Arsenic Removal and Iron Recovery from Arsenic-Bearing Iron Ores by Calcification-Magnetic Roasting and Magnetic Separation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Technical Route for CMR-LMS
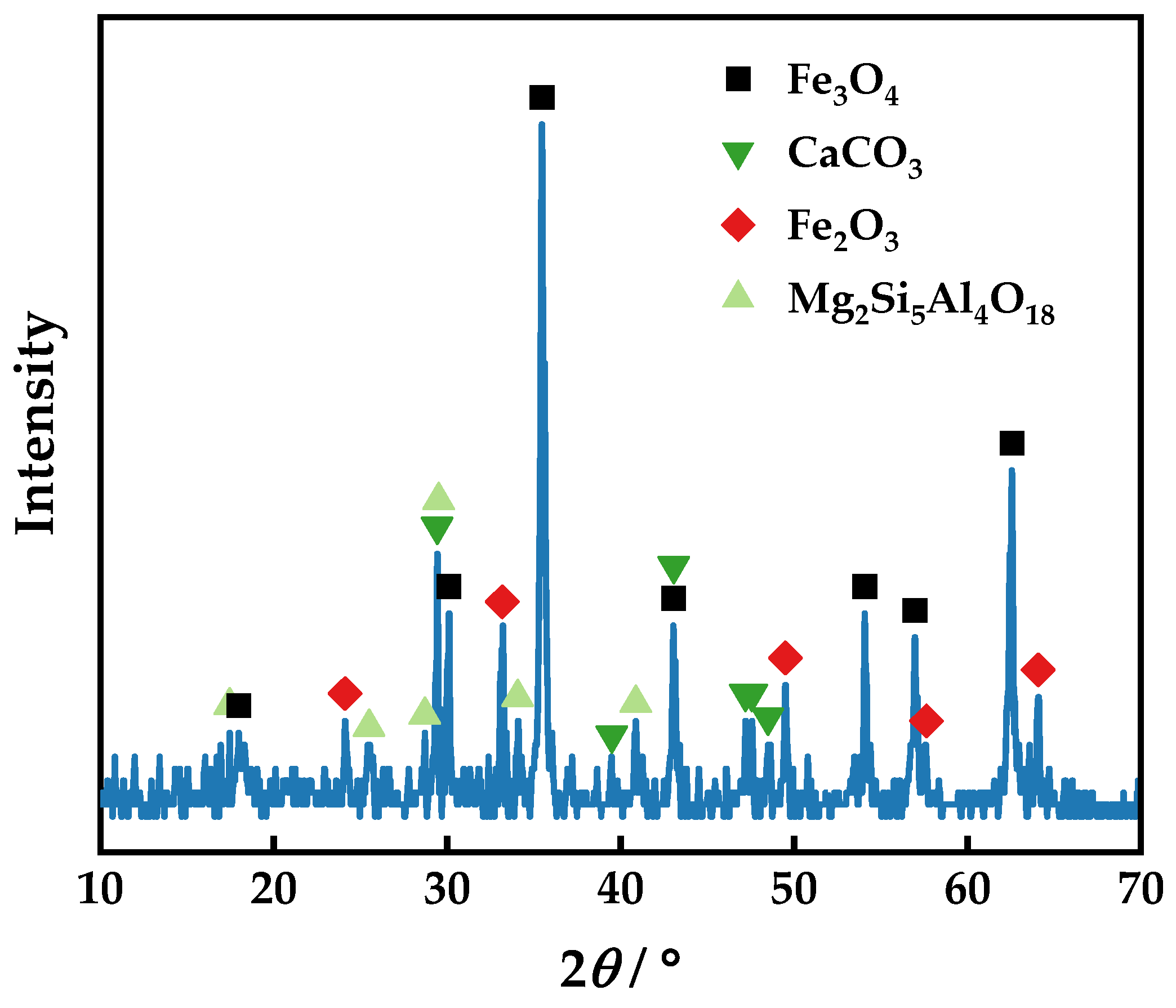
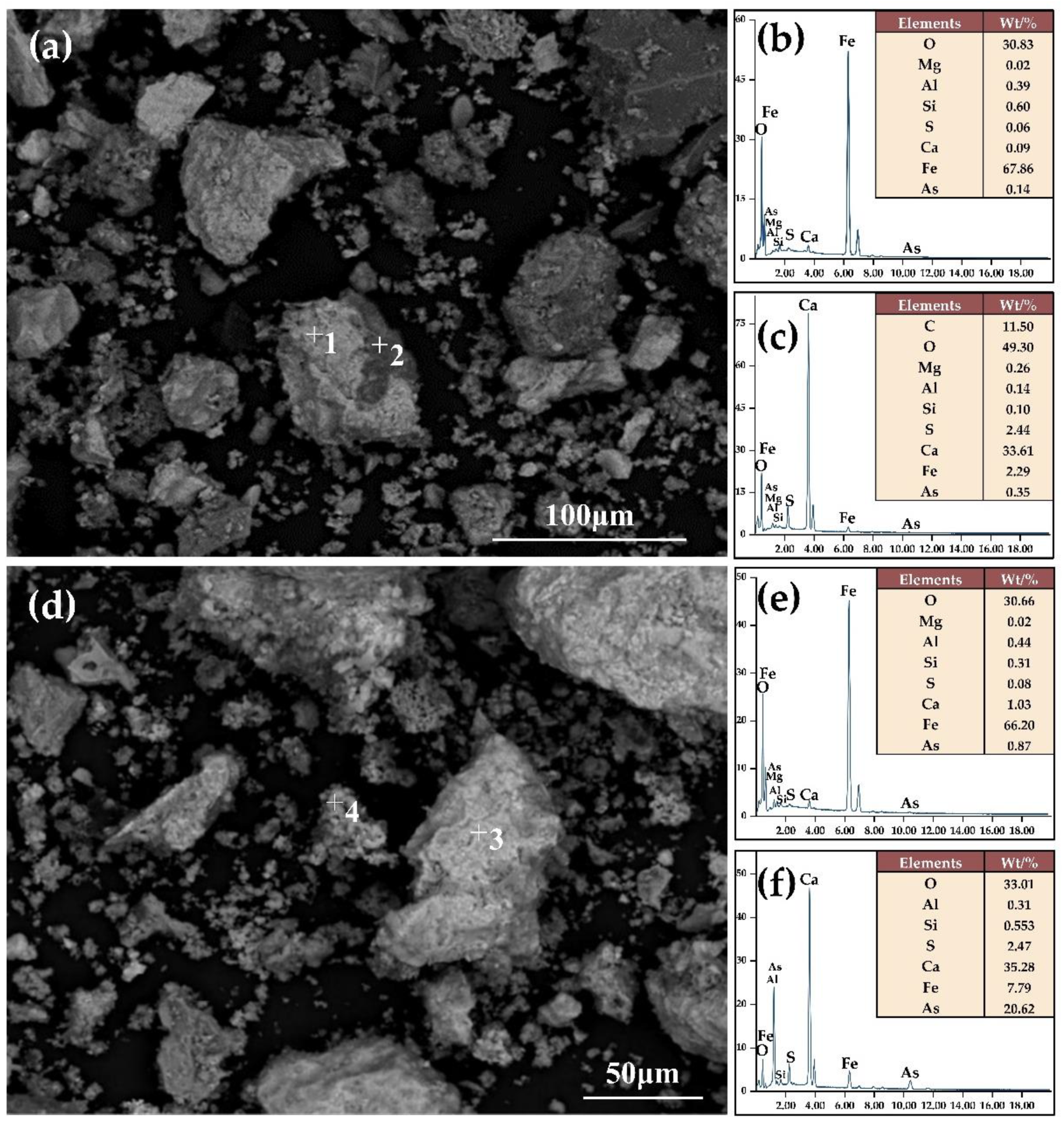
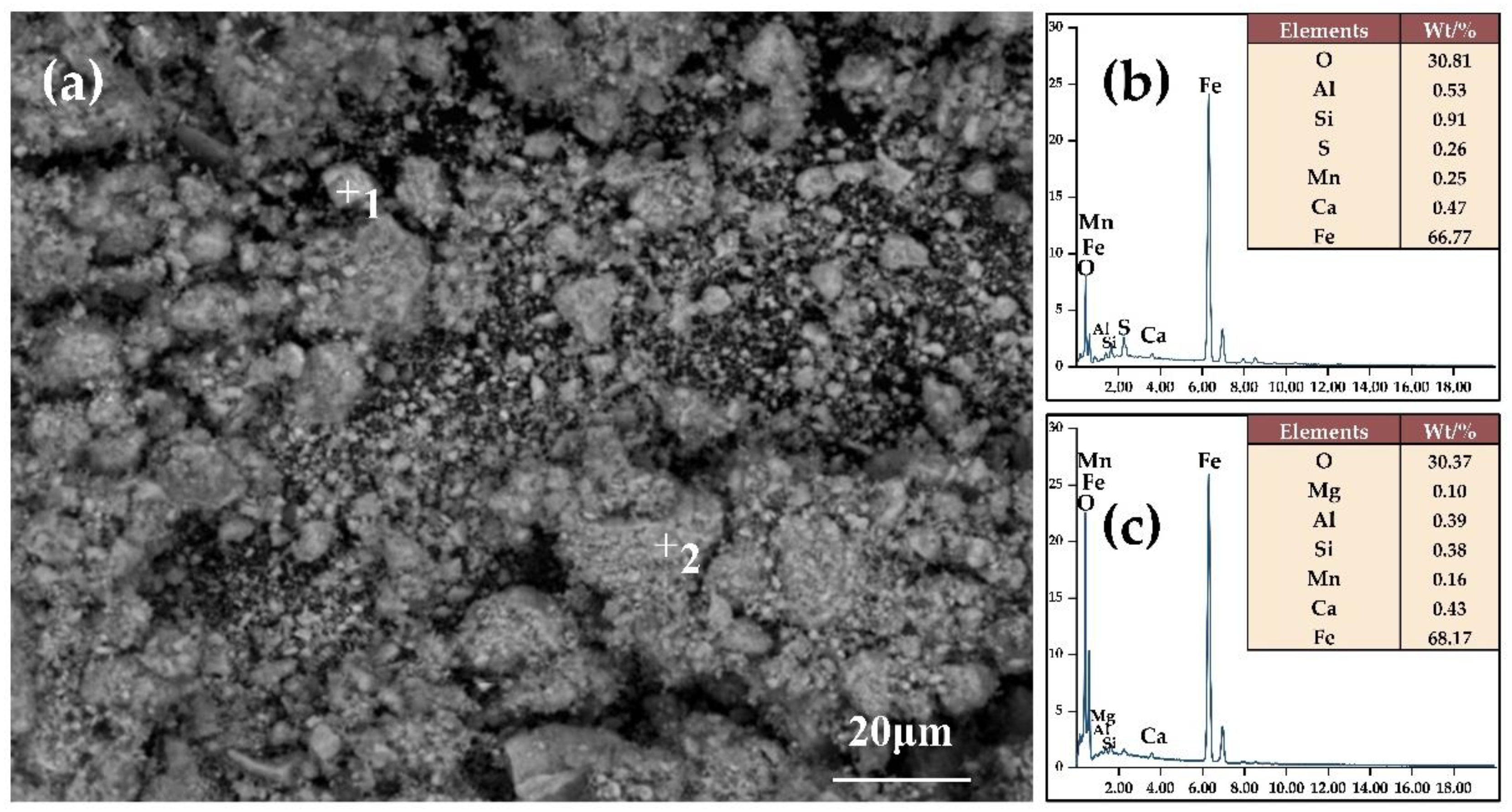
2.2.2. Characterization Test
3. Results and Discussion
3.1. Main Factors of Calcification-Magnetic Roasting
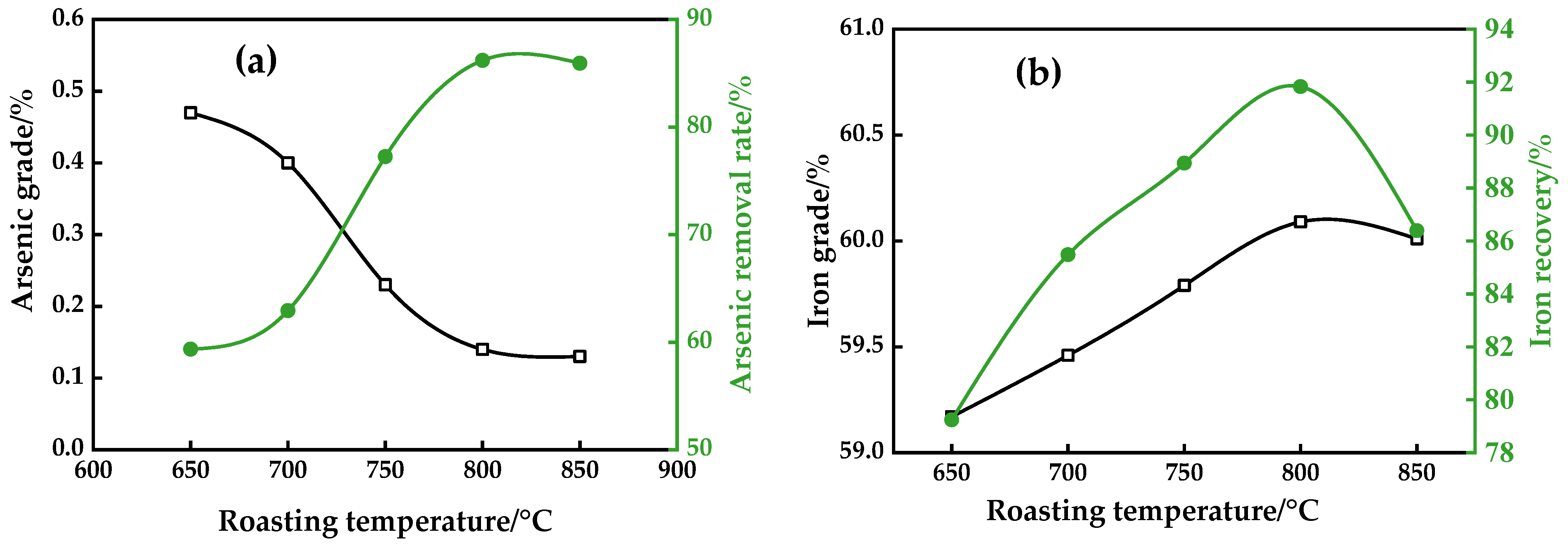
3.1.1. Roasting Temperature
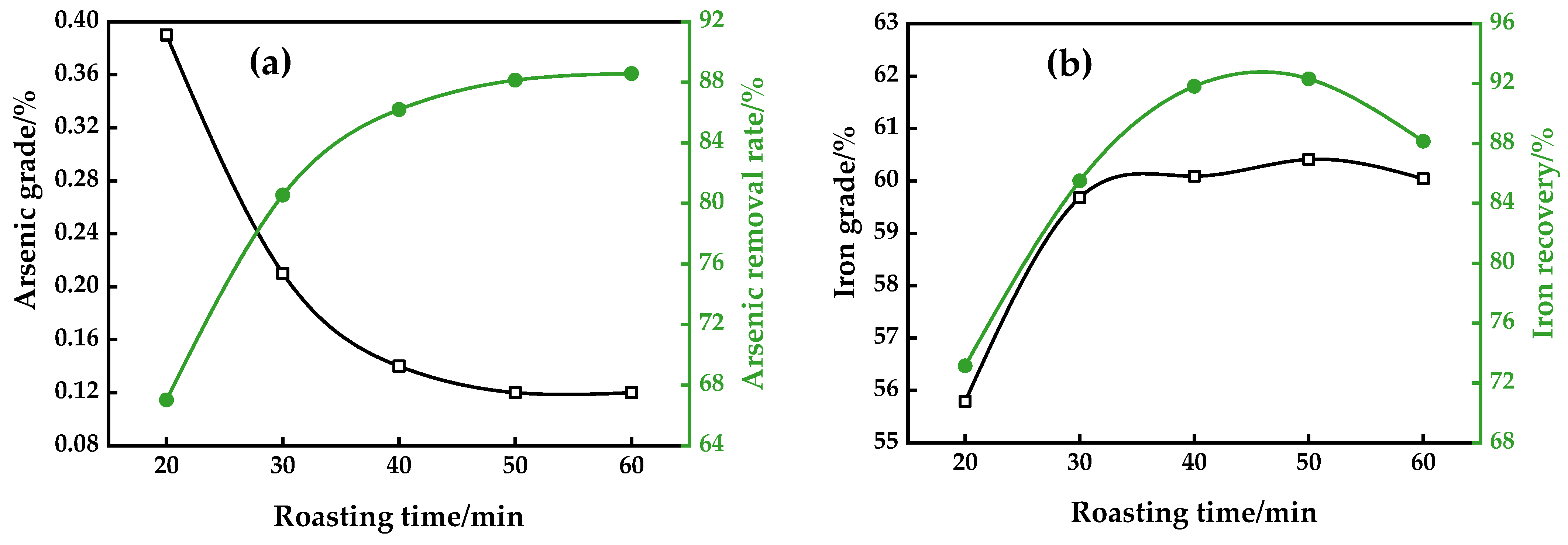
3.1.2. Roasting Time
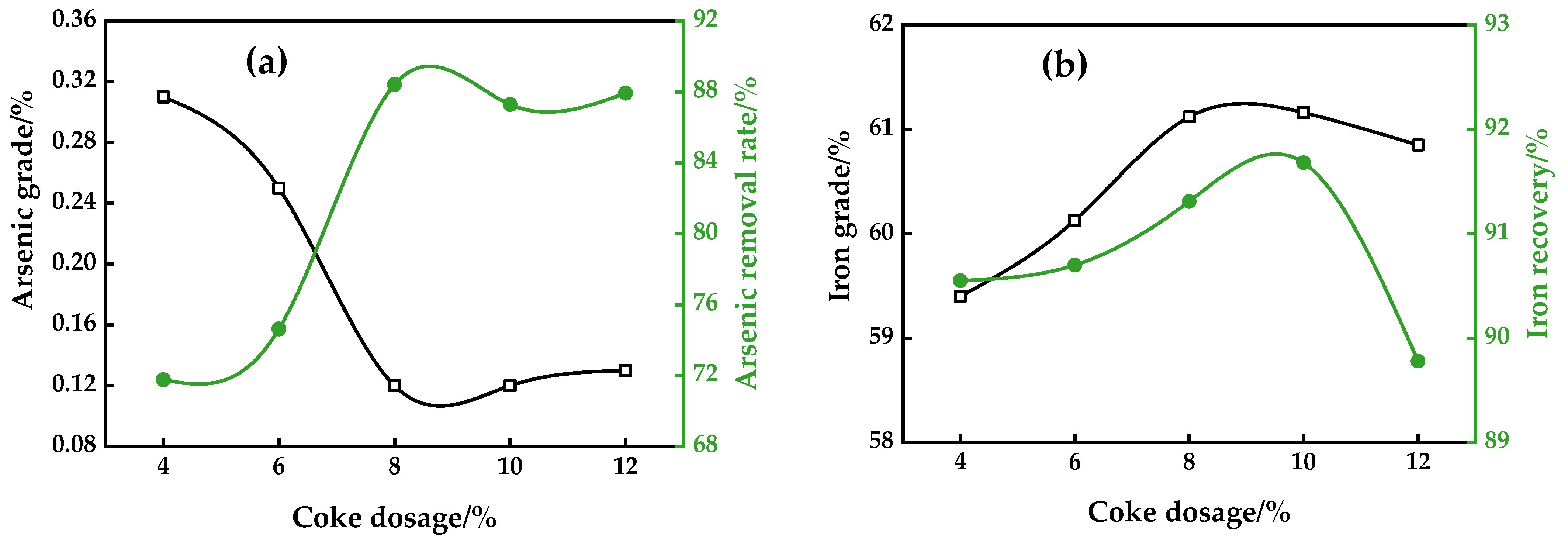
3.1.3. Coke Dosage
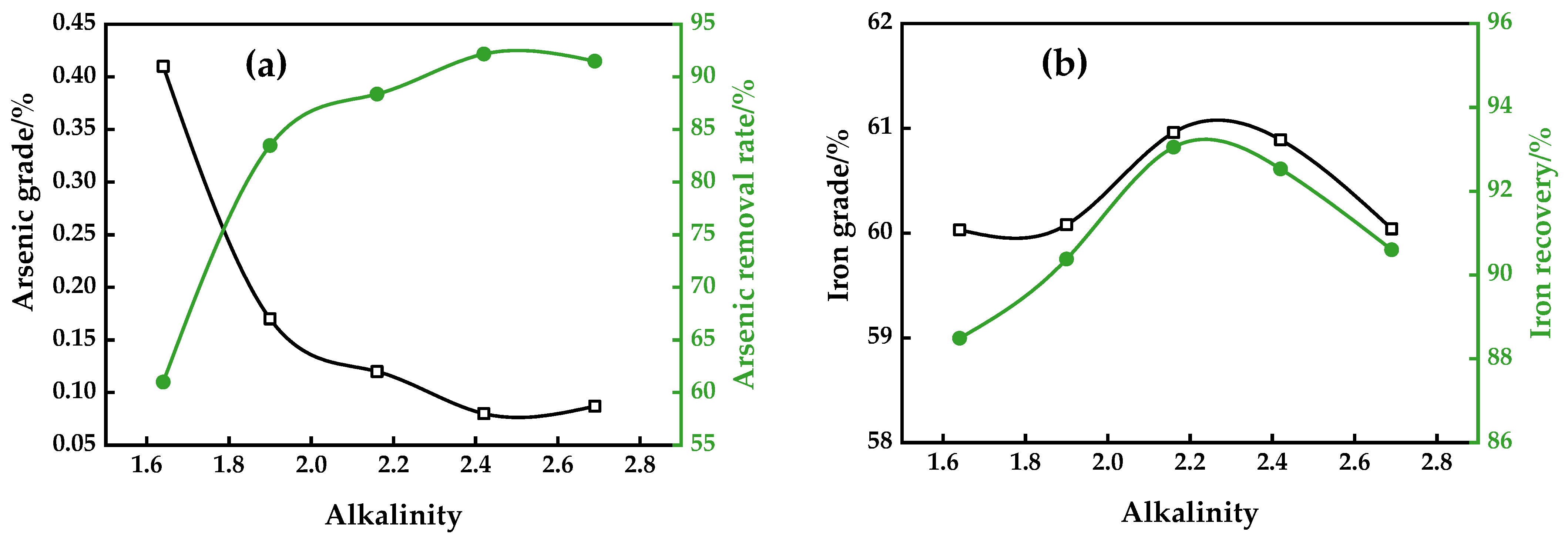
3.1.4. Alkalinity
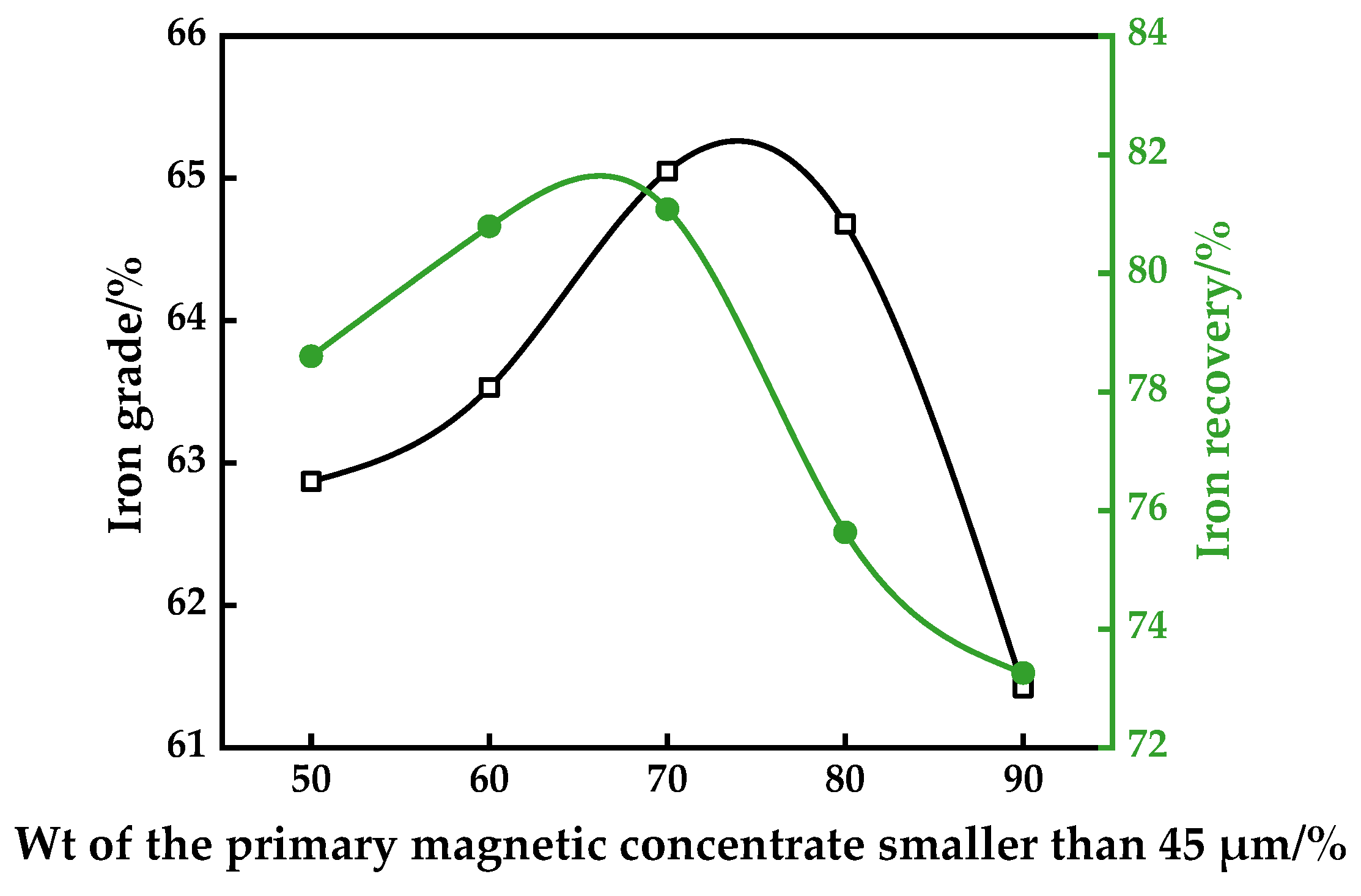
3.2. Effect of Grinding Fineness of the Second-Staged Magnetic Separation
3.3. Beneficiation of Arsenic and Iron Minerals with the CMR-LMS Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riveros, P.A.; Dutrizac, J.E.; Spencer, P. Arsenic disposal practices in the metallurgical industry. Can. Metall. Q. 2001, 40, 395–420. [Google Scholar] [CrossRef]
