023. "The Ablation Performance of Silicon Nitride/Boron Nitride Fibrous Monolithic Ceramics under an Oxyacetylene Combustion Torch" Materials 16, no. 20: 6703. https://doi.org/10.3390/ma16206703






