Experimental Studies on Thermal Oxidation and Laser Ignition Properties of Al-Mg-Li Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Sizing and Imaging
2.2. TG-DSC Characterization
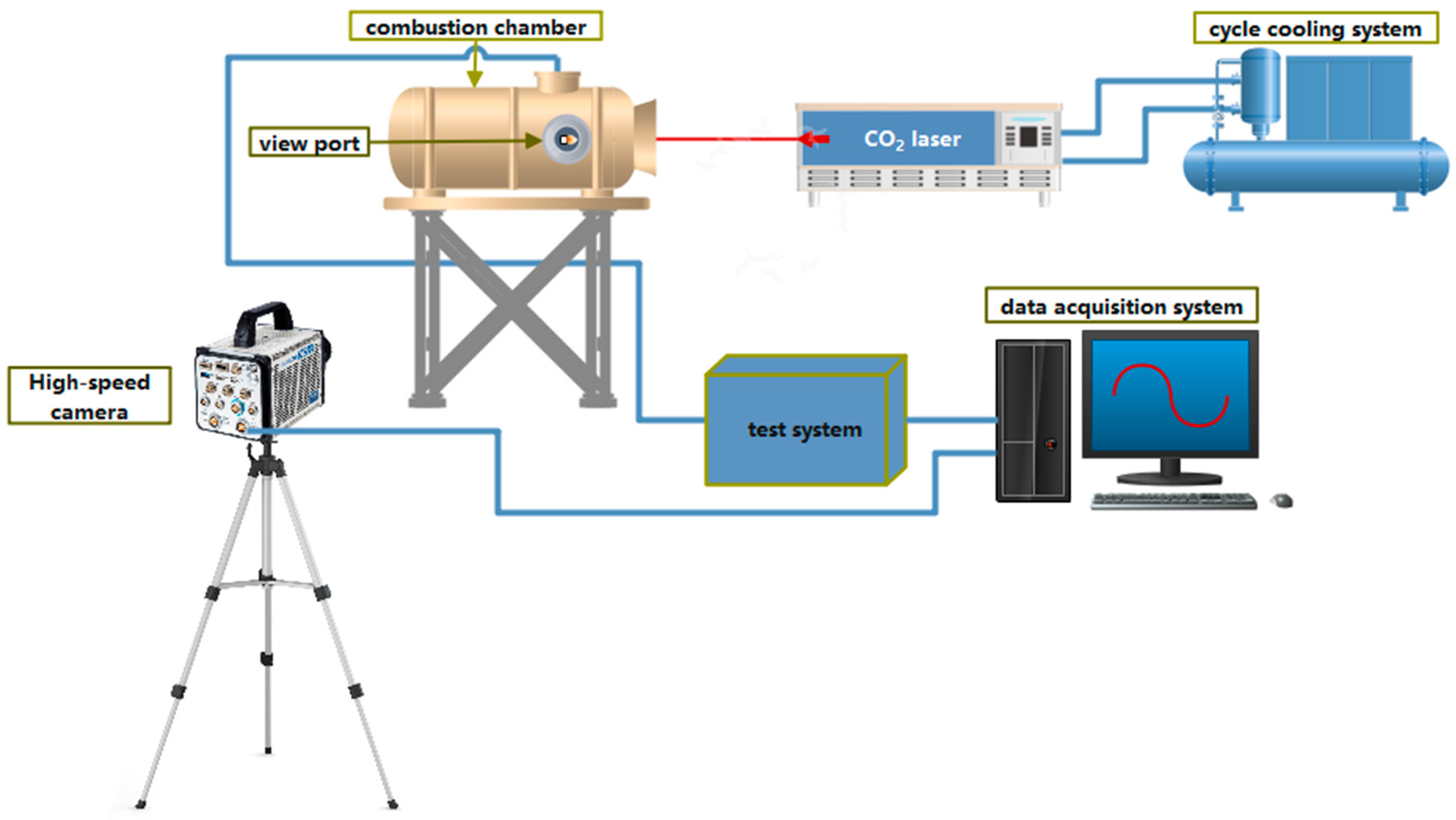
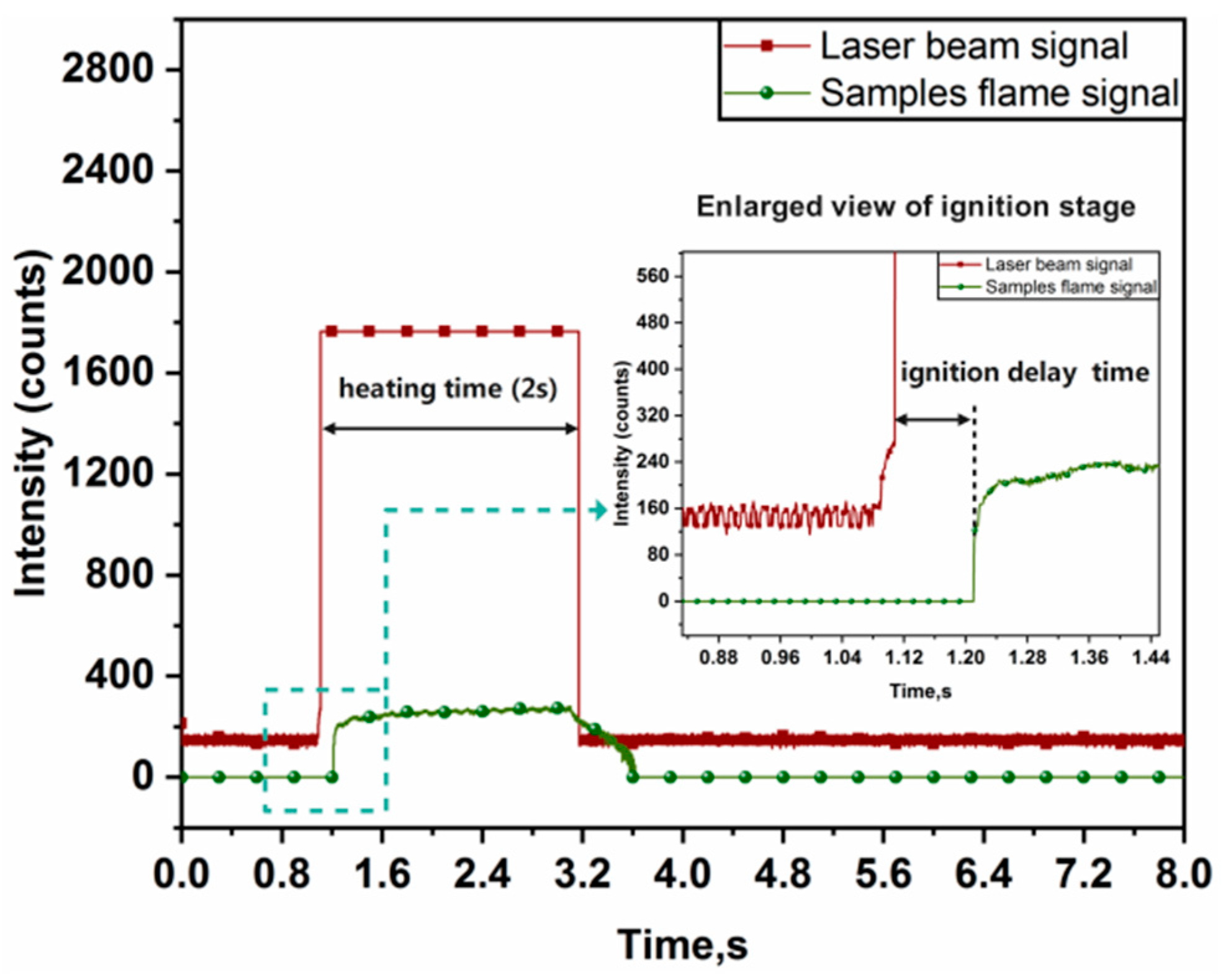
2.3. Laser Ignition System
3. Results and Discussion
3.1. Oxidation Procedures in Different Heating Rates
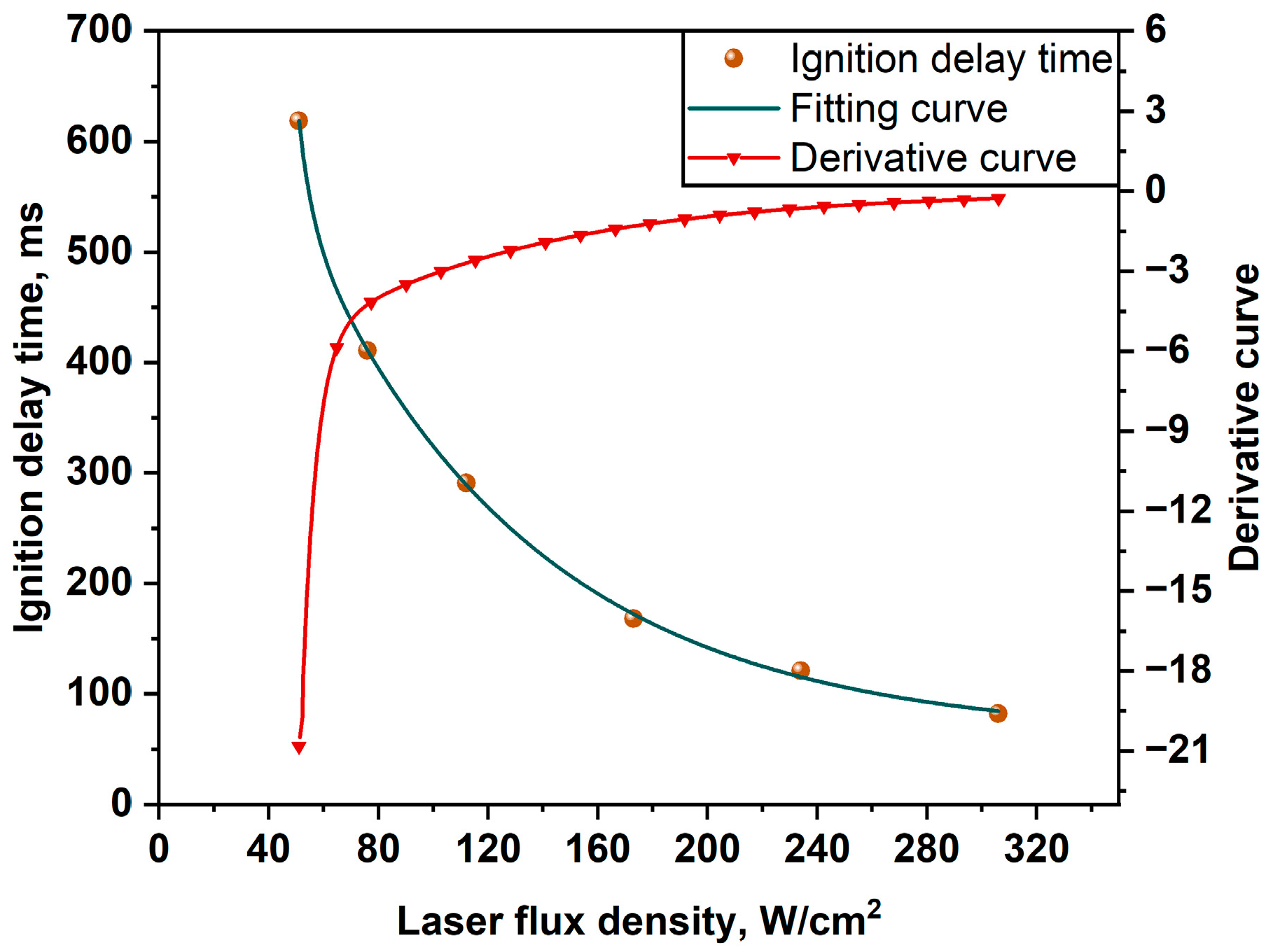
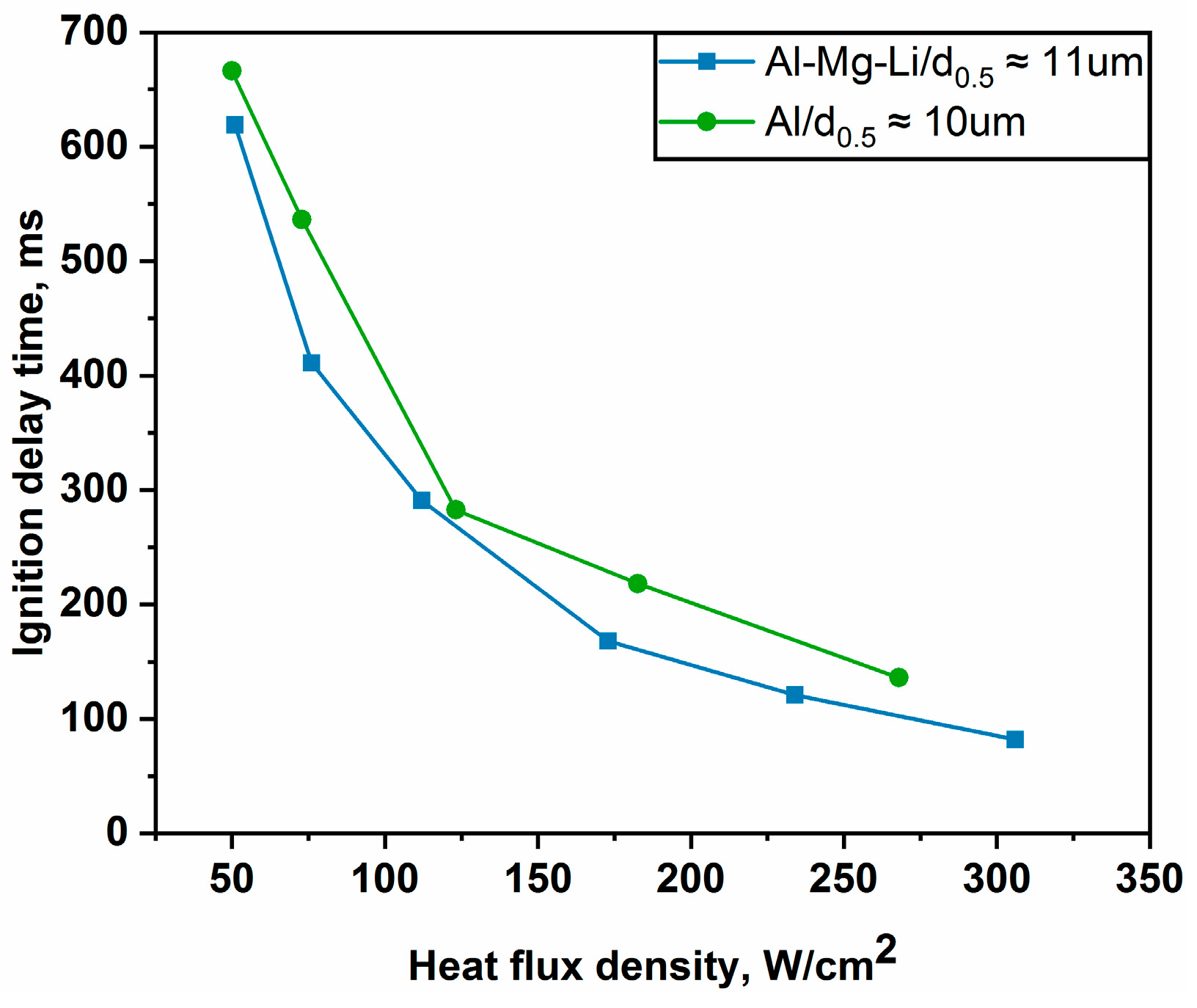
3.2. Ignition Delay Time of Alloy Powders in Different Laser Flux Densities
- x, the lase flux density, W/cm2;
- y, the ignition delay time, ms;
- A1 and A2, dimensionless coefficients;
- E1 and E2, parameters related to ignition energy of Li and Mg;
- y0, constant related to laser ignition delay time, ms;
- R2, coefficient of determination, measuring the closeness of the model to the data points.
3.3. Ignition and Combustion Process at Different Laser Powers
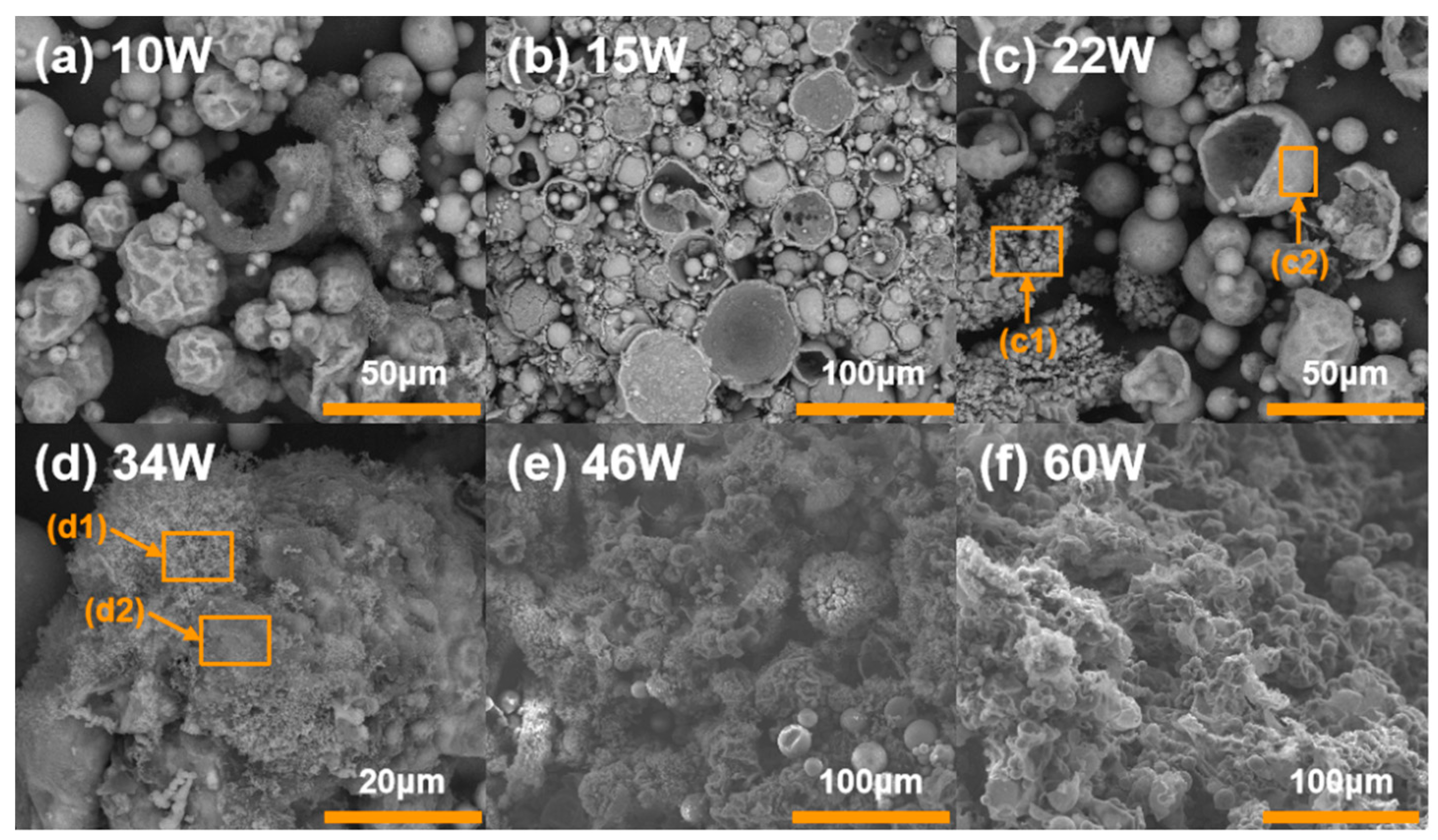
3.4. CCPs Characterization and Analysis
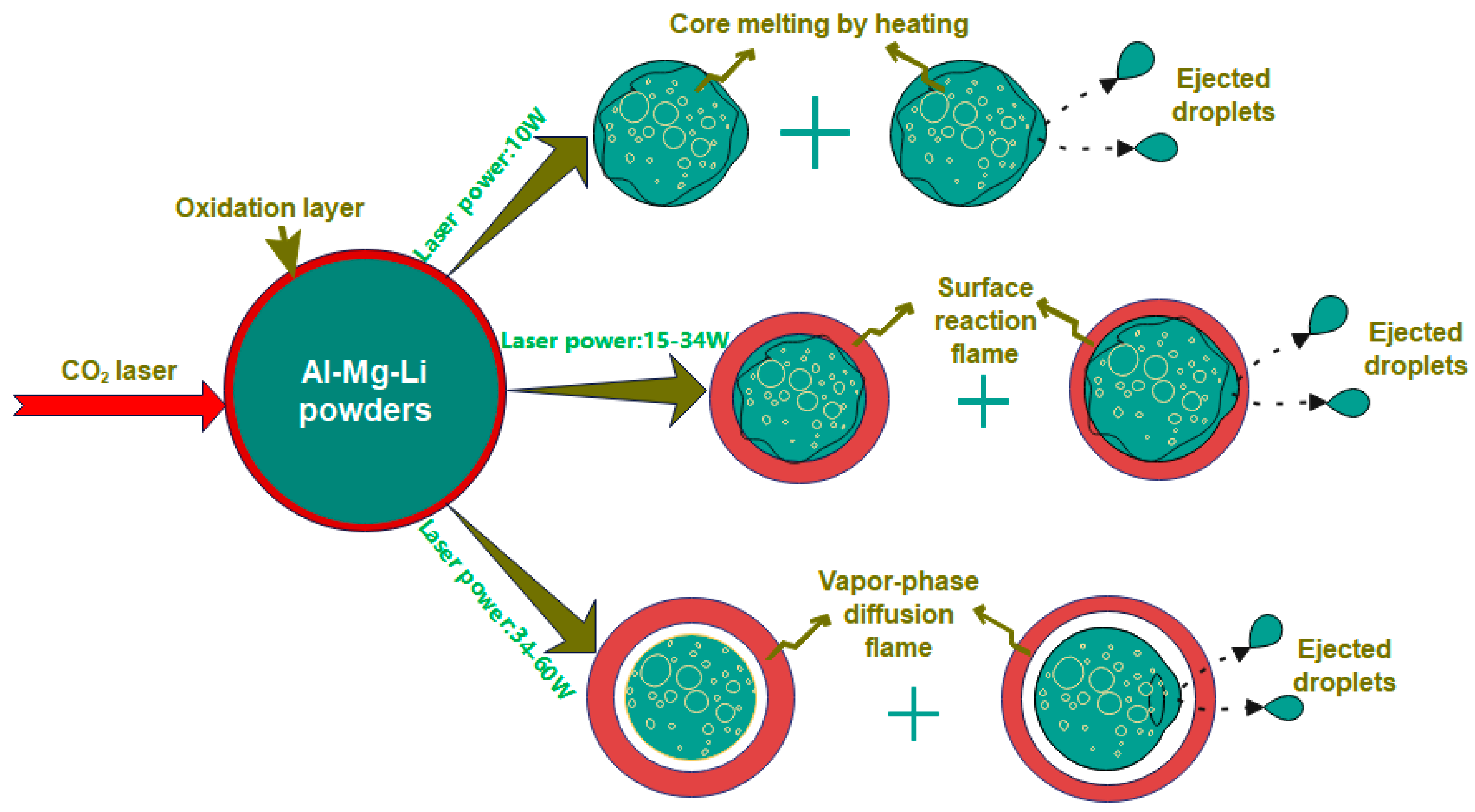
3.5. Ignition Mechanism at Different Laser Powers
4. Conclusions
- The thermal oxidation behaviors of the powder samples were significantly correlated with the heating rates at different heating rates. At the lower heating rate of 10 K/min, the powder’s thermal hysteresis is less and the powder energy release in stage I is more concentrated. However, the degree of heat release concentration approached a similar level at the heating rates of 30 K and 50 K.
