Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications
Abstract
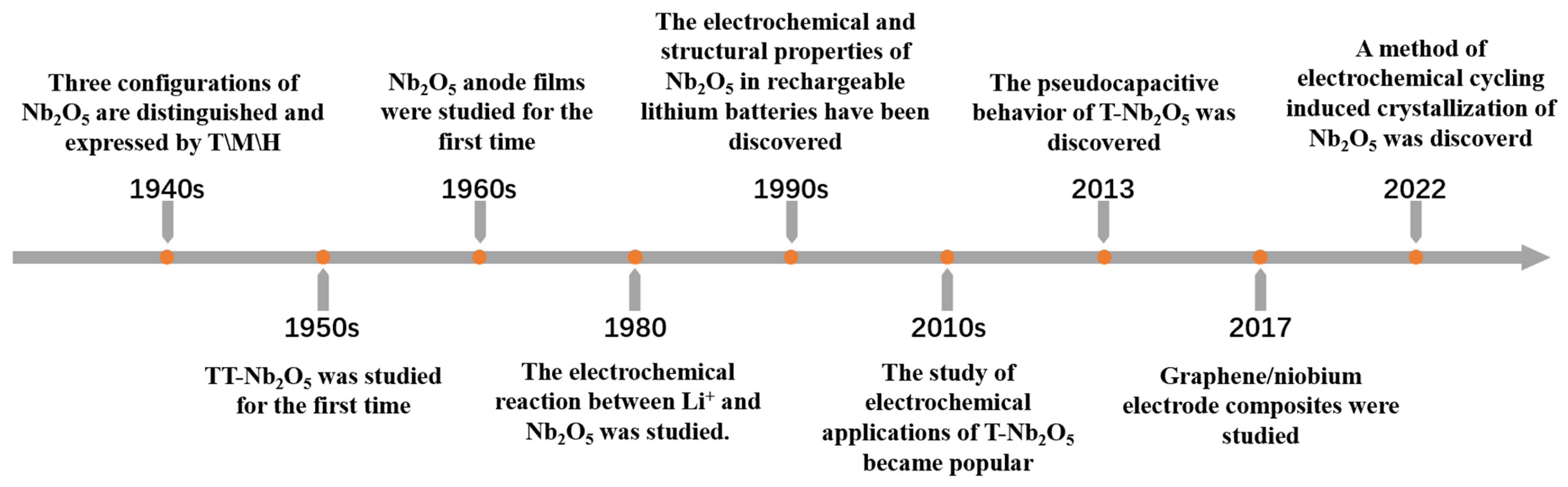
1. Introduction
2. Polymorphs of Nb2O5 and Synthesis Methods
2.1. Polymorphs of Nb2O5
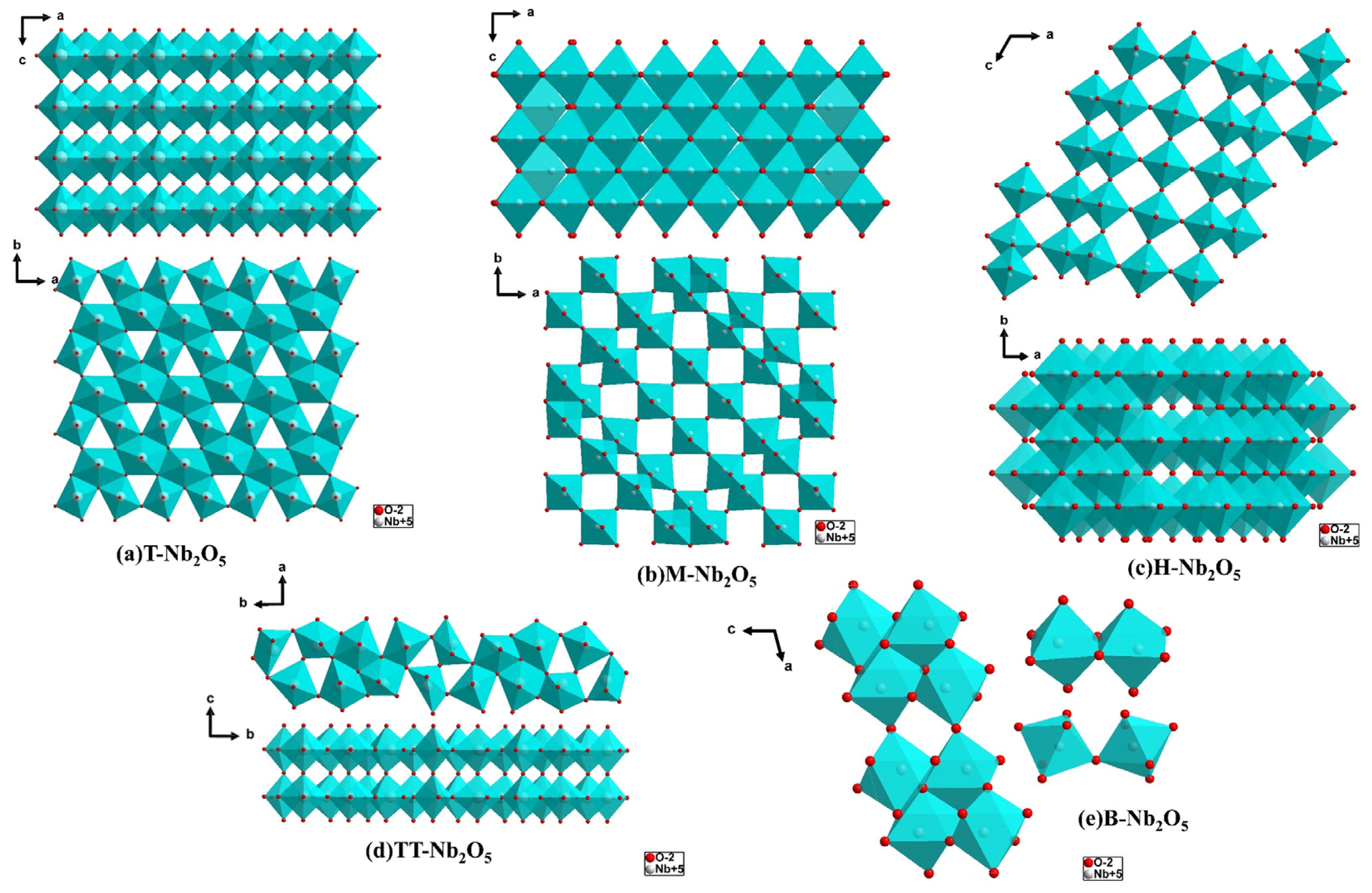
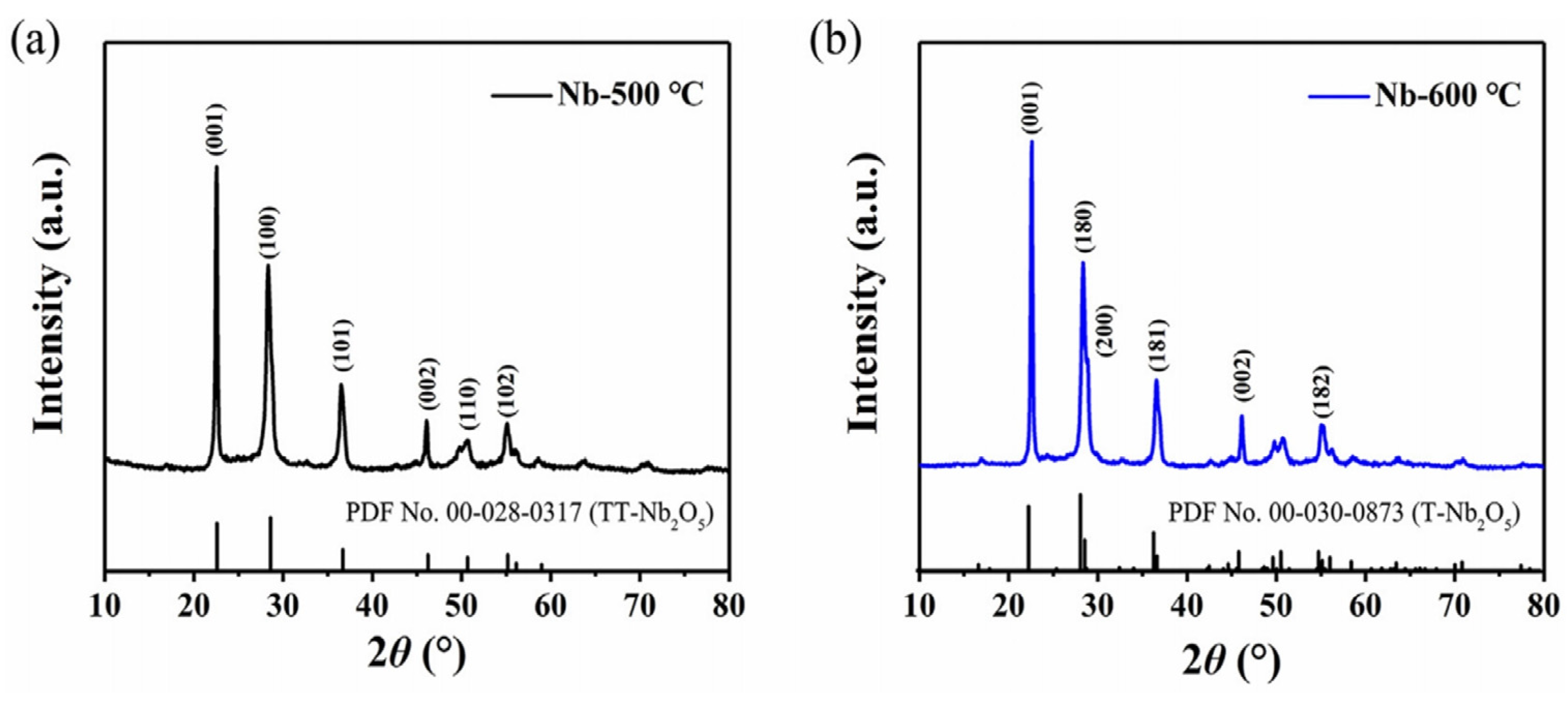
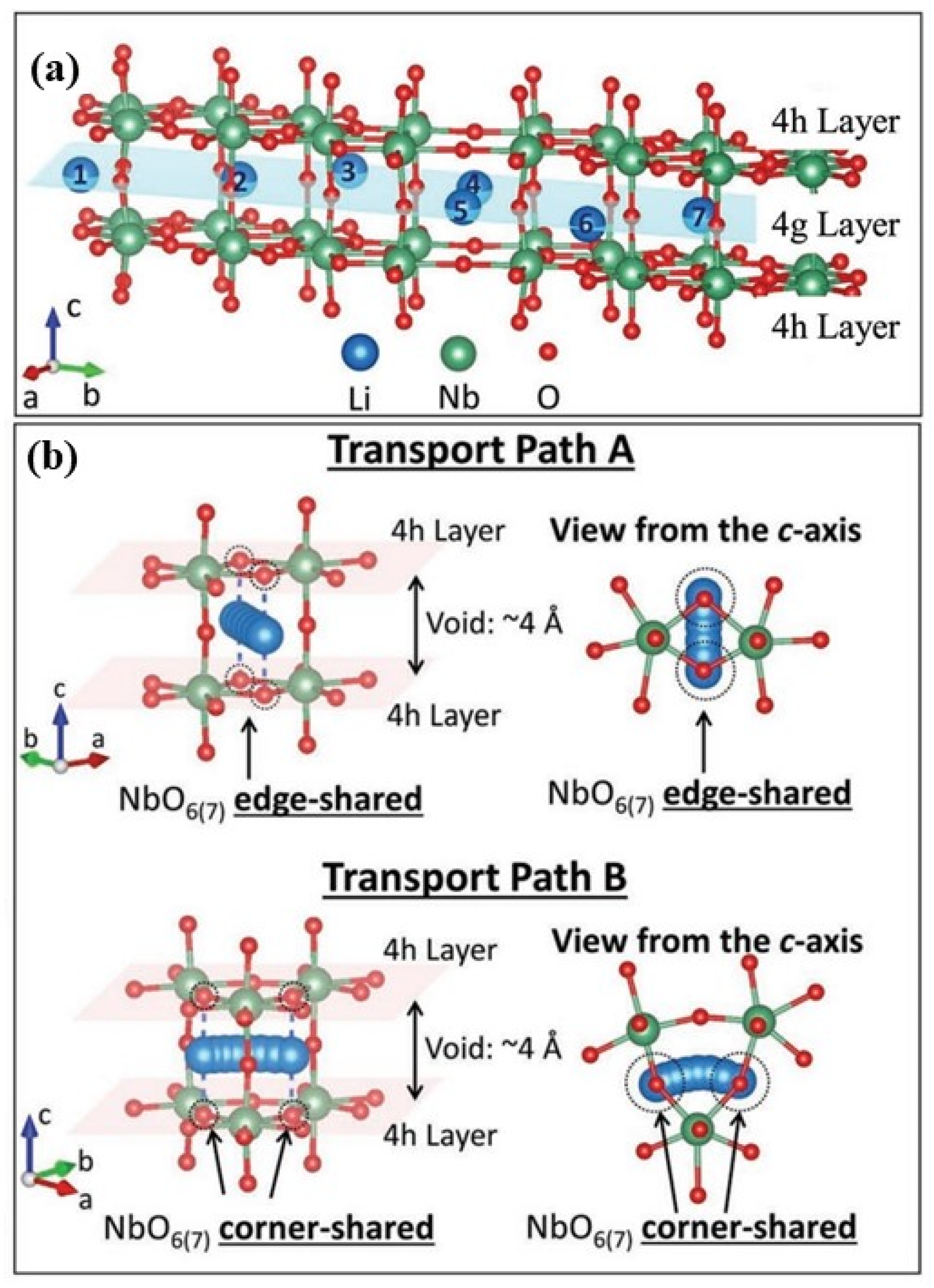
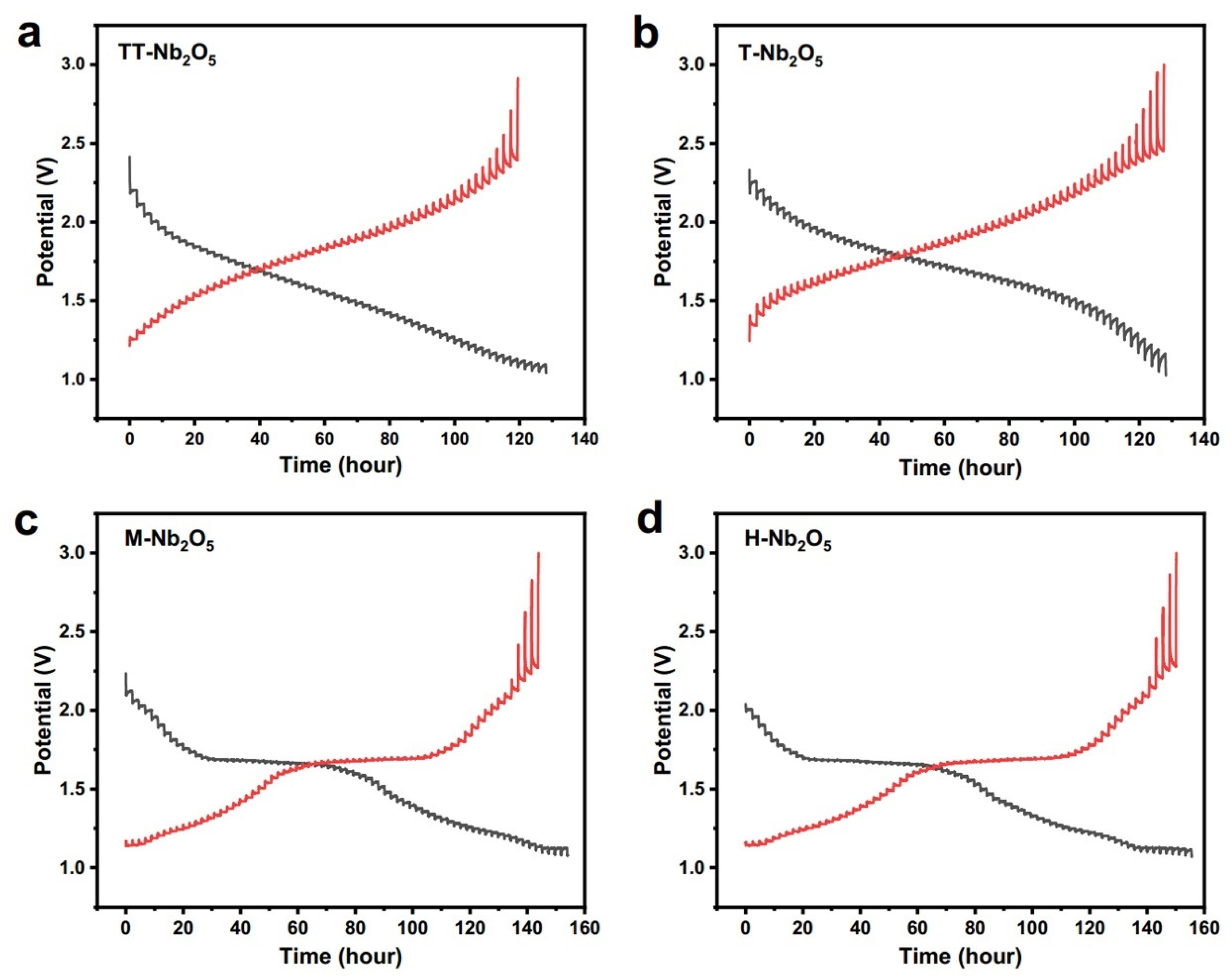
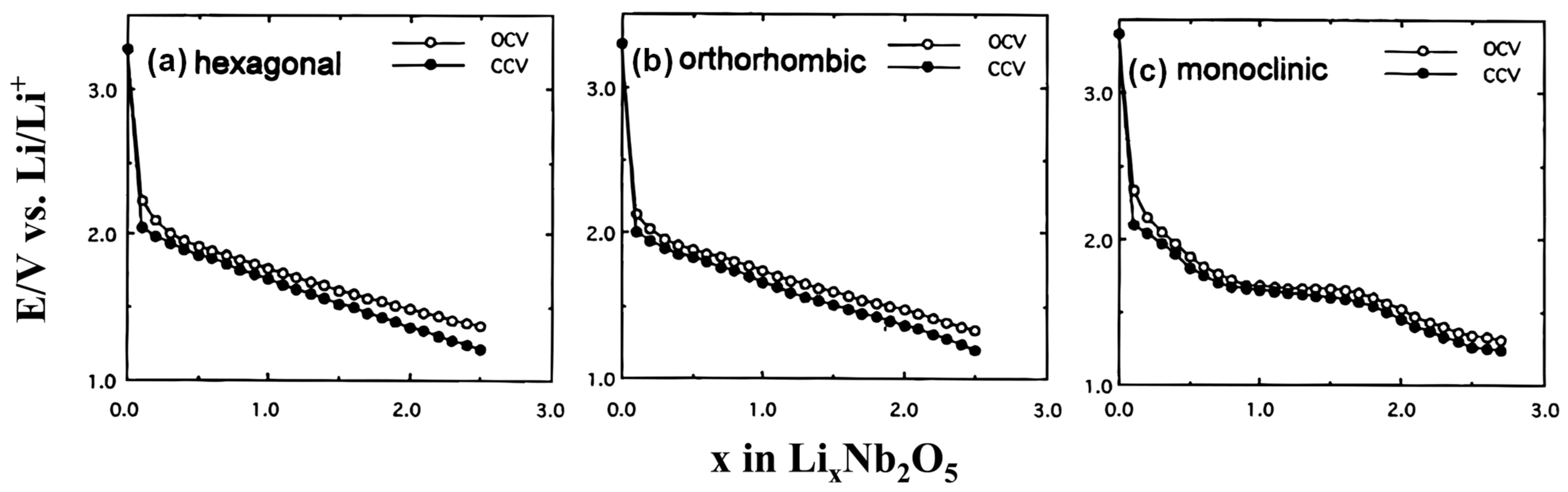
2.1.1. T- Nb2O5
2.1.2. TT-Nb2O5
2.1.3. H-Nb2O5
2.1.4. M-Nb2O5
2.1.5. B-Nb2O5
2.1.6. Other Nb2O5 Phases
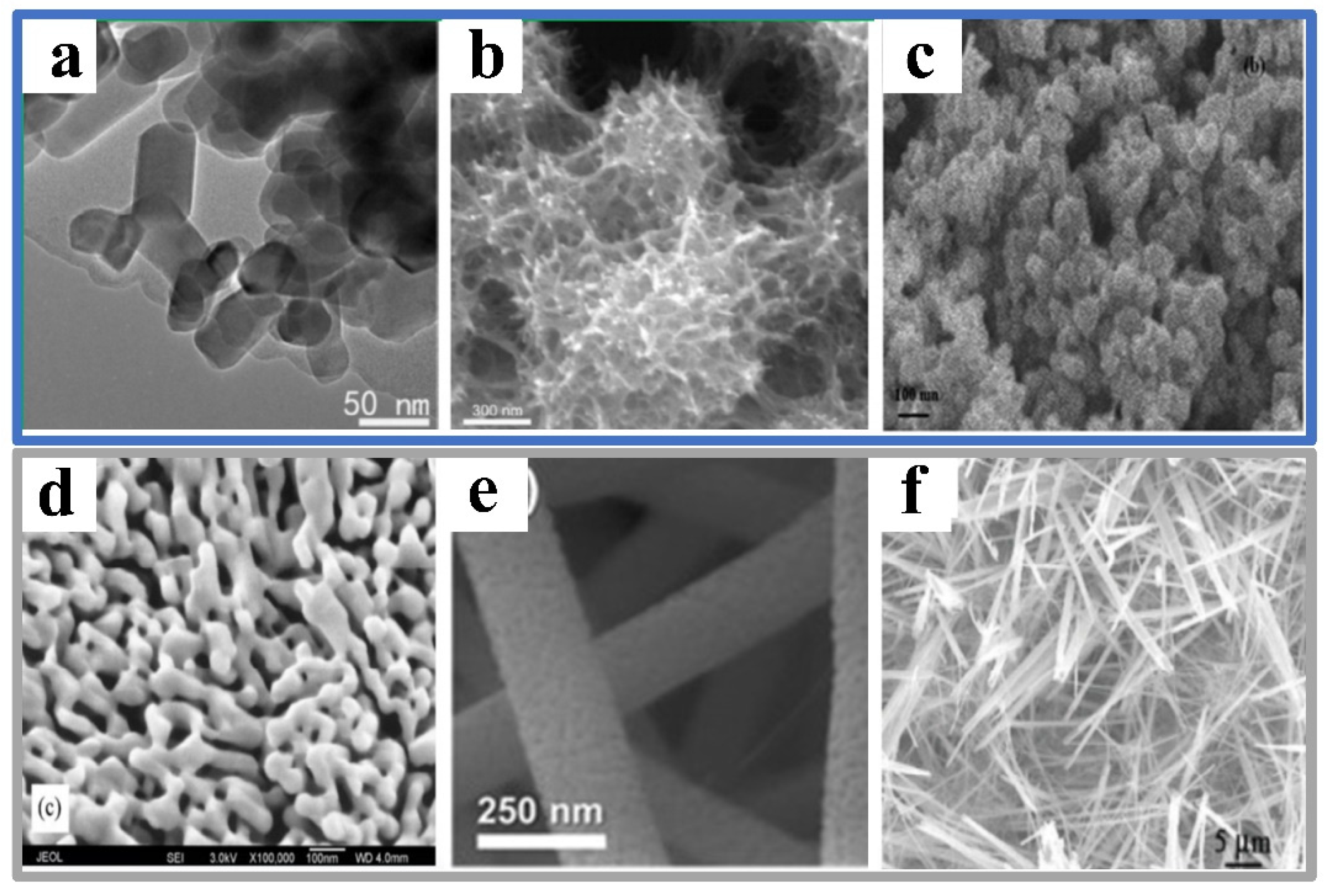
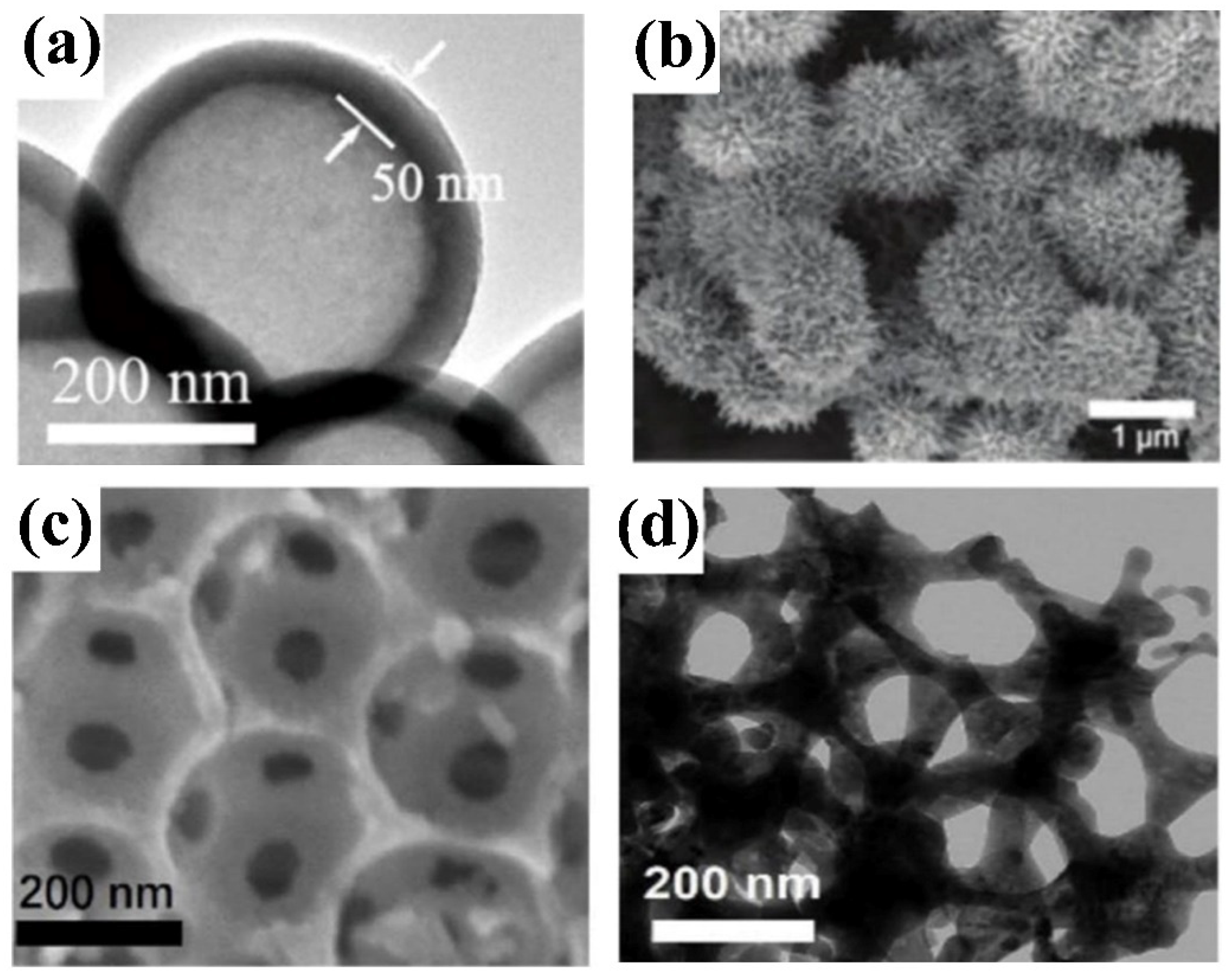
2.2. Synthesis Methods
2.2.1. Hydrothermal and Solvothermal Methods
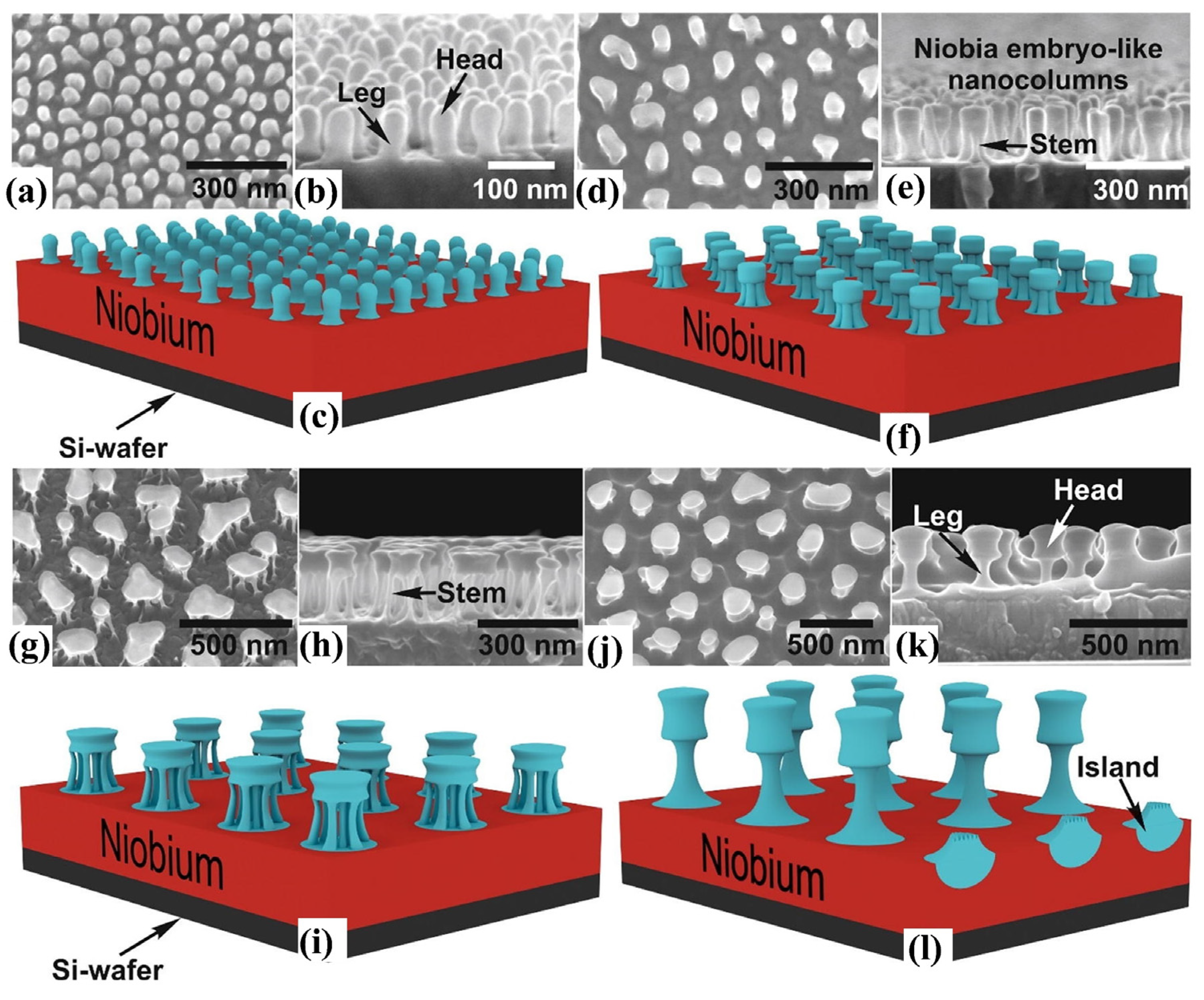
2.2.2. Anodization Method
2.2.3. Sol-Gel Methods
2.2.4. Electrodeposition
2.2.5. Vapor Phase Deposition
2.2.6. Thermal Oxidation
3. Properties of Nb2O5
3.1. Electrical Properties
3.2. Optical Properties
3.3. Mechanical Properties
4. Application of Nb2O5
4.1. Lithium-Ion Battery
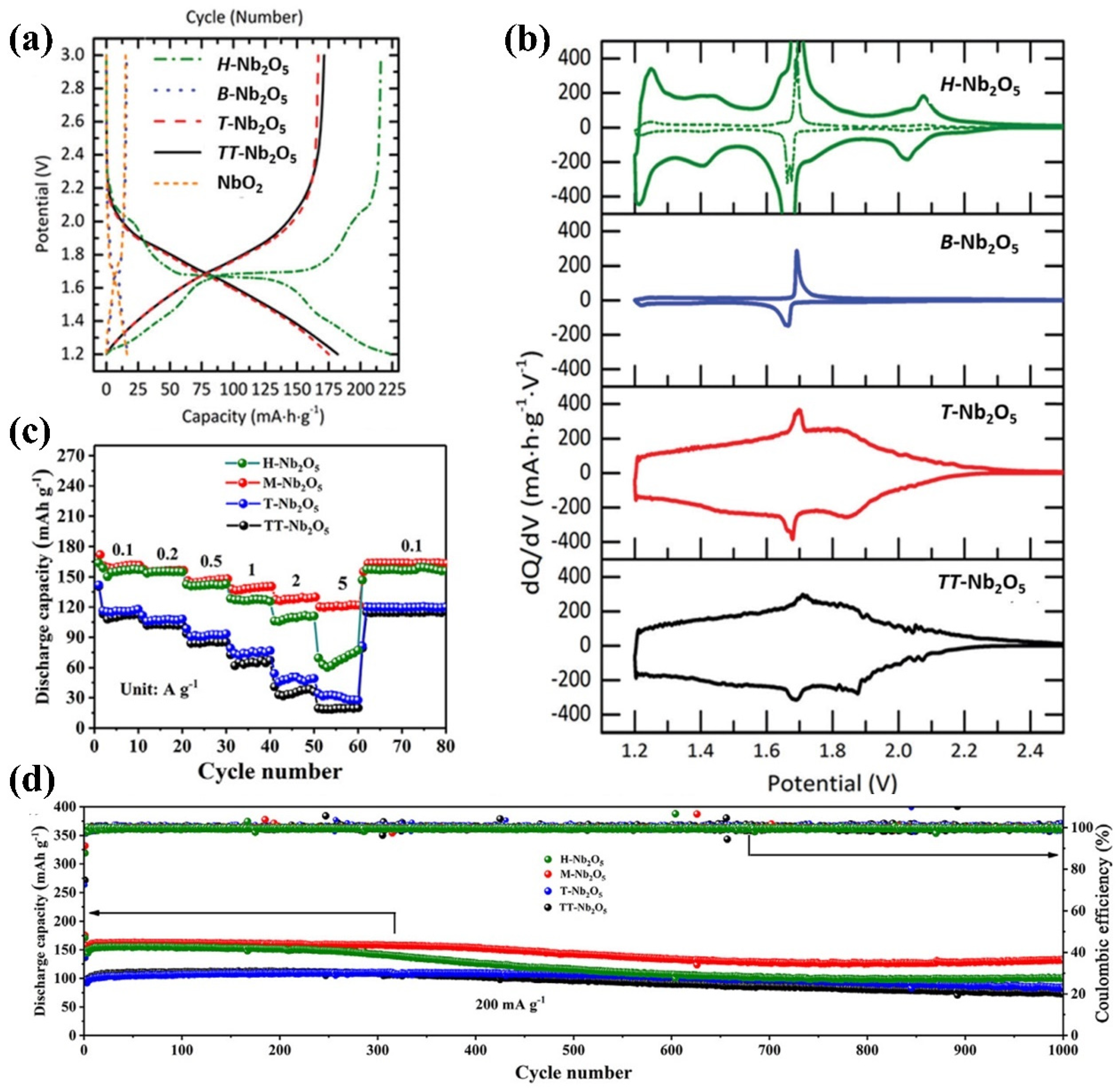
4.1.1. Lithium-Ion Battery Anode Performance
4.1.2. Lithium Storage Mechanism
4.1.3. Effect of Nb2O5 Nanostructures
4.2. Supercapacitor
4.2.1. High Rate Electrode
4.2.2. Hybrid Supercapacitors
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar]
- Wu, J.; Xue, D. Localized crystallization: A chemical transformation of Nb2O5 rod-like arrays into ordered niobate arrays. CrystEngComm 2011, 13, 1966–1975. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, L.; Liebau, M.; Ren, Y.; Luo, C.; Liu, B.; Zhang, S.; Gläser, R. Promotion effect of niobium on ceria catalyst for selective catalytic reduction of NO with NH3. J. Rare Earths 2022, 40, 1535–1545. [Google Scholar]
- Brauer, G. Die Oxyde des Niobs. Anorg. Allg. Chem. 1941, 248, 1–31. [Google Scholar] [CrossRef]
- Liu, M.; Xue, D. Large-scale fabrication of H2(H2O)Nb2O6 and Nb2O5 hollow microspheres. Mater. Res. Bull. 2010, 45, 333. [Google Scholar] [CrossRef][Green Version]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-Based Photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar]
- Yi, T.F.; Sari, H.M.K.; Li, X.; Wang, F.; Zhu, Y.R.; Hu, J.; Zhang, J.; Li, X. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021, 85, 105955. [Google Scholar]
- Ohzuku, T.; Sawai, K.; Hirai, T. Electrochemistry of L-niobium pentoxide a lithium/non-aqueous cell. J. Power Sources 1987, 19, 287–299. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar]
- Dhawan, S.; Dhawan, T.; Vedeshwar, A.G. Residual stress induced crystalline to amorphous phase transformation in Nb2O5 quantum dots. J. Appl. Phys. 2014, 116, 043503. [Google Scholar]
- Schäfer, H.; Gruehn, R.; Schulte, F. The Modifications of Niobium Pentoxide. Angew. Chem. Int. Ed. 1966, 5, 40–52. [Google Scholar] [CrossRef]
- Ikeya, T.; Senna, M. Change in the structure of niobium pentoxide due to mechanical and thermal treatments. J. Non-Cryst. Solids 1988, 105, 243–250. [Google Scholar] [CrossRef]
- Huang, H.; Kuai, H.; Ding, X.; Hu, B.; Chen, Y.; Zhou, Q.; Xiong, X. Single crystal H-Nb2O5 growing along the [001] crystal direction for ultrafast lithium storage. J. Mater. Chem. A 2023, 11, 642–648. [Google Scholar] [CrossRef]
- Pinto, M.B.; Soares, A.L., Jr.; Quintão, M.C.; Duarte, H.A.; De Abreu, H.A. Unveiling the Structural and Electronic Properties of the B-Nb2O5 Surfaces and Their Interaction with H2O and H2O2. J. Phys. Chem. C 2018, 122, 6618–6628. [Google Scholar] [CrossRef]
- Mertin, W.; Andersson, S.; Gruehn, R. Über die Kristallstruktur von M-Nb2O5. J. Solid State Chem. 1970, 1, 419–424. [Google Scholar] [CrossRef]
- Kato, K. Structure refinement of H-Nb2O5. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 764–767. [Google Scholar] [CrossRef]
- Andersson, S. The Crystal Structure of N-Nb2O5, prepared in the presence of small amounts of LiF. Z. Anorg. Allg. Chem. 1967, 351, 106–112. [Google Scholar] [CrossRef]
- Israelsson, M.; Kihlborg, L. The crystal structure of monoclinic wolfram vanadium oxide, W3V5O20, an OD structure related to R-Nb2O5. J. Solid State Chem. 1970, 1, 469–477. [Google Scholar] [CrossRef]
- Reznichenko, L.A.; Akhnazarova, V.V.; Shilkina, L.A.; Razumovskaya, O.N.; Dudkina, S.I. Invar effect in N-Nb2O5, αht-Nb2O5, and L-Nb2O5. Crystallogr. Rep. 2009, 54, 483–491. [Google Scholar] [CrossRef]
- Bowman, A.; Wallace, T.; Yarnell, J.; Wenzel, R. The crystal structure of niobium monoxide. Acta Crystallogr. 1966, 21, 843. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Song, T. High capacity monoclinic Nb2O5 and semiconducting NbO2 composite as high-power anode material for Li-Ion batteries. J. Power Sources 2019, 414, 377–382. [Google Scholar] [CrossRef]
- Griffith, K.J.; Forse, A.C.; Griffin, J.M.; Grey, C.P. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J. Am. Chem. Soc. 2016, 138, 8888–8899. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.I.; Weissman, J.G. Structures of niobium pentoxide and their implications on chemical behavior. Catal. Today 1990, 8, 27–36. [Google Scholar] [CrossRef]
- Pinto, M.B.; Soares, A.L.; Orellana, A.M.; Duarte, H.A.; De Abreu, H.A. Structural, Electronic, and Thermodynamic Properties of the T and B Phases of Niobia: First-Principle Calculations. J. Phys. Chem. A 2017, 121, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef]
- Serghiou, G.C.; Winters, R.R.; Hammack, W.S. Pressure-induced amorphization and reduction of T-Nb2O5. Phys. Rev. Lett. 1992, 68, 3311–3314. [Google Scholar] [CrossRef]
