Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization
Abstract
:1. Introduction
2. History of Biocompatibility
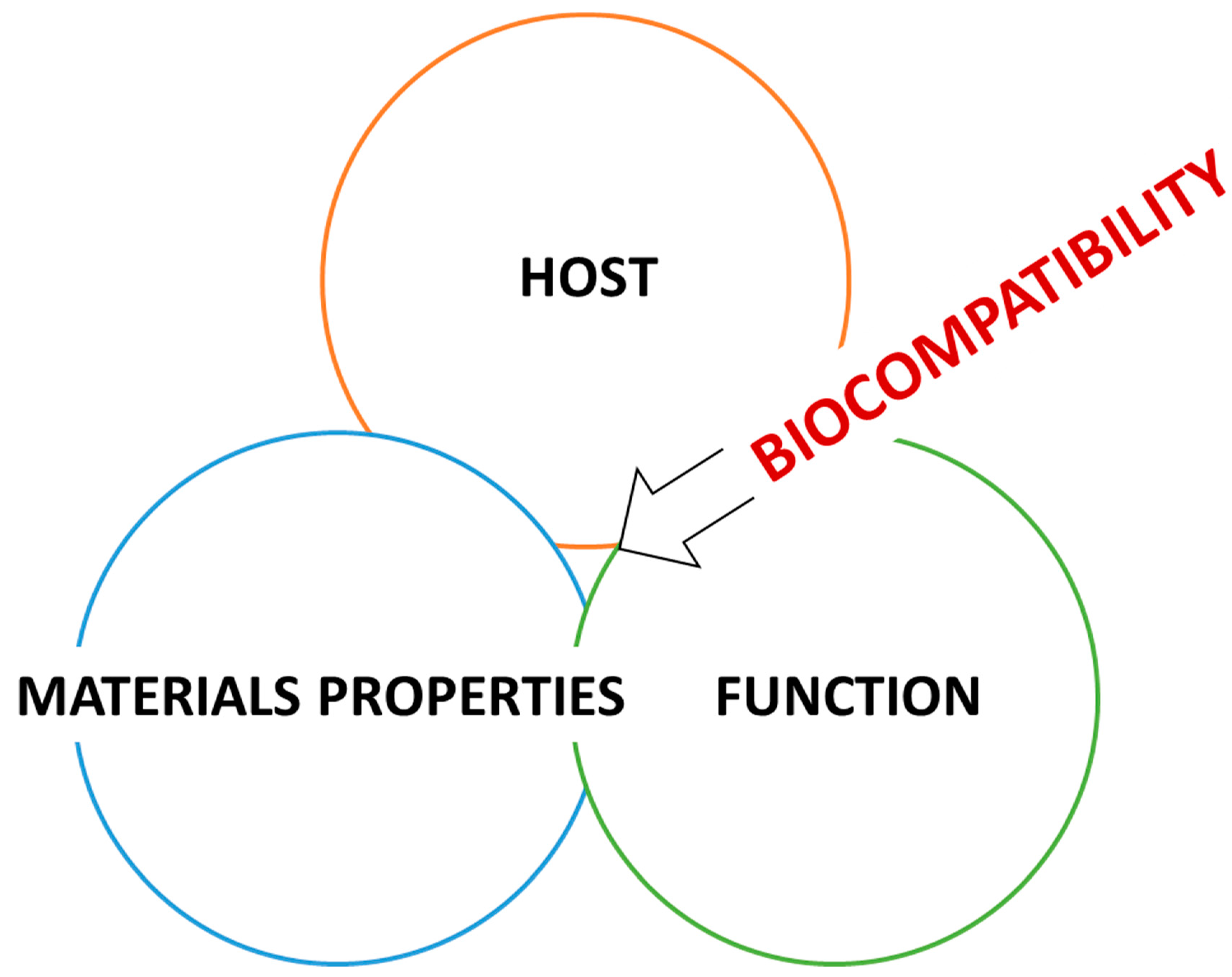
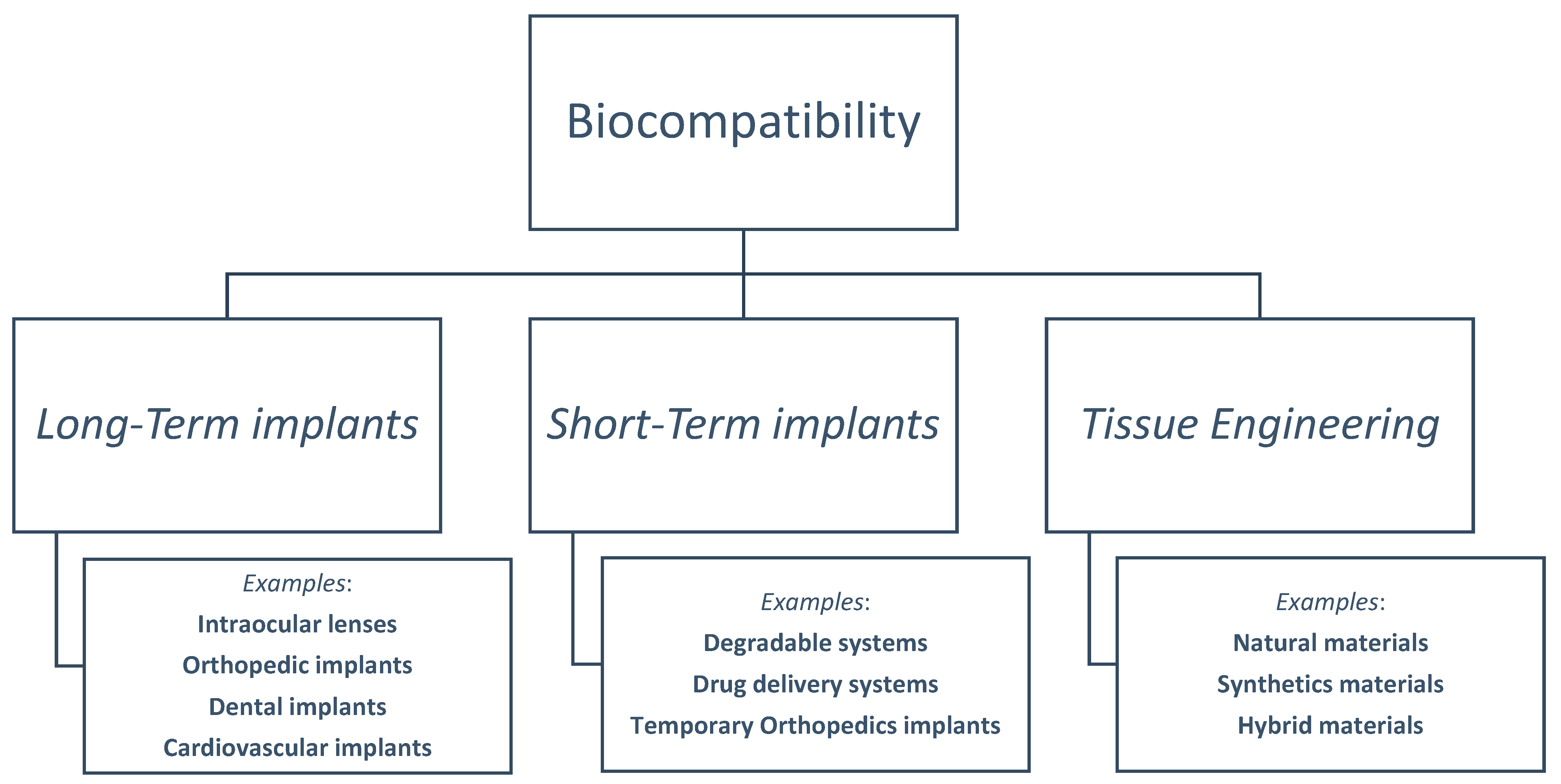
3. Definition and Uses of Biocompatibility in Different Fields
3.1. Long-Term Implants
3.1.1. Cardiovascular Implants
Artificial Heart Valves
Stents
3.1.2. Intraocular Lenses
3.1.3. Orthopedics
Total Joint Replacement
Spinal Implants
3.1.4. Dentistry and Prosthetic Implants
Dental Implants
Endosseous Tooth Implants
Subperiosteal and Staple/Transosteal Implants
Dental Restoration
Amalgam
Resin-Based Composites
3.1.5. Biocompatible Alloys