- Cheng, R.J.; Zhang, H.; Ni, H.W. Arsenic removal from arsenopyrite-bearing iron ore and arsenic recovery from dust ash by roasting method. Processes 2019, 7, 754. [Google Scholar] [CrossRef]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; Zhang, X.K.; Bai, S.C. Mechanism research on arsenic removal from arsenopyrite ore during a sintering process. Int. J. Miner. Metall. Mater. 2017, 24, 353–359. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107–133. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Davey, K.J.; Jorgensen, F.R.A.; Wright, S.; Brew, D.R.M.; Haque, N.; Vance, E.R. Development and evaluation of an early removal process for the beneficiation of arsenic-bearing copper ores. Miner. Eng. 2010, 23, 1167–1173. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.J.; Bradshaw, D. Flotation separation of copper sulphides from arsenic minerals at Rosebery copper concentrator. Miner. Eng. 2014, 66–68, 207–214. [Google Scholar] [CrossRef]
- Zhai, Q.; Liu, R.; Wang, C.; Wen, X.; Li, J.; Xie, Z.; Sun, W. Mineralogical characteristics of copper smelting slag affecting the synchronous flotation enrichment of copper and arsenic. J. Environ. Chem. Eng. 2022, 10, 108871. [Google Scholar] [CrossRef]
- Wang, L.T.; Wang, H.C.; Xu, F.; Chen, P.F. Experimental study of microwave treating high arsenic ore. Metall. Eng. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- You, Z.X.; Wen, P.D.; Li, G.H.; Zhang, Y.B.; Jiang, T. Effect of roasting atmosphere on arsenic volatilization behavior of As-bearing iron concentrate pellet. Chin. J. Nonferrous Met. 2016, 26, 1523–1530. [Google Scholar] [CrossRef]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; He, H.Y.; Yang, H.H.; Xiong, S. Experimental study on arsenic removal from low arsenic-bearing iron ore with sintering Process. Sinter. Pelletizing 2016, 41, 13–16. [Google Scholar] [CrossRef]
- Charcraborti, N.; Lyhch, D.C. Thermodynamics of roasting arsenopyrite. Metall. Mater. Trans. B 1982, 14, 239–251. [Google Scholar] [CrossRef]