- Li, L.; Deng, J.; Yu, R.; Chen, J.; Wang, X.; Xing, X. Phase evolution in low-dimensional niobium oxide synthesized by a topochemical method. Inorg. Chem. 2010, 49, 1397–1403. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruna, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Kong, L.P.; Zhang, C.F.; Zhang, S.M.; Wang, J.T.; Cai, R.; Lv, C.X.; Qiao, W.M.; Ling, L.C.; Long, D.H. High-power and high-energy asymmetric supercapacitors based on Li+-intercalation into a T-Nb2O5/graphene pseudocapacitive electrode. J. Mater. Chem. A 2014, 2, 17962–17970. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.-H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.-S.; Roh, K.C.; et al. Facile Synthesis of Nb2O5@Carbon Core–Shell Nanocrystals with Controlled Crystalline Structure for High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef]
- Liu, F.F.; Cheng, X.L.; Xu, R.; Wu, Y.; Jiang, Y.; Yu, Y. Binding Sulfur-Doped Nb2O5 Hollow Nanospheres on Sulfur-Doped Graphene Networks for Highly Reversible Sodium Storage. Adv. Funct. Mater. 2018, 28, 1800394. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Tang, Y.B. Hierarchical T-Nb2O5 nanostructure with hybrid mechanisms of intercalation and pseudocapacitance for potassium storage and high-performance potassium dual-ion batteries. J. Mater. Chem. A 2018, 6, 17889–17895. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, G.R.; Luo, D.; Zhao, Y.; Zhang, Y.G.; Zhou, G.F.; Shui, L.L.; Wang, X. Amorphous-crystalline-heterostructured niobium oxide as two-in-one host matrix for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2021, 9, 11160–11167. [Google Scholar] [CrossRef]
- Hu, Z.; He, Q.; Liu, Z.; Liu, X.; Qin, M.; Wen, B.; Shi, W.; Zhao, Y.; Li, Q.; Mai, L. Facile formation of tetragonal-Nb2O5 microspheres for high-rate and stable lithium storage with high areal capacity. Sci. Bull. 2020, 65, 1154–1162. [Google Scholar] [CrossRef]
- Liao, J.; Tan, R.; Kuang, Z.; Cui, C.; Wei, Z.; Deng, X.; Yan, Z.; Feng, Y.; Li, F.; Wang, C. Controlling the morphology, size and phase of Nb2O5 crystals for high electrochemical performance. Chin. Chem. Lett. 2018, 29, 1785–1790. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.-H.; Chou, T.-F.; Zhao, B.; El-Sayed, M.A.; Liu, M. Unraveling the nature of anomalously fast energy storage in T-Nb2O5. J. Am. Chem. Soc. 2017, 139, 7071–7081. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Qiao, W.; Wang, J.; Ling, L. Oxygen vacancies enhance the lithium ion intercalation pseudocapacitive properties of orthorhombic niobium pentoxide. J. Colloid Interface Sci. 2020, 562, 193–203. [Google Scholar] [CrossRef]
- Holtzberg, F.; Reisman, A.; Berry, M.; Berkenblit, M. Chemistry of the Group VB Pentoxides. VI. The Polymorphism of Nb2O5. J. Am. Chem. Soc. 1957, 79, 2039–2043. [Google Scholar] [CrossRef]
- Shafer, M.W.; Roy, R. The Polymorphism of Nb2O5. Z. Kristallogr Cryst. Mater. 1958, 110, 241–248. [Google Scholar] [CrossRef]
- Terao, N. Structures des Oxydes de Niobium. Jpn. J. Appl. Phys. 1963, 2, 156. [Google Scholar] [CrossRef]
- Tamura, S. High-pressure phase research on Nb2O5. J. Mater. Sci. 1972, 7, 298–302. [Google Scholar] [CrossRef]
- Weissman, J.G.; Ko, E.I.; Wynblatt, P.; Howe, J.M. High-resolution electron microscopy and image simulation of TT-,T-, and H-niobia and model silica-supported niobium surface oxides. Chem. Mater. 1989, 1, 187–193. [Google Scholar] [CrossRef]
- Košutová, T.; Horák, L.; Pleskunov, P.; Hanuš, J.; Nikitin, D.; Kúš, P.; Cieslar, M.; Gordeev, I.; Burazer, S.; Choukourov, A.; et al. Thermally-driven morphogenesis of niobium nanoparticles as witnessed by in-situ X-ray scattering. Mater. Chem. Phys. 2022, 277, 125466. [Google Scholar] [CrossRef]
- Taques Tractz, G.; Staciaki da Luz, F.; Regina Masetto Antunes, S.; do Prado Banczek, E.; Taras da Cunha, M.; Rogério Pinto Rodrigues, P. Nb2O5 synthesis and characterization by Pechini method to the application as electron transport material in a solar device. Sol. Energy 2021, 216, 1–6. [Google Scholar] [CrossRef]
- Schäfer, H.; Schulte, F.; Gruehn, R. Neue Nb2O5-Modifikationen. Angew. Chem. 1964, 76, 536–537. [Google Scholar] [CrossRef]
- Uyeda, N.; Fujiyoshi, Y.; Ishizuka, K. High resolution imaging and interpretation of regular and irregular structures in N-niobium pentoxide crystal. Ultramicroscopy 1984, 15, 139–149. [Google Scholar] [CrossRef]
- Barnes, P.; Zuo, Y.; Dixon, K.; Hou, D.; Lee, S.; Ma, Z.; Connell, J.G.; Zhou, H.; Deng, C.; Smith, K. Electrochemically induced amorphous-to-rock-salt phase transformation in niobium oxide electrode for Li-ion batteries. Nat. Mater. 2022, 21, 795–803. [Google Scholar] [CrossRef]
- Shi, C.; Xiang, K.; Zhu, Y.; Zhou, W.; Chen, X.; Chen, H. Box-implanted Nb2O5 nanorods as superior anode materials in lithium ion batteries. Ceram. Int. 2017, 43, 12388–12395. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, F.; Yin, X.; Song, Y.; Xiang, T.; Wang, J. Arginine-Assisted Hydrothermal Synthesis of Urchin-like Nb2O5 Nanostructures Composed of Nanowires and Their Application in Cyclohexanone Ammoximation. J. Phys. Chem. C 2018, 122, 2155–2164. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, Z.; Lü, M.; Zhang, A.; Ma, Q. Preparation and characterization of porous Nb2O5 nanoparticles. Mater. Res. Bull. 2008, 43, 1363–1368. [Google Scholar] [CrossRef]
