Microbubble Oxidation for Fe2+ Removal from Hydrochloric Acid Laterite Ore Leachate
Abstract
1. Introduction
2. Experimental
2.1. Samples and Methods Used
2.2. Analytical Method
3. Results and Discussion
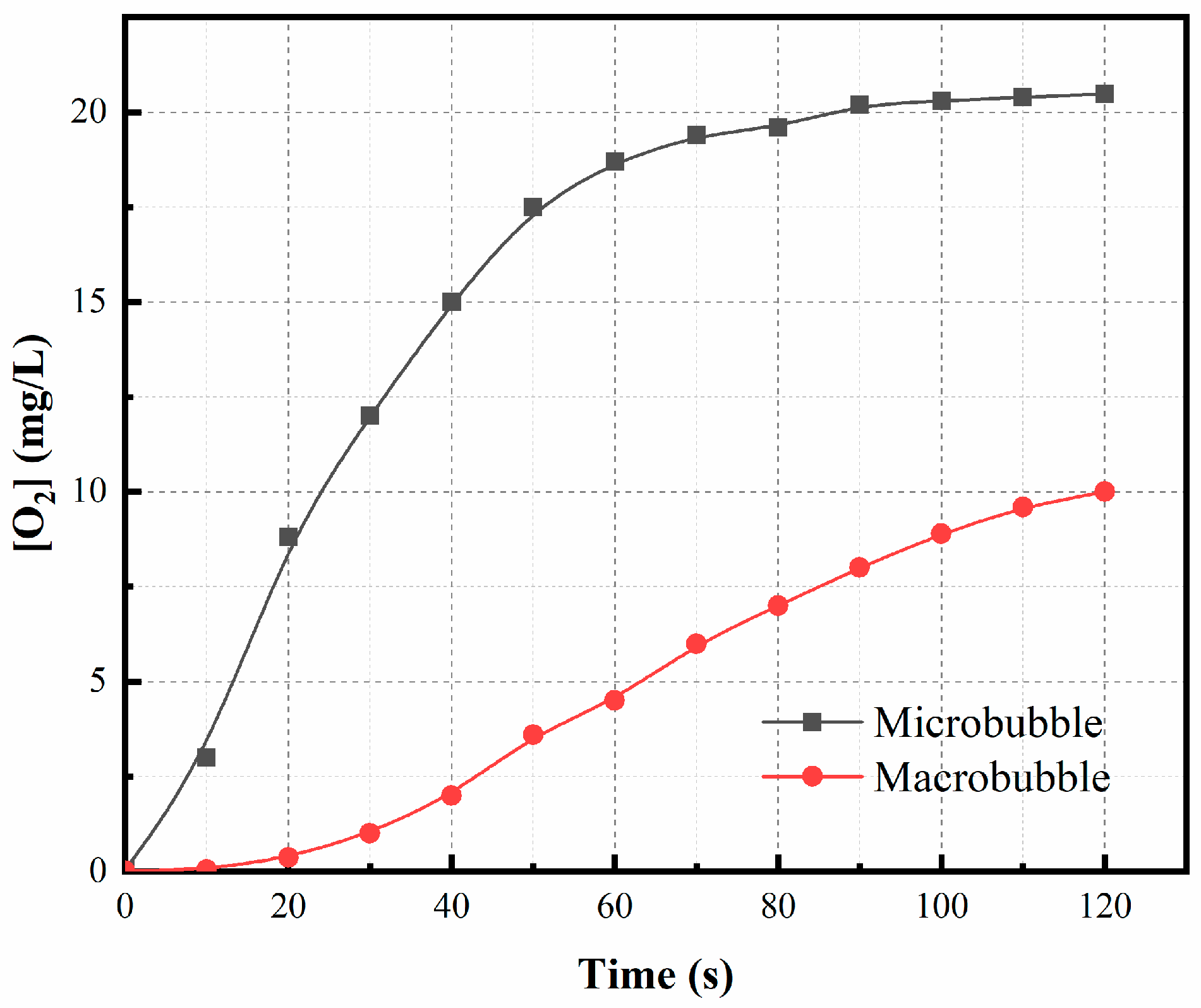
3.1. Effect of Oxygen Mass Transfer by Microbubble Aeration
3.2. Effects of Experimental Conditions on Fe2+ Oxidation
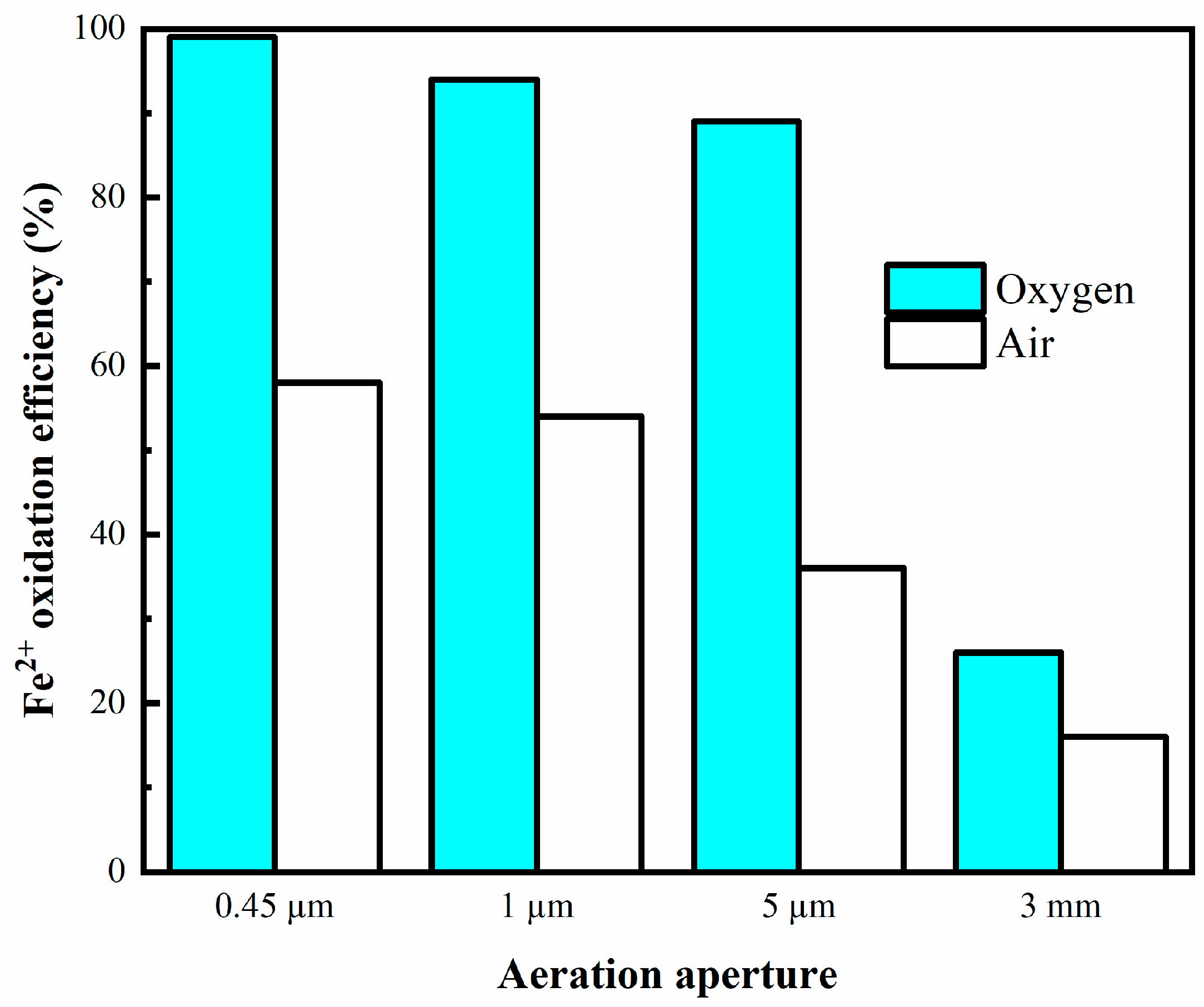
3.2.1. Effect of Aeration Aperture
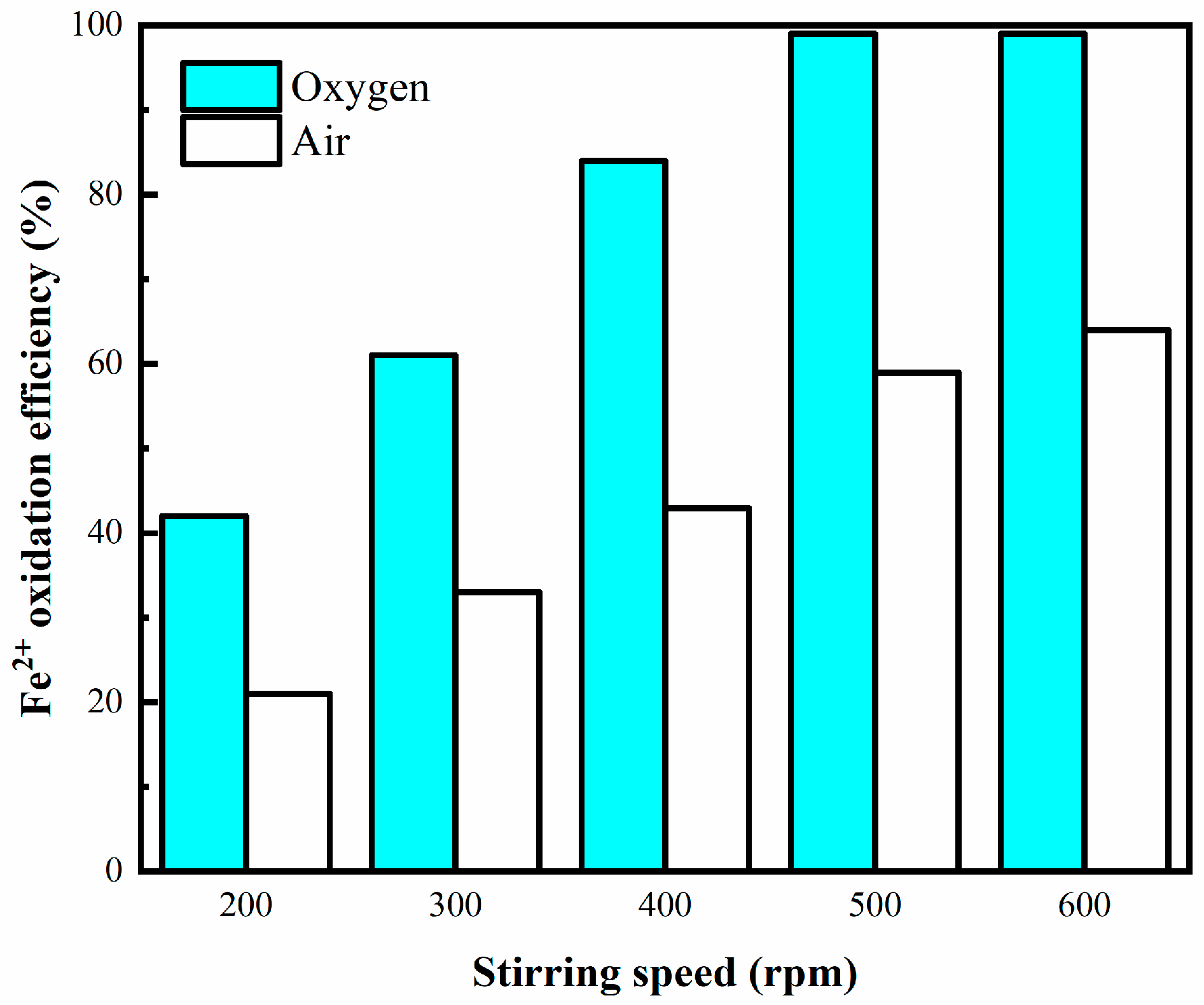
3.2.2. Effect of Stirring Speed
3.2.3. Effect of Bubbling Flow Rate
3.2.4. Effect of pH
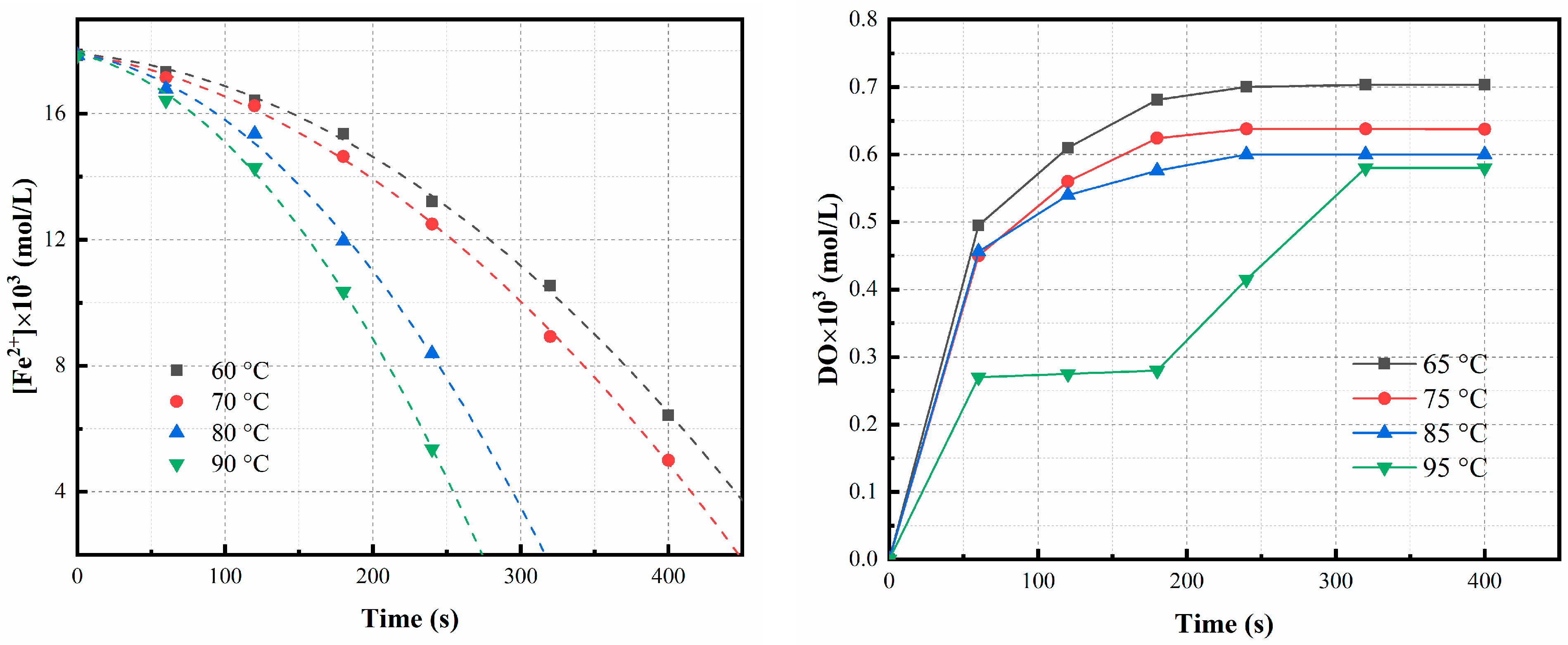
3.2.5. Effect of Temperature
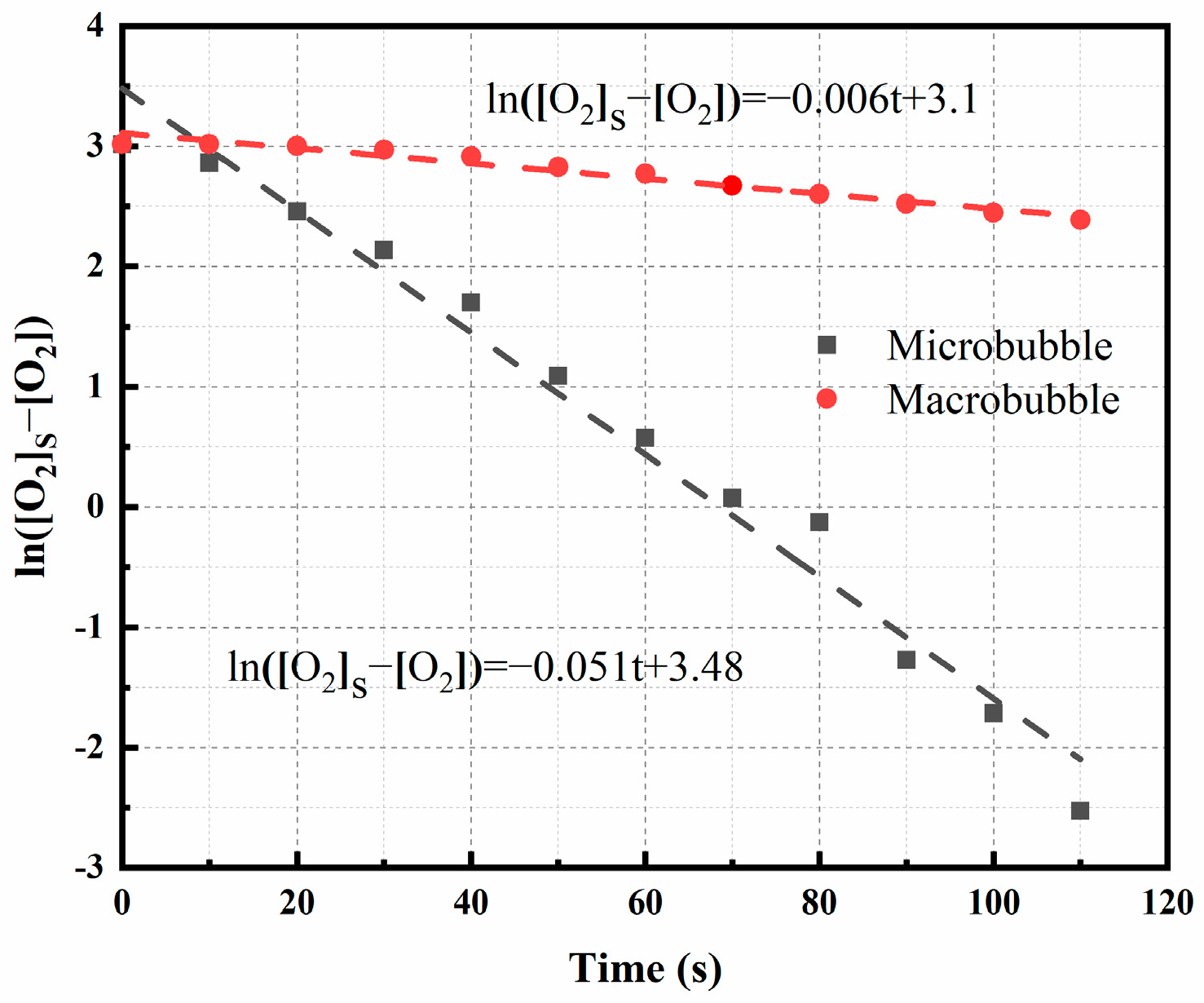
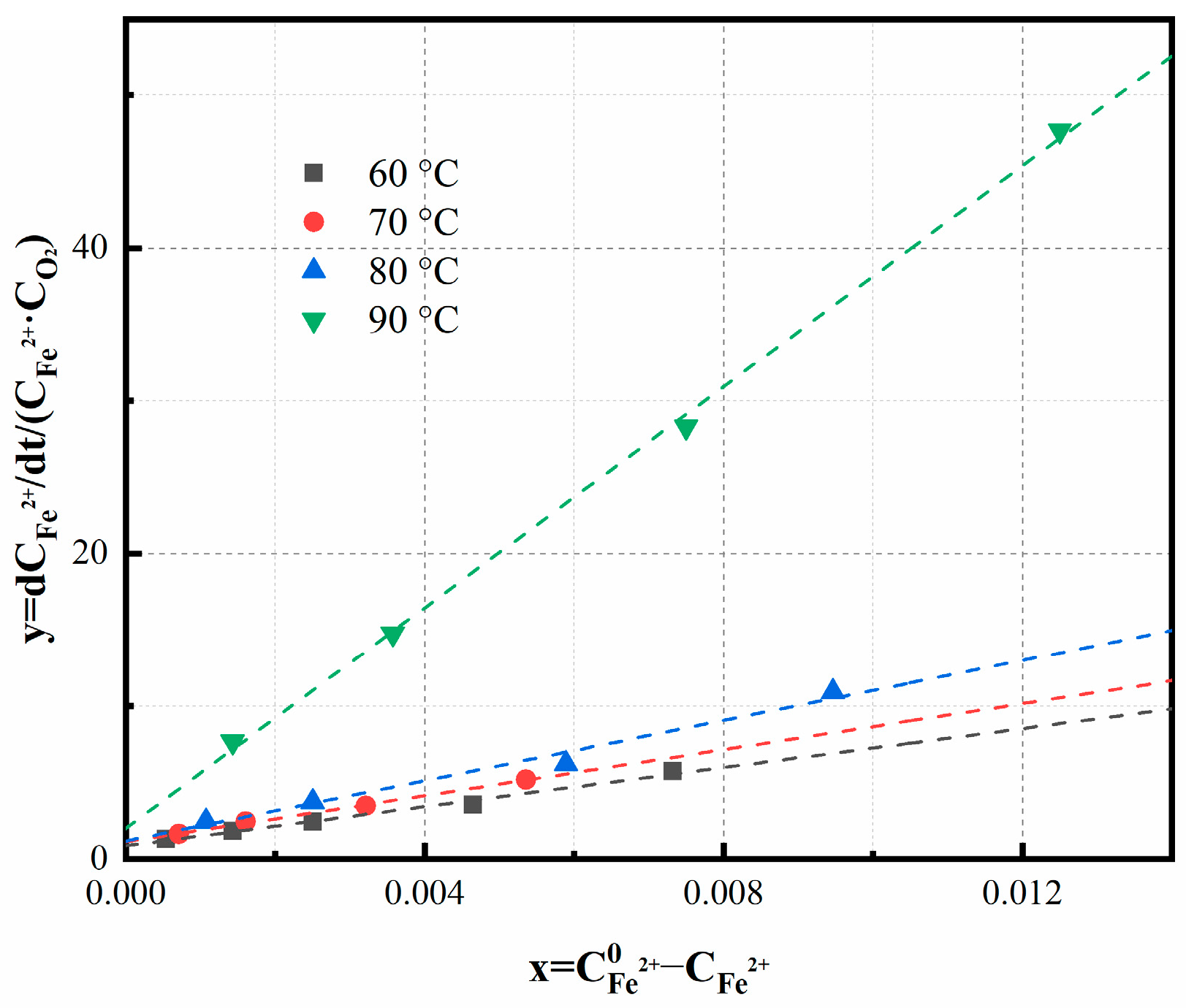
3.3. Study of Macroscale Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Guo, Q.; Wei, G.; Qu, J.; Qi, T. Precipitation of α-Fe2O3 and recovery of Ni and Co from synthetic laterite-leaching solutions. Hydrometallurgy 2015, 153, 21–29. [Google Scholar]
- Ilyas, S.; Srivastava, R.R.; Kim, H.; Ilyas, N.; Sattar, R. Extraction of nickel and cobalt from a laterite ore using the carbothermic reduction roasting-ammoniacal leaching process. Sep. Purif. Technol. 2020, 232, 971. [Google Scholar]
