Histological Comparison of Collagenated Cancellous Equine Bone Blocks Used as Inlay or Onlay for Lateral Bone Augmentation in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
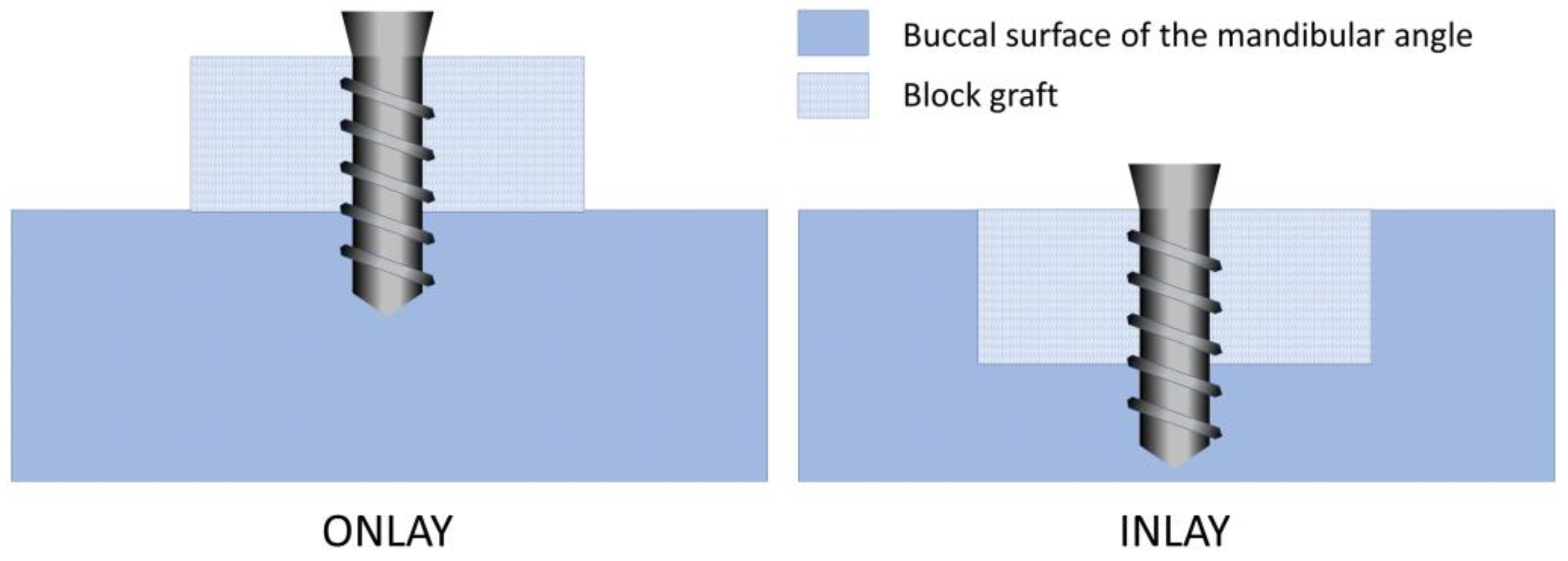
2.2. Study Design
2.3. Experimental Animals and Sample Size
2.4. Randomization and Allocation Concealment
2.5. Biomaterials
2.6. Anesthetic Procedures
2.7. Surgical Procedure
2.8. Animal Maintenance
2.9. Euthanasia
2.10. Histological Processing
2.11. Histomorphometric Evaluation
2.12. Experimental Outcomes and Statistical Methods
3. Results
3.1. Clinical Outcomes
3.2. Descriptive Histological Evaluation
3.3. Histomorphometric Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Kotagudda Ranganath, S.; Schlund, M.; Delattre, J.; Ferri, J.; Chai, F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater. Today Biol. 2022, 14, 100267. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Marx, R.E. Bone and bone graft healing. Oral Maxillofac. Surg. Clin. North. Am. 2007, 19, 55–66. [Google Scholar] [CrossRef]
- Habal, M.B. Bone grafting in craniofacial surgery. Clin. Plast. Surg. 1994, 21, 349–363. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahl, E.; Enbom, L.; Engevall, S.; Engquist, B.; Eriksson, A.R.; Feldmann, G.; Freiberg, N.; Glantz, P.O.; Kjellman, O.; et al. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J. Periodontol. 1988, 59, 287–296. [Google Scholar] [CrossRef]
- Jemt, T.; Lekholm, U.; Gröndahl, K. 3-year followup study of early single implant restorations ad modum Brånemark. Int. J. Periodontics Restor. Dent. 1990, 10, 340–349. [Google Scholar]
- Misch, C.M.; Misch, C.E.; Resnik, R.R.; Ismail, Y.H. Reconstruction of maxillary alveolar defects with mandibular symphysis grafts for dental implants: A preliminary procedural report. Int. J. Oral Maxillofac. Implant. 1992, 7, 360–366. [Google Scholar]
- Misch, C.E. Divisions of available bone in implant dentistry. Int. J. Oral Implantol. 1990, 7, 9–17. [Google Scholar] [PubMed]
- Salvato, G.; Agliardi, E. Calvarial bone grafts in severe maxillary atrophy: Preprosthetic surgery with sedation. Implant. Dent. 2007, 16, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Burstein, J.; Sedghizadeh, P.P. Cortical tenting grafting technique in the severely atrophic alveolar ridge for implant site preparation. Implant. Dent. 2008, 17, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.G.; Dickerson, N.C.; Mitchell, J.C. Calvarial bone harvest and grafting techniques for maxillary and mandibular implant surgery. Atlas Oral Maxillofac. Surg. Clin. N. Am. 1994, 2, 109–122. [Google Scholar] [CrossRef]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Lateral ridge augmentation and implant placement: An experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int. J. Oral Maxillofac. Implant. 2001, 16, 343–354. [Google Scholar]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Buser, D. Lateral ridge augmentation using different bone fillers and barrier membrane application. A histologic and histomorphometric pilot study in the canine mandible. Clin. Oral Implant. Res. 2001, 12, 260–269. [Google Scholar] [CrossRef]
- Chiriac, G.; Herten, M.; Schwarz, F.; Rothamel, D.; Becker, J. Autogenous bone chips: Influence of a new piezoelectric device (Piezosurgery) on chip morphology, cell viability and differentiation. J. Clin. Periodontol. 2005, 32, 994–999. [Google Scholar] [CrossRef]
- Nowzari, H.; Aalam, A.A. Mandibular cortical bone graft part 2: Surgical technique, applications, and morbidity. Compend. Contin. Educ. Dent. 2007, 28, 274–280, quiz 281-2. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 136–159. [Google Scholar] [CrossRef]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Schultze-Mosgau, S.; Radespiel-Tröger, M.; Kloss, F.; Neukam, F.W. Morbidity of harvesting of chin grafts: A prospective study. Clin. Oral Implant. Res. 2001, 12, 495–502. [Google Scholar] [CrossRef]
