The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Microstructure Characterization
2.3. Actuation Measurements
3. Results and Discussion
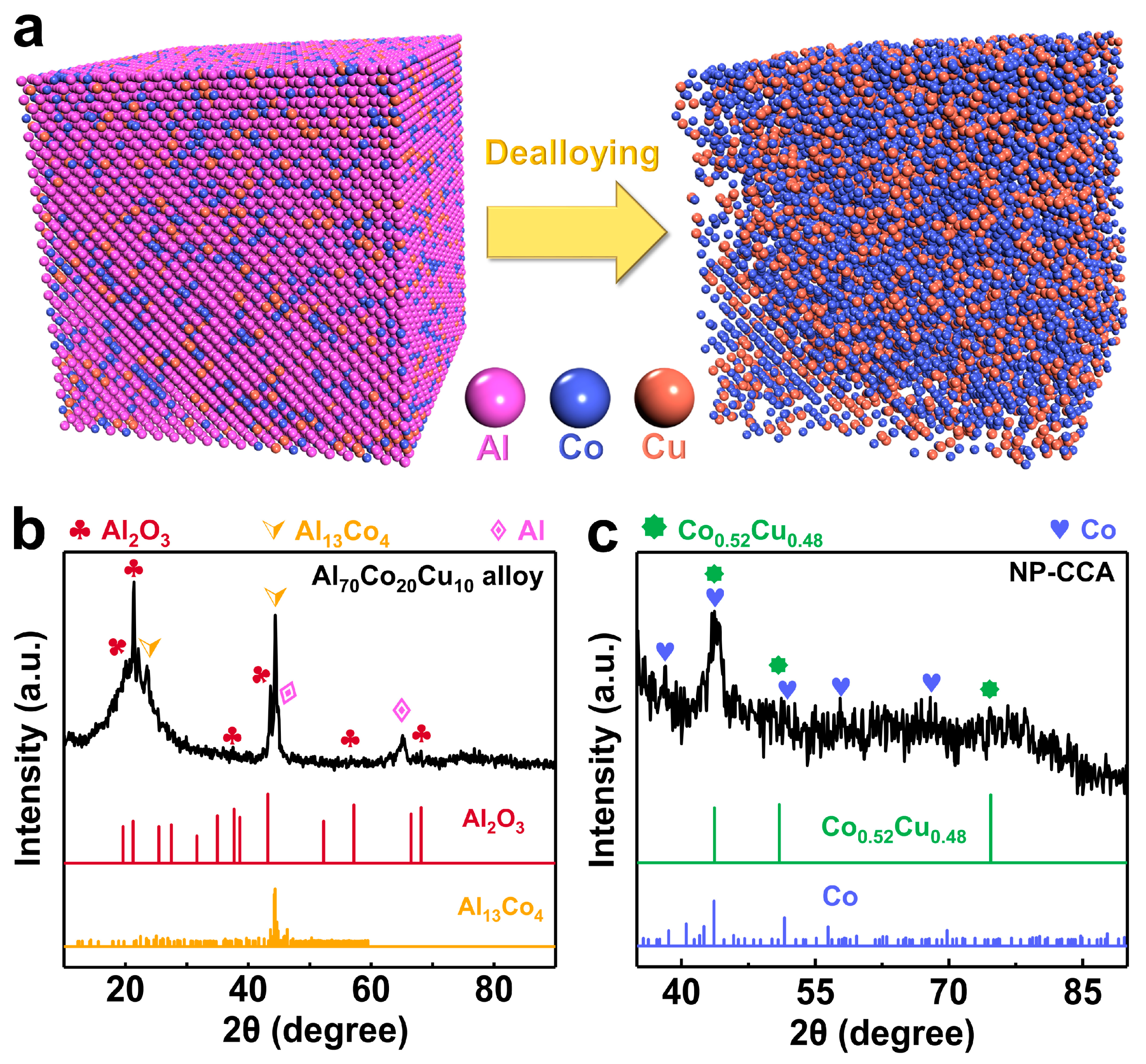
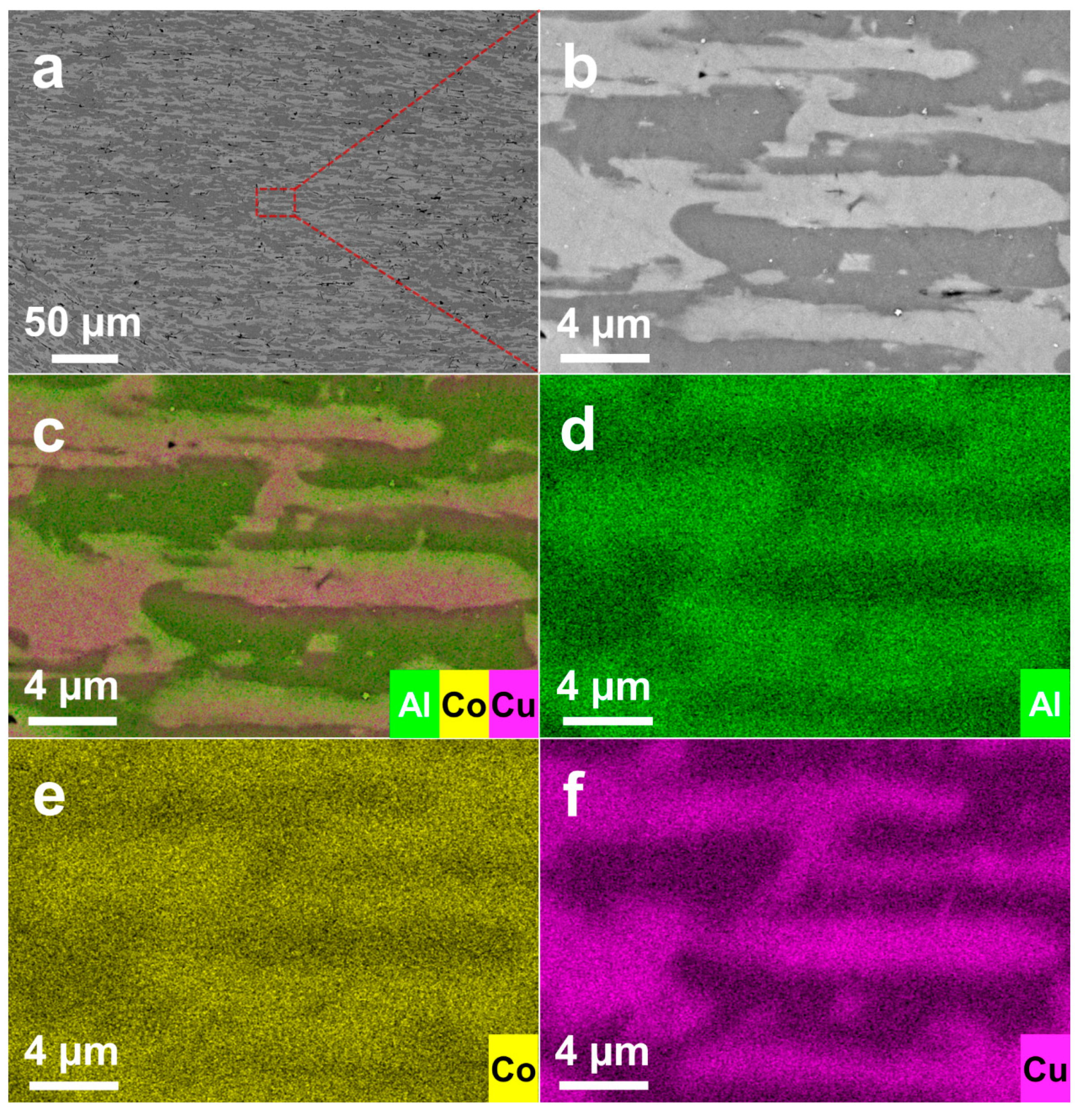
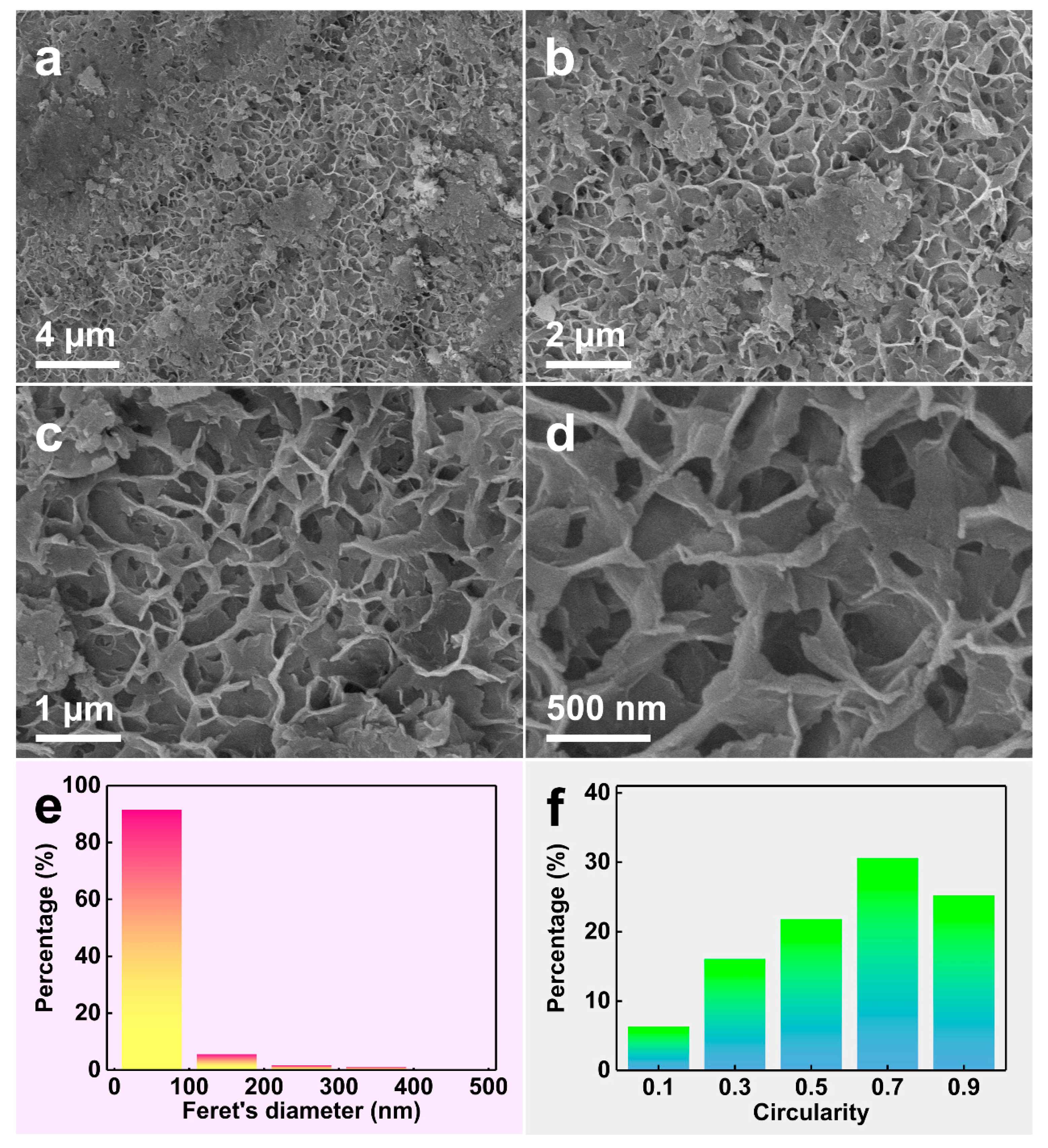
3.1. Microstructures of Al-Co-Cu Precursor Alloy and NP-CCA
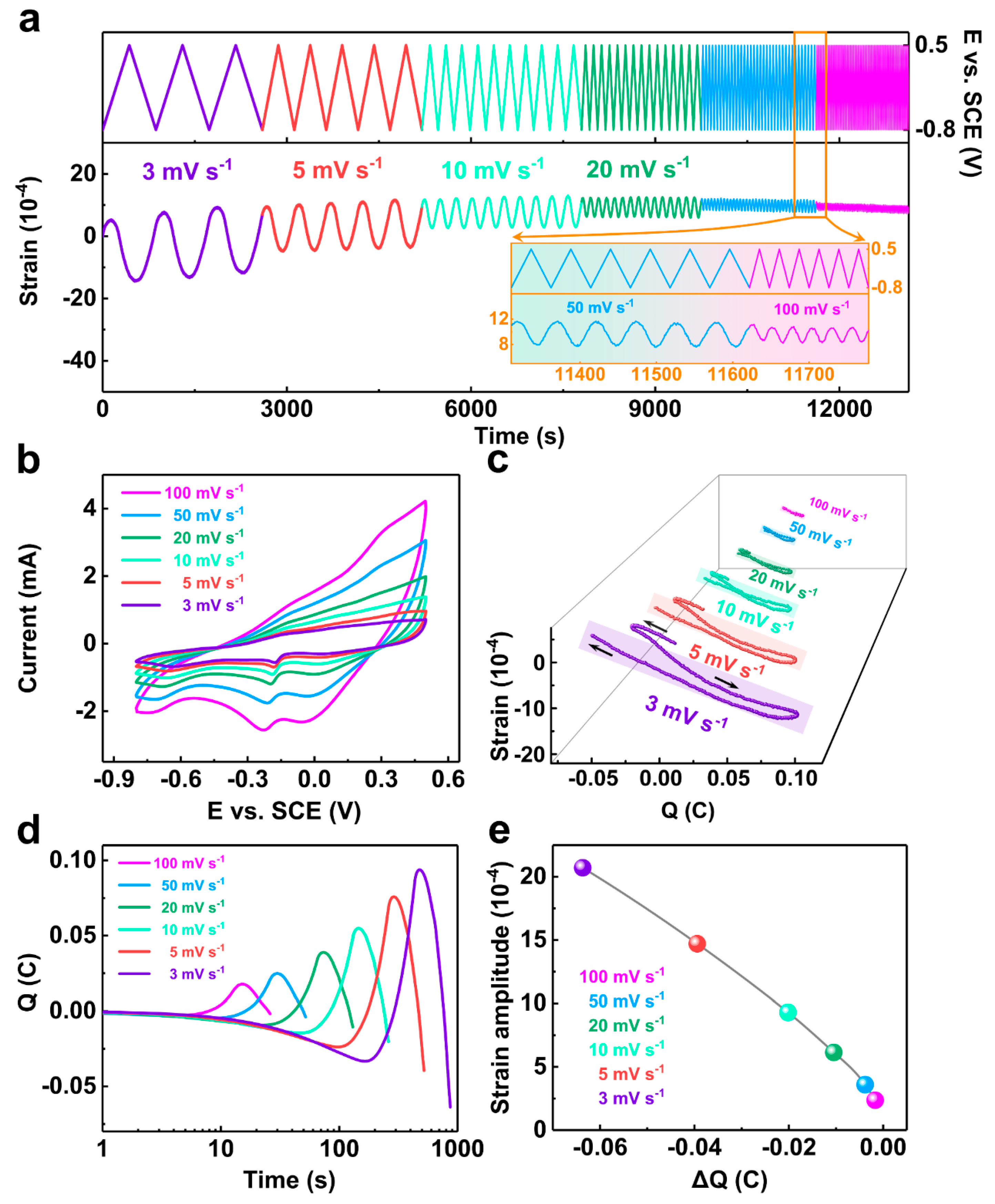
3.2. Electrochemical Actuation Properties of the NP-CCA Sample
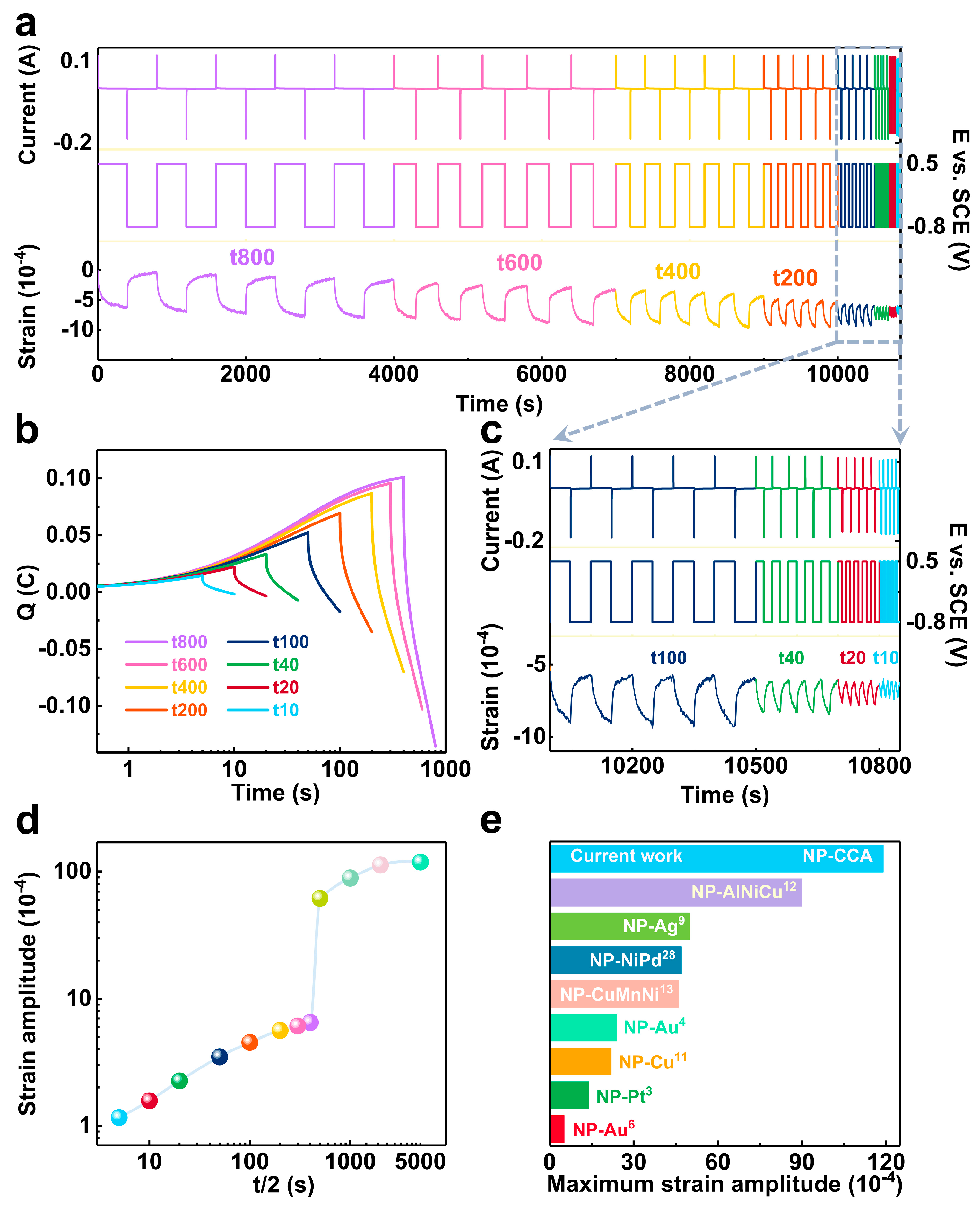
3.2.1. Electrochemical Actuation Stability Test
3.2.2. Capacitive Capacity of Materials
3.2.3. Maximum Strain Amplitude
3.2.4. Comparison of Actuation Performance with Other NP-Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Ma, D.; Wang, Y.; Xie, Y.; Yu, Z.; Cheng, J.; Li, L.; Sun, L.; Dong, S.; Wang, H. Kirigami-origami-inspired lead-free piezoelectric ceramics. Adv. Sci. 2023, 10, 2207059. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.-F.; Yang, H. Electrically driven crosslinked liquid crystal polymers. Chin. J. Chem. 2023, 41, 2925–2938. [Google Scholar] [CrossRef]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef]
- Jin, H.-J.; Parida, S.; Kramer, D.; Weissmüller, J. Sign-inverted surface stress-charge response in nanoporous gold. Surf. Sci. 2008, 602, 3588–3594. [Google Scholar] [CrossRef]