- Mihajlovic, I.; Strbac, N.; Nikolic, D.; Živkovic, Z. Potential metallurgical treatment of Copper concentrates with high Arsenic contents. J. S. Afr. Inst. Min. Metall. 2011, 111, 409–416. [Google Scholar]
- Yin, Z.L.; Lu, W.H.; Xiao, H. Arsenic removal from copper-silver ore by roasting in vacuum. Vacuum 2014, 101, 350–353. [Google Scholar] [CrossRef]
- Lu, W.H.; Yin, Z.L. Study on thermal decomposition and Arsenic removal of a silver bearing Copper ore. Int. J. Miner. Process 2016, 153, 1–7. [Google Scholar] [CrossRef]
- Nakazawa, S.; Yazawa, A.; Jorgensen, F.R.A. Simulation of the removal of Arsenic during the roasting of Copper concentrate. Metall. Mater. Trans. B 1999, 30, 393–401. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.H.; Hu, X. Study on removal Arsenic from iron ore with Arsenic in sintering process. Adv. Mater. Res. 2011, 284, 238–241. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Nong, Z. Mineral phases and release behaviors of As in the process of sintering residues containing As at high temperature. Sci. World J. 2014, 2014, 260504. [Google Scholar] [CrossRef]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; Jia, S.K.; Xiong, S. Thermodynamics of arsenic removal from arsenic-bearing iron ores with sintering process and dust ash by roasting. Iron Steel 2017, 52, 26–39. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.H.; Hu, X. Experimental study on removal arsenic in iron ore with arsenic sintering process. Iron Steel 2010, 45, 7–11. [Google Scholar] [CrossRef]
- Zhang, S.H.; Lü, Q.; Hu, X. Thermodynamics of arsenic removal from arsenic-bearing iron ores. Chin. J. Nonferrous Met. 2011, 21, 1705–1712. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, H.M.; Yu, X.J. Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213–214, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Riveros, G.; Utigard, T.A. Disposal of arsenic in copper discharge slags. J. Hazard. Mater. 2000, 77, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.