- Ristić, M.; Popović, S.; Musić, S. Sol–gel synthesis and characterization of Nb2O5 powders. Mater. Lett. 2004, 58, 2658–2663. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Jung, J.-W.; Youn, D.-Y.; Kim, C.; Yu, S.; Cho, S.-H.; Yoon, K.R.; Kim, I.-D. Mesoporous orthorhombic Nb2O5 nanofibers as pseudocapacitive electrodes with ultra-stable Li storage characteristics. J. Power Sources 2017, 360, 434–442. [Google Scholar] [CrossRef]
- Yan, C.; Nikolova, L.; Dadvand, A.; Harnagea, C.; Sarkissian, A.; Perepichka, D.F.; Xue, D.; Rosei, F. Multiple NaNbO3/Nb2O5 heterostructure nanotubes: A new class of ferroelectric/semiconductor nanomaterials. Adv. Mater. 2010, 22, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xue, D. Controlled fabrication of Nb2O5 hollow nanospheres and nanotubes. Modern Phys. Lett. B 2009, 23, 3769–3775. [Google Scholar] [CrossRef]
- Liu, F.; Xue, D. Fabrication of Nb2O5 nanotrees with controlled branching degrees. Phys. Scr. 2010, T139, 014074. [Google Scholar] [CrossRef]
- Yan, C.; Xue, D. Formation of Nb2O5 Nanotube Arrays through Phase Transformation. Adv. Mater. 2008, 20, 1055–1058. [Google Scholar] [CrossRef]
- Draper, P.H.G.; Harvey, J. The structure of anodic films—I. An electron diffraction examination of the products of anodic oxidation on tantalum, niobium and zirconium. Acta Metall. 1963, 11, 873–879. [Google Scholar] [CrossRef]
- Arora, M.R.; Kelly, R. The structure and stoichiometry of anodic films on V, Nb, Ta, Mo and W. J. Mater. Sci. 1977, 12, 1673–1684. [Google Scholar] [CrossRef]
- Aladjem, A.; Brandon, D.G.; Yahalom, J.; Zahavi, J. Electron-beam crystallization of anodic oxide films. Electrochim. Acta 1970, 15, 663–671. [Google Scholar] [CrossRef]
- Habazaki, H.; Oikawa, Y.; Fushimi, K.; Aoki, Y.; Shimizu, K.; Skeldon, P.; Thompson, G.E. Importance of water content in formation of porous anodic niobium oxide films in hot phosphate–glycerol electrolyte. Electrochim. Acta 2009, 54, 946–951. [Google Scholar] [CrossRef]
- Ou, J.Z.; Rani, R.A.; Ham, M.-H.; Field, M.R.; Zhang, Y.; Zheng, H.; Reece, P.; Zhuiykov, S.; Sriram, S.; Bhaskaran, M.; et al. Elevated Temperature Anodized Nb2O5: A Photoanode Material with Exceptionally Large Photoconversion Efficiencies. ACS Nano 2012, 6, 4045–4053. [Google Scholar] [CrossRef] [PubMed]
- Karlinsey, R.L. Preparation of self-organized niobium oxide microstructures via potentiostatic anodization. Electrochem. Commun. 2005, 7, 1190–1194. [Google Scholar] [CrossRef]
- Karlinsey, R.L. Self-assembled Nb2O5 microcones with tailored crystallinity. J. Mater. Sci. 2006, 41, 5017–5020. [Google Scholar] [CrossRef]
- Wei, W.; Lee, K.; Shaw, S.; Schmuki, P. Anodic formation of high aspect ratio, self-ordered Nb2O5 nanotubes. Chem. Commun. 2012, 48, 4244–4246. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yang, Y.; Yang, M.; Schmuki, P. Formation of Highly Ordered Nanochannel Nb Oxide by Self-Organizing Anodization. Chem.–A Eur. J. 2012, 18, 9521–9524. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rani, R.; Zoolfakar, A.S.; Subbiah, J.; Ou, J.Z.; Kalantar-zadeh, K. Highly ordered anodized Nb2O5 nanochannels for dye-sensitized solar cells. Electrochem. Commun. 2014, 40, 20–23. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; Ou, J.Z.; Kadir, R.A.; Nili, H.; Latham, K.; Sriram, S.; Bhaskaran, M.; Zhuiykov, S.; Kaner, R.B.; et al. Reduced impurity-driven defect states in anodized nanoporous Nb2O5: The possibility of improving performance of photoanodes. Chem. Commun. 2013, 49, 6349–6351. [Google Scholar] [CrossRef]
- Yao, D.D.; Rani, R.A.; O’Mullane, A.P.; Kalantar-zadeh, K.; Ou, J.Z. High Performance Electrochromic Devices Based on Anodized Nanoporous Nb2O5. J. Phys. Chem. C 2014, 118, 476–481. [Google Scholar] [CrossRef]
- Liu, X.L.; Yuan, R.L.; Liu, Y.S.; Zhu, S.; Lin, J.; Chen, X.F. Niobium pentoxide nanotube powder for efficient dye-sensitized solar cells. New J. Chem. 2016, 40, 6276–6280. [Google Scholar] [CrossRef]
- Khairir, N.S.; Rani, R.A.; Ab Kadir, R.; Soin, N.; Abdullah, W.F.H.; Mamat, M.H.; Rusop, M.; Zoolfakar, A.S. Electrical Behavior of a Nanoporous Nb2O5/Pt Schottky Contact at Elevated Temperatures. J. Electron. Mater. 2019, 48, 611–620. [Google Scholar] [CrossRef]
- Pligovka, A.; Yunin, P.; Hoha, A.; Korolyov, S.; Gorokh, G.; Skorokhodov, E. Morphology and Structure of Defected Niobium Oxide Nonuniform Arrays Formed by Anodizing Bilayer Al/Nb Systems. Tech. Phys. 2020, 65, 1771–1776. [Google Scholar] [CrossRef]
- Gorokh, G.G.; Pligovka, A.N.; Lozovenko, A. Columnar niobium oxide nanostructures: Mechanism of formation, microstructure, and electrophysical properties. Tech. Phys. 2019, 64, 1657–1665. [Google Scholar] [CrossRef]
- Pligovka, A.; Hoha, A.; Turavets, U.; Poznyak, A.; Zakharau, Y. Formation features, morphology and optical properties of nanostructures via anodizing Al/Nb on Si and glass. Mater. Today Proc. 2021, 37, A8–A15. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Zakhlebayeva, A. Electro-physical properties of niobia columnlike nanostructures via the anodizing of Al/Nb layers. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-Level 3D Column-like Nanofilms with Hexagonally–Packed Tantalum Fabricated via Anodizing of Al/Nb and Al/Ta Layers—A Potential Nano-Optical Biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef]
- Matsuda, A.; Kawamura, G. Sol-Gel Nano-/Micropatterning Process. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ulrich, D.R. Prospects of sol-gel processes. J. Non-Cryst. Solids 1988, 100, 174–193. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Nistico, R.; Scalarone, D.; Magnacca, G. Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Microporous Mesoporous Mater. 2017, 248, 18–29. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer Science & Business Media: Cham, Switzerland, 2009. [Google Scholar]