3.2. Short-Term Implants
3.2.1. Biodegradable Implanted Systems
3.2.2. Drug Delivery Systems
3.2.3. Temporary Orthopedic Implants
3.3. Tissue Engineering: Advancing Biocompatibility in Regenerative Medicine
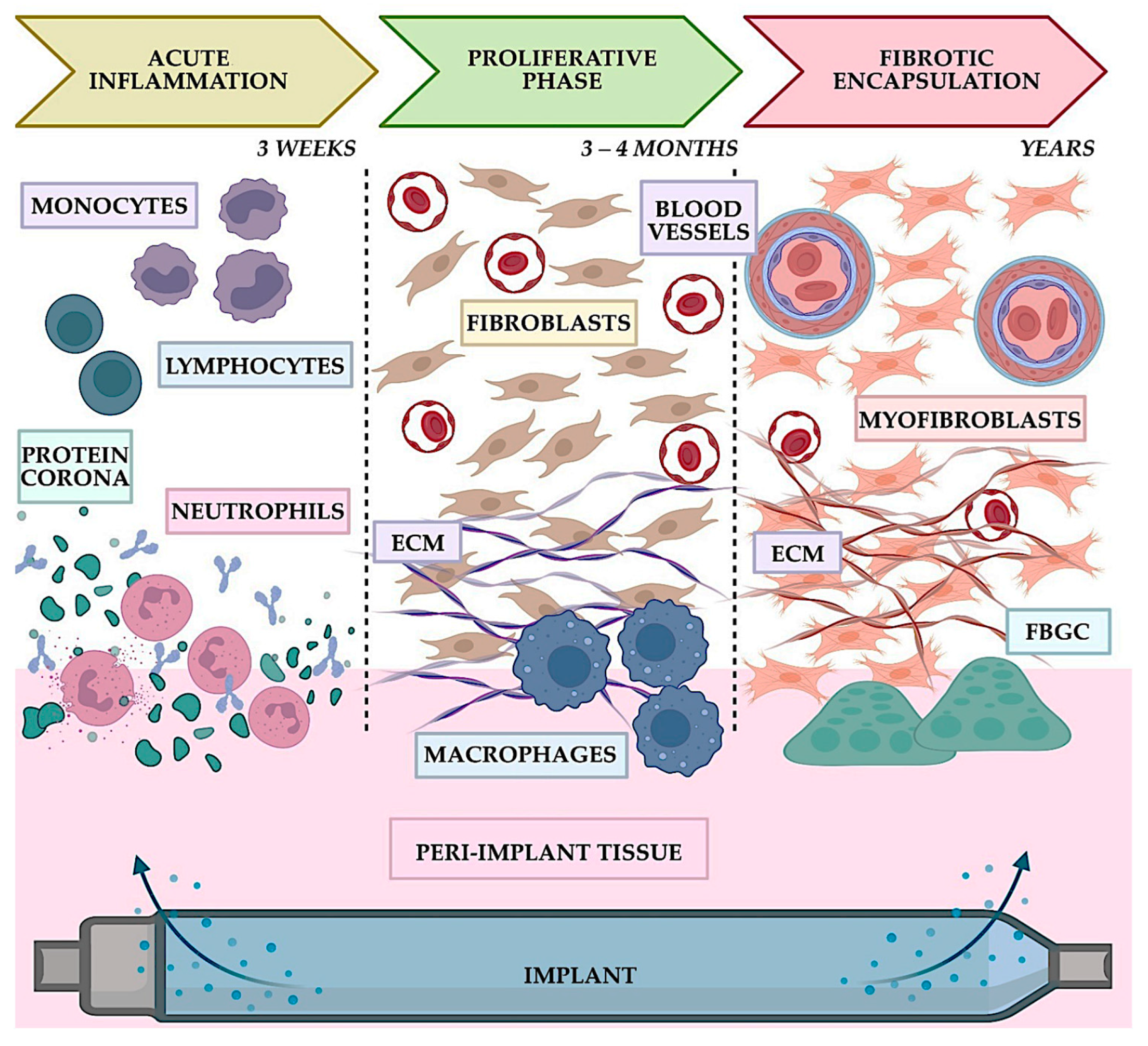
4. Biocompatibility Testing: Assessing Compatibility and Ensuring the Safety of Hosts
4.1. In Vivo vs. In Vitro Testing: Unveiling Material Safety
4.2. Tests of Various Material Properties: Evaluating the Biocompatibility
4.3. Mechanical Properties Assessment: Ensuring Performance and Durability
4.3.1. Chemical Testing
General Steps and Uses
Tests
4.3.2. Biological Testing or Assessment