- Yin, F.; Ruan, S.F.; Jiang, P.H.; Wang, C.Y.; Chen, Y.Q. Experimental study on roasted ore of poor nickeliferous laterite ore with ammonia leaching technology. Min. Metall. 2007, 16, 29–32. [Google Scholar]
- Ma, B.; Yang, W.; Yang, B.; Wang, C.; Chen, Y.; Zhang, Y. Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores. Hydrometallurgy 2015, 155, 88–94. [Google Scholar]
- Liu, K.; Chen, Q.; Hu, H.; Ding, Z.; Yin, Z. Characteristics of scales formed from pressure leaching of Yuanjiang laterite. Hydrometallurgy 2011, 109, 131–139. [Google Scholar]
- Meng, L.; Qu, J.; Guo, Q.; Xie, K.; Zhang, P.; Han, L.; Zhang, G.; Qi, T. Recovery of Ni, Co, Mn, and Mg from nickel laterite ores using alkaline oxidation and hydrochloric acid leaching. Sep. Purif. Technol. 2015, 143, 80–87. [Google Scholar] [CrossRef]
- McDonald, R.; Whittington, B. Atmospheric acid leaching of nickel laterites review Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar]
- McDonald, R.; Whittington, B. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies. Hydrometallurgy 2008, 91, 56–69. [Google Scholar]