- Alquier, C.; Vandenborre, M.T.; Henry, M. Synthesis of niobium pentoxide gels. J. Non-Cryst. Solids 1986, 79, 383–395. [Google Scholar] [CrossRef]
- Schmitt, M.; Heusing, S.; Aegerter, M.A.; Pawlicka, A.; Avellaneda, C. Electrochromic properties of Nb2O5 sol-gel coatings. Sol. Energy Mater. Sol. Cells 1998, 54, 9–17. [Google Scholar] [CrossRef]
- Melo, L.; Avellaneda, C.O.; Caram, R.; Sichieri, E.; Pawlicka, A. Electrochromic Properties of Sol-gel Coating of Nb2O5 and Nb2O5: Li+. Mater. Res. 2002, 5, 43–46. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Shen, X.M.; Wu, X.R.; Cao, F.B.; Li, L.S. Effect of crystallization degree on optical characteristics of Nb2O5 by sol-gel process. Mod. Phys. Lett. B 2021, 35, 2150171. [Google Scholar] [CrossRef]
- Pu, J.; Shen, Z.H.; Zhong, C.L.; Zhou, Q.W.; Liu, J.Y.; Zhu, J.; Zhang, H.G. Electrodeposition Technologies for Li-Based Batteries: New Frontiers of Energy Storage. Adv. Mater. 2020, 32, 1903808. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Kim, T.-Y.; Kim, N.-J.; Lee, C.-H.; Park, E.-M.; Park, C.; Suh, S.-J. Preparation and characterization of Nb2O5–Al2O3 composite oxide formed by cathodic electroplating and anodizing. Mater. Sci. Eng. B 2011, 176, 1505–1508. [Google Scholar] [CrossRef]
- Jha, G.; Tran, T.; Qiao, S.P.; Ziegler, J.M.; Ogata, A.F.; Dai, S.; Xu, M.J.; Le Thai, M.; Chandran, G.T.; Pan, X.Q.; et al. Electrophoretic Deposition of Mesoporous Niobium(V)Oxide Nanoscopic Films. Chem. Mater. 2018, 30, 6549–6558. [Google Scholar] [CrossRef]
- RobertáLee, G. Studies on the electrochemical deposition of niobium oxide. J. Mater. Chem. 1996, 6, 187–192. [Google Scholar]
- Zhitomirsky, I. Electrolytic deposition of oxide films in the presence of hydrogen peroxide. J. Eur. Ceram. Soc. 1999, 19, 2581–2587. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Electrolytic deposition of niobium oxide films. Mater. Lett. 1998, 35, 188–193. [Google Scholar] [CrossRef]
- Kamada, K.; Mukai, M.; Matsumoto, Y. Anodic dissolution of tantalum and niobium in acetone solvent with halogen additives for electrochemical synthesis of Ta2O5 and Nb2O5 thin films. Electrochim. Acta 2004, 49, 321–327. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Ye, C.; Zhang, L.; Li, C.L.; Sun, K.N. T-Nb2O5 quantum dots prepared by electrodeposition for fast Li ion intercalation/deintercalation. Nanotechnology 2017, 28, 215401. [Google Scholar] [CrossRef]
- Yang, D. Nanocomposite Films for Gas Sensing; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Yap, Y. Physical Vapor Deposition, Encyclopedia of Nanotechnology: Springer Reference; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Moreto, J.; Gelamo, R.; Nascimento, J.; Taryba, M.; Fernandes, J. Improving the corrosion protection of 2524-T3-Al alloy through reactive sputtering Nb2O5 coatings. Appl. Surf. Sci. 2021, 556, 149750. [Google Scholar] [CrossRef]
- Çetinörgü-Goldenberg, E.; Klemberg-Sapieha, J.-E.; Martinu, L. Effect of postdeposition annealing on the structure, composition, and the mechanical and optical characteristics of niobium and tantalum oxide films. Appl. Opt. 2012, 51, 6498–6507. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.-L. Effect of sputtering anisotropic ejection on the optical properties and residual stress of Nb2O5 thin films. Appl. Surf. Sci. 2010, 257, 481–486. [Google Scholar] [CrossRef]
- Reichman, B.; Bard, A.J. Electrochromism at niobium pentoxide electrodes in aqueous and acetonitrile solutions. J. Electrochem. Soc. 1980, 127, 241–242. [Google Scholar] [CrossRef]
- Vinnichenko, M.; Rogozin, A.; Grambole, D.; Munnik, F.; Kolitsch, A.; Möller, W.; Stenzel, O.; Wilbrandt, S.; Chuvilin, A.; Kaiser, U. Highly dense amorphous Nb2O5 films with closed nanosized pores. Appl. Phys. Lett. 2009, 95, 081904. [Google Scholar] [CrossRef]
- Pillis, M.F.; Geribola, G.A.; Scheidt, G.; de Araújo, E.G.; de Oliveira, M.C.L.; Antunes, R.A. Corrosion of thin, magnetron sputtered Nb2O5 films. Corros. Sci. 2016, 102, 317–325. [Google Scholar] [CrossRef]
- Coşkun, Ö.D.; Demirela, S. The optical and structural properties of amorphous Nb2O5 thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 2013, 277, 35–39. [Google Scholar] [CrossRef]
- Chen, K.-N.; Hsu, C.-M.; Liu, J.; Liou, Y.-C.; Yang, C.-F. Investigation of antireflection Nb2O5 thin films by the sputtering method under different deposition parameters. Micromachines 2016, 7, 151. [Google Scholar] [CrossRef]
- Al-Baradi, A.M.; El-Nahass, M.; Hassanien, A.; Atta, A.; Alqahtani, M.S.; Aldawsari, A.O. Influence of RF sputtering power on structural and optical properties of Nb2O5 thin films. Optik 2018, 168, 853–863. [Google Scholar] [CrossRef]
- Dhar, A.; Alford, T. Optimization of Nb2O5/Ag/Nb2O5 multilayers as transparent composite electrode on flexible substrate with high figure of merit. J. Appl. Phys. 2012, 112, 103113. [Google Scholar] [CrossRef]
- Venkataraj, S.; Drese, R.; Kappertz, O.; Jayavel, R.; Wuttig, M. Characterization of niobium oxide films prepared by reactive DC magnetron sputtering. Phys. Status Solidi A 2001, 188, 1047–1058. [Google Scholar] [CrossRef]