Cytotoxicity
Sensitization Assays
Irritation Tests
Subchronic Toxicity Tests
Genotoxicity
Implantation Tests
Hemocompatibility
Carcinogenicity Tests
Reproductive and Developmental Toxicity Tests
Biodegradation Tests
Toxicokinetic Studies or Chronic Toxicity Tests
5. Regulatory Affairs Organizations That Deal with Biocompatibility and Their Focus
5.1. ISO, FDA, and TÜV SÜD
5.2. The Focus of Biocompatibility Evaluation
5.2.1. FDA and ISO 10993
5.2.2. TÜV SÜD
6. Comments, Points for Consideration, and What Is New in This Review
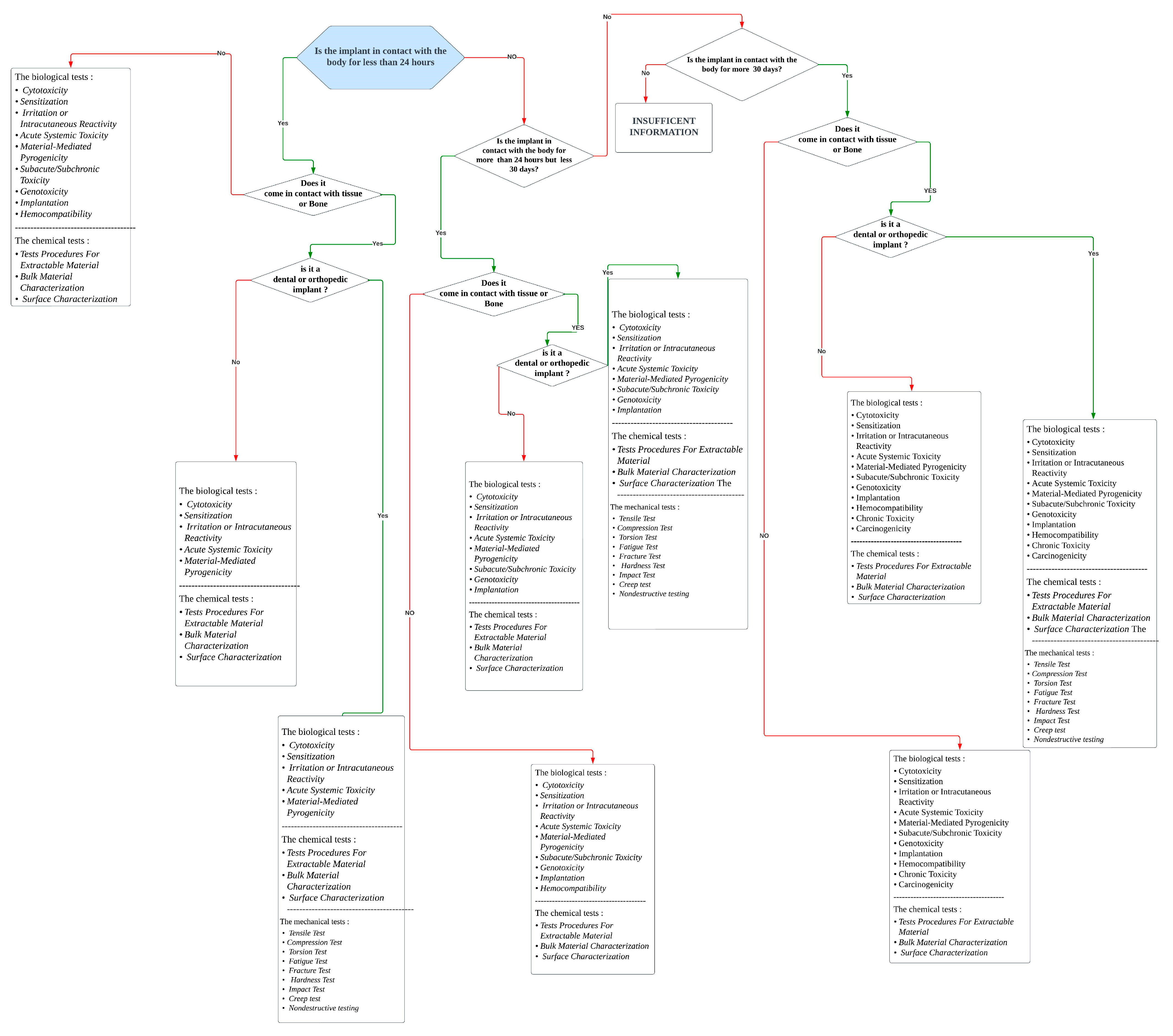
- The flowchart uses information from the FDA and ISO.