- Wang, K.; Li, J.; McDonald, R.; Browner, R. The effect of iron precipitation upon nickel losses from synthetic atmospheric nickel laterite leach solutions: Statistical analysis and modelling. Hydrometallurgy 2011, 109, 140–152. [Google Scholar]
- Zhang, W.; Muir, D. Oxidation of Fe(II) in a synthetic nickel laterite leach liquor with SO2/air. Miner. Eng. 2010, 23, 40–44. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, Y.; Le, H.; Li, C. Separation and recovery of Zn, Fe and Mn in acid mine drainage. J. Cent. South Univ. Sci. Technol. 2011, 42, 1858–1864. [Google Scholar]
- Du, X.; Yang, W.; Liu, Y.; Zhang, W.; Wang, Z.; Nie, J.; Li, G.; Liang, H. Removal of manganese, ferrous and antibiotics from groundwater simultaneously using peroxymonosulfate-assisted in-situ oxidation/coagulation integrated with ceramic membrane process. Sep. Purif. Technol. 2020, 252, 117492. [Google Scholar] [CrossRef]
- Sugimori, H.; Kanzaki, Y.; Yokota, K.; Murakami, T. Nonlinear dependence of the oxidation rate of Fe(II) on dissolved oxygen under low-O2 conditions in aqueous solutions. J. Miner. Pet. Sci. 2011, 106, 142–152. [Google Scholar] [CrossRef][Green Version]