- Bussamara, R.; Eberhardt, D.; Feil, A.; Migowski, P.; Wender, H.; de Moraes, D.P.; Machado, G.; Papaléo, R.M.; Teixeira, S.R.; Dupont, J. Sputtering deposition of magnetic Ni nanoparticles directly onto an enzyme surface: A novel method to obtain a magnetic biocatalyst. Chem. Commun. 2013, 49, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Kuzminykh, Y.; Dabirian, A.; Reinke, M.; Hoffmann, P. High vacuum chemical vapour deposition of oxides: A review of technique development and precursor selection. Surf. Coat. Technol. 2013, 230, 13–21. [Google Scholar] [CrossRef]
- Jung, S.-C.; Imaishi, N.; Park, H.-C. Reaction engineering modeling of low-pressure metalorganic chemical vapor deposition of Nb2O5 thin film. Jpn. J. Appl. Phys. 1995, 34, L775. [Google Scholar] [CrossRef]
- Breiland, W.G.; Coltrin, M.E.; Creighton, J.R.; Hou, H.Q.; Moffat, H.K.; Tsao, J.Y. Organometallic vapor phase epitaxy (OMVPE). Mater. Sci. Eng. R Rep. 1999, 24, 241–274. [Google Scholar] [CrossRef]
- Mueller, N.S.; Morfa, A.J.; Abou-Ras, D.; Oddone, V.; Ciuk, T.; Giersig, M. Growing graphene on polycrystalline copper foils by ultra-high vacuum chemical vapor deposition. Carbon 2014, 78, 347–355. [Google Scholar] [CrossRef]
- Baum, T.H.; Larson, C.E.; Jackson, R.L. Laser-induced chemical vapor deposition of aluminum. Appl. Phys. Lett. 1989, 55, 1264–1266. [Google Scholar] [CrossRef]
- O’Neill, S.A.; Parkin, I.P.; Clark, R.J.; Mills, A.; Elliott, N. Atmospheric pressure chemical vapour deposition of thin films of Nb2O5 on glass. J. Mater. Chem. 2003, 13, 2952–2956. [Google Scholar] [CrossRef]
- de Oliveira, A.F.; da Silveira, C.B.; de Campos, S.D.; de Campos, E.A.; Carasek, E. Niobium (V) oxide coated on thin glass–ceramic rod as a solid phase microextraction fiber. Talanta 2005, 66, 74–79. [Google Scholar] [CrossRef]
- da Silveira, C.; de Oliveira, A.; de Campos, S.; de Campos, E.; Fraporti, A. Nb2O5 coating of glass fibres applied by chemical vapour deposition. Surf. Eng. 2012, 28, 68–72. [Google Scholar] [CrossRef]
- Kovendhan, M.; Joseph, D.P.; Manimuthu, P.; Ganesan, S.; Sambasivam, S.; Maruthamuthu, P.; Suthanthiraraj, S.A.; Venkateswaran, C.; Mohan, R. Spray deposited Nb2O5 thin film electrodes for fabrication of dye sensitized solar cells. Trans. Indian Inst. Met. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- Xia, J.; Masaki, N.; Jiang, K.; Yanagida, S. Fabrication and characterization of thin Nb2O5 blocking layers for ionic liquid-based dye-sensitized solar cells. J. Photochem. Photobiol. A 2007, 188, 120–127. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Wang, W.; Zhang, X.; Wang, B.; Tian, H.; Wang, Y.; Guan, J.; Gu, H. Fast and highly-sensitive hydrogen sensing of Nb2O5 nanowires at room temperature. Int. J. Hydrogen Energy 2012, 37, 4526–4532. [Google Scholar] [CrossRef]
- Tamang, R.; Varghese, B.; Mhaisalkar, S.G.; Tok, E.S.; Sow, C.H. Probing the photoresponse of individual Nb2O5 nanowires with global and localized laser beam irradiation. Nanotechnology 2011, 22, 115202. [Google Scholar] [CrossRef] [PubMed]
- Nico, C.; Rino, L.; Matos, M.; Monteiro, R.; Costa, F.M.; Monteiro, T.; Graça, M.P. NbO/Nb2O5 core–shells by thermal oxidation. J. Eur. Ceram. Soc. 2013, 33, 3077–3083. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, J. Formation of niobium oxide nanowires by thermal oxidation. J. Ind. Eng. Chem. 2009, 15, 860–864. [Google Scholar] [CrossRef]
- De Sá, A.; Rangel, C.; Skeldon, P.; Thompson, G. Semiconductive properties of anodic niobium oxides. Port. Electrochim. Acta 2006, 24, 305. [Google Scholar] [CrossRef]
- Luo, H.; Song, W.; Hoertz, P.G.; Hanson, K.; Ghosh, R.; Rangan, S.; Brennaman, M.K.; Concepcion, J.J.; Binstead, R.A.; Bartynski, R.A. A sensitized Nb2O5 photoanode for hydrogen production in a dye-sensitized photoelectrosynthesis cell. Chem. Mater. 2013, 25, 122–131. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Meireles, A.; Nico, C.; Valente, M.A. Nb2O5 nanosize powders prepared by sol–gel–Structure, morphology and dielectric properties. J. Alloys Compd. 2013, 553, 177–182. [Google Scholar] [CrossRef]
- Jiayu, D.; Zhefei, W.; Zhijie, G.; Yuan, X.; Lixi, W.; ZHANG, Q. Optimization on dielectric properties of Y2Ti2O7 ceramics with Bi2O3-Nd2O3-Nb2O5 co-doping. J. Rare Earths 2011, 29, 683–687. [Google Scholar]
- Clima, S.; Pourtois, G.; Van Elshocht, S.; De Gendt, S.; Heyns, M.M.; Wouters, D.J.; Kittl, J.A. Dielectric response of Ta2O5, NbTaO5 and Nb2O5 from first-principles investigations. ECS Trans. 2009, 19, 729. [Google Scholar] [CrossRef]
- García, H.; Castán, H.; Perez, E.; Dueñas, S.; Bailón, L.; Blanquart, T.; Niinistö, J.; Kukli, K.; Ritala, M.; Leskelä, M. Influence of growth and annealing temperatures on the electrical properties of Nb2O5-based MIM capacitors. Semicond. Sci. Technol. 2013, 28, 055005. [Google Scholar] [CrossRef]
- Soares, M.; Leite, S.; Nico, C.; Peres, M.; Fernandes, A.; Graça, M.; Matos, M.; Monteiro, R.; Monteiro, T.; Costa, F. Effect of processing method on physical properties of Nb2O5. J. Eur. Ceram. Soc. 2011, 31, 501–506. [Google Scholar] [CrossRef]
- Greener, E.; Hirthe, W. Electrical Conductivity of Nonstoichiometric α-Nb2O5. J. Electrochem. Soc. 1962, 109, 600. [Google Scholar] [CrossRef]
- Nanda, G.; Awin, E.W.; Gasyak, T.; Koroleva, E.; Filimonov, A.; Vakhrushev, S.; Sujith, R.; Kumar, R. Temperature dependent conductivity and broadband dielectric response of precursor-derived Nb2O5. Ceram. Int. 2020, 46, 9512–9518. [Google Scholar] [CrossRef]
- Lazarova, K.; Vasileva, M.; Marinov, G.; Babeva, T. Optical characterization of sol–gel derived Nb2O5 thin films. Opt. Laser Technol. 2014, 58, 114–118. [Google Scholar] [CrossRef]