- The flowchart simplifies the understanding of the recommended testing framework.
- The flowchart lacks specific documents for the ISO 10933 series. It is based on an understanding of the concepts and preferred characteristics.
- The flowchart specifically focuses on the tests required for implantable devices.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, 11. [Google Scholar] [CrossRef]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Bengt Kasemo QSense Overview What Is Biocompatibility. Available online: https://www.biolinchina.com/wp-content/uploads/2019/11/QSense-Overview-What-is-biocompatibility.pdf (accessed on 20 November 2022).
- Huzum, B.; Puha, B.; Necoara, R.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.; Alexa, O. Biocompatibility Assessment of Biomaterials Used in Orthopedic Devices: An Overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Chen, S.T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 271. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a Key Functional Requirement of Next-Generation Medical Devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Gordon, S. Elie Metchnikoff, the Man and the Myth. J. Innate Immun. 2016, 8, 223–227. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. A History of Biomaterials; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- History of Linen and Why Linen|Fek’s Bedding. Available online: https://www.feksbedding.com/services-7 (accessed on 21 February 2023).
- Kim, H.; Hwang, K.; Yun, S.M. Catgut and Its Use in Plastic Surgery. J. Craniofac Surg. 2020, 31, 876–878. [Google Scholar] [CrossRef]
- Schiappa, J.; Van Hee, R. From Ants to Staples: History and Ideas Concerning Suturing Techniques. Acta Chir. Belg. 2012, 112, 395–402. [Google Scholar] [CrossRef]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Hernigou, P. Earliest Times before Hip Arthroplasty: From John Rhea Barton to Themistocles Glück. Int. Orthop. 2013, 37, 2313–2318. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty-over 100 Years of Operative History. Orthop. Rev. 2011, 3, e16. [Google Scholar]
- Singh, H.; Kaur, M.; Dhillon, J.S.; Mann, J.S.; Kumar, A. Evolution of Restorative Dentistry from Past to Present. Indian. J. Dent. Sci. 2017, 9, 38. [Google Scholar] [CrossRef]
- Knosp, H.; Holliday, R.J.; Corti, C.W. Gold in dentistry: Alloys, uses and performance. Gold Bull. 2003, 36, 93–102. [Google Scholar] [CrossRef]
- Muchtar, A.; Amat, N.F.; Yahaya, N.; Ghazali, M.J. A Review of Zirconia as a Dental Restorative Material. Aust. J. Basic. Appl. Sci. 2012, 6, 9–13. [Google Scholar]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-Alloys for Long-Term Implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Jeewandara, T.M.; Wise, S.G.; Ng, M.K.C. Biocompatibility of Coronary Stents. Materials 2014, 7, 769–786. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Heart Valve Disease—What Does That Mean? Available online: https://drtejaspatel.com/heart-valve-disease-diagnosis-treatment/ (accessed on 22 July 2023).
- Stanisławska, A. Biomaterials and Implants in Cardiac and Vascular Surgery—Review. Adv. Mater. Sci. 2014, 14, 5–17. [Google Scholar] [CrossRef]
- Heart Valve Repair and Replacement|Thoracic Key. Available online: https://thoracickey.com/heart-valve-repair-and-replacement/ (accessed on 23 July 2023).
- Özyol, P.; Özyol, E.; Karel, F. Biocompatibility of Intraocular Lenses. Turk. Oftalmoloiji Derg. 2017, 47, 221–225. [Google Scholar] [CrossRef]
- Explained: Hydrophobic and Hydrophilic | MIT News | Massachusetts Institute of Technology. Available online: https://news.mit.edu/2013/hydrophobic-and-hydrophilic-explained-0716 (accessed on 13 February 2023).
- Corrosionpedia What Is a Creep Strength?—Definition from Corrosionpedia. Available online: https://www.corrosionpedia.com/definition/6260/creep-strength (accessed on 26 December 2022).
- Katti, K.S. Biomaterials in Total Joint Replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- John, K.R.S. Biocompatibility of Dental Materials. Dent. Clin. N. Am. 2007, 51, 747–760. [Google Scholar] [CrossRef]
- Noble, A. The Biocompatibility of Various Dental Materials. Sci. J. Lander Coll. Arts Sci. 2020, 13, 13–20. [Google Scholar]
- Park, J.B.; Lakes, R.S. Biomaterials: An Introduction; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
- Park, J.B. Biomaterials Science and Engineering; InTech: Vienna, Austria, 2012. [Google Scholar]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An Update. J. Indian. Prosthodont. Soc. 2013, 13, 2. [Google Scholar] [CrossRef]
- What Is an Endosseous Implant|ICOI. Available online: https://www.icoi.org/glossary/endosseous-implant/ (accessed on 31 March 2023).