- Jones, A.M.; Griffin, P.J.; Waite, T.D. Ferrous iron oxidation by molecular oxygen under acidic conditions: The effect of citrate, EDTA and fulvic acid. Geochim. Cosmochim. Acta 2015, 160, 117–131. [Google Scholar] [CrossRef]
- Brown, E.R.; Mazzarella, J.D. Mechanism of oxidation of ferrous polydentate complexes by dioxygen. J. Electroanal. Chem. Interfacial Electrochem. 1987, 222, 173–192. [Google Scholar] [CrossRef]
- Frank, J.; Izaguirre, M.M. Effect of ionic strength and ionic interactions on the oxidation of Fe(II). J. Solut. Chem. 1989, 18, 585–599. [Google Scholar]
- Kirby, C.; Dennis, A.; Kahler, A. Aeration to degas CO2, increase pH, and increase iron oxidation rates for efficient treatment of net alkaline mine drainage. Appl. Geochem. 2009, 24, 1175–1184. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Pressure oxidation of ferrous ions by oxygen and hematite precipitation from concentrated solution of calcium, copper and iron chlorides. Hydrometallurgy 2013, 140, 59–65. [Google Scholar] [CrossRef]
- Stumm, W.; Lee, G.F. Oxygenation of Ferrous Iron. Ind. Eng. Chem. 1961, 53, 143–146. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Nagayama, M. The effect of ferric hydroxide on the oxygenation of ferrous ions in neutral solutions. Chem. Informationsdienst 1976, 7, 14. [Google Scholar] [CrossRef]
