Improved Thermoelectric Performance of Sb2Te3 Nanosheets by Coating Pt Particles in Wide Medium-Temperature Zone
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of Sb2Te3 NCs
2.2. Synthesis of Sb2Te3 NCs Coated Pt Nanoparticles
2.3. Characterization and Thermoelectric Measurements
2.4. DFT Calculations
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of Electronic Bands for High Performance Bulk Thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance Bulk Thermoelectrics with All-Scale Hierarchical Architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.-D.; Kanatzidis, M.G. Rationally Designing High-Performance Bulk Thermoelectric Materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef]
- Park, J.; Dylla, M.; Xia, Y.; Wood, M.; Snyder, G.J.; Jain, A. When Band Convergence Is Not Beneficial for Thermoelectrics. Nat. Commun. 2021, 12, 3425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Slade, T.J.; Hu, L.; Tan, X.Y.; Luo, Y.; Luo, Z.-Z.; Xu, J.; Yan, Q.; Kanatzidis, M.G. Defect Engineering in Thermoelectric Materials: What Have We Learned? Chem. Soc. Rev. 2021, 50, 9022–9054. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow Thermal Conductivity and High Thermoelectric Figure of Merit in Snse Crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Hinterleitner, B.; Knapp, I.; Poneder, M.; Shi, Y.; Müller, H.; Eguchi, G.; Eisenmenger-Sittner, C.; Stöger-Pollach, M.; Kakefuda, Y.; Kawamoto, N.; et al. Thermoelectric Performance of a Metastable Thin-Film Heusler Alloy. Nature 2019, 576, 85–90. [Google Scholar] [CrossRef]