- von Arx, T.; Häfliger, J.; Chappuis, V. Neurosensory disturbances following bone harvesting in the symphysis: A prospective clinical study. Clin. Oral Implant. Res. 2005, 16, 432–439. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontology 2000 2017, 73, 84–102. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Particulate vs. block bone grafts: Three-dimensional changes in graft volume after reconstruction of the atrophic maxilla, a 2-year radiographic follow-up. J. Craniomaxillofac. Surg. 2012, 40, 654–659. [Google Scholar] [CrossRef]
- Nyström, E.; Ahlqvist, J.; Legrell, P.E.; Kahnberg, K.E. Bone graft remodelling and implant success rate in the treatment of the severely resorbed maxilla: A 5-year longitudinal study. Int. J. Oral Maxillofac. Surg. 2002, 31, 158–164. [Google Scholar] [CrossRef]
- Gordh, M.; Alberius, P. Some basic factors essential to autogeneic nonvascularized onlay bone grafting to the craniofacial skeleton. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1999, 33, 129–146. [Google Scholar] [CrossRef]
- Pistilli, R.; Felice, P.; Piatelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: Preliminary data from a pilot randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 153–171. [Google Scholar] [PubMed]
- Al Ruhaimi, K.A. Bone graft substitutes: A comparative qualitative histologic review of current osteoconductive grafting materials. Int. J. Oral Maxillofac. Implant. 2001, 16, 105–114. [Google Scholar]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.; Balan, V.F.; Botticelli, D.; Soldini, C.; Okamoto, R.; Xavier, S.P. Histomorphometric, Immunohistochemical and Microtomographic Comparison between Autogenous and Xenogenous Bone Blocks for Mandibular Lateral Augmentation in Rabbits. Materials 2021, 14, 6049. [Google Scholar] [CrossRef]
- Romito, G.A.; Villar, C.C.; Sapata, V.M.; Soares, H.H.; Fonseca, M.A.; Conde, M.; Hammerle, C.H.F.; Schwarz, F. Autogenous bone block versus collagenated xenogeneic bone block in the reconstruction of the atrophic alveolar ridge: A non-inferiority randomized clinical trial. J. Clin. Periodontol. 2022, 49, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Romito, G.A.; Soares, H.H.; do Amaral, G.C.L.S.; Fonseca, M.A.; Sapata, V.M.; Conde, M.C.; Hammerle, C.H.F.; Schwarz, F.; Villar, C.C. Radiographic outcomes of ridge reconstruction with autogenous bone block versus collagenated xenogeneic bone block: A randomized clinical trial. Clin. Oral Implant. Res. 2023, 34, 863–871. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants—A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar]
- Xuan, F.; Lee, C.U.; Son, J.S.; Fang, Y.; Jeong, S.M.; Choi, B.H. Vertical ridge augmentation using xenogenous bone blocks: A comparison between the flap and tunneling procedures. J. Oral Maxillofac. Surg. 2014, 72, 1660–1670. [Google Scholar] [CrossRef]
- Nyström, E.; Legrell, P.E.; Forssell, A.; Kahnberg, K.E. Combined use of bone grafts and implants in the severely resorbed maxilla. Postoperative evaluation by computed tomography. Int. J. Oral Maxillofac. Surg. 1995, 24 Pt 1, 20–25. [Google Scholar] [CrossRef]
