3D Printing of Individualized Microfluidic Chips with DLP-Based Printer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Microfluidic Chips with a DLP Printer
- (1)
- Use three-dimensional modeling software, such as Solidworks (Solidworks 2021), to establish a three-dimensional model of the microfluidic chip. In the chip model, reserve structures such as chip interfaces. Import the chip model into the DLP printer software (Sprintray V2.15) in STL file format, and after layer slicing, obtain the printing control code.
- (2)
- Depending on the application scenario of the printed chip, select suitable chip printing materials and machine printing accuracy settings. Use the obtained printing code to manufacture the chip.
- (3)
- After the chip printing is completed, remove the chip from the printing platform and perform ultrasonic cleaning using a dedicated cleaning solution or anhydrous ethanol in an ultrasonic cleaner for 10 min.
- (4)
- Rinse the residual cleaning solution or anhydrous ethanol from the chip’s surface with water and dry it by blowing or in a drying oven.
- (5)
- Use 405 nm ultraviolet light for secondary curing of the chip and check the chip effect. Each irradiation lasts for 3 min. Repeat this process until the chip reaches the best state. Save the microfluidic chip for future use.
- (1)
- Based on the initial design of the microfluidic chip, modify the channel design from resin encapsulation to an open channel design, and reserve a flat structure for channel encapsulation.
- (2)
- Import the open channel model into the DLP printing software and generate the printing code.
- (3)
- Depending on the application scenario of the printed chip, select suitable chip printing materials and printing accuracy settings. Especially for microstructures, choose the appropriate printing direction to avoid structural distortion caused by improper selection of the Z printing direction. Use the obtained printing code to manufacture the chip.
- (4)