- Wang, K.; Stenner, C.; Weissmüller, J. A nanoporous gold-polypyrrole hybrid nanomaterial for actuation. Sensor. Actuat. B-Chem. 2017, 248, 622–629. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L. Robust metallic actuators based on nanoporous gold rapidly dealloyed from gold-nickel precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Si, C.; Bai, Q.; Ma, W.; Gao, H.; Zhang, Z. Electrochemical actuation behaviors of bulk nanoporous palladium in acid and alkaline solutions. Electrochim. Acta 2016, 220, 91–97. [Google Scholar] [CrossRef]
- Tan, F.; Yu, B.; Wang, Y.; Bai, Q.; Zhang, Z. Hierarchically structured nanoporous palladium with ordered/disordered channels for ultrahigh and fast strain. Nano Lett. 2023, 23, 505–513. [Google Scholar] [CrossRef]
- Detsi, E.; Sellès, M.S.; Onck, P.R.; De Hosson, J.T.M. Nanoporous silver as electrochemical actuator. Scr. Mater. 2013, 69, 195–198. [Google Scholar] [CrossRef]
- Tan, F.; Yu, B.; Yan, X.; Zhang, Y.; Bai, Q.; Zhang, J.; Zhang, Z. Electrochemical actuation behaviors of bulk nanoporous copper with a hierarchical structure. J. Alloys Compd. 2022, 923, 166469. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Y.; Tan, F.; Zhang, Z. Eutectic-derived synthesis of hierarchically nanoporous copper for electrochemical actuation and solar steam generation. Nano Res. 2023. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, C.; Tan, F.; Zhang, Z. High-performance, low-cost nanoporous alloy actuators by one-step dealloying of Al-Ni-Cu precursors. Intermetallics 2022, 145, 107537. [Google Scholar] [CrossRef]
- Tan, F.; Yan, X.; Yang, W.; Sun, Y.; Bai, Q.; Zhang, Z. Microstructural/compositional regulations and actuation properties of nanoporous ternary CuMnNi alloys fabricated by electrochemical dealloying. Phys. Status Solidi A 2022, 219, 2200053. [Google Scholar] [CrossRef]
- Matsumoto, K.; Irisawa, T.; Kitamura, M.; Yokoyama, E.; Kumagai, Y.; Koukitu, A. Effective distribution coefficients of an ideal solid solution crystal: Monte carlo simulation. J. Cryst. Growth 2005, 276, 635–642. [Google Scholar] [CrossRef]
- Qiu, H.J.; Peng, L.; Li, X.; Xu, H.T.; Wang, Y. Using corrosion to fabricate various nanoporous metal structures. Corros. Sci. 2015, 92, 16–31. [Google Scholar] [CrossRef]
- Zhevnenko, S.N.; Gershman, E.I. Grain boundary phase transformation in Cu-Co solid solutions. J. Alloys Compd. 2012, 536, S554–S558. [Google Scholar] [CrossRef]
- Huang, J.Y.; Yu, Y.D.; Wu, Y.K.; Li, D.X.; Ye, H.Q. Microstructure and homogeneity of nanocrystalline Co-Cu supersaturated solid solutions prepared by mechanical alloying. J. Mater. Res. 1997, 12, 936–946. [Google Scholar] [CrossRef]