- The ignition delay time reduces with increasing laser flux density, according to the results of the laser ignition tests. In addition, there is a clear nonlinear link between ignition delay time and laser flux density, which can be adequately described by the ExpDec2 model.
- The ignition and combustion mode of Al-Mg-Li alloy powders changes from surface to gas phase combustion with the increase in ignition power. At low laser power (10 W), the powders are mostly heated only, with a small amount of active metal ejected outside, and the combustion flame is not obvious. At higher laser powers (15–34 W), the powders showed obvious combustion flame, accompanied by active metal droplet ejection, and the powders were considered to be mainly burning on the surface according to the product morphologies. At much higher laser power (34–60 W), the flame exhibits a clear gas phase profile and the microscopic morphology of the products is less likely to have a spherical shell. Therefore, it is considered that the powders mainly undergo gas-phase combustion with shell fragmentation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Bu, Y.P.; Xu, X.; Yang, Q.C. Experimental Investigation on the Thrust Regulation of a Mg-CO2 Martian Ramjet. Acta Astronaut. 2022, 197, 191–199. [Google Scholar] [CrossRef]
- Luo, S.B.; Feng, Y.B.; Song, J.W.; Xu, D.Q.; Xia, K.X. Progress and Challenges in Exploration of Powder Fueled Ramjets. Appl. Therm. Eng. 2022, 213, 118776. [Google Scholar]
- Zhang, T.F.; Yao, N.; Zhou, C.Y.; Li, Y.; Zhao, Y.P.; Pang, A.M.; Wu, S.X. Combustion Characteristics of Cross-linked Fluorinated Polymer Supported Aluminum/oxidizer Microsphere in HTPB Propellant. Fire. Phys. Chem. 2022, 2, 20–27. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, Y.H.; Zhang, J.; Liang, J.Y.; Yang, Y.L.; Lin, K.F.; Pang, A.M.; Shuai, Y. Capsule Structured Al/FeF3/AP Energetic Microspheres with Enhanced Combustion Performance and Energy Release Efficiency by A Microexplosion Reaction. Fuel 2023, 340, 127546. [Google Scholar]
- Maggi, F.; Dossi, S.; Paravan, C.; DeLuca, L.T.; Liljedahl, M. Activated Aluminum Powders for Space Propulsion. Powder Technol. 2021, 394, 782–790. [Google Scholar] [CrossRef]
- Griego, C.; Yilmaz, N.; Atmanli, A. Analysis of Aluminum Particle Combustion in a Downward Burning Solid Rocket Propellant. Fuel 2019, 237, 405–412. [Google Scholar] [CrossRef]
- Chong, F.; Li, S.F. Experimental Research of The Effects of Superfine Aluminum Powders on the Combustion Characteristics of NEPE Propellants. Propell. Explos. Pyrot. 2002, 27, 34–38. [Google Scholar]
- Li, C.; Hu, C.B.; Xin, X.; Li, Y.; Sun, H.J. Experimental Study on the Operation Characteristics of Aluminum Powder Fueled Ramjet. Acta Astronaut. 2016, 129, 74–81. [Google Scholar] [CrossRef]
- Jung, W.; Baek, S.; Park, J.; Kwon, S. Combustion Characteristics of Ramjet Fuel Grains with Boron and Aluminum Additives. J. Propuls. Power 2018, 34, 1070–1079. [Google Scholar] [CrossRef]
- Feng, Y.B.; Luo, S.B.; Song, J.W.; Xia, K.X.; Xu, D.Q. Numerical Investigation on the Combustion Characteristics of Aluminum Powder Fuel in a Supersonic Cavity-Based Combustor. Appl. Therm. Eng. 2023, 221, 119842. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, W.; Zou, X.R.; Shi, B.L.; Wang, N.F. Ignition And Combustion of a Dense Powder Jet of Micron-Sized Aluminum Particles in Hot Gas. Proc. Combust. Inst. 2023, 39, 3625–3636. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, Z.L.; Wang, M.J. Investigation of Nozzle Two-Phase Flow Characteristics for Nanometer Aluminum Powder Combustion in a Metal Fuel Motor. Powder Technol. 2018, 339, 446–458. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Chakravarthy, S.R.; Ganesan, S.; Jayaraman, K. Effect of Aluminum Particle Size on Primary Combustion and Performance of Water Ramjet. AIAA 2018, 4959, 8165632. [Google Scholar]
- Dreizin, E.L. Experimental Study of Stages in Aluminium Particle Combustion in Air. Combust. Flame 1996, 105, 541–556. [Google Scholar] [CrossRef]
- Yu, W.H.; Li, S.P.; Liu, M.Y.; Han, L.; Song, R.; Wang, N.F.; Deng, Z. Effect of Oxidant Concentration on The Combustion Characteristics of Aluminum Particle-Laden Flow. Powder Technol. 2021, 389, 235–242. [Google Scholar] [CrossRef]
- Sarou-Kanian, V.; Rifflet, J.C.; Millot, F.; Millot, F.; Gökalp, I. Aluminum Combustion in Wet and Dry CO2: Consequences for Surface Reactions. Combust. Flame 2006, 145, 220–230. [Google Scholar] [CrossRef]
- Feng, Y.C.; Xia, Z.X.; Huang, L.Y.; Yan, X.T. Experimental Investigation on the Combustion Characteristics of Aluminum in Air. Acta Astronaut. 2016, 129, 1–7. [Google Scholar] [CrossRef]
- Olsen, S.E.; Beckstead, M.W. Burn Time Measurements of Single Aluminum Particles in Steam and CO2 Mixtures. J. Prop. Power 2011, 12, 662–671. [Google Scholar] [CrossRef]
- Shi, H.; Ren, J.Q.; Tang, W.Q.; Li, J.M.; Yang, R.J. Effect of Fast-Burning Compound ACP and Catalyst on the Combustion Performance of Al/Mg-Based Fuel-Rich HTPB Propellants. Fire. Phys. Chem. 2022, 2, 28–35. [Google Scholar] [CrossRef]