- After the chip printing is completed, remove the chip from the printing platform and perform ultrasonic cleaning using a dedicated cleaning solution or anhydrous ethanol in an ultrasonic cleaner for 10 min. Then rinse the residual cleaning solution or anhydrous ethanol from the chip’s surface with water and blow dry, or dry in a drying oven.
- (5)
- Use a 405 nm ultraviolet light for secondary curing of the chip and check the chip effect. Each irradiation lasts for 3 min. Repeat this process until the chip reaches the optimal state.
- (6)
- Place the chip after secondary curing in close contact with transparent adhesive tape (Scotch, 3M, Tonawanda, NY, USA) and remove any air bubbles between the bonding surfaces to ensure a flat and clean encapsulation plane.
- (7)
- Heat the encapsulated chip in an oven at a temperature lower than the resin’s thermal deformation threshold for 1–2 h to ensure stable bonding. After confirming the encapsulation effect, retain the chip.
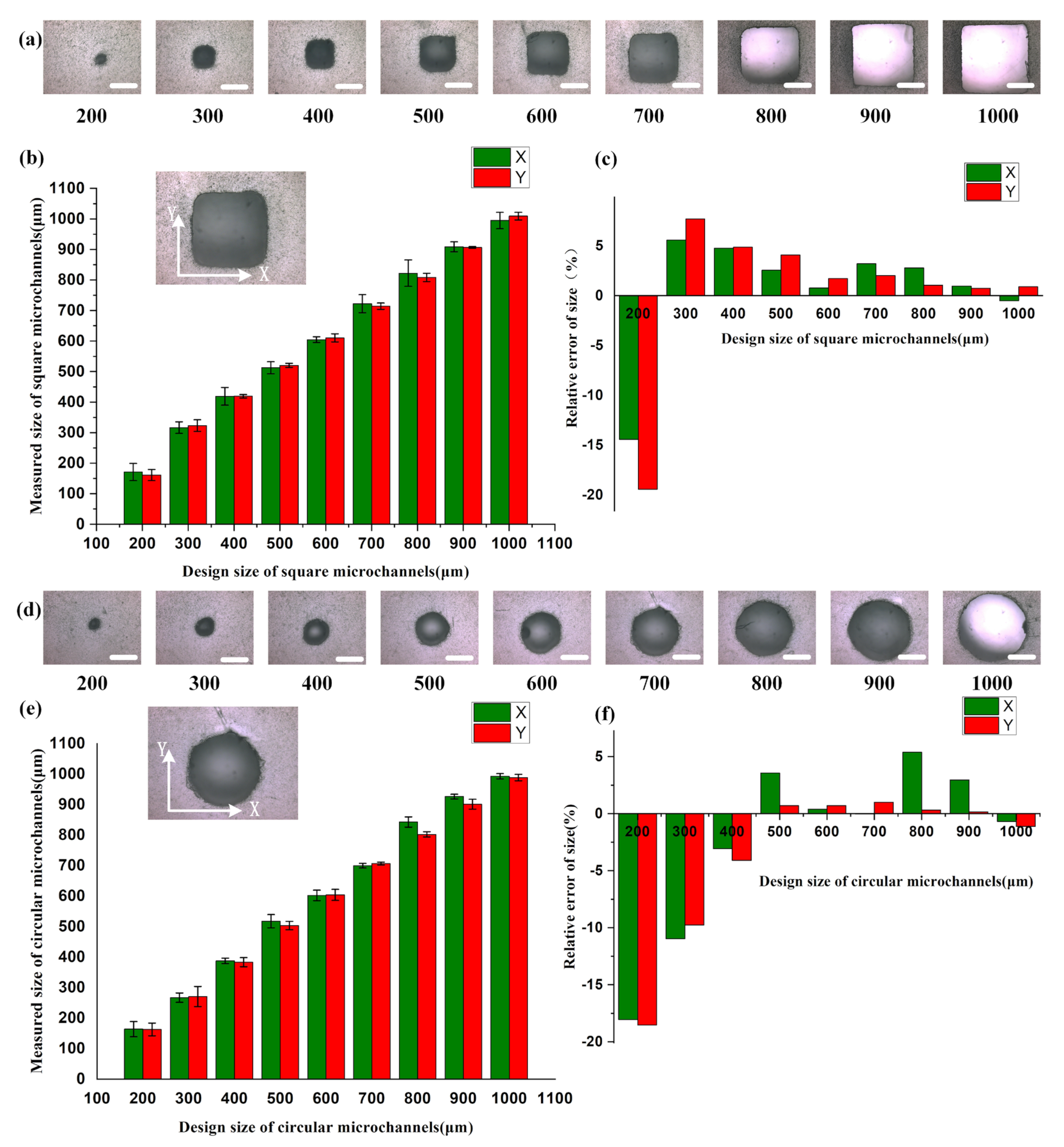
2.2. Characterization of Printing Accuracy of Microfluidic Chips
2.3. Characterization of Surface Quality of 3D-Printed Chips
2.4. Characterization of Printing Materials for 3D-Printed Chips
3. Results and Discussions
3.1. Analysis of Printing Accuracy of Microfluidic Chips
3.2. Analysis of Surface Quality of Printed Chips
3.3. Analysis of Printing Materials’ Properties
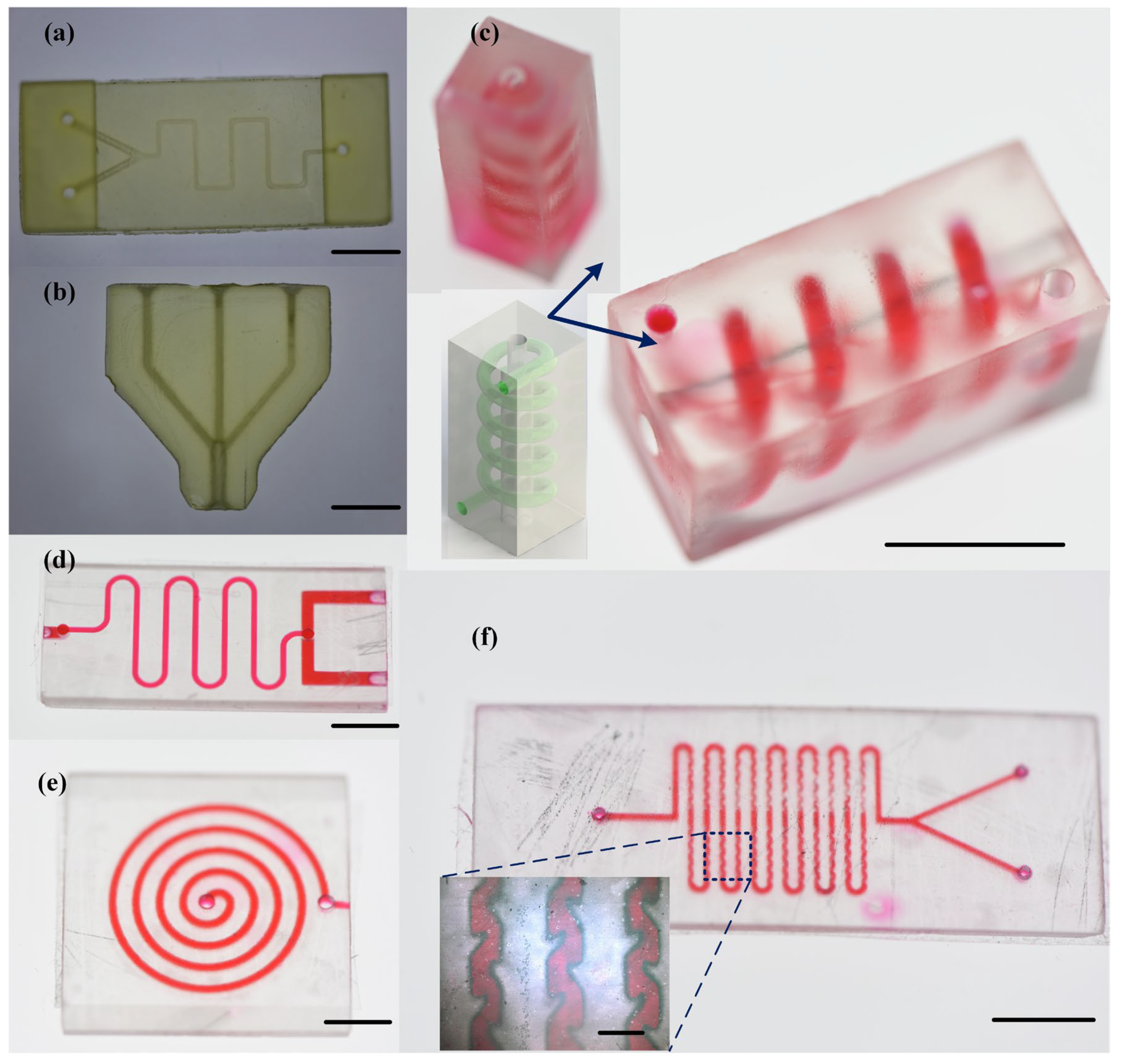
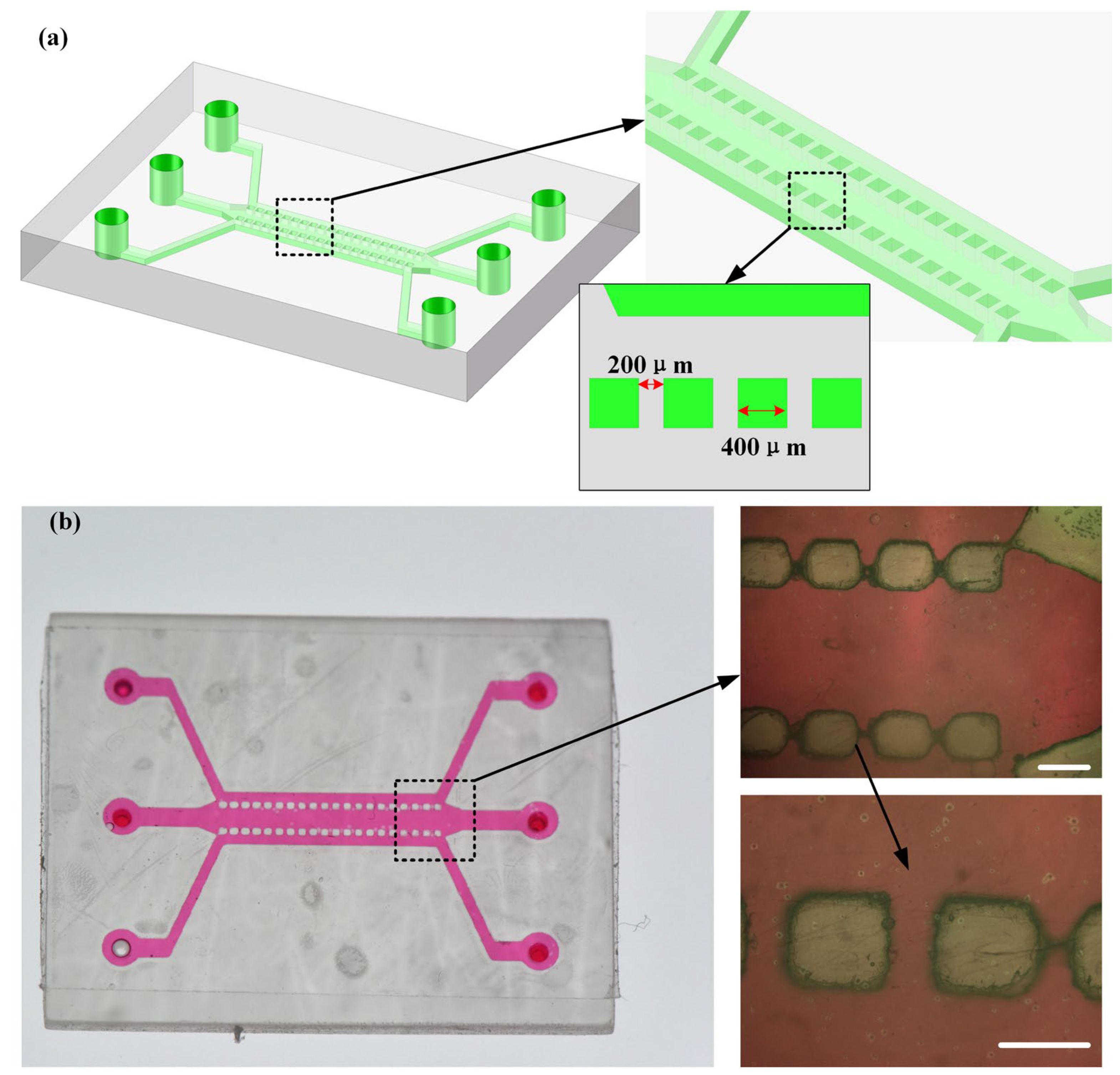
3.4. Three-Dimentional-Printed Microfluidic Chips and Their Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; I Solvas, X.C.; Wootton, R.C.R.; DeMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Quoc Pho, H.; Zong, L.; Li, S.; Pourali, N.; Rebrov, E.; Nghiep Tran, N.; Ostrikov, K.K.; Hessel, V. Microfluidic plasmas: Novel technique for chemistry and chemical engineering. Chem. Eng. J. 2021, 417, 129355. [Google Scholar] [CrossRef]
- Frey, N.; Sönmez, U.M.; Minden, J.; LeDuc, P. Microfluidics for understanding model organisms. Nat. Commun. 2022, 13, 3195. [Google Scholar] [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef]