- Schüth, F.; Schmidt, W. Microporous and mesoporous materials. Adv. Mater. 2002, 14, 629–638. [Google Scholar] [CrossRef]
- Czarnecki, S.; Lewiński, T. On material design by the optimal choice of young’s modulus distribution. Int. J. Solids Struct. 2017, 110–111, 315–331. [Google Scholar] [CrossRef]
- Jin, H.-J.; Wang, X.-L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au-Pt alloys as large strain electrochemical actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Pandey, S.K.; Yadav, T.P.; Srivastava, O.N. Influence of chemical leaching on Al-Cu-Co decagonal quasicrystals. Mater. Chem. Phys. 2017, 200, 23–32. [Google Scholar]
- Tsai, A.P.; Tsurui, T.; Memezawa, A.; Aoki, K.; Indue, A.; Masumoto, T. Formation of quasicrystals through peritectoid reactions from a non-equilibrium cubic phase prepared by mechanical alloying. Phil. Mag. Lett. 1993, 67, 393–398. [Google Scholar] [CrossRef]
- Dražić, S.; Sladoje, N.; Lindblad, J. Estimation of feret’s diameter from pixel coverage representation of a shape. Pattern Recogn. Lett. 2016, 80, 37–45. [Google Scholar] [CrossRef]
- Takashimizu, Y.; Iiyoshi, M. New parameter of roundness R: Circularity corrected by aspect ratio. Prog. Earth Planet. Sc. 2016, 3, 2. [Google Scholar] [CrossRef]
