The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite
Abstract
:1. Introduction
2. Materials and Methods
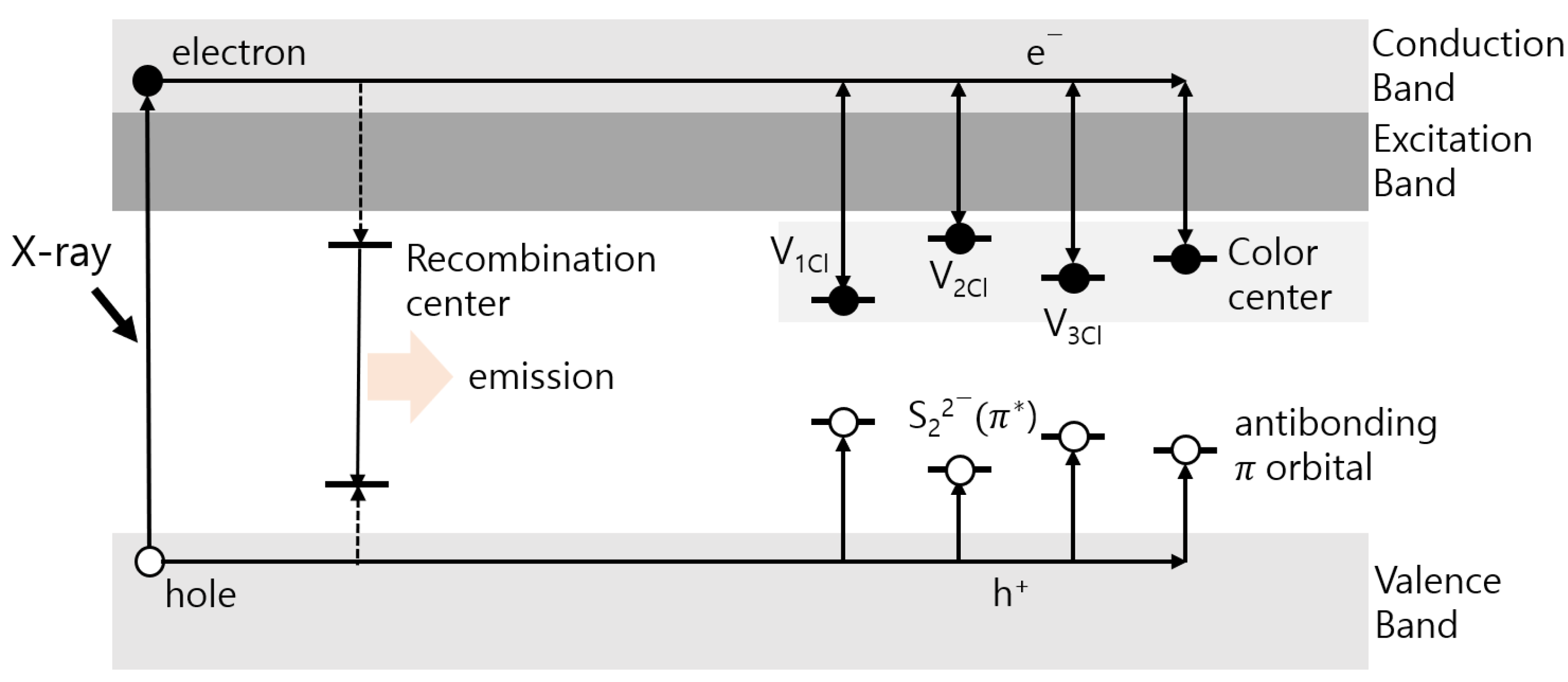
2.1. Theory
2.2. X-ray Diffraction (XRD) of Na8Al6Si6O24(Cl,S)2 Hackmanite
2.3. TL Reader
3. Results and Discussion
3.1. Measurement of the TL Glow Curve of Na8Al6Si6O24(Cl,S)2
3.2. Analyzed TL Trap Parameters through the De-Convolution Method
3.3. Analysis of TL Trap Parameters through the Peak Shape Method
3.4. Analysis of TL Trap Parameters through the Initial Rise Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agamah, C.; Vuori, S.; Colinet, P.; Norrbo, I.; de Carvalho, J.M.; Nakamura, L.K.O.; Lindblom, J.; van Goethem, L.; Emmermann, A.; Saarinen, T.; et al. Hackmanite-The Natural Glow-in-the-Dark Material. Chem. Mater. 2020, 32, 8895–8905. [Google Scholar] [CrossRef]
- Norrbo, I.; Gluchowski, P.; Hyppänen, I.; Laihinen, T.; Laukkanen, P.; Mäkelä, J.; Mamedov, F.; Santos, H.S.; Sinkkonen, J.; Tuomisto, M.; et al. Mechanisms of Tenebrescence and Persistent Luminescence in Synthetic Hackmanite Na8Al6Si6O24(Cl,S)2. ACS Appl. Mater. Interfaces 2016, 8, 11592–11602. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.R.; Simmonds, A.; Armstrong, J.A.; Weller, M.T. Compositional and structural control of tenebrescence. J. Mater. Chem. 2010, 20, 10883–10887. [Google Scholar] [CrossRef]
- Vuori, S.; Colinet, P.; Norrbo, I.; Steininger, R.; Saarinen, T.; Palonen, H.; Paturi, P.; Rodrigues, L.C.V.; Göttlicher, J.; Le Bahers, T.; et al. Detection of X-Ray Doses with Color-Changing Hackmanites: Mechanism and Application. Adv. Opt. Mater. 2021, 9, 2100762. [Google Scholar] [CrossRef]
- Medved, D.B. Hackmanite and its tenebrescent properties. J. Earth Planet. Mater. 1954, 39, 615–629. [Google Scholar]
- Norrbo, I.; Gluchowski, P.; Paturi, P.; Sinkkonen, J.; Lastusaari, M. Persistent Luminescence of Tenebrescent Na8Al6Si6O24(Cl,S)2: Multifunctional Optical Markers. Inorg. Chem. 2015, 54, 7717–7724. [Google Scholar] [CrossRef]