- Abood, M.; Wahid, M.; Saimon, J.; Salim, E. Physical properties of Nb2O5 thin films prepared at 12M ammonium concentration. Int. J. Nanoelectron. Mater. 2018, 11, 237–244. [Google Scholar]
- Sun, S.; Mottram, L.; Hyatt, N.C. On the existence of the compound “Ce3NbO7+δ” prepared under air atmosphere. J. Rare Earths 2021, 39, 596–599. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Al-Douri, Y.; Hashim, U.; Salim, E.T. Optical investigations of photonics lithium niobate. Sol. Energy 2015, 120, 381–388. [Google Scholar] [CrossRef]
- Le Viet, A.; Jose, R.; Reddy, M.; Chowdari, B.; Ramakrishna, S. Nb2O5 photoelectrodes for dye-sensitized solar cells: Choice of the polymorph. J. Phys. Chem. C 2010, 114, 21795–21800. [Google Scholar] [CrossRef]
- Abe, S. Formation of Nb2O5 matrix and Vis-NIR absorption in Nb-Ge-O thin film. Nanoscale Res. Lett. 2012, 7, 1–6. [Google Scholar]
- Liu, J.; Xue, D.; Li, K. Single-crystalline nanoporous Nb2O5 nanotubes. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar]
- Brayner, R.; Bozon-Verduraz, F. Niobium pentoxide prepared by soft chemical routes: Morphology, structure, defects and quantum size effect. Phys. Chem. Chem. Phys. 2003, 5, 1457–1466. [Google Scholar] [CrossRef]
- Agarwal, G.; Reddy, G. Study of surface morphology and optical properties of Nb2O5 thin films with annealing. J. Mater. Sci. Mater. Electron. 2005, 16, 21–24. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Schmitt, M.; Guo, Y. Sol-gel niobium pentoxide coatings: Applications to photovoltaic energy conversion and electrochromism. Int. J. Photoenergy 2002, 4, 108237. [Google Scholar] [CrossRef]
- Xiao, X.; Dong, G.; Xu, C.; He, H.; Qi, H.; Fan, Z.; Shao, J. Structure and optical properties of Nb2O5 sculptured thin films by glancing angle deposition. Appl. Surf. Sci. 2008, 255, 2192–2195. [Google Scholar] [CrossRef]
- Shcherbina, O.; Palatnikov, M.; Efremov, V. Mechanical properties of Nb2O5 and Ta2O5 prepared by different procedures. Inorg. Mater. 2012, 48, 433–438. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin films and nanostructures of niobium pentoxide: Fundamental properties, synthesis methods and applications. J. Mater. Chem. A 2014, 2, 15683–15703. [Google Scholar]
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on improving the safety and sustainability of high voltage lithium-ion batteries through the electrolyte and separator region. Adv. Energy Mater. 2022, 12, 2200147. [Google Scholar]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [PubMed]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar]
- Ren, J.; Ren, R.P.; Lv, Y.K. A New Anode for Lithium-Ion Batteries Based on Single-Walled Carbon Nanotubes and Graphene: Improved Performance through a Binary Network Design. Chem.–A Asian J. 2018, 13, 1223–1227. [Google Scholar]
- Abdollahifar, M.; Molaiyan, P.; Perovic, M.; Kwade, A. Insights into Enhancing Electrochemical Performance of Li-Ion Battery Anodes via Polymer Coating. Energies 2022, 15, 8791. [Google Scholar]
- Molaiyan, P.; Dos Reis, G.S.; Karuppiah, D.; Subramaniyam, C.M.; Garcia-Alvarado, F.; Lassi, U. Recent progress in biomass-derived carbon materials for Li-ion and Na-ion batteries—A review. Batteries 2023, 9, 116. [Google Scholar]
- Välikangas, J.; Laine, P.; Hu, T.; Tynjälä, P.; Selent, M.; Molaiyan, P.; Jürgen, K.; Lassi, U. Effect of Secondary Heat Treatment after a Washing on the Electrochemical Performance of Co-Free LiNi0.975Al0.025O2 Cathodes for Li-Ion Batteries. Small 2023, 2305349. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar]
- Deng, Q.; Fu, Y.; Zhu, C.; Yu, Y. Niobium-based oxides toward advanced electrochemical energy storage: Recent advances and challenges. Small 2019, 15, 1804884. [Google Scholar] [CrossRef]
- Zhou, Z.; Lou, S.; Cheng, X.; Cui, B.; Si, W.; Ding, F.; Ma, Y.; Zuo, P.; Du, C.; Wang, J. Superior Electrochemical Performance of WNb2O8 Nanorods Triggered by Ultra-Efficient Li+ Diffusion. ChemistrySelect 2020, 5, 1209–1213. [Google Scholar] [CrossRef]
- Lin, J.; Yuan, Y.; Su, Q.; Pan, A.; Dinesh, S.; Peng, C.; Cao, G.; Liang, S. Facile synthesis of Nb2O5/carbon nanocomposites as advanced anode materials for lithium-ion batteries. Electrochim. Acta 2018, 292, 63–71. [Google Scholar]
- Shen, F.; Sun, Z.; He, Q.; Sun, J.; Kaner, R.B.; Shao, Y. Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage. Mater. Horiz. 2021, 8, 1130–1152. [Google Scholar]
- Griffith, K.J.; Wiaderek, K.M.; Cibin, G.; Marbella, L.E.; Grey, C.P. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature 2018, 559, 556–563. [Google Scholar] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [PubMed]
- Ding, H.; Song, Z.; Zhang, H.; Zhang, H.; Li, X. Niobium-based oxide anodes toward fast and safe energy storage: A review. Mater. Today Nano 2020, 11, 100082. [Google Scholar]
- Yang, M.; Li, S.; Huang, J. Three-Dimensional Cross-Linked Nb2O5 Polymorphs Derived from Cellulose Substances: Insights into the Mechanisms of Lithium Storage. ACS Appl. Mater. Interfaces 2021, 13, 39501–39512. [Google Scholar]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar]
- Reichman, B.; Bard, A.J. The Application of Nb2O5 as a Cathode in Nonaqueous Lithium Cells. J. Electrochem. Soc. 1981, 128, 344. [Google Scholar] [CrossRef]
- Kumagai, N.; Tanno, K.; Nakajima, T.; Watanabe, N. Structural changes of Nb2O5 and V2O5 as rechargeable cathodes for lithium battery. Electrochim. Acta 1983, 28, 17–22. [Google Scholar]
- Kodama, R.; Terada, Y.; Nakai, I.; Komaba, S.; Kumagai, N. Electrochemical and in situ XAFS-XRD investigation of Nb2O5 for rechargeable lithium batteries. J. Electrochem. Soc. 2006, 153, A583. [Google Scholar] [CrossRef]