- Snyder, G.J.; Ursell, T.S. Thermoelectric Efficiency and Compatibility. Phys. Rev. Lett. 2003, 91, 148301. [Google Scholar] [CrossRef]
- Zoui, M.A.; Bentouba, S.; Stocholm, J.G.; Bourouis, M. A Review on Thermoelectric Generators: Progress and Applications. Energies 2020, 13, 3606. [Google Scholar] [CrossRef]
- Song, S.; Mao, J.; Shuai, J.; Zhu, H.; Ren, Z.; Saparamadu, U.; Tang, Z.; Wang, B.; Ren, Z. Study on Anisotropy of N-Type Mg3sb2-Based Thermoelectric Materials. Appl. Phys. Lett. 2018, 112, 092103. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, J.; Liu, H.; Chen, Z.; Lin, L.; Ren, D.; Liu, B.; Ang, R. Optimized Strategies for Advancing N-Type Pbte Thermoelectrics: A Review. ACS Appl. Mater. Interfaces 2020, 12, 49323–49334. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.P.; Zhu, T.J.; Yue, X.Q.; Liu, X.H.; Wang, Y.G.; Xu, Z.J.; Zhao, X.B. Enhanced Figure of Merit in Antimony Telluride Thermoelectric Materials by In–Ag Co-Alloying for Mid-Temperature Power Generation. Acta Mater. 2015, 85, 270–278. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Zheng, W.; Guo, J.; Xiong, R.; Wang, Z.; Shi, J. Dramatically Improving Thermoelectric Performance of Topological Half-Heusler Compound Luptsb Via Hydrostatic Pressure. J. Mater. Chem. A 2018, 6, 20069–20075. [Google Scholar] [CrossRef]
- Wan, C.; Wang, Y.; Wang, N.; Norimatsu, W.; Kusunoki, M.; Koumoto, K. Development of Novel Thermoelectric Materials by Reduction of Lattice Thermal Conductivity. Sci. Technol. Adv. Mater. 2010, 11, 044306. [Google Scholar] [CrossRef] [PubMed]