- Liu, L.; Ao, W.; Wen, Z.; Wang, Y.; Long, Y.X.; Liu, P.J.; He, G.Q.; Li, L.K.B. Modifying the Ignition, Combustion and Agglomeration Characteristics of Composite Propellants Via Al-Mg Alloy Additives. Combust. Flame 2022, 238, 111926. [Google Scholar] [CrossRef]
- Barnett, D.S.; Kazimi, M.S. Modeling Lithium Reactions with Steam-Air Mixtures. Fusion Eng. Des. 1989, 11, 321–334. [Google Scholar]
- Barnett, D.S.; Gil, T.K.; Kazimi, M.S. Lithium-Mixed Gas Reactions. Fusion Technol. 1989, 15, 967–972. [Google Scholar] [CrossRef]
- Jeppson, D.W.; Ballif, J.L.; Yuan, W.W.; Chou, B.E. Lithium Literature Review: Lithium’s Properties and Interactions. 1978. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/09/410/9410560.pdf?r=1 (accessed on 21 October 2023).
- Diez, G.A.; Manship, T.D.; Terry, B.C.; Gunduz, L.E.; Son, S.F. Characterization of an Aluminum-Lithium-Alloy-Based Composite Propellant at Elevated Pressures. J. Prop. Power 2020, 37, 332–337. [Google Scholar] [CrossRef]
- Terrya, B.C.; Gunduz, I.E.; Pfeil, M.A.; Sippel, T.R.; Son, S.F. A Mechanism for Shattering Micro-Explosions and Dispersive Boiling Phenomena in Aluminum–Lithium Alloy Based Solid Propellant. Proc. Combust. Inst. 2017, 36, 2309–2316. [Google Scholar] [CrossRef]
- Popov, E.I.; Kashporov, L.Y.; Mal’tsev, V.M.; Breiter, A.L. Combustion Mechanism of Aluminum—Magnesium Alloy Particles. Combust. Explos. Shock Waves 1973, 9, 204–208. [Google Scholar] [CrossRef]