- Han, J.-T.; Huang, Y.-H.; Goodenough, J.B. New anode framework for rechargeable lithium batteries. Chem. Mater. 2011, 23, 2027–2029. [Google Scholar] [CrossRef]
- Nakazawa, H.; Sano, K.; Abe, T.; Baba, M.; Kumagai, N. Charge–discharge characteristics of all-solid-state thin-filmed lithium-ion batteries using amorphous Nb2O5 negative electrodes. J. Power Sources 2007, 174, 838–842. [Google Scholar] [CrossRef]
- Kumagai, N.; Koishikawa, Y.; Komaba, S.; Koshiba, N. Thermodynamics and Kinetics of Lithium Intercalation into Nb2O5 Electrodes for a 2 V Rechargeable Lithium Battery. J. Electrochem. Soc. 1999, 146, 3203. [Google Scholar] [CrossRef]
- Come, J.; Augustyn, V.; Kim, J.W.; Rozier, P.; Taberna, P.-L.; Gogotsi, P.; Long, J.W.; Dunn, B.; Simon, P. Electrochemical kinetics of nanostructured Nb2O5 electrodes. J. Electrochem. Soc. 2014, 161, A718. [Google Scholar] [CrossRef]
- Liu, C.-P.; Zhou, F.; Ozolins, V. First principles study for lithium intercalation and diffusion behavior in orthorhombic Nb2O5 electrochemical supercapacitor. In APS March Meeting Abstracts; American Physical Society: College Park, MD, USA, 2012; p. B26-003. [Google Scholar]
- Kim, J.W.; Augustyn, V.; Dunn, B. The effect of crystallinity on the rapid pseudocapacitive response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Song, Z.; Li, H.; Liu, W.; Zhang, H.; Yan, J.; Tang, Y.; Huang, J.; Zhang, H.; Li, X. Ultrafast and stable Li-(de) intercalation in a large single crystal H-Nb2O5 anode via optimizing the homogeneity of electron and ion transport. Adv. Mater. 2020, 32, 2001001. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; Johnson, I.D.; Brett, D.J.L.; Shearing, P.R.; Liu, Z.; Darr, J.A. High power nano-Nb2O5 negative electrodes for lithium-ion batteries. Electrochim. Acta 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, W.; Wang, J.; Dong, C.; Lin, Y.; Chen, I.-W.; Huang, F. Orthorhombic Nb2O5−x for durable high-rate anode of Li-ion batteries. iScience 2020, 23, 100767. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Wu, Y.; Zhang, T.; Guo, Y.; Zhang, Q.; Zeng, Y.; Lu, J. Nanostructured Nb2O5 cathode for high-performance lithium-ion battery with Super-P and graphene compound conductive agents. J. Electroanal. Chem. 2018, 827, 112–119. [Google Scholar] [CrossRef]
- Ye, R.; Ohta, K.; Baba, M. Electrochemical properties of amorphous Nb2O5 thin film and its application to rechargeable thin film lithium ion batteries. ECS Trans. 2016, 73, 49. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Zhang, Y. Fabrication of Nb2O5 nanosheets for high-rate lithium ion storage applications. Sci. Rep. 2015, 5, 8326. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Zettsu, N.; Hirata, K.; Kami, K.; Teshima, K. High-voltage capabilities of ultra-thin Nb2O5 nanosheet coated LiNi1/3Co1/3Mn1/3O2 cathodes. RSC Adv. 2016, 6, 67514–67519. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Chen, H.; Ma, J.; Zhang, R. Facile synthesis of Nb2O5 nanobelts assembled from nanorods and their applications in lithium ion batteries. J. Phys. Chem. Solids 2017, 111, 8–11. [Google Scholar] [CrossRef]
- Huang, C.; Fu, J.; Song, H.; Li, X.; Peng, X.; Gao, B.; Zhang, X.; Chu, P.K. General fabrication of mesoporous Nb2O5 nanobelts for lithium ion battery anodes. RSC Adv. 2016, 6, 90489–90493. [Google Scholar] [CrossRef]
- Wei, M.; Wei, K.; Ichihara, M.; Zhou, H. Nb2O5 nanobelts: A lithium intercalation host with large capacity and high rate capability. Electrochem. Commun. 2008, 10, 980–983. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Piao, J.-Y.; Hu, L.-L.; Bin, D.-S.; Lin, X.-J.; Duan, S.-Y.; Cao, A.-M.; Wan, L.-J. Controlling the reaction of nanoparticles for hollow metal oxide nanostructures. J. Am. Chem. Soc. 2018, 140, 9070–9073. [Google Scholar] [CrossRef]
- Lu, H.; Xiang, K.; Bai, N.; Zhou, W.; Wang, S.; Chen, H. Urchin-shaped Nb2O5 microspheres synthesized by the facile hydrothermal method and their lithium storage performance. Mater. Lett. 2016, 167, 106–108. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Cai, Z.; Fang, G.; Pan, A.; Liang, S. Nb2O5 microstructures: A high-performance anode for lithium ion batteries. Nanotechnology 2016, 27, 46LT01. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Liu, Y.; Sun, R.; Ma, J.; Guo, J.; Hu, M. Urchin-like hierarchical H-Nb2O5 microspheres: Synthesis, formation mechanism and their applications in lithium ion batteries. Dalton Trans. 2017, 46, 10935–10940. [Google Scholar] [CrossRef]
- Liu, G.; Jin, B.; Bao, K.; Xie, H.; Guo, J.; Ji, X.; Zhang, R.; Jiang, Q. Facile synthesis of porous Nb2O5 microspheres as anodes for lithium-ion batteries. Int. J. Hydrogen Energy 2017, 42, 6065–6071. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Li, S.; Schmidt, C.N.; Xu, Q.; Cao, X.; Cao, G. Macroporous nanostructured Nb2O5 with surface Nb4+ for enhanced lithium ion storage properties. ChemNanoMat 2016, 2, 675–680. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Zhang, X.; Liu, B.; Xu, L.; Zhang, Z.; Zhang, Y. 2D ultrathin nanosheet-assembled Nb2O5 microflowers for lithium ion batteries. Mater. Lett. 2018, 227, 112–115. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Hu, X.; Fan, F.; Cai, S.; Zheng, C.; Stucky, G.D. Review on comprehending and enhancing the initial Coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries. Nano Energy 2020, 77, 105143. [Google Scholar]