- Eivari, H.A.; Sohbatzadeh, Z.; Mele, P.; Assadi, M.H.N. Low Thermal Conductivity: Fundamentals and Theoretical Aspects in Thermoelectric Applications. Mater. Today Energy 2021, 21, 100744. [Google Scholar] [CrossRef]
- Soleimani, Z.; Zoras, S.; Ceranic, B.; Shahzad, S.; Cui, Y. A Review on Recent Developments of Thermoelectric Materials for Room-Temperature Applications. Sustain. Energy Technol. Assess. 2020, 37, 100604. [Google Scholar] [CrossRef]
- Dong, J.; Sun, F.-H.; Tang, H.; Pei, J.; Zhuang, H.-L.; Hu, H.-H.; Zhang, B.-P.; Pan, Y.; Li, J.-F. Medium-Temperature Thermoelectric Gete: Vacancy Suppression and Band Structure Engineering Leading to High Performance. Energy Environ. Sci. 2019, 12, 1396–1403. [Google Scholar] [CrossRef]
- Hong, M.; Chasapis, T.C.; Chen, Z.-G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. N-Type Bi2Te3−XSeX Nanoplates with Enhanced Thermoelectric Efficiency Driven by Wide-Frequency Phonon Scatterings and Synergistic Carrier Scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lim, K.H.; Ibáñez, M.; Ortega, S.; Li, M.; David, J.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; et al. High Thermoelectric Performance in Crystallographically Textured N-Type Bi2Te3−XSeX Produced from Asymmetric Colloidal Nanocrystals. ACS Nano 2018, 12, 7174–7184. [Google Scholar] [CrossRef]
- Du, B.; Lai, X.; Liu, Q.; Liu, H.; Wu, J.; Liu, J.; Zhang, Z.; Pei, Y.; Zhao, H.; Jian, J. Spark Plasma Sintered Bulk Nanocomposites of Bi2Te2.7Se0.3 Nanoplates Incorporated Ni Nanoparticles with Enhanced Thermoelectric Performance. ACS Appl. Mater. Interfaces 2019, 11, 31816–31823. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.M.F.; Pires, A.L.; Silva, J.P.B.; Magalhães, V.H.; Grilo, J.; Brito, F.P.; Silva, M.F.; Pereira, A.M.; Goncalves, L.M. High-Performance Μ-Thermoelectric Device Based on Bi2Te3/SB2Te3 P–N Junctions. ACS Appl. Mater. Interfaces 2019, 11, 38946–38954. [Google Scholar] [CrossRef]
- Hu, L.; Meng, F.; Zhou, Y.; Li, J.; Benton, A.; Li, J.; Liu, F.; Zhang, C.; Xie, H.; He, J. Leveraging Deep Levels in Narrow Bandgap Bi0.5Sb1.5Te3 for Record-High Ztave near Room Temperature. Adv. Funct. Mater. 2020, 30, 2005202. [Google Scholar] [CrossRef]
- Meroz, O.; Elkabets, N.; Gelbstein, Y. Enhanced Thermoelectric Properties of N-Type Bi2Te3−XSeX Alloys Following Melt-Spinning. ACS Appl. Energy Mater. 2020, 3, 2090–2095. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.-D.; Gao, H.; Wang, L.-J.; Li, M.; Shi, X.-L.; Hong, M.; Wang, H.; Zou, J.; Chen, Z.-G. High Porosity in Nanostructured N-Type Bi2Te3 Obtaining Ultralow Lattice Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 31237–31244. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Han, W.; Sun, J.; Chen, G.; Chen, J.; Yang, X.; Chen, G.; Ma, F. Realizing the Interface Tuned Thermoelectric Transport Performance in Bi2Te3-Based Hierarchical Nanostructures. J. Phys. Chem. C 2019, 123, 23817–23825. [Google Scholar] [CrossRef]
- Bahk, J.-H.; Shakouri, A. Minority Carrier Blocking to Enhance the Thermoelectric Figure of Merit in Narrow-Band-Gap Semiconductors. Phys. Rev. B 2016, 93, 165209. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Zhang, Q.; Zhu, T.; Zhai, R.; Zhao, X. Liquid-Phase Hot Deformation to Enhance Thermoelectric Performance of N-Type Bismuth-Telluride-Based Solid Solutions. Adv. Sci. 2019, 6, 1901702. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, G.; Tan, C.; Xiong, C.; Guo, Z.; Yin, Y.; Yu, B.; Xiao, Y.; Hu, H.; Liu, G.; et al. Phonon Engineering for Thermoelectric Enhancement of P-Type Bismuth Telluride by a Hot-Pressing Texture Method. ACS Appl. Mater. Interfaces 2020, 12, 31612–31618. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wang, H. A Descriptive Model of Thermoelectric Transport in a Resonant System of Pbse Doped with Tl. J. Mater. Chem. A 2019, 7, 12859–12868. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Yang, J.; Duan, B.; Wu, L.; Zhang, W.; Yang, J. High Thermoelectric Performance in Te-Free (Bi,Sb)2Se3via Structural Transition Induced Band Convergence and Chemical Bond Softening. Energy Environ. Sci. 2016, 9, 3436–3447. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in Pbte by Distortion of the Electronic Density of States. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Luo, Y.; Liu, Y.; Shi, J.; Xiong, R.; Wang, Z. Synergistical Tuning Interface Barrier and Phonon Propagation in Au–Sb2Te3 Nanoplate for Boosting Thermoelectric Performance. J. Phys. Chem. Lett. 2019, 10, 4903–4909. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.; Xiao, H.; Gao, Z.; Liu, Z.; Zu, X.; Li, S.; Qiao, L. Band Degeneracy Enhanced Thermoelectric Performance in Layered Oxyselenides by First-Principles Calculations. NPJ Comput. Mater. 2021, 7, 18. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Z.; Miao, L.; Shi, J.; Xiong, R. Extremely Anisotropic Thermoelectric Properties of Snse under Pressure. Energy Environ. Mater. 