- Aly, Y.; Schoenitz, M.; Dreizin, E.L. Ignition and Combustion of Mechanically Alloyed Al-Mg Powders with Customized Particle Sizes. Combust. Flame 2013, 160, 835–842. [Google Scholar] [CrossRef]
- Belal, H.; Han, C.W.; Gunduz, I.E.; Ortalan, V.; Son, S.F. Ignition and Combustion Behavior of Mechanically Activated Al–Mg Particles in Composite Solid Propellants. Combust. Flame 2018, 194, 410–418. [Google Scholar] [CrossRef]
- Zenin, A.A.; Kuznetsov, G.P.; Kolesnikov, V.I. Combustion of Aluminum-Magnesium Alloy Particles Under Microgravity Conditions. Russ. J. Phys. Chem. B 2011, 5, 84–96. [Google Scholar] [CrossRef]
- Noirfontaine, M.N.; Baldinozzi, G.; Barthés-Labrousse, M.G.; Kusinski, J.; Boëmare, G.; Herinx, M.; Feuerbacher, M. High Temperature Oxidation of the Al3Mg2 Complex Metallic Alloy. Oxid. Met. 2010, 73, 219–232. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Liang, L.X.; Liu, H.Y.; Liu, Y.Q.; Li, F.S. Thermal Reactivity of Nanostructure Al0.8Mg0.2 Alloy Powder Used in Thermites. Rare Meta Mat. Eng. 2012, 41, 9–13. [Google Scholar]
- Hu, A.B.; Cai, S.Z. Spatial Phase Structure and Oxidation Process of Al-W Alloy Powder with High Sphericity. J. Mater. Sci. Technol. 2022, 114, 62–72. [Google Scholar] [CrossRef]
- Hu, A.B.; Cai, S.Z. Research on the Novel Al-W Alloy Powder with High Volumetric Combustion Enthalpy. J. Mater. Sci. Technol. 2021, 13, 311–320. [Google Scholar] [CrossRef]
- MOORE, J.T.; Turns, S.R.; Yetter, R.A. Combustion of Lithium–Aluminum Alloys. Combust. Sci. Technol. 2005, 177, 627–669. [Google Scholar] [CrossRef]
- Partridge, P.G. Oxidation of Aluminium-Lithium Alloys in the Solid and Liquid States. Int. Mater. Rev. 1990, 35, 37–58. [Google Scholar] [CrossRef]