- Grandin, H.M.; Berner, S.; Dard, M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Dental Implants Periodontist Explains: Types of Dental Implants. Available online: https://appledentalimplantcentre.com/types-of-dental-implants/ (accessed on 7 July 2023).
- Aalam, A.A.; Krivitsky, A.; Kurtzman, G.M. Decision Making with Zygomatic and Pterygoid Dental Implants in the Severely Atrophic Maxilla: A Narrative Review. Dent. Rev. 2022, 2, 100054. [Google Scholar] [CrossRef]
- Roy, M.; Corti, A.; Dominici, S.; Pompella, A.; Cerea, M.; Chelucci, E.; Dorocka-Bobkowska, B.; Daniele, S. Biocompatibility of Subperiosteal Dental Implants: Effects of Differently Treated Titanium Surfaces on the Expression of ECM-Related Genes in Gingival Fibroblasts. J. Funct. Biomater. 2023, 14, 59. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Nuvvula, S.; Matinlinna, J.P.; Yiu, C.K.; King, N.M. Biocompatibility of Various Dental Materials in Contemporary Dentistry: A Narrative Insight. J. Investig. Clin. Dent. 2013, 4, 9–19. [Google Scholar] [CrossRef]
- Rathore, M.; Singh, A.; Pant, V.A. The Dental Amalgam Toxicity Fear: A Myth or Actuality. Toxicol. Int. 2012, 19, 81. [Google Scholar] [CrossRef]
- Britannica, The Editors of Encyclopaedia. “alloy”. Encyclopedia Britannica, 20 July 2023. Available online: https://www.britannica.com/technology/alloy (accessed on 13 October 2023).
- Bərbîņə, A.C.; Mareci, D.; Chelariu, R.; Bolat, G.; Munteanu, C.; Cho, K.; Niinomi, M. The Estimation of Corrosion Behavior of New TiNbTaZr Alloys for Biomedical Applications. Mater. Corros. 2014, 65, 1017–1023. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Țugui, C.A.; Perju, M.C.; Benchea, M.; Spataru, M.C.; Sandu, A.V.; Vizureanu, P. Biocompatible Titanium Alloys Used in Medical Applications. Rev. Chim. 2019, 70, 1302–1306. [Google Scholar] [CrossRef]
- Sing, S.L. Perspectives on Additive Manufacturing Enabled Beta-Titanium Alloys for Biomedical Applications. Int. J. Bioprint 2022, 8, 478. [Google Scholar] [CrossRef]
- Dart, A.J.; Dart, C.M. Suture Material: Conventional and Stimuli Responsive. Compr. Biomater. 2011, 6, 573–587. [Google Scholar] [CrossRef]
- Fleck, S.K.C. Biodegradable Magnesium Alloys as Temporary Orthopaedic Implants: A Review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Biocompatibility and Drug Delivery Systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Jin, W.; Chu, P.K. Orthopedic Implants. Encycl. Biomed. Eng. 2017, 425–439. [Google Scholar] [CrossRef]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic Implants and Devices for Bone Fractures and Defects: Past, Present and Perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C.; Gad-Mcdonald, S. Biomaterials, Medical Devices, and Combination Products Biocompatibility Testing and Safety Assessment; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Learn About the Types of Biocompatibility Tests—Pacific BioLabs. Available online: https://pacificbiolabs.com/types-of-biocompatibility-tests/ (accessed on 26 December 2022).
- In Vivo vs. In Vitro: Definition, Examples, and More. Available online: https://www.healthline.com/health/in-vivo-vs-in-vitro#definitions (accessed on 26 December 2022).
- Physical and Chemical Characterization of Medical Devices|Element. Available online: https://www.element.com/nucleus/2020/physical-and-chemical-characterization-of-medical-devices (accessed on 1 April 2023).
- Mechanical Testing of Biomaterials and Tissues; CBSET Inc.: Lexington, MA, USA, 2012; Available online: https://cbset.org/wp-content/uploads/2016/03/CBSETMechanicalTestingFlyer.pdf (accessed on 1 April 2023).
- Mechanical Testing: Different Types of Testing for Mechanical Properties. Available online: https://www.rapiddirect.com/blog/what-is-mechanical-testing/ (accessed on 1 April 2023).
- The More You Know, the More You Save: A Smart Approach to Chemical Characterization. Available online: https://cdnmedia.eurofins.com/european-west/media/1925429/9417-mdt-smart-approach-to-chemical-characterization_web.pdf (accessed on 17 April 2023).
- Biocompatibility Chemical Characterization—Pacific BioLabs. Available online: https://pacificbiolabs.com/biocompatibility-chemical-charaterization (accessed on 17 April 2023).
- Characterization and Analytical Techniques—Latest Research and News|Nature. Available online: https://www.nature.com/subjects/characterization-and-analytical-techniques (accessed on 6 May 2023).
- Introduction to Biocompatibility Testing—Pacific BioLabs. Available online: https://pacificbiolabs.com/biocompatibility-testing (accessed on 2 May 2023).
- Biocompatibility Test Methods—Pacific BioLabs. Available online: https://pacificbiolabs.com/biocompatibility-test-methods (accessed on 1 April 2023).
- Biological Evaluation of Medical Devices-Copyright Protected Document. 2009. fda.org. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 6 May 2023).