2023, 6, e12361. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, X.; Huang, S.; Guo, J.; Bi, P.; Shi, J.; Zheng, W.; Wang, Z.; Xiong, R. Porosity Induced Thermoelectric Performance Optimization for Antimony Telluride. Ceram. Int. 2018, 44, 21421–21427. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, D.; Wei, W.; Liu, F.; Tang, X.; Shi, J.; Wang, Z.; Xiong, R. Decoupling Electrical and Thermal Properties in Antimony Telluride Based Artificial Multilayer for High Thermoelectric Performance. Appl. Phys. Lett. 2015, 107, 203901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum Espresso. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Prandini, G.; Marrazzo, A.; Castelli, I.E.; Mounet, N.; Marzari, N. Precision and Efficiency in Solid-State Pseudopotential Calculations. NPJ Comput. Mater. 2018, 4, 72. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Singh, D.J. BoltzTraP: A Code for Calculating Band-Structure Dependent Quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. Vesta 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Fang, T.; Li, X.; Hu, C.; Zhang, Q.; Yang, J.; Zhang, W.; Zhao, X.; Singh, D.J.; Zhu, T. Complex Band Structures and Lattice Dynamics of Bi2Te3-Based Compounds and Solid Solutions. Adv. Funct. Mater. 2019, 29, 1900677. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, H.-J.; Xiang, B.; Fu, L.-W.; Zhu, H.; Chai, D.; Zhu, B.; Yu, Y.; Gao, N.; Huang, Z.-Y.; et al. Thermoelectric Performance of Sb2Te3-Based Alloys Is Improved by Introducing PN Junctions. ACS Appl. Mater. Interfaces 2018, 10, 23277–23284. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, J.; Cui, B.; Xie, L.; Wang, W.; Yin, L.; Qin, D.; Cai, W.; Zhang, Q.; Sui, J. Achieving a High Average zT Value in Sb2Te3-Based Segmented Thermoelectric Materials. ACS Appl. Mater. Interfaces 2020, 12, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ming, H.; Li, D.; Qin, X.; Zhang, J.; Huang, L.; Song, C.; Wang, L. Ultralow Thermal Conductivity and High Thermoelectric Performance of N-Type Bi2Te2.7Se0.3-Based Composites Incorporated with Gaas Nanoinclusions. ACS Appl. Mater. Interfaces 2020, 12, 37155–37163. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Dharmaiah, P.; Kim, D.H.; Yoon, D.K.; Kim, T.H.; Song, S.H.; Hong, S.-J. Synergistic Optimization of the Thermoelectric and Mechanical Properties of Large-Size Homogeneous Bi0.5Sb1.5Te3 Bulk Samples Via Carrier Engineering for Efficient Energy Harvesting. ACS Appl. Mater. Interfaces 2022, 14, 10394–10406. [Google Scholar] [CrossRef]
- Ma, S.; Li, C.; Wei, P.; Zhu, W.; Nie, X.; Sang, X.; Zhang, Q.; Zhao, W. High-Pressure Synthesis and Excellent Thermoelectric Performance of Ni/BiTeSe Magnetic Nanocomposites. J. Mater. Chem. A 2020, 8, 4816–4826. [Google Scholar] [CrossRef]
- Musah, J.-D.; Guo, C.; Novitskii, A.; Serhiienko, I.; Adesina, A.E.; Khovaylo, V.; Wu, C.-M.L.; Zapien, J.A.; Roy, V.A.L. Ultralow Thermal Conductivity in Dual-Doped N-Type Bi2Te3 Material for Enhanced Thermoelectric Properties. Adv. Electron. Mater. 2021, 7, 2000910. [Google Scholar] [CrossRef]
- Budak, S.; Guner, S.; Minamisawa, R.A.; Muntele, C.I.; Ila, D. Thermoelectric Properties of Zn4Sb3/CeFe(4−x)CoxSb12 Nano-Layered Superlattices Modified by MeV Si Ion Beam. Appl. Surf. Sci. 2014, 310, 226–229. [Google Scholar] [CrossRef]