- Han, X.; Meng, Q.; Wan, X.; Sun, B.Y.; Zhang, Y.; Shen, B.C.; Gao, J.L.; Ma, Y.L.; Zuo, P.J.; Lou, S.F.; et al. Intercalation pseudocapacitive electrochemistry of Nb-based oxides for fast charging of lithium-ion batteries. Nano Energy 2021, 81, 105635. [Google Scholar] [CrossRef]
- Yan, L.; Rui, X.; Chen, G.; Xu, W.; Zou, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar]
- Lubimtsev, A.A.; Kent, P.R.; Sumpter, B.G.; Ganesh, P. Understanding the origin of high-rate intercalation pseudocapacitance in Nb2O5 crystals. J. Mater. Chem. A 2013, 1, 14951–14956. [Google Scholar] [CrossRef]
- Chen, P.-C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano 2010, 4, 4403–4411. [Google Scholar] [CrossRef]
- Lim, E.; Kim, H.; Jo, C.; Chun, J.; Ku, K.; Kim, S.; Lee, H.I.; Nam, I.-S.; Yoon, S.; Kang, K. Advanced hybrid supercapacitor based on a mesoporous niobium pentoxide/carbon as high-performance anode. ACS Nano 2014, 8, 8968–8978. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Yan, J.; Sumboja, A.; Lee, P.S. Orthorhombic niobium oxide nanowires for next generation hybrid supercapacitor device. Nano Energy 2015, 11, 765–772. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Chen, Z.; Augustyn, V.; Ma, X.; Wang, G.; Dunn, B.; Lu, Y. High-performance supercapacitors based on nanocomposites of Nb2O5 nanocrystals and carbon nanotubes. Adv. Energy Mater. 2011, 1, 1089–1093. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Constructing T-Nb2O5@Carbon hollow core-shell nanostructures for high-rate hybrid supercapacitor. J. Power Sources 2018, 396, 88–94. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Cai, Z.; Fang, G.; Cai, Y.; Pan, A.; Liang, S. Nb2O5 quantum dots embedded in MOF derived nitrogen-doped porous carbon for advanced hybrid supercapacitor applications. J. Mater. Chem. A 2016, 4, 17838–17847. [Google Scholar]
- Ding, Y.; Zeng, M.; Fu, L. Low-temperature synthesis of sp2 carbon nanomaterials. Sci. Bull. 2019, 64, 1817–1829. [Google Scholar]
- Wang, X.; Li, G.; Tjandra, R.; Fan, X.; Xiao, X.; Yu, A. Fast lithium-ion storage of Nb2O5 nanocrystals in situ grown on carbon nanotubes for high-performance asymmetric supercapacitors. RSC Adv. 2015, 5, 41179–41185. [Google Scholar]
- Ma, Y.; Han, J.; Wang, M.; Chen, X.; Jia, S. Electrophoretic deposition of graphene-based materials: A review of materials and their applications. J. Mater. 2018, 4, 108–120. [Google Scholar]
- Chen, Z.; Li, H.; Lu, X.; Wu, L.; Jiang, J.; Jiang, S.; Wang, J.; Dou, H.; Zhang, X. Nitrogenated Urchin-like Nb2O5 Microspheres with Extraordinary Pseudocapacitive Properties for Lithium-Ion Capacitors. ChemElectroChem 2018, 5, 1516–1524. [Google Scholar]
- Wang, J.; Li, H.; Shen, L.; Dong, S.; Zhang, X. Nb2O5 nanoparticles encapsulated in ordered mesoporous carbon matrix as advanced anode materials for Li ion capacitors. RSC Adv. 2016, 6, 71338–71344. [Google Scholar] [CrossRef]
- Zheng, J.; Cygan, P.; Jow, T. Hydrous ruthenium oxide as an electrode material for electrochemical capacitors. J. Electrochem. Soc. 1995, 142, 2699. [Google Scholar] [CrossRef]
- Arunkumar, P.; Ashish, A.G.; Babu, B.; Sarang, S.; Suresh, A.; Sharma, C.H.; Thalakulam, M.; Shaijumon, M.M. Nb2O5/graphene nanocomposites for electrochemical energy storage. RSC Adv. 2015, 5, 59997–60004. [Google Scholar] [CrossRef]
- Zhang, C.J.; Maloney, R.; Lukatskaya, M.R.; Beidaghi, M.; Dyatkin, B.; Perre, E.; Long, D.; Qiao, W.; Dunn, B.; Gogotsi, Y. Synthesis and electrochemical properties of niobium pentoxide deposited on layered carbide-derived carbon. J. Power Sources 2015, 274, 121–129. [Google Scholar] [CrossRef]
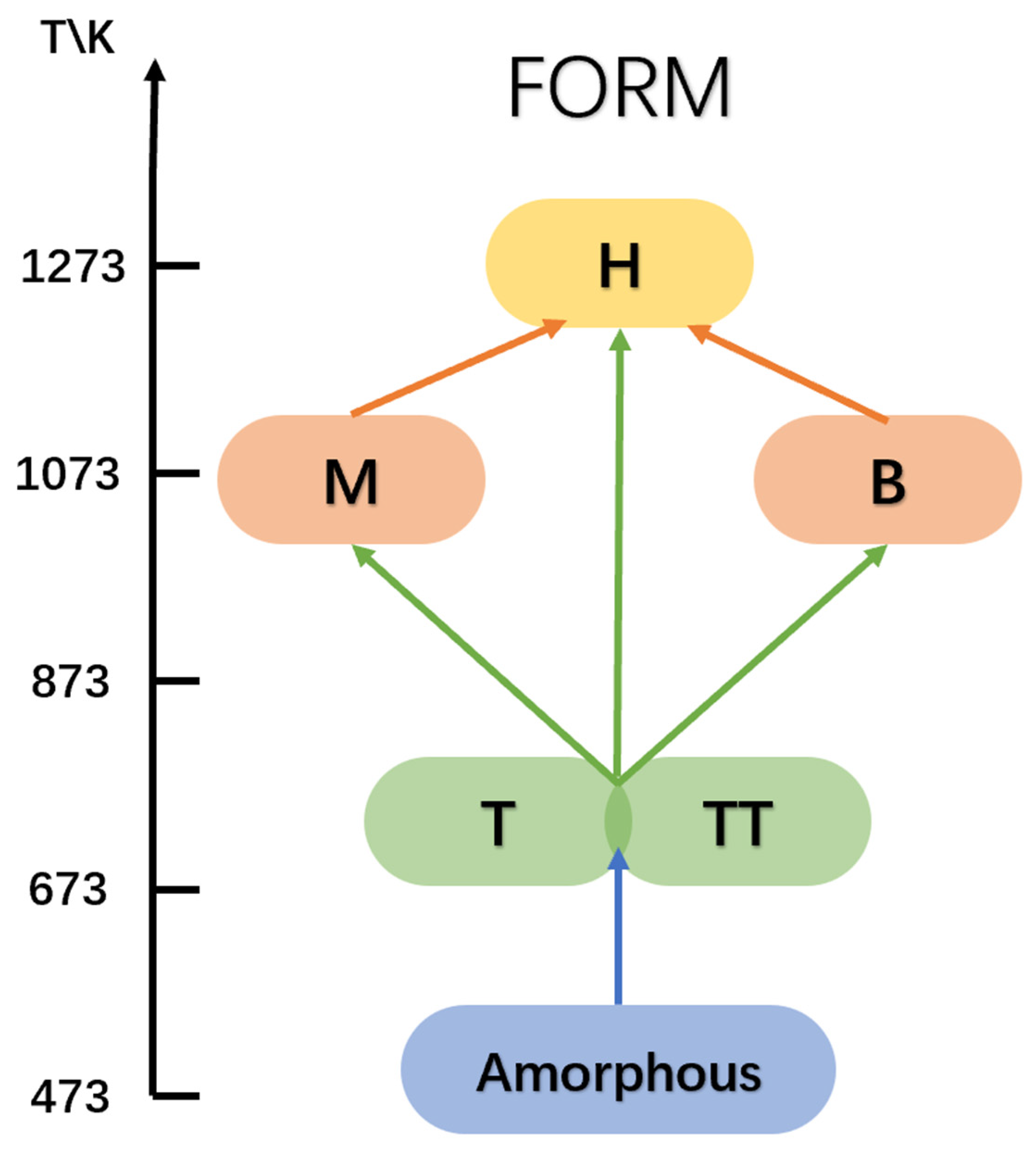
| Polymorphism | Designation of the Nb2O5 Form | Crystallization Temperature (K) | Ref. |
|---|---|---|---|
| TT-Nb2O5 | Tief-tief | 773–873 | [11] |
| T-Nb2O5 | Tief (γ) | 873–1073 | [12,13] |
| B-Nb2O5 | Blätter (ζ) | 1023–1123 | [14] |
| M-Nb2O5 | Medium (β) | 1173–1223 | [15] |
| H-Nb2O5 | High (α) | 1273 | [16] |
| N-Nb2O5 | Needles | 1103 | [17] |
| P-Nb2O5 | Prisms (η) | 1023 | [11] |
| R-Nb2O5 | neutral | - | [18] |
| ε-Nb2O5 | - | 1708 | [11] |
| Nb2O5-I-high | - | 1558 | [11] |
| Nb2O5-II | - | 1153–1223 | [11] |
| The oxI to oxVI Forms of—Nb2O5 | oxidation | 1573 | [11] |
| Materials | Crystal Structure | Cell Parameter | Space Group | Ref. |
|---|---|---|---|---|
| TT-Nb2O5 | Pseudohexagonal | a = b = 3.607 Å/a = b = 3.600 Å c = 3.925 Å/c = 3.919 Å | P6/mmm (No. 191) | [12,20] |
| T-Nb2O5 | Orthorhombic | a = 6.75 Å/a = 6.144 Å b = 29.175 Å/b = 29.194 Å c = 3.930 Å/c = 3.940 Å | Pbam (No. 55) | [12,13] |
| M-Nb2O5 | Tetragonal | a = b = 20.44 Å c = 3.832 Å | I4/mmm (No. 139) | [21] |
| H-Nb2O5 | Monoclinic | a = 21.153 Å/a = 21.163 Å b = 3.8233 Å/b = 3.824 Å c = 19.356 Å/c = 19.355 Å | P2/m (No. 10) | [13,21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, R.; Wang, Z.; Li, J.; Chen, K. Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications. Materials 2023, 16, 6956. https://doi.org/10.3390/ma16216956
Pang R, Wang Z, Li J, Chen K. Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications. Materials. 2023; 16(21):6956. https://doi.org/10.3390/ma16216956
Chicago/Turabian StylePang, Rui, Zhiqiang Wang, Jinkai Li, and Kunfeng Chen. 2023. "Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications" Materials 16, no. 21: 6956. https://doi.org/10.3390/ma16216956
APA StylePang, R., Wang, Z., Li, J., & Chen, K. (2023). Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications. Materials, 16(21), 6956. https://doi.org/10.3390/ma16216956