- Rokade, A.A.; Jin, Y.E.; Park, S.S. Facile synthesis of plate-like CuS nanoparticles and their optical and photo-thermal properties. Mater. Chem. Phys. 2018, 207, 465–469. [Google Scholar]
- Mirvakili, S.M.; Hunter, I.W. Artificial muscles: Mechanisms, applications, and challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar]
- Deng, Q.; Jia, H.; An, C.; Wu, S.; Zhao, S.; Hu, N. Progress and prospective of electrochemical actuator materials. Compos. Part A 2023, 165, 107336. [Google Scholar]
- Zhang, J.; Lv, L.; Gao, H.; Bai, Q.; Zhang, C.; Zhang, Z. Electrochemical actuation behaviors and mechanisms of bulk nanoporous Ni-Pd alloy. Scr. Mater. 2017, 137, 73–77. [Google Scholar] [CrossRef]
- Smith, J.R.; Campbell, S.A.; Walsh, F.C. Cyclic voltammetry at metal electrodes. T. I. Met. Finish 1995, 73, 72–78. [Google Scholar] [CrossRef]
- Tan, F.; Yu, B.; Bai, Q.; Zhang, Z. Potentiostatic dealloying fabrication and electrochemical actuation performance of bulk nanoporous palladium. Metals 2022, 12, 2153. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L.; Krekeler, T.; Ritter, M.; Weissmüller, J. Semiordered hierarchical metallic network for fast and large charge-induced strain. Nano Lett. 2017, 17, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Markmann, J.; Weissmüller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar]