- Corcoran, A.L.; Wang, S.; Aly, Y.; Dreizin, E.L. Combustion of Mechanically Alloyed Al-Mg Powders in Products of a Hydrocarbon Flame. Combust. Sci. Technol. 2015, 187, 807–825. [Google Scholar] [CrossRef]
- Xia, Z.X.; Shen, H.J.; Hu, J.X.; Liu, B. Experimental Investigation of Powdered Metals Fuel Ramjet. AIAA/ASME/SAE/ASEE Joint. Prop. Conf. 2008, 44, 5131. [Google Scholar]
- Shoshin, Y.L.; Mudrry, R.S.; Dreizin, E.L. Preparation and Characterization of Energetic Al-Mg Mechanical Alloy Powders. Combust. Flame. 2002, 128, 259–269. [Google Scholar] [CrossRef]
- Xiao, F.; McLuckie, W.M. Laser Ignitibility of Insensitive Secondary Explosive 1,1-diamino- 2,2-dinitroethene (FOX-7). J. Hazard. Mater. 2015, 285, 375–382. [Google Scholar]
- Ma, H.R.; Mao, D.; Zhang, H.L.; Pang, A.M.; Yang, M. Preparation and Properties of Al-Mg Alloy Powders as Combustion Agent. J. Solid Rocket. Technol. 2016, 39, 649–654. (In Chinese) [Google Scholar]
- Schoenitz, M.; Patel, B.; Agboh, O.; Dreizin, E.L. Oxidation of Aluminum Powders at High Heating Rates. Thermochim. Acta 2009, 507, 115–122. [Google Scholar]
- Chen, D.N.; Chen, X.L.; Bao, Y.X.; Zong, J.; Ma, K.J.; Yu, R.R.; Zhuang, C.J. Experimental Research on Minimum Ignition Energy of Lycopodium/Coal/ Ultrafine Powder Mixed System. J. Phys. Conf. Ser. 2021, 1, 12–37. [Google Scholar] [CrossRef]
- Addai, E.K.; Gabel, D.; Kamal, M.; Krause, U. Minimum Ignition Energy of Hybrid Mixtures of Combustible Dusts and Gases. Process Saf. Environ. 2016, 102, 503–512. [Google Scholar]