- Tüfekci, N.; Sarikaya, H.Z. Catalytic effects of high Fe(III) concentrations on Fe(II) oxidation. Water Sci. Technol. 1996, 34, 389–396. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, L.; Xia, Z. Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials 2013, 6, 3676–3687. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Li, P.; Takahashi, M.; Chiba, K. Degradation of phenol by the collapse of microbubbles. Chemosphere 2009, 75, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Wada, T.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y.; Kukizaki, M. Degradation of methyl orange using short-wavelength UV irradiation with oxygen microbubbles. J. Hazard. Mater. 2009, 162, 1103–1110. [Google Scholar] [CrossRef]
- Edma, B.; Gb, C.; Av, C.; Ekea, B.; Acm, C.; Epfa, C. Fundamentals and applications of nanobubbles. Interface Sci. Technol. 2019, 30, 69–99. [Google Scholar]
- Kukizaki, M.; Goto, M. Spontaneous formation behavior of uniform-sized microbubbles from Shirasu porous glass (SPG) membranes in the absence of water-phase flow. Colloids Surf. A Physicochem. Eng. Asp. 2007, 296, 174–181. [Google Scholar] [CrossRef]
- Vogel, A.I. A Text-Book of Quantitative Inorganic Analysis—Theory and Practice; Longman: London, UK, 1955. [Google Scholar]
- Lakshmanan, D.; Clifford, D.A.; Samanta, G. Ferrous and Ferric Ion Generation During Iron Electrocoagulation. Environ. Sci. Technol. 2009, 43, 3853. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L. Treatment of Organics Contaminated Wastewater by Ozone Micro-Nano-Bubbles. Water 2018, 11, 55. [Google Scholar] [CrossRef]
- Huffman, R.E.; Davidson, N. Kinetics of the Ferrous Iron-Oxygen Reaction in Sulfuric Acid Solution. J. Am. Chem. Soc. 1956, 78, 4836–4842. [Google Scholar] [CrossRef]
- Chu, L.-B.; Xing, X.-H.; Yu, A.-F.; Zhou, Y.-N.; Sun, X.-L.; Jurcik, B. Enhanced ozonation of simulated dyestuff wastewater by microbubbles. Chemosphere 2007, 68, 1854–1860. [Google Scholar] [PubMed]
- Izawa, S.; Inoue, Y.; Kimura, A. Oxidative stress response in yeast: Effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 1995, 368, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Bal, V.; Gayasen, V.; Bibals, R.; Avhad, A.P.; Chakrabarty, D.; Bandyopadhyaya, R. Modeling and experiments on release of metofluthrin from a thin cellulosic-polymer film. Chem. Eng. Res. Des. 2016, 118, 31–40. [Google Scholar] [CrossRef]