- Bukowski, B.C.; Keil, F.J.; Ravikovitch, P.I.; Sastre, G.; Snurr, R.Q.; Coppens, M.-O. Connecting theory and simulation with experiment for the study of diffusion in nanoporous solids. Adsorption 2021, 27, 683–760. [Google Scholar]
- Jayaraman, T.R.; Venkatesan, V.K.; Udupa, H.V.K. Cyclic voltammetric studies of electroless cobalt in NaOH. Electrochim. Acta 1975, 20, 209–213. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Y.; Zhang, J.; Ding, Y.; Peng, Z.; Zhang, Z. Hierarchically nanoporous nickel-based actuators with giant reversible strain and ultrahigh work density. J. Mater. Chem. C 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, C.; Tan, F.; Wu, F.; Zhang, Z. Nanoporous copper as an inexpensive electrochemical actuator responsive to sub-volt voltages. Electrochem. Commun. 2021, 124, 106940. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L.; Krekeler, T. Simultaneous enhancement of actuation strain and mechanical strength of nanoporous Ni-Mn actuators. Adv. Electron. Mater. 2021, 7, 2100381. [Google Scholar] [CrossRef]
- Kramer, D. Dependence of surface stress, surface energy and surface tension on potential and charge. Phys. Chem. Chem. Phys. 2008, 10, 168–177. [Google Scholar] [CrossRef]
- Marichev, V.A. The adsorption of hydroxide ions on metals. Electrochim. Acta 1998, 43, 2203–2214. [Google Scholar] [CrossRef]