- TÜV SÜD Your Challenges. Available online: https://www.tuvsud.com/en/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-testing/chemical-or-biological-testing-of-medical-devices/biocompatibility-testing (accessed on 11 March 2023).
- Administration of Drugs and Experimental Compounds in Mice and Rats|Research Support. Available online: https://www.bu.edu/researchsupport/compliance/animal-care/working-with-animals/procedures/administration-of-drugs-and-experimental-compounds-in-mice-and-rats/ (accessed on 4 May 2023).
- Anderson, J.M. Biocompatibility. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 363–383. [Google Scholar] [CrossRef]
- A Practical Guide to ISO 10993-4: Hemocompatibility. Available online: https://www.mddionline.com/news/practical-guide-iso-10993-4-hemocompatibility (accessed on 6 May 2023).
- Carcinogenicity. Available online: https://joint-research-centre.ec.europa.eu/eu-reference-laboratory-alternatives-animal-testing-eurl-ecvam/alternative-methods-toxicity-testing/validated-test-methods-health-effects/carcinogenicity_en (accessed on 6 May 2023).
- Biocompatibility Testing for Medical Devices|TÜV SÜD. Available online: https://www.tuvsud.com/en/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-testing/chemical-or-biological-testing-of-medical-devices/biocompatibility-testing (accessed on 11 March 2023).
- Biocompatibility Evaluation Endpoints by Device Category|FDA. Available online: https://www.fda.gov/medical-devices/biocompatibility-assessment-resource-center/biocompatibility-evaluation-endpoints-device-category#implant-tissuebone (accessed on 1 April 2023).
- GLP and Device Studies—Pacific BioLabs. Available online: https://pacificbiolabs.com/glp-medical-device (accessed on 1 April 2023).


| Application | Material | Who and When | Additional Notes |
|---|---|---|---|
| Sutures | Linen | Early Egyptian civilization [10]. | Linen was discovered to be extremely complimentary to human cells. It was extremely absorbent and capable of reducing fever as it is antibacterial. In addition, it can keep dust from passing and other properties that let it be handy [11]. |
| Catgut | Used by Europeans during the Middle Ages [10]. | Catgut’s capacity for withstanding tension and its ability to last make it the perfect material for sutures [12]. | |
| Heads of large biting ants | Famously seen in South Africa and India [10]. | Ants were used as “suturing devices”, because they had powerful mandibles. They were put extremely close to the wound and bit directly against both margins of the cut, reducing the distance between them. The ant’s body would split after a certain period, leaving the head and bite firmly in place to preserve a closed wound [13]. | |
| Hip prostheses | Wood | The first attempt at repairing a broken hip is made in 1840 [14]. | A wooden block did not replace any biological tissue, but it was inserted between the broken ends of the hip! Following such a procedure, more and more foreign and biological elements were inserted [15]. |
| Ivory | Glück’s implant was a ball and socket prosthesis in 1880 [14]. | Ivory is a substance found in various biological components, including elephant tusks, boar teeth, and many more. It is recognized to have various desirable features, including mechanical properties, machinability, and homogeneity [8]. | |
| Glass | Smith-Petersen used to fit with the hip joint in 1925 [10]. | Despite its moderate biocompatibility, glass was unsuccessful as a hip prosthesis material. The smooth surface of the 1925 glass as an entire hip prosthesis quickly cracked under the pressures exerted by the joint [16]. | |
| Dental Restorative materials | Gold | In 1795, Robert Wolfendale was the first to employ gold (gold foil) for tooth repair.Later, in 1855, gold foil was found to be a cohesive substanc [17]. | Gold foil is composed of pure gold and is desirable to use in the tooth restoration process with cold work. This temperature range is beneficial, which results in an exact filling. However, gold mechanical resistance is incompatible with the application, so it was only employed for extremely tiny holes [18]. |
| Zirconia | In 1789, German chemists learned how to use it [19]. | Approximately 20 years ago, zirconia emerged as a promising restorative dental material due to its superior mechanical qualities. The decision to employ zirconia was primarily driven by its exceptional strength, making it suitable for load-bearing applications. However, despite its advantageous mechanical properties, zirconia fell short in terms of aesthetic appearance due to its opaque coloration [19]. |
| Component | Description | Examples |
|---|---|---|
| Locking Element | One or more moving parts that facilitate the valve’s opening and closing. |
|
| Cover | The cage or ring that houses the locking element, allowing it to move. |
|
| Base | A ring-shaped component bordered with synthetic fabric, providing the foundation for the valve’s assembly. |
|
| Device | Purpose | Materials | Biomechanical Properties | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Cage | Used as a stabilizer to distribute forces between vertebral bodies and to restore space between intervertebral and foramina space. | It is typically made from metal, ceramic, plastic, most commonly PEEK, titanium, and stainless steel. | Elastic modulus is similar to bone; radiolucent; good load-sharing; minimally invasive; preserves normal spinal anatomy. | They provide a graft for vertebrae to refuse and heal when the intervertebral disc has failed. Because of their porosity, they allow the bone to grow through them. | Some materials might be hydrophobic and unable to bond to bone for solid fusion. |