- Faria, P.E.; Okamoto, R.; Bonilha-Neto, R.M.; Xavier, S.P.; Santos, A.C.; Salata, L.A. Immunohistochemical, tomographic and histological study on onlay iliac grafts remodeling. Clin. Oral Implant. Res. 2008, 19, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Yonezawa, D.; Silva, E.R.; Xavier, S.P. The Impact on the Healing of Bioactivation with Argon Plasma of a Xenogeneic Graft with Adequate Fixation but Poor Adaptation to the Recipient Site: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 2021, 36, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sager, M.; Ferrari, D.; Mihatovic, I.; Becker, J. Influence of recombinant human platelet-derived growth factor on lateral ridge augmentation using biphasic calcium phosphate and guided bone regeneration: A histomorphometric study in dogs. J. Periodontol. 2009, 80, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. The jumping distance revisited: An experimental study in the dog. Clin. Oral Implant. Res. 2003, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Berglundh, T.; Lindhe, J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. A experimental study in labrador dogs. J. Clin. Periodontol. 2002, 29, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri-implant bone. An experimental study in the dog. J. Clin. Periodontol. 2004, 31, 309–317. [Google Scholar] [CrossRef]
- Araújo, M.G.; Sonohara, M.; Hayacibara, R.; Cardaropoli, G.; Lindhe, J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial: An experiment in the dog. J. Clin. Periodontol. 2002, 29, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- De Santis, E.; Lang, N.P.; Scala, A.; Viganò, P.; Salata, L.A.; Botticelli, D. Healing outcomes at implants installed in grafted sites: An experimental study in dogs. Clin. Oral Implant. Res. 2012, 23, 340–350. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Favero, G.; Beolchini, M.; Morelli, F.; Botticelli, D. Healing at mandibular block-grafted sites: An experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 516–522. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Deluiz, D.; Tinoco, E.M.B. Horizontal Alveolar Ridge Augmentation with Allogeneic Bone Block Graft Compared with Autogenous Bone Block Graft: A Systematic Review. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef]
- Donkiewicz, P.; Benz, K.; Kloss-Brandstätter, A.; Jackowski, J. Survival Rates of Dental Implants in Autogenous and Allogeneic Bone Blocks: A Systematic Review. Medicina 2021, 57, 1388. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Vitenson, J.; Deluiz, D.; Østergaard, K.B.; Tinoco, E.M.B. Lateral Alveolar Ridge Augmentation with Autogenous Tooth Block Graft Compared with Autogenous Bone Block Graft: A Systematic Review. J. Oral Maxillofac. Res. 2022, 13, e1. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Zhao, R.; Guo, Y.; Li, J.; Ma, N.; Gong, J. Efficacy of autogenous tooth block for lateral ridge augmentation compared with autogenous bone block: A systematic review and meta-analysis. Medicine 2023, 102, e35326. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Botticelli, D.; Carneiro Martins, E.N.; Caneva, M.; Lang, N.P.; Xavier, S.P. Healing at the interface between recipient sites and autologous block bone grafts affixed by either position or lag screw methods: A histomorphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 1484–1491. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, R.; Xavier, S.P.; Morinaga, K.; Botticelli, D.; Silva, E.R.; Nakajima, Y.; Baba, S. Histological Comparison of Collagenated Cancellous Equine Bone Blocks Used as Inlay or Onlay for Lateral Bone Augmentation in Rabbits. Materials 2023, 16, 6742. https://doi.org/10.3390/ma16206742
Sakaguchi R, Xavier SP, Morinaga K, Botticelli D, Silva ER, Nakajima Y, Baba S. Histological Comparison of Collagenated Cancellous Equine Bone Blocks Used as Inlay or Onlay for Lateral Bone Augmentation in Rabbits. Materials. 2023; 16(20):6742. https://doi.org/10.3390/ma16206742
Chicago/Turabian StyleSakaguchi, Ryuichi, Samuel Porfirio Xavier, Kenzo Morinaga, Daniele Botticelli, Erick Ricardo Silva, Yasushi Nakajima, and Shunsuke Baba. 2023. "Histological Comparison of Collagenated Cancellous Equine Bone Blocks Used as Inlay or Onlay for Lateral Bone Augmentation in Rabbits" Materials 16, no. 20: 6742. https://doi.org/10.3390/ma16206742