- Mishra, A.K.; Pradhan, D. Hierarchical urchin-like cobalt-doped CuO for enhanced electrocatalytic oxygen evolution reaction. ACS Appl. Energy Mater. 2021, 4, 9412–9419. [Google Scholar] [CrossRef]
- Shi, N.; Sun, S.; Zhang, B.; Du, Q.; Liao, Y.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Chen, X. Co(OH)2 nanosheets decorated Cu(OH)2 nanorods for highly sensitive nonenzymatic detection of glucose. Nanotechnology 2020, 31, 325502. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xie, L.; Liu, T.; Song, B.; Pang, S.; Wang, X. An equivalent strain energy density model for fatigue life prediction under large compressive mean stress. Int. J. Fatigue 2023, 177, 107899. [Google Scholar] [CrossRef]
- Kapfer, S.; Sporer, S.; Hyde, S.T.; Mecke, K.; Schroeder-Turk, G.E. Elastic and morphological properties of porous biomaterials. Biophys. J. 2010, 98, 571a. [Google Scholar] [CrossRef][Green Version]
- Mehrabian, A. The poroelastic constants of multiple-porosity solids. Int. J. Eng. Sci. 2018, 132, 97–104. [Google Scholar] [CrossRef]
- Pia, G.; Delogu, F. A phenomenological approach to yield strength in nanoporous metal foams. Scr. Mater. 2015, 103, 26–29. [Google Scholar]