| Pedicle Screws | Provide rigid attachment between vertebrae and rod; allows for precise correction and alignment. Allow the redirection of forces. | Titanium, especially TiAl4V, stainless steel, cobalt-chromium. | High bending and torsional strength; low profile; rigid fixation; improved fusion rates; reduced rates of pseudarthrosis. | They can withstand significant forces and loads which are used in scoliosis. | There is a high possibility of loosening the screw, pulling out, or breaking, that might affect bone healing. |
| Spinal Rods | Adds stability to spinal implant structure; contoured to the patient’s spine. | Titanium, PEEK, stainless steel, cobalt-chromium, nitinol. | Biocompatible; improved biomechanical properties; minimal artifact on imaging; improved sagittal realignment. | The choice of material provides the patient with a wide range or customized characteristics. | Risk of fatigue, fractures, deformation; notch sensitivity; difficulty in identifying faults or breaks; risk of pseudarthrosis; the possibility of leaving weakness that affects overall durability. |
| Spinal Plates | Adds stability to spinal implant structure; screws into vertebral bodies to help restore normal alignment. | Titanium, stainless steel. | Rigid fixation; improved fusion rates. | — | — |
| The Key Properties | The Purpose | Actions Taken |
|---|---|---|
| Mechanical properties | Ensure endurance and functionality under operating conditions. |
|
| Wear resistance | Minimize implant failure due to wear debris and osteolysis. |
|
| Corrosion resistance | Ensure implant longevity and prevent the release of harmful substances. |
|
| Biocompatibility | Avoid toxicity and immune system-triggered complications. |
|
| osseointegration | Facilitate integration with a neighboring bone for stability. |
|
| Mechanical Test | Description | Additional Information |
|---|---|---|
| Tensile Test | Examining the stress, strain, and yield deformation of materials under tension. A sample is pulled until it breaks while measuring the applied force and deformation. | The test standards vary depending on the material, such as ASTM D638 / ISO 527-2 for reinforced plastics, ASTM D412 / ISO 37 for vulcanized and thermoplastic rubber, and ASTM E8 / ASTM A370/ISO 6892 for metals [60]. |
| Compression Test | Determine compressive strength, stiffness, and deformation of materials. A sample is compressed until it breaks while measuring the applied force and deformation. | ASTM D3574 covers flexible cellular materials, ASTM D695-15 covers rigid plastics, AITM 0010 covers 2-Inch Concrete Cubes, and ISO 844 covers rigid cellular plastics [60]. |
| Torsion Test | Measures the behavior of materials under torsional load (angular) to determine their torsional strength, stiffness, and ductility. The test provides information about shear modulus of elasticity, shear yield strength, shear strength, and more. | Various types of torsion tests are conducted, including torsion only, axial torsion, and failure tests, depending on the specific requirements of the material or device being tested. |
| Fatigue Test | Measures the behavior of materials under cyclic load applied at different angles to determine their fatigue strength and fatigue life. A sample is subjected to repeated loading and unloading cycles until it fails while measuring the applied stress and number of cycles. | The results of fatigue tests are typically presented in the form of a graph showing the number of cycles to failure plotted against the amplitude of the cyclic stress. |
| Fracture Test | Measures the energy required to cause an already cracked material to break fully. This test helps determine the material’s ability to resist fracturing and provides insights into brittle fracture behavior and grain size examination. | Fracture tests are conducted to assess the fracture toughness and brittleness of the material and to study the grain structure and any potential defects. |
| Hardness Test | Measures the ability of materials to resist indentation, scratching, or deformation. Different hardness tests, such as Brinell, Rockwell, and Vickers, employ different methods to measure hardness. | Hardness tests assess the material’s resistance to indentation or deformation, with specific test methods chosen based on the material and the desired hardness scale. |
| Impact Test | Measures the behavior of materials under sudden impact or shock load to determine their impact strength and toughness. A sample is subjected to a sudden impact or shock while measuring the energy absorbed by the sample. | There are two common impact tests: the Charpy and Izod tests. Both involve fracturing the material and measuring the energy absorbed during fracture to determine its impact resistance. |
| Creep test | Also known as a stress–relaxation test, it provides insights into the behavior of a material under constant stress. | Creep tests involve subjecting the material to constant stress or load for an extended period and measuring the resulting deformation or relaxation over time. Creep behavior is important for understanding long-term material performance. |
| Nondestructive testing | Nondestructive testing methods assess a material’s mechanical properties without damaging the original material. | Nondestructive testing techniques, such as acoustic emission testing, electromagnetic testing, and leak testing, are employed to evaluate the mechanical properties of materials without causing any permanent damage. These tests are valuable for quality control and inspection purposes. |
| Category | Examples |
|---|---|
| Traditional Extractable Material Characterization | USP (United States Pharmacopeia) Physicochemical Test Panel for Elastomeric Closures for Injections USP Polyethylene Containers Tests–Heavy Metals and Nonvolatile Residues Indirect Food Additives and Polymers Extractables (21CFR Part 177 Sterilant Residues–Ethylene Oxide, Ethylene Chlorohydrin, Ethylene Glycol |