- Rahim, F.A.; Miller, A.; McLaughlin, W.L. Response of Radiation Monitoring Labels to Gamma Rays and Electrons. Radiat. Phys. Chem. 1985, 25, 767–775. [Google Scholar] [CrossRef]
- Badour, Y.; Jubera, V.; Andron, I.; Frayret, C.; Gaudon, M. Photochromism in inorganic crystallised compounds. Opt. Mater. X 2021, 12, 100110. [Google Scholar] [CrossRef]
- Vuori, S.; Byron, H.; Norrbo, I.; Tuomisto, M.; Lastusaari, M. Photochromic photography with hackmanite obtained by large-scale synthesis. J. Ind. Eng. Chem. 2023, 120, 361–373. [Google Scholar] [CrossRef]
- Zhang, L.-T.; Fu, Z.-X.; Yin, J.-C.; Liu, M.; Zhang, Y.-Q.; Lan, L.; Li, N.; Bu, X.-H. UV and X-ray dual-induced photochromism in a benzophenone-based metal–organic framework for inkless erasable printing. CrystEngComm 2023, 25, 4019–4023. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X. Calix-Like Metal-Organic Complex for High-Sensitive X-Ray-Induced Photochromism. Adv. Sci. 2015, 3, 1500224. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xie, J.; Wang, X.Y.; Wang, Y.; Li, Z.J.; Diefenbach, K.; Pan, Q.J.; Qian, Y.; Wang, J.Q.; Wang, S.; et al. Visible colorimetric dosimetry of UV and ionizing radiations by a dual-module photochromic nanocluster. Nat. Commun. 2021, 12, 2798. [Google Scholar] [CrossRef] [PubMed]
- Byron, H.; Norrbo, I.; Lastusaari, M. A zeolite-free synthesis of luminescent and photochromic hackmanites. J. Alloys Compd. 2021, 872, 159671. [Google Scholar] [CrossRef]
- El-Faramawy, N.A.; El-Kameesy, S.U.; El-Agramy, A.; Metwally, G. The dosimetric properties of in-house prepared copper doped lithium borate examined using the TL-technique. Radiat. Phys. Chem. 2000, 58, 9–13. [Google Scholar] [CrossRef]
- Mori, Y.; Kuroda, I.; Nakajima, S.; Sasaki, T.; Nakai, S. New nonlinear optical crystal: Cesium lithium borate. Appl. Phys. Lett. 1995, 67, 1818–1820. [Google Scholar] [CrossRef]
- Basun, S.; Imbusch, G.F.; Jia, D.D.; Yen, W.M. The analysis of thermoluminescence glow curves. J. Lumin. 2003, 104, 283–294. [Google Scholar] [CrossRef]