- Jain, A.; Shin, Y.; Persson, K.A. Computational Predictions of Energy Materials Using Density Functional Theory. Nat. Rev. Mater. 2016, 1, 15004. [Google Scholar] [CrossRef]

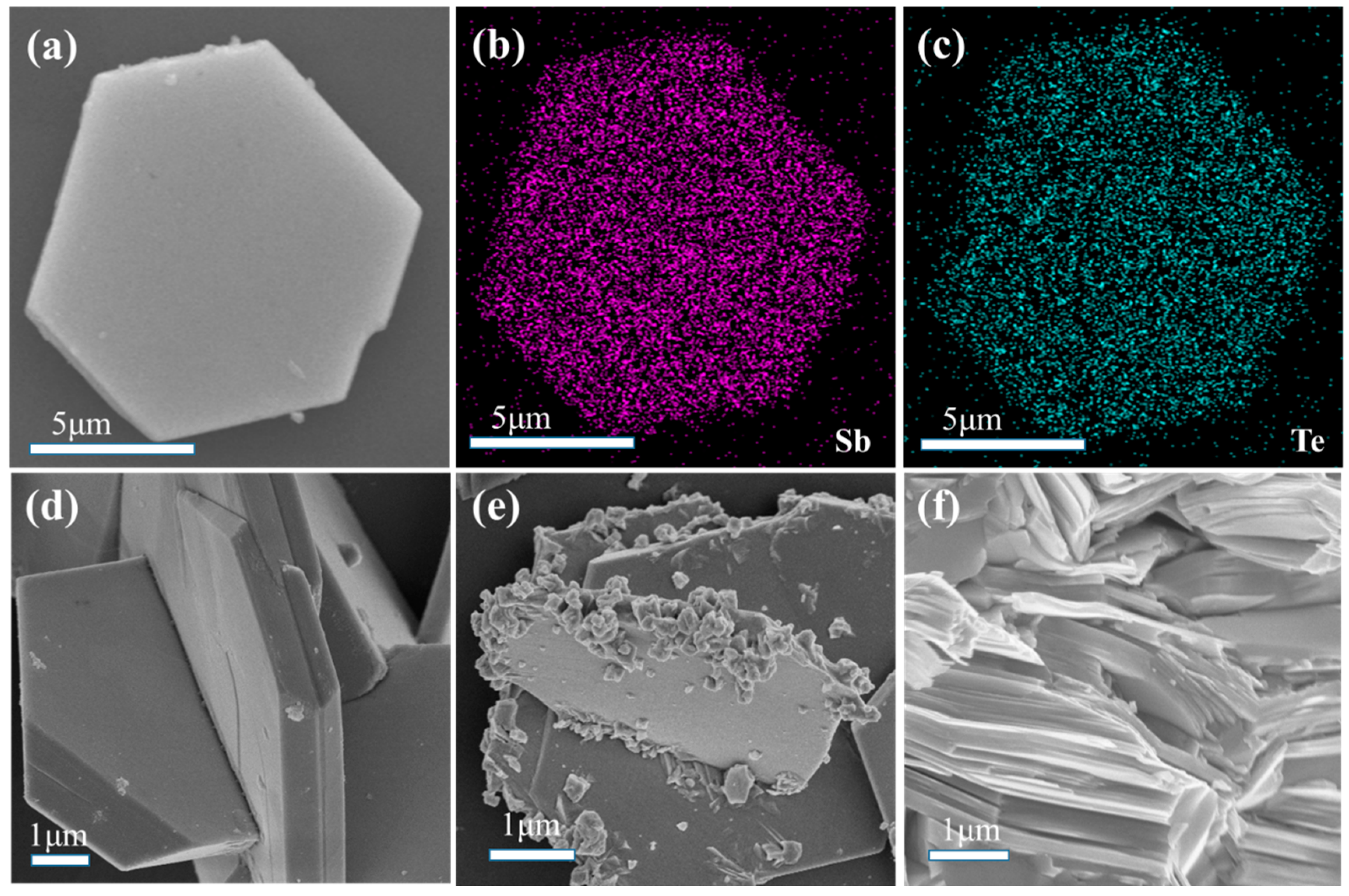
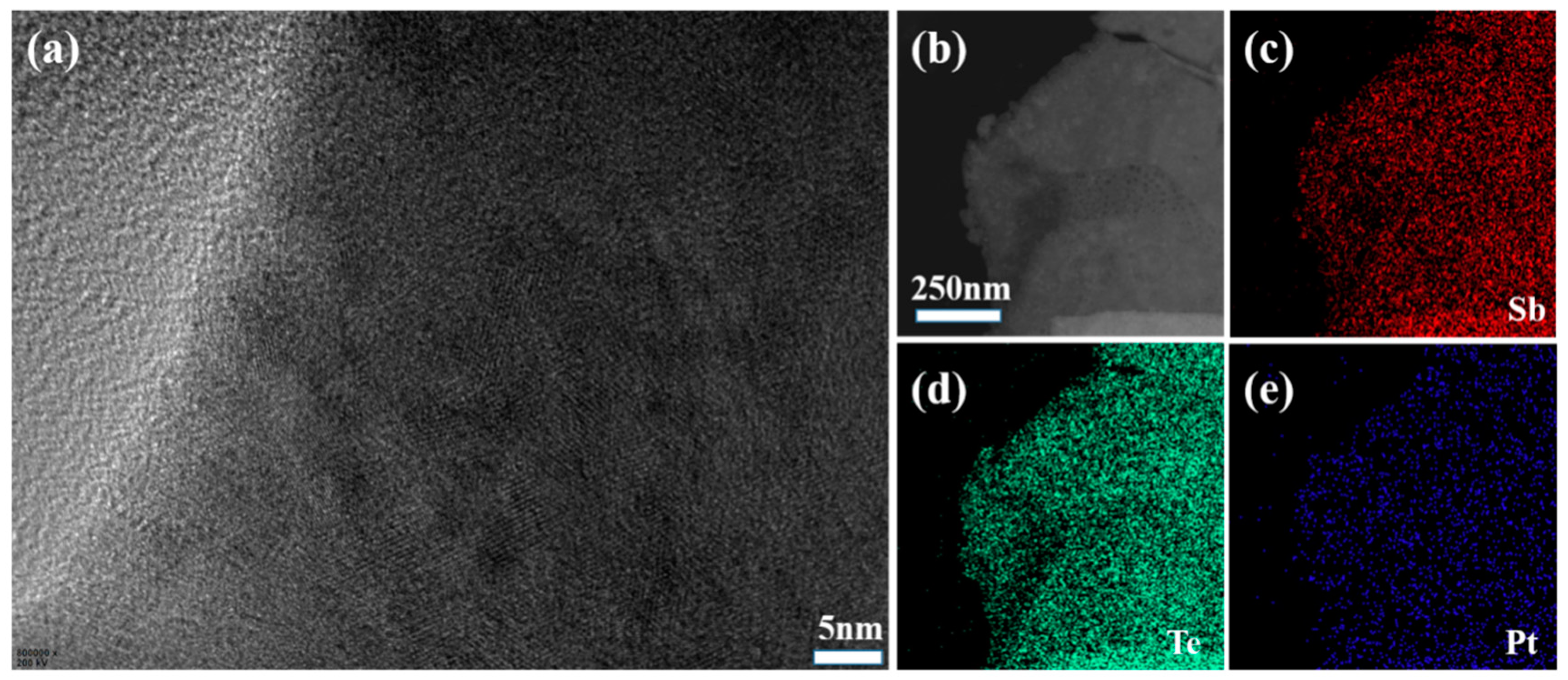
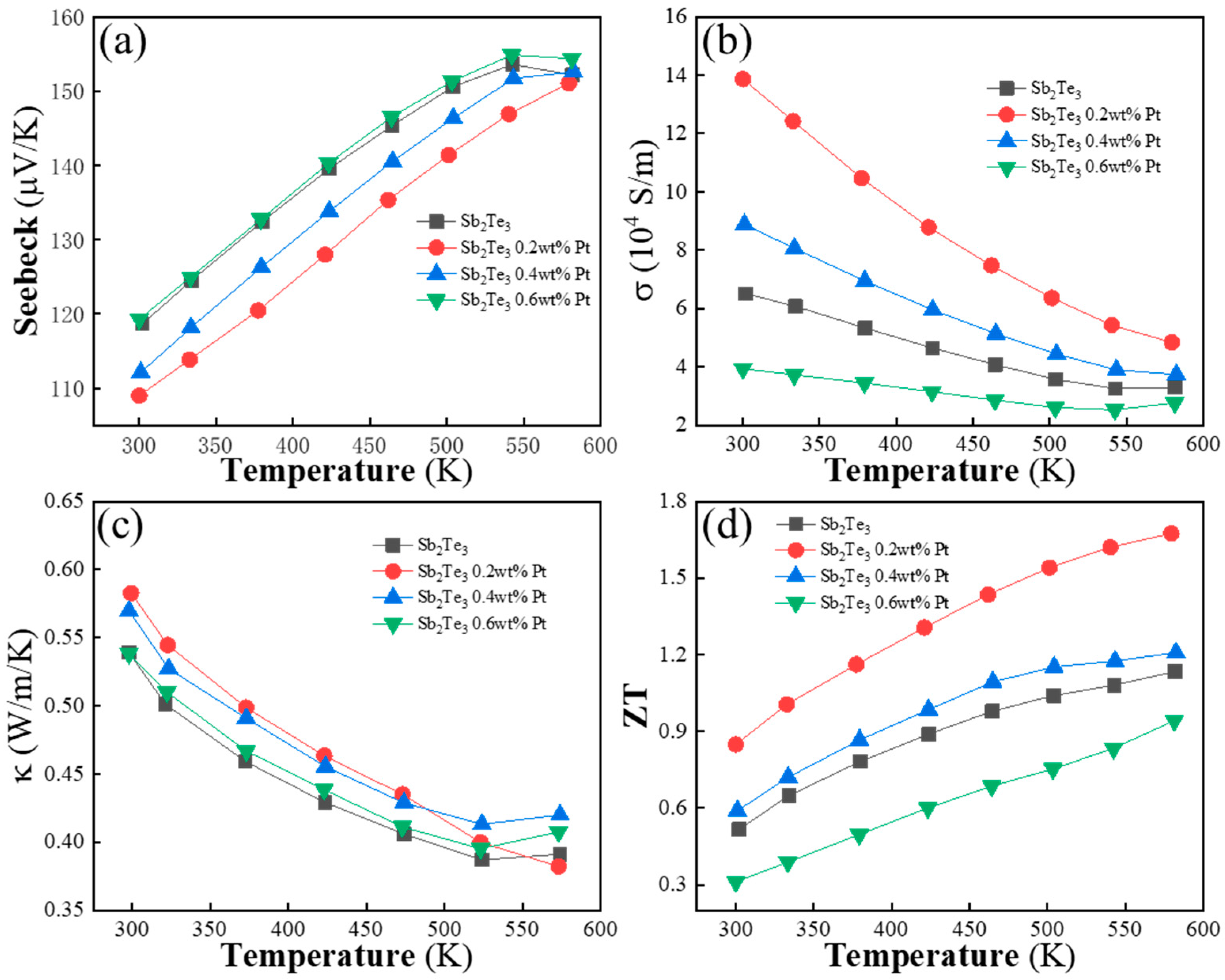

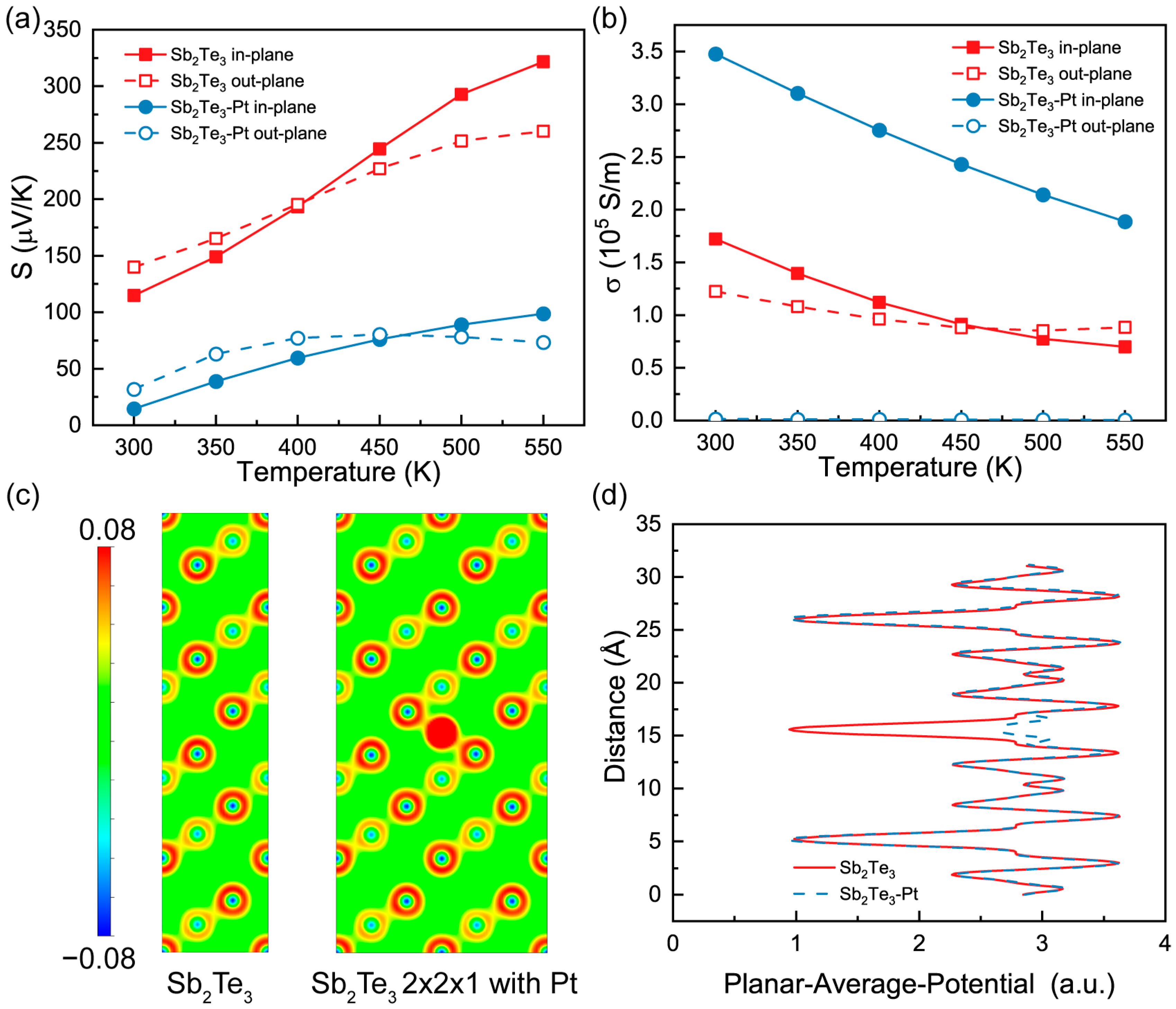
| Element | Mass (%) | Atom (%) |
|---|---|---|
| Sb | 36.34 | 37.74 |
| Te | 61.25 | 60.70 |
| Pt | 2.41 | 1.56 |
| Sample | P/N Type | Carrier Concentration (cm−3) | Carrier Mobility (cm2/V/S) | Electrical Conductivity (T = 300 K S/m) |
|---|---|---|---|---|
| ST-1 | P | 8.76 × 1018 | 4.63 × 102 | 6.46 × 104 |
| ST-2 | P | 2.37 × 1019 | 3.63 × 102 | 13.8 × 104 |
| ST-3 | P | 1.87 × 1019 | 2.79 × 102 | 8.9 × 104 |
| ST-4 | P | 9.8 × 1018 | 2.5 × 102 | 3.93 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Q.-L.; Dong, L.-Q.; Hao, Q. Improved Thermoelectric Performance of Sb2Te3 Nanosheets by Coating Pt Particles in Wide Medium-Temperature Zone. Materials 2023, 16, 6961. https://doi.org/10.3390/ma16216961
Guan Q-L, Dong L-Q, Hao Q. Improved Thermoelectric Performance of Sb2Te3 Nanosheets by Coating Pt Particles in Wide Medium-Temperature Zone. Materials. 2023; 16(21):6961. https://doi.org/10.3390/ma16216961
Chicago/Turabian StyleGuan, Qing-Ling, Li-Quan Dong, and Qun Hao. 2023. "Improved Thermoelectric Performance of Sb2Te3 Nanosheets by Coating Pt Particles in Wide Medium-Temperature Zone" Materials 16, no. 21: 6961. https://doi.org/10.3390/ma16216961