-B.; Gu, B.-S.; Ruan, Z.-Y.; Wang, Z.-H.; Luo, B.-C.; Chun, T.-J. Influence of specific surface area on the strength of iron oxidized pellets. Minerals 2022, 12, 921. [Google Scholar] [CrossRef]
- Uwadiale, G.G.O.O. Magnetizing reduction of iron ores. Miner. Process. Extr. Metall. Rev. 1992, 11, 1–19. [Google Scholar] [CrossRef]
- Dai, M.; Gu, B.; Ma, X.; Chun, T. Nitrogen monoxide reduction by carbon monoxide to combustion control with calcium ferrite redox in iron ore sintering. Fuel 2023, 337, 127172. [Google Scholar] [CrossRef]
- Yuan, S.; Xiao, H.; Wang, R.; Li, Y.; Gao, P. Improved iron recovery from low-grade iron ore by efficient suspension magnetization roasting and magnetic separation. Miner. Eng. 2022, 186, 107761. [Google Scholar] [CrossRef]
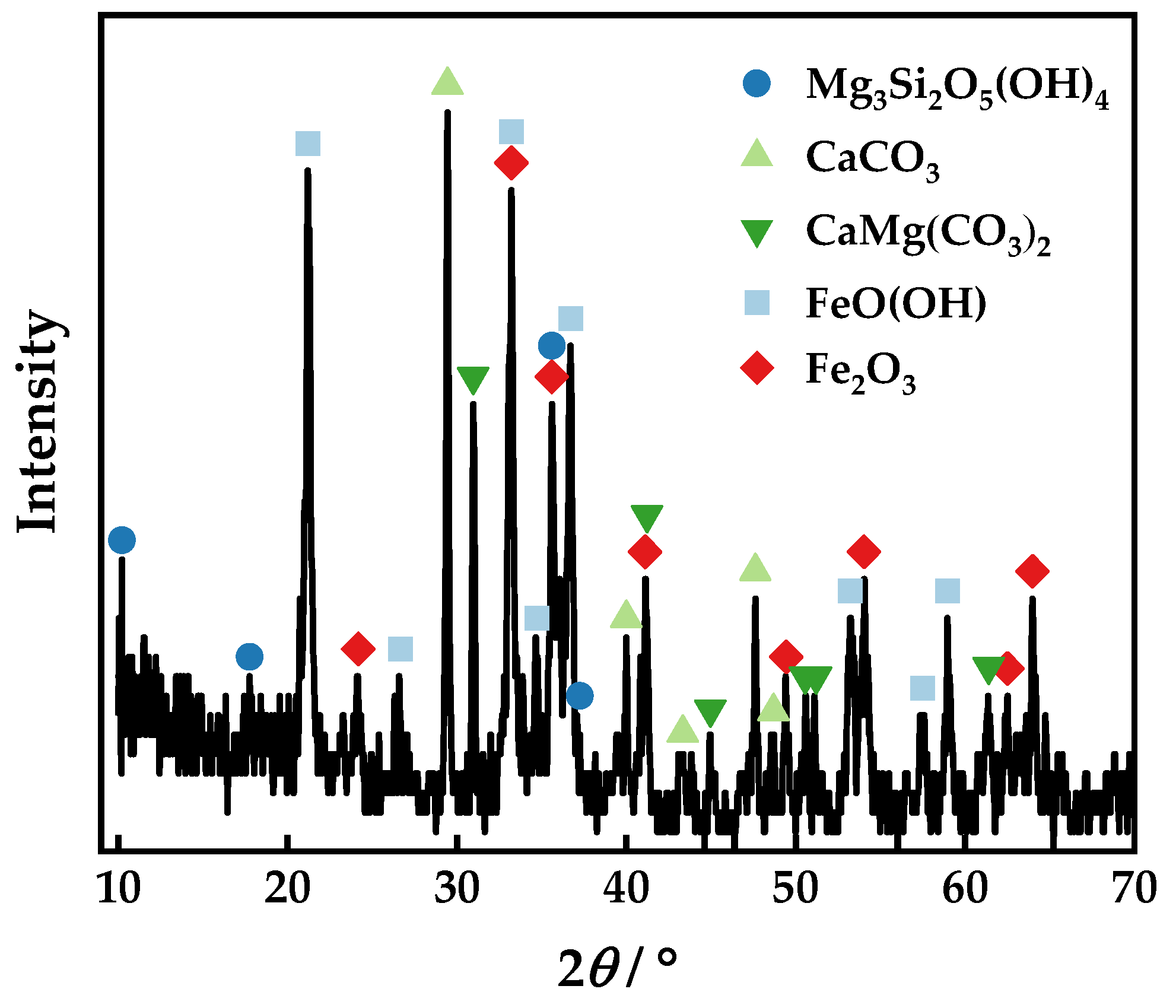
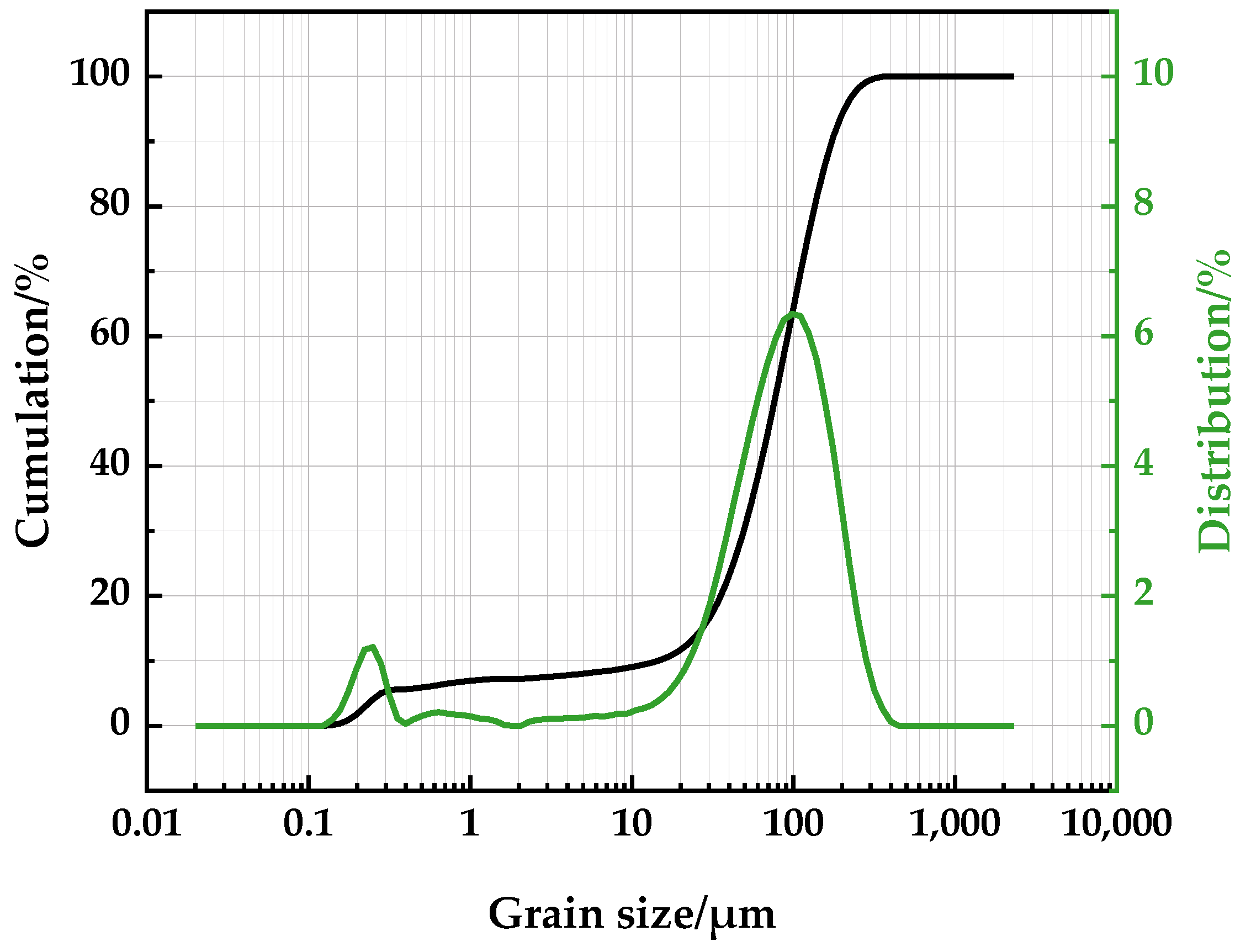
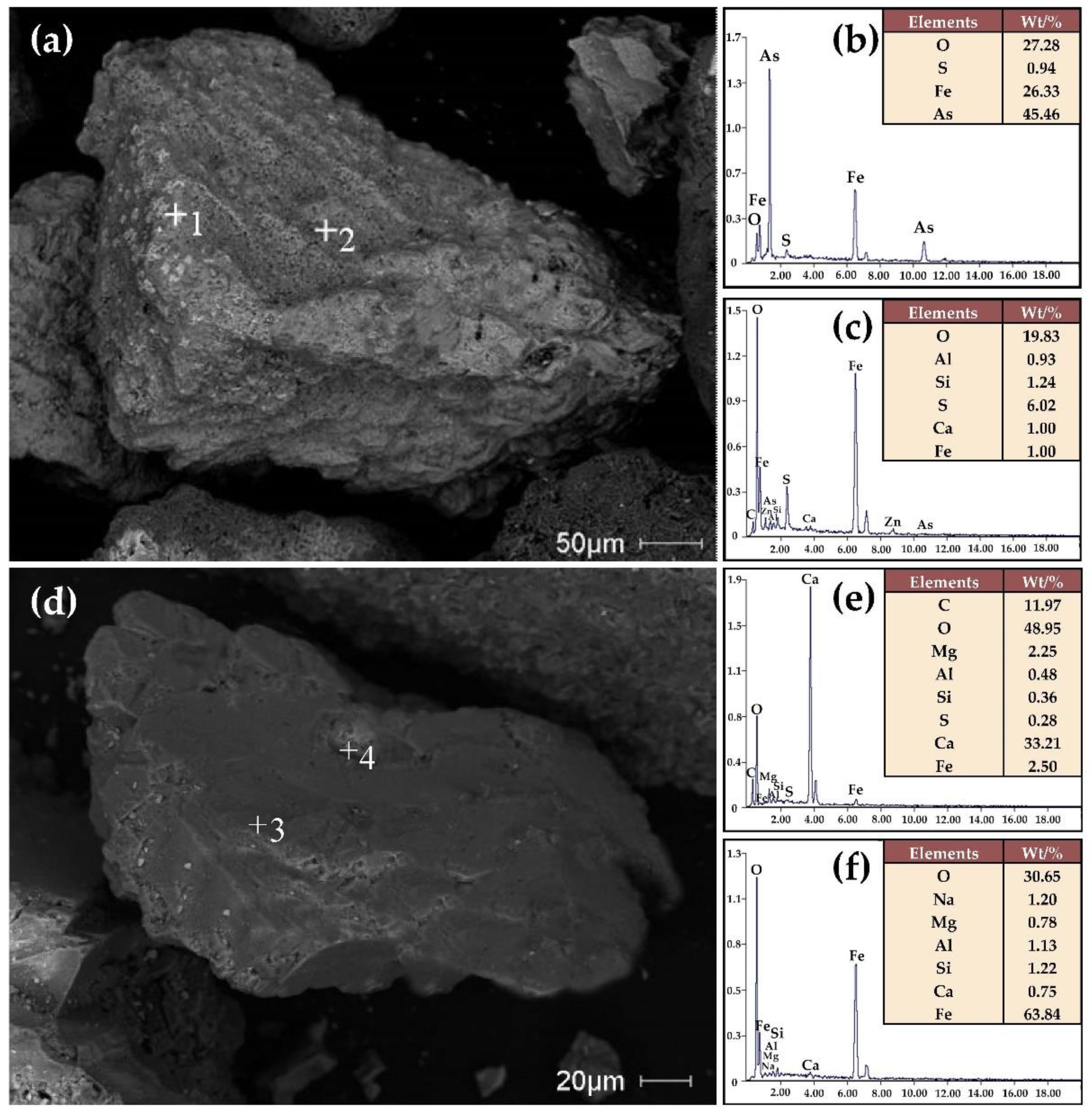
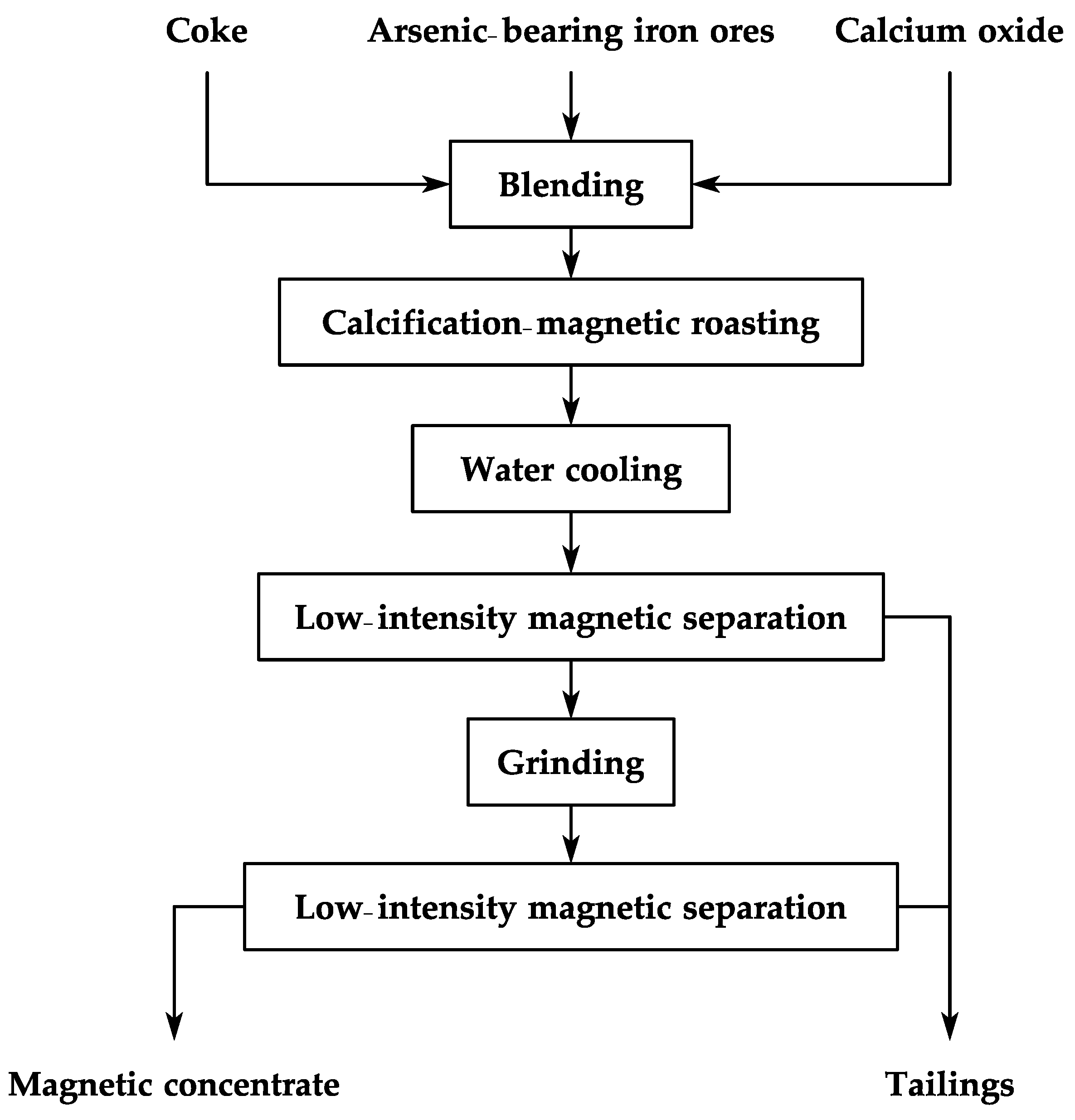