- Bal, V.; Bandyopadhyaya, R. Generalized Model for Nano- and Submicron Particle Formation in Liquid Phase, Incorporating Reaction Kinetics and Hydrodynamic Interaction: Experiment, Modeling, and Simulation. J. Phys. Chem. C 2018, 122, 20489–20499. [Google Scholar] [CrossRef]
- Bal, V.; Bandyopadhyaya, R. Mechanistic aspects in the formation of nano- and submicron particles in a batch and a continuous microfluidic reactor: Experiment, modeling and simulation. Chem. Eng. J. 2019, 371, 1385–8947. [Google Scholar]
- Terasaka, K.; Hirabayashi, A.; Nishino, T.; Fujioka, S.; Kobayashi, D. Development of microbubble aerator for waste water treatment using aerobic activated sludge. Chem. Eng. Sci. 2011, 66, 3172–3179. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar]
- Fogg, P.; Gerrard, W. Solubility of Gases in Liquids: A Critical Evaluation of Gas/Liquid Systems in Theory and Practice; Wiley: London, UK, 1991. [Google Scholar]
- Sung, W.; Morgan, J.J. Kinetics and product of ferrous iron oxygenation in aqueous systems. Environ. Sci. Technol. 1980, 14, 561–568. [Google Scholar] [CrossRef]