| Tests Procedures for Extractable Material | Liquid Chromatography Infrared Spectroscopy (IR) Mass Spectrometry Residual Solvents Atomic Absorption Spectroscopy (AAS) Inductively coupled Plasma Spectroscopy (ICP) |
| Bulk Material Characterization | Atomic Absorption Spectroscopy (AAS) Inductively coupled Plasma Spectroscopy (ICP) Thermal Analysis Infrared Spectroscopy Analysis to identify and estimate the Gross Composition (For example, Reflectance Spectroscopy, Transmission Spectroscopy |
| Surface Characterization | IR Reflectance Spectroscopy Scanning Electron Microscopy (SEM) |
| Type of Test | Description |
|---|---|
| Ames Test | Detects point mutations using Salmonella typhimurium bacterial strains sensitive to mutagens. |
| Mouse Lymphoma Assay | It uses mammalian cells to detect point mutations and can detect clastogenic lesions in genes. |
| HGPRT Assay | It uses mammalian cells to detect point mutations. |
| Unscheduled DNA Synthesis (UDS) Assay | Detects DNA damage and repair using both in vitro and in vivo methods. |
| Chromosomal Aberration Assay | Allows direct observation of chromosome damage using both in vitro and in vivo methods. |
| Mouse Micronucleus Assay | Detects chromosome damage using mammalian cells. |
| Test Name | Recommended for | Purpose |
|---|---|---|
| Hemolysis assay | All devices except those that do not have direct contact with blood cells | Measures the damage to red blood cells when exposed to materials or their extracts and compares it to positive and negative controls. |
| Coagulation assays | All devices with blood | Measures the effect of the test article on human blood coagulation time. |
| Prothrombin Time Assay | All devices with blood | General screening test for the detection of coagulation abnormalities in the extrinsic pathway. |
| Partial Thromboplastin Time Assay | All devices with blood | Detects coagulation abnormalities in the intrinsic pathway. |
| Thrombogenicity test | Devices unsuited to in vivo | Required tests in coagulation, platelets, hematology, and complement system categories. The most common test for thrombogenicity is the in vivo method. |
| Complement activation | Implant devices | In vitro assay to measure complement activations in the human plasma due to exposure of the plasma to the test article or an extract. Measures complement activation. |
| Device Examples | Test Category | ||||
|---|---|---|---|---|---|
| Thrombosis | Coagulation | Platelets | Hematology | Complement System | |
| Annuloplasty rings, mechanical heart valves | x | x a | |||
| Intra-aortic balloon pumps | x | x | x | x | x |
| Total artificial hearts, ventricular-assist devices | x | x | |||
| Embolization devices | x a | ||||
| Endovascular grafts | x | x a | |||
| Implantable defibrillators and cardioverters | x | x a | |||
| Pacemaker leads | x | x a | |||
| Leukocyte removal filter | x | x | x a | ||
| Prosthetic (synthetic) vascular grafts and patches, including arteriovenous shunts | x | x a | |||
| Category | Contact Location | The Duration of Contact | |
|---|---|---|---|
| Meaning |
|
|
|
| Categories |
|
|
|
| Biological Effect | Limited Duration | Prolonged Duration | Permanent |
|---|---|---|---|
| Cytotoxicity | ✔ | ✔ | ✔ |
| Sensitization | ✔ | ✔ | ✔ |
| Irritation or Intracutaneous Reactivity | ✔ | ✔ | ✔ |
| Acute Systemic Toxicity | ✔ | ✔ | ✔ |
| Material-Mediated Pyrogenicity | ✔ | ✔ | ✔ |
| Subacute/Subchronic Toxicity | ✔ | ✔ | |
| Genotoxicity | ✔ | ✔ | |
| Implantation | ✔ | ✔ | |
| Chronic Toxicity | ✔ | ||
| Carcinogenicity | ✔ |
| Biological Effect | Limited Duration | Prolonged Duration | Permanent |
|---|---|---|---|
| Cytotoxicity | ✔ | ✔ | ✔ |
| Sensitization | ✔ | ✔ | ✔ |
| Irritation or Intracutaneous Reactivity | ✔ | ✔ | ✔ |
| Acute Systemic Toxicity | ✔ | ✔ | ✔ |
| Material-Mediated Pyrogenicity | ✔ | ✔ | ✔ |
| Subacute/Subchronic Toxicity | ✔ | ✔ | |
| Genotoxicity | ✔ | ✔ | ✔ |
| Implantation | ✔ | ✔ | ✔ |
| Hemocompatibility | ✔ | ✔ | ✔ |
| Chronic Toxicity | ✔ | ||
| Carcinogenicity | ✔ |
| Test | Standards | What Does It Evaluate? |
|---|---|---|
| Cytotoxicity | ISO 10993-5 [67] | Test for toxicity of medical device or material on cell culture. |
| Genotoxicity | ISO 10993-3 and FDA [67] | Test for toxins that affect the genetic material of cells. |
| Hemocompatibility | ISO 10993-4 and ASTM [67] | Test for effects of blood-contacting medical devices on blood. |
| Irritation and Sensitization | ISO 10993-10 [67] | Test for skin irritability and adverse cutaneous reactions. |
| Systemic Effects | ISO 10993-11 and ASTM [67] | Test for effects of medical devices on the body, for example, the possibility of fever and toxicity. |
| Implantation | ISO 10993-6 [67] | Test for effects of medical devices on surrounding tissue at various levels of visibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zyoud, W.; Haddadin, D.; Hasan, S.A.; Jaradat, H.; Kanoun, O. Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials 2023, 16, 6881. https://doi.org/10.3390/ma16216881
Al-Zyoud W, Haddadin D, Hasan SA, Jaradat H, Kanoun O. Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials. 2023; 16(21):6881. https://doi.org/10.3390/ma16216881
Chicago/Turabian StyleAl-Zyoud, Walid, Dana Haddadin, Sameer Ahmad Hasan, Hussamaldeen Jaradat, and Olfa Kanoun. 2023. "Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization" Materials 16, no. 21: 6881. https://doi.org/10.3390/ma16216881
APA StyleAl-Zyoud, W., Haddadin, D., Hasan, S. A., Jaradat, H., & Kanoun, O. (2023). Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials, 16(21), 6881. https://doi.org/10.3390/ma16216881