- Majewska, N.; Leśniewki, T.; Mahlik, S.; Grinberg, M.; Chruścińska, A.; Michalik, D.; Sopicka-Lizer, M. Study of persistent luminescence and thermoluminescence in SrSi2N2O2:Eu2+,Ce3+,Dy3+,Nd3+. Phys. Chem. Chem. Phys. 2020, 22, 17152–17159. [Google Scholar] [CrossRef]
- Sadek, A.M.; Eissa, H.M.; Basha, A.M.; Kitis, G. Development of the peak fitting and peak shape methods to analyze the thermoluminescence glow-curves generated with exponential heating function. Nucl. Instrum. Methods Phys. Res. B 2014, 330, 103–107. [Google Scholar] [CrossRef]
- Rawat, N.S.; Kulkarni, M.S.; Mishra, D.R.; Bhatt, B.C.; Sunta, C.M.; Gupta, S.K.; Sharma, D.N. Use of initial rise method to analyze a general-order kinetic thermoluminescence glow curve. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 3475–3479. [Google Scholar] [CrossRef]
- Chen, R.; Huntley, D.J.; Berger, G.W. Analysis of Thermoluminescence Data Dominated by Second-Order Kinetics. Phys. Status Solidi 1983, 79, 251–261. [Google Scholar] [CrossRef]
- Abd El-Hafez, A.I.; Yasin, M.N.; Sadek, A.M. GCAFIT—A new tool for glow curve analysis in thermoluminescence nanodosimetry. Nucl. Instrum. Methods Phys. Res. A 2011, 637, 158–163. [Google Scholar] [CrossRef]
- Sang, N.D.; Thi, H.H.Q. Using the genetic algorithm to detect kinetic parameters of thermoluminescence glow curves. Nucl. Instrum. Methods Phys. Res. B 2022, 517, 33–42. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Weller, M.T. Structural observation of photochromism. Chem. Commun. 2006, 10, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Goettlicher, J.; Kotelnikov, A.; Suk, N.; Kovalski, A.; Vitova, T.; Steininger, R. Sulfur Kα X-ray absorption near-edge structure spectroscopy on the photochrome sodalite variety hackmanite. Cryst. Mater. 2013, 228, 157–171. [Google Scholar] [CrossRef]
- Chung, K.S.; Choe, H.S.; Lee, J.I.; Kim, J.L. A new method for the numerical analysis of thermoluminescence glow curve. Radiat. Meas. 2007, 42, 731–734. [Google Scholar] [CrossRef]