- Chmielewski, T.; Charewicz, W.A. The oxidation of Fe(II) in aqueous sulphuric acid under oxygen pressure. Hydrometallurgy 1984, 12, 21–30. [Google Scholar] [CrossRef]
- Hove, M.; van Hille, R.P.; Lewis, A.E. Iron solids formed from oxidation precipitation of ferrous sulfate solutions. AIChE J. 2007, 53, 2569–2577. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Wang, Y.; Zhu, B.; Wei, G.; Ma, F.; Yu, Z.; Qu, J. Microbubble Oxidation for Fe2+ Removal from Hydrochloric Acid Laterite Ore Leachate. Materials 2023, 16, 6951. https://doi.org/10.3390/ma16216951
Xu Z, Wang Y, Zhu B, Wei G, Ma F, Yu Z, Qu J. Microbubble Oxidation for Fe2+ Removal from Hydrochloric Acid Laterite Ore Leachate. Materials. 2023; 16(21):6951. https://doi.org/10.3390/ma16216951
Chicago/Turabian StyleXu, Ziyang, Yu Wang, Boyuan Zhu, Guangye Wei, Fei Ma, Zhihui Yu, and Jingkui Qu. 2023. "Microbubble Oxidation for Fe2+ Removal from Hydrochloric Acid Laterite Ore Leachate" Materials 16, no. 21: 6951. https://doi.org/10.3390/ma16216951
APA StyleXu, Z., Wang, Y., Zhu, B., Wei, G., Ma, F., Yu, Z., & Qu, J. (2023). Microbubble Oxidation for Fe2+ Removal from Hydrochloric Acid Laterite Ore Leachate. Materials, 16(21), 6951. https://doi.org/10.3390/ma16216951