- Daniele, M.A.; Boyd, D.A.; Adams, A.A.; Ligler, F.S. Microfluidic Strategies for Design and Assembly of Microfibers and Nanofibers with Tissue Engineering and Regenerative Medicine Applications. Adv. Healthc. Mater. 2015, 4, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Yan, H.; Liu, C.; Zhu, X.; Chen, B. Microfluidics as an Emerging Platform for Exploring Soil Environmental Processes: A Critical Review. Environ. Sci. Technol. 2022, 56, 711–731. [Google Scholar] [CrossRef]
- Patze, S.; Huebner, U.; Liebold, F.; Weber, K.; Cialla-May, D.; Popp, J. SERS as an analytical tool in environmental science: The detection of sulfamethoxazole in the nanomolar range by applying a microfluidic cartridge setup. Anal. Chim. Acta 2017, 949, 1–7. [Google Scholar] [CrossRef]
- Urbanski, J.P.; Thies, W.; Rhodes, C.; Amarasinghe, S.; Thorsen, T. Digital microfluidics using soft lithography. Lab Chip 2006, 6, 96–104. [Google Scholar] [CrossRef]
- Lin, T.; Do, T.; Kwon, P.; Lillehoj, P.B. 3D printed metal molds for hot embossing plastic microfluidic devices. Lab Chip 2017, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Weisgrab, G.; Ovsionikov, A.; Costa, P.F. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef]
- Bacha, T.W.; Manuguerra, D.C.; Marano, R.A.; Stanzione, J.F. Hydrophilic modification of SLA 3D printed droplet generators by photochemical grafting. Rsc. Adv. 2021, 11, 21745–21753. [Google Scholar] [CrossRef] [PubMed]
- Quero, R.F.; Costa, B.M.D.C.; Da Silva, J.A.F.; de Jesus, D.P. Using multi-material fused deposition modeling (FDM) for one-step 3D printing of microfluidic capillary electrophoresis with integrated electrodes for capacitively coupled contactless conductivity detection. Sens. Actuators B Chem. 2022, 365, 131959. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1213–1993. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 45–65. [Google Scholar] [CrossRef]