- Bleistein, T.; Jung, A.; Diebels, S. A microsphere-based material model for open cell metal foams. Contin. Mech. Therm. 2020, 32, 255–267. [Google Scholar] [CrossRef]
- Ouyang, B.; Peng, D.; Jiao, J.; Ye, J.; Jin, N. First-principle investigation of the stability and mechanical properties of the binder phase B(B=Co and Ni) with the maximum solubility of the transition-group elements M(M=V, Ti, Ta, Mo, W and Cr). Mater. Today Commun. 2023, 34, 105217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Tan, F.; Wang, J.; Zhao, K.; Wang, Y.; Zhang, J.; Liu, H. The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure. Materials 2023, 16, 6942. https://doi.org/10.3390/ma16216942
Chen X, Tan F, Wang J, Zhao K, Wang Y, Zhang J, Liu H. The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure. Materials. 2023; 16(21):6942. https://doi.org/10.3390/ma16216942
Chicago/Turabian StyleChen, Xiao, Fuquan Tan, Jianfeng Wang, Kunpeng Zhao, Yaoguang Wang, Jie Zhang, and Haixia Liu. 2023. "The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure" Materials 16, no. 21: 6942. https://doi.org/10.3390/ma16216942
APA StyleChen, X., Tan, F., Wang, J., Zhao, K., Wang, Y., Zhang, J., & Liu, H. (2023). The Electrochemical Actuation Performances of Nanoporous Ternary AlCoCu Alloy with a Unique Nanosheet Structure. Materials, 16(21), 6942. https://doi.org/10.3390/ma16216942