- Addai, E.K.; Gabel, D.; Kamal, M.; Krause, U. Experimental Investigation on the Minimum Ignition Temperature of Hybrid Mixtures of Dusts And Gases or Solvents. J. Hazard. Mater. 2015, 301, 314–326. [Google Scholar] [CrossRef]
- Kofstad, P. Oxidation of Metals: Determination of Activation Energies. Nature 1957, 179, 1362–1363. [Google Scholar] [CrossRef]
- Bazyn, T.; Krier, H.; Glumac, N. Evidence for the Transition from The Diffusion-Limit in Aluminum Particle Combustion. Proc. Combust. Inst. 2007, 31, 2021–2028. [Google Scholar]
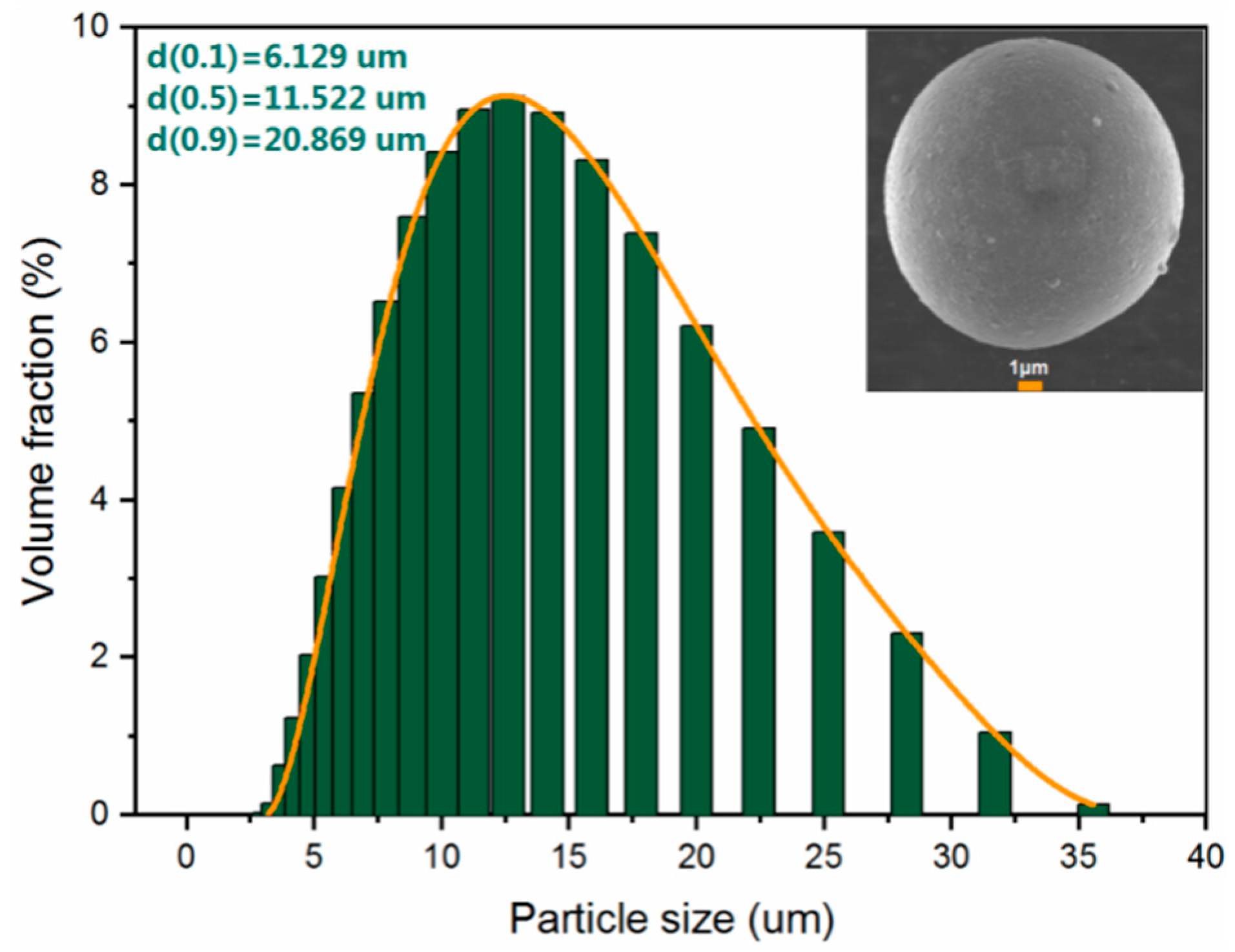
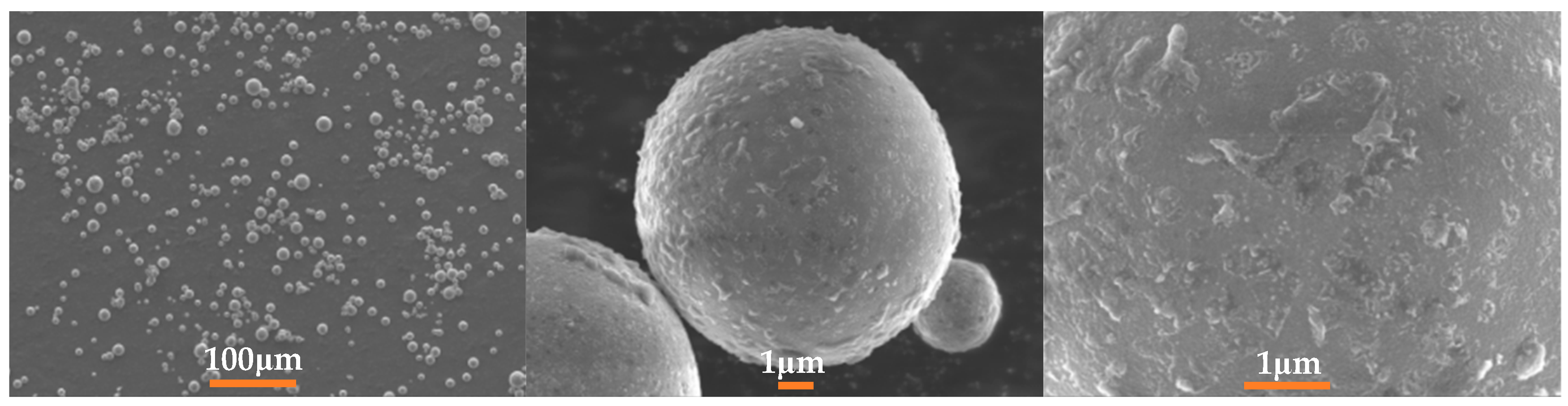
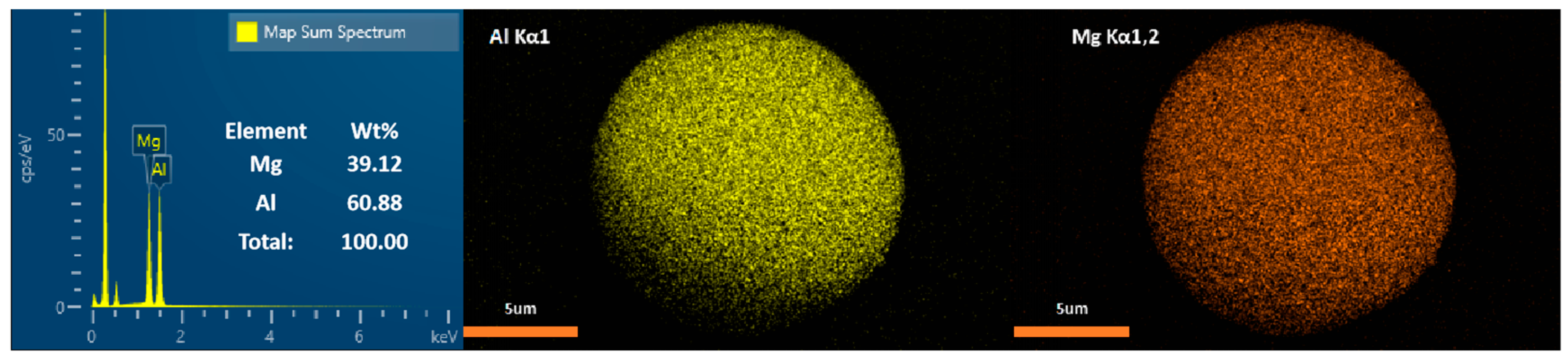





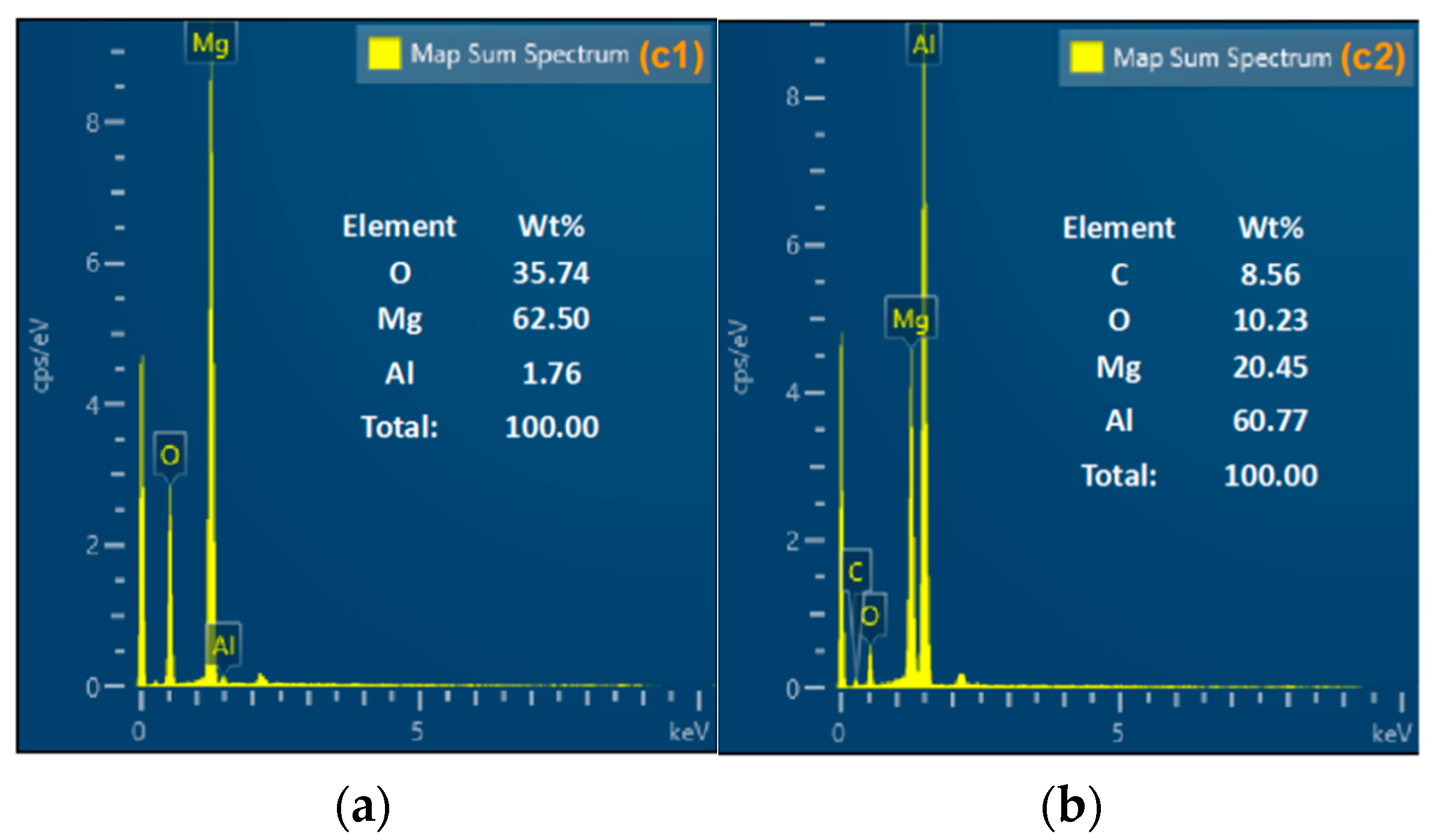
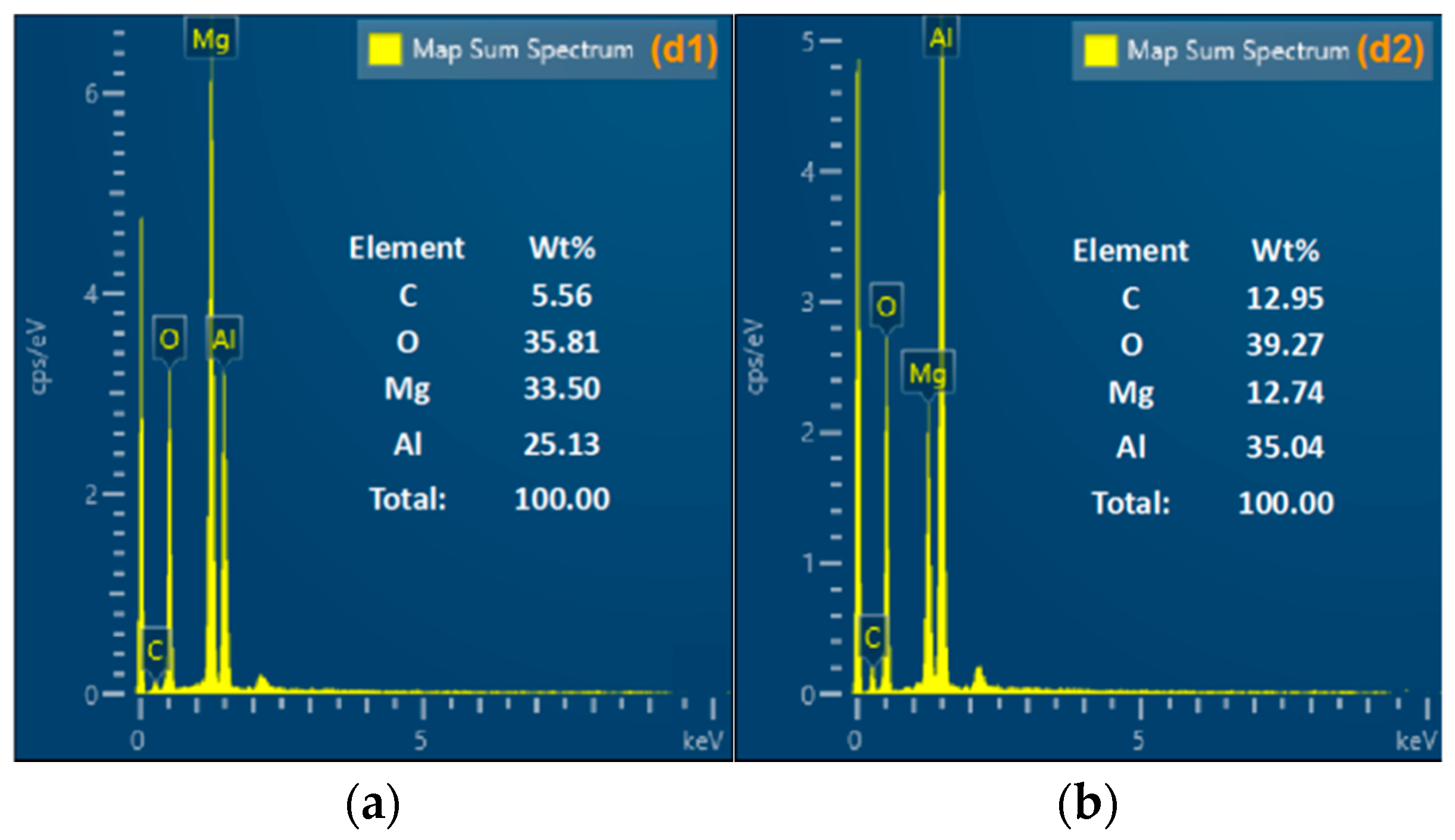
| Element | wt% |
|---|---|
| Al | 61.986% |
| Mg | 30.846% |
| Li | 9.768% |
| Values of Parameters | |||||
|---|---|---|---|---|---|
| A1 | A2 | E1 | E2 | y0 | R2 |
| 1.9 × 106 | 857.6 | 5.1 | 84.6 | 61.5 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Ma, K.; Guo, C.; Jiang, M.; Wu, C.; Li, S.; Hu, S. Experimental Studies on Thermal Oxidation and Laser Ignition Properties of Al-Mg-Li Powders. Materials 2023, 16, 6931. https://doi.org/10.3390/ma16216931
Lu Y, Ma K, Guo C, Jiang M, Wu C, Li S, Hu S. Experimental Studies on Thermal Oxidation and Laser Ignition Properties of Al-Mg-Li Powders. Materials. 2023; 16(21):6931. https://doi.org/10.3390/ma16216931
Chicago/Turabian StyleLu, Yingying, Kai Ma, Changchao Guo, Ming Jiang, Chengfeng Wu, Shipeng Li, and Shaoqing Hu. 2023. "Experimental Studies on Thermal Oxidation and Laser Ignition Properties of Al-Mg-Li Powders" Materials 16, no. 21: 6931. https://doi.org/10.3390/ma16216931