- Ozbolat, V.; Dey, M.; Ayan, B.; Ozbolat, I.T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication 2019, 11, 34101. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-Based 3D Printing of Microfluidic Devices for Chemical and Biomedical Applications: A Topical Review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D bioprinted organ-on-chips. Aggregate 2023, 4, e197. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.C.; Pereira, I.; Maciel, L.I.L.; Vaz, B.G.; Coltro, W.K.T. 3D printed microfluidic mixer for real-time monitoring of organic reactions by direct infusion mass spectrometry. Anal. Chim. Acta 2022, 1190, 339252. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.; Rein, C.; Konrat, E.; Meermeyer, S.L.; Lee-Thedieck, C.; Kotz-Helmer, F.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene. Micromachines 2021, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Childs, E.H.; Latchman, A.V.; Lamont, A.C.; Hubbard, J.D.; Sochol, R.D. Additive Assembly for PolyJet-Based Multi-Material 3D Printed Microfluidics. J. Microelectromech. Syst. 2020, 29, 1094–1096. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.F.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K.; et al. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab Chip 2016, 16, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Zips, S.; Hiendlmeier, L.; Weiß, L.J.K.; Url, H.; Teshima, T.F.; Schmid, R.; Eblenkamp, M.; Mela, P.; Wolfrum, B. Biocompatible, Flexible, and Oxygen-Permeable Silicone-Hydrogel Material for Stereolithographic Printing of Microfluidic Lab-On-A-Chip and Cell-Culture Devices. ACS Appl. Polym. Mater. 2021, 3, 243–258. [Google Scholar] [CrossRef]
- Guerra, M.G.; Volpone, C.; Galantucci, L.M.; Percoco, G. Photogrammetric measurements of 3D printed microfluidic devices. Addit. Manuf. 2018, 21, 53–62. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Van der Linden, P.J.E.M.; Popov, A.M.; Pontoni, D. Accurate and rapid 3D printing of microfluidic devices using wavelength selection on a DLP printer. Lab Chip 2020, 2, 4128–4414. [Google Scholar] [CrossRef]