- Chung, K.S.; Lee, J.I.; Kim, J.L. A computer program for the deconvolution of the thermoluminescence glow curves by employing the interactive trap model. Radiat. Meas. 2012, 47, 766–769. [Google Scholar] [CrossRef]
- Misra, S.K.; Eddy, N.W. IFOM, A formula for universal assessment of goodness-of-fit of gamma ray spectra. Nucl. Instr. Methods 1979, 166, 537–540. [Google Scholar] [CrossRef]






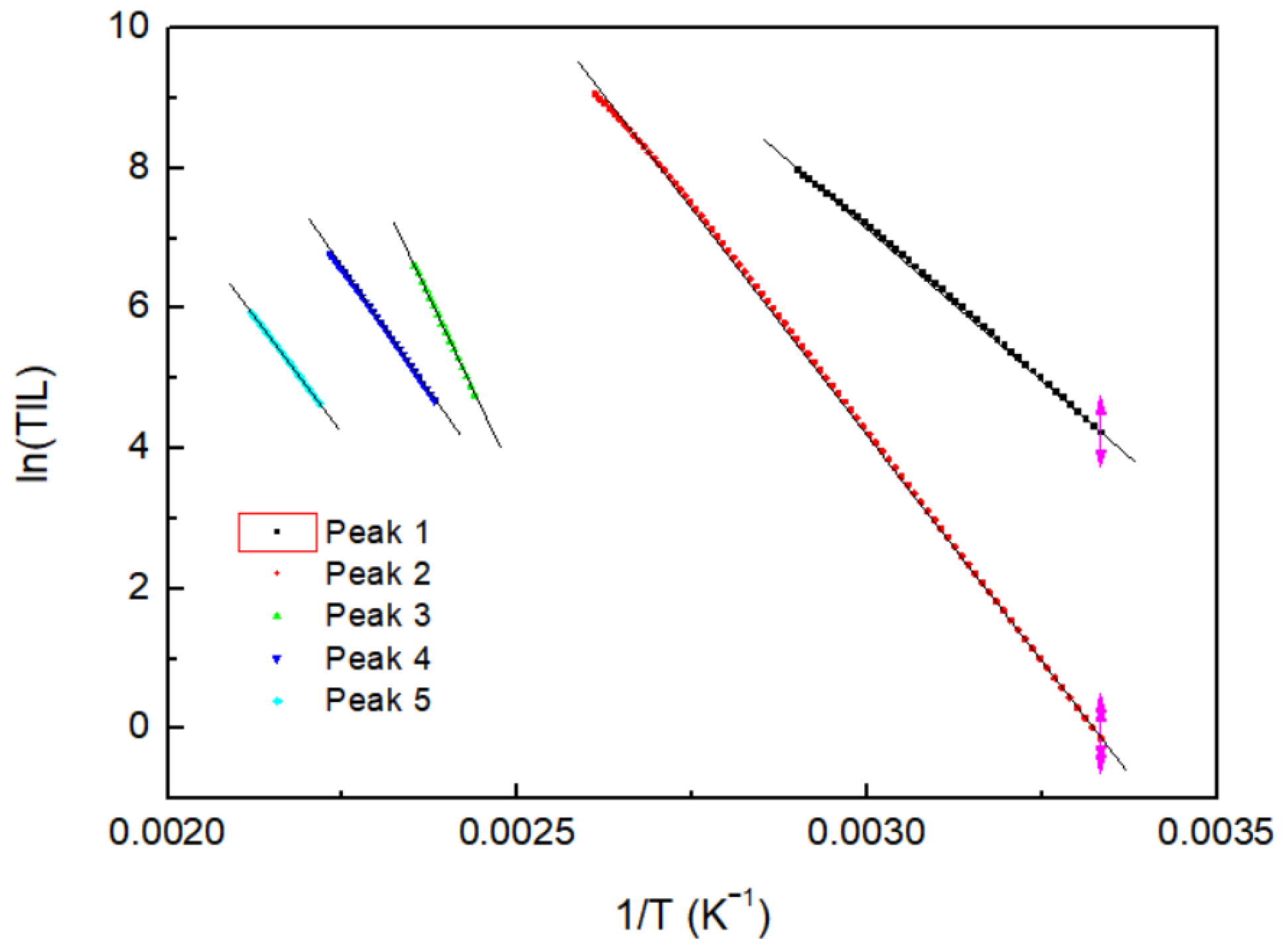
| Peak | Activation Energy (E) (eV) | Frequency Factor (s)(s−1) |
|---|---|---|
| 1 | 0.78 | 1.33 × 1010 |
| 2 | 1.13 | 4.94 × 1013 |
| 3 | 1.94 | 2.57 × 1021 |
| 4 | 1.3 | 1.51 × 1013 |
| 5 | 1.19 | 1.18 × 1011 |
| Peak | Trap Lifetime (s) | Temperature (K) | μg | Activation Energy (eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Tg | T2 | ω | τ | δ | Peak Shape | Initialize (R2 = 0.998) | Deconv. Method * | Avg. | |||
| 1 | 3.59 | 346.5 | 368 | 386.6 | 40.1 | 21.5 | 18.6 | 0.46 | 0.75 | 0.78 | ||
| 2 | 2.71 | 382.9 | 403 | 425.6 | 42.7 | 20.1 | 22.6 | 0.53 | 1.11 | 1.13 | ||
| 3 | 1.47 | 435.4 | 453 | 479.8 | 44.4 | 17.6 | 26.8 | 0.60 | 1.85 | 1.94 | ||
| 4 | 3.34 | 453.6 | 478 | 504.3 | 50.7 | 24.4 | 26.3 | 0.52 | 1.21 | 1.3 | ||
| 5 | 3.91 | 488.3 | 514 | 533.1 | 44.8 | 25.7 | 19.1 | 0.43 | 1.14 | 1.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Kim, S.-C. The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite. Materials 2023, 16, 6776. https://doi.org/10.3390/ma16206776
Kim S-H, Kim S-C. The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite. Materials. 2023; 16(20):6776. https://doi.org/10.3390/ma16206776
Chicago/Turabian StyleKim, Sung-Hwan, and Seon-Chil Kim. 2023. "The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite" Materials 16, no. 20: 6776. https://doi.org/10.3390/ma16206776
APA StyleKim, S.-H., & Kim, S.-C. (2023). The Kinetics of Carrier Trap Parameters in Na8Al6Si6O24(Cl,S)2 Hackmanite. Materials, 16(20), 6776. https://doi.org/10.3390/ma16206776