| Element | Fetotal | As | CaO | MgO | SiO2 | Al2O3 | S | P | Loss | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Contents | 45.32 | 0.70 | 9.46 | 3.58 | 7.62 | 3.86 | 0.61 | 0.06 | 28.79 | 100.00 |
| Iron Minerals | Magnetite | Siderite | Ferrosilite | Pyrite | Hematite and Limonite | Total |
|---|---|---|---|---|---|---|
| Distribution | 4.18 | 0.29 | 5.29 | 4.85 | 85.39 | 100.00 |
| Physical Property | True Density/(g/cm3) | Blain Specific Surface Area/(cm2/g) | Grain Size Mode/μm |
|---|---|---|---|
| Arsenic-bearing iron ore | 3.79 | 603.8 | 105.4 |
| Standard cement sample | 3.18 | 3870 | - |
| Product | Yield | Grade | Recovery | ||
|---|---|---|---|---|---|
| As | Fe | As | Fe | ||
| Magnetic concentrate | 55.27 | 0.085 | 65.65 | 6.71 | 80.06 |
| Tailings | 44.73 | 1.46 | 20.20 | 93.29 | 19.94 |
| Feed | 100.00 | 100.00 | 45.32 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.; Zhou, Y.; Xiao, Q.; Lv, J.; Huang, L.; Xie, X.; Hu, Y.; Tong, X.; Chun, T. Arsenic Removal and Iron Recovery from Arsenic-Bearing Iron Ores by Calcification-Magnetic Roasting and Magnetic Separation Process. Materials 2023, 16, 6884. https://doi.org/10.3390/ma16216884
Dai M, Zhou Y, Xiao Q, Lv J, Huang L, Xie X, Hu Y, Tong X, Chun T. Arsenic Removal and Iron Recovery from Arsenic-Bearing Iron Ores by Calcification-Magnetic Roasting and Magnetic Separation Process. Materials. 2023; 16(21):6884. https://doi.org/10.3390/ma16216884
Chicago/Turabian StyleDai, Mengbo, Yongcheng Zhou, Qingfei Xiao, Jinfang Lv, Lingyun Huang, Xian Xie, Yiming Hu, Xiong Tong, and Tiejun Chun. 2023. "Arsenic Removal and Iron Recovery from Arsenic-Bearing Iron Ores by Calcification-Magnetic Roasting and Magnetic Separation Process" Materials 16, no. 21: 6884. https://doi.org/10.3390/ma16216884
APA StyleDai, M., Zhou, Y., Xiao, Q., Lv, J., Huang, L., Xie, X., Hu, Y., Tong, X., & Chun, T. (2023). Arsenic Removal and Iron Recovery from Arsenic-Bearing Iron Ores by Calcification-Magnetic Roasting and Magnetic Separation Process. Materials, 16(21), 6884. https://doi.org/10.3390/ma16216884