- Amini, A.; Guijt, R.M.; Themelis, T.; De Vos, J.; Eeltink, S. Recent developments in digital light processing 3D-printing techniques for microfluidic analytical devices. J. Chromatogr. A 2023, 1692, 463842. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Y.; Hui, Y.; Zhang, L.; Zhou, N.; Ju, F. A 3D-printed microfluidic gradient concentration chip for rapid antibiotic-susceptibility testing. Bio-Des. Manuf. 2022, 5, 210–219. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Zuo, T.; Wang, Q.; Wang, H.; Liu, Z. High-accuracy DLP 3D printing of closed microfluidic channels based on a mask option strategy. Int. J. Adv. Manuf. Technol. 2023, 127, 4001–4012. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, S.; Kim, I.; Kim, K.; Kwon, S.K.; Hwang, N.S. Gelatin-based micro-hydrogel carrying genetically engineered human endothelial cells for neovascularization. Acta Biomater. 2019, 95, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.A.; Zhang, H.; Zhang, Y.; Cui, W. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021, 1, 20210036. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Li, J.; Guo, Z.; Zhang, Y.; Nie, B.; Qi, G.; Zhang, X.; Zhang, J.; Wei, R. 3D Printing of Individualized Microfluidic Chips with DLP-Based Printer. Materials 2023, 16, 6984. https://doi.org/10.3390/ma16216984
Qiu J, Li J, Guo Z, Zhang Y, Nie B, Qi G, Zhang X, Zhang J, Wei R. 3D Printing of Individualized Microfluidic Chips with DLP-Based Printer. Materials. 2023; 16(21):6984. https://doi.org/10.3390/ma16216984
Chicago/Turabian StyleQiu, Jingjiang, Junfu Li, Zhongwei Guo, Yudong Zhang, Bangbang Nie, Guochen Qi, Xiang Zhang, Jiong Zhang, and Ronghan Wei. 2023. "3D Printing of Individualized Microfluidic Chips with DLP-Based Printer" Materials 16, no. 21: 6984. https://doi.org/10.3390/ma16216984
APA StyleQiu, J., Li, J., Guo, Z., Zhang, Y., Nie, B., Qi, G., Zhang, X., Zhang, J., & Wei, R. (2023). 3D Printing of Individualized Microfluidic Chips with DLP-Based Printer. Materials, 16(21), 6984. https://doi.org/10.3390/ma16216984





