Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of Red Mud
2.2. Red Mud Characterization
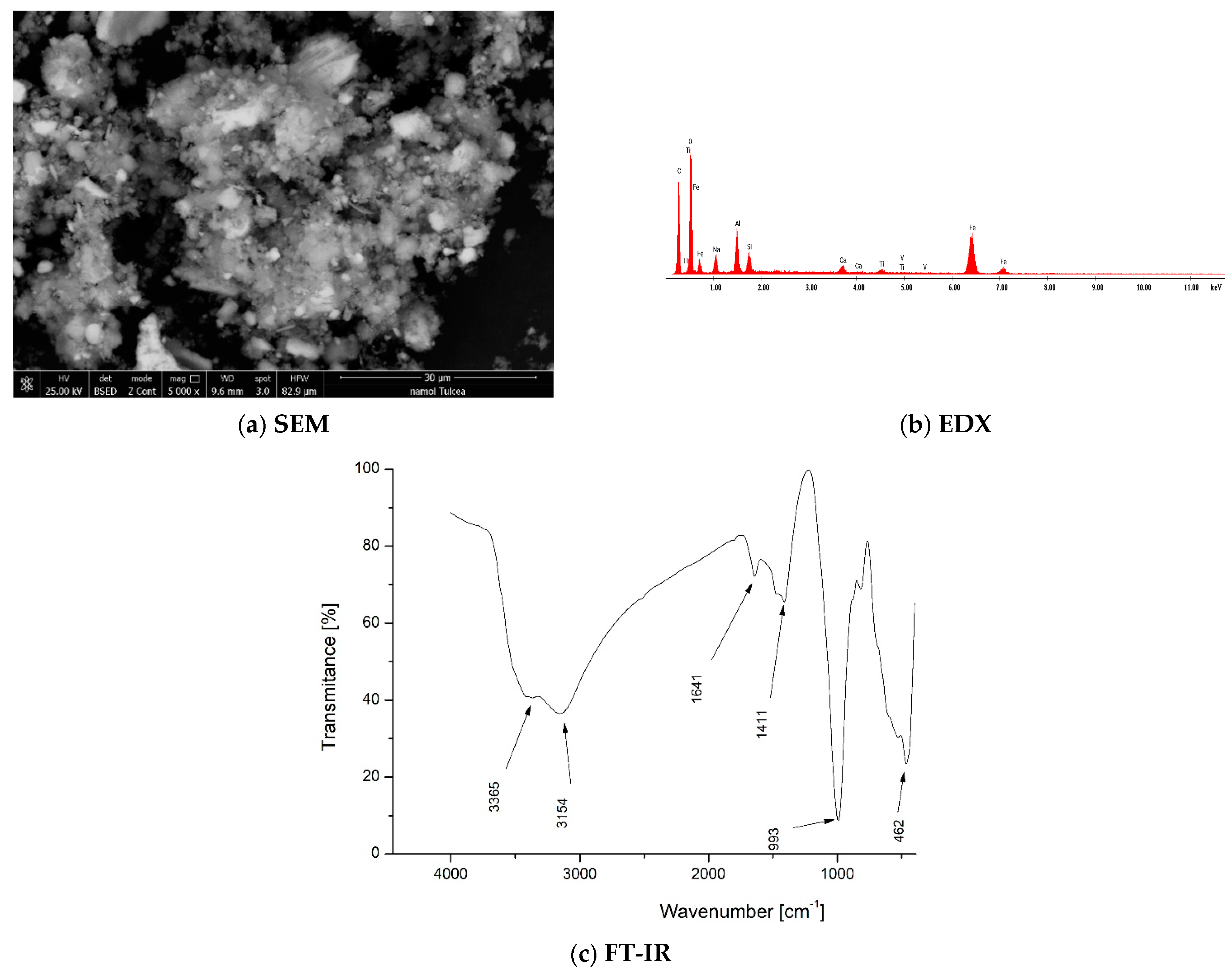
Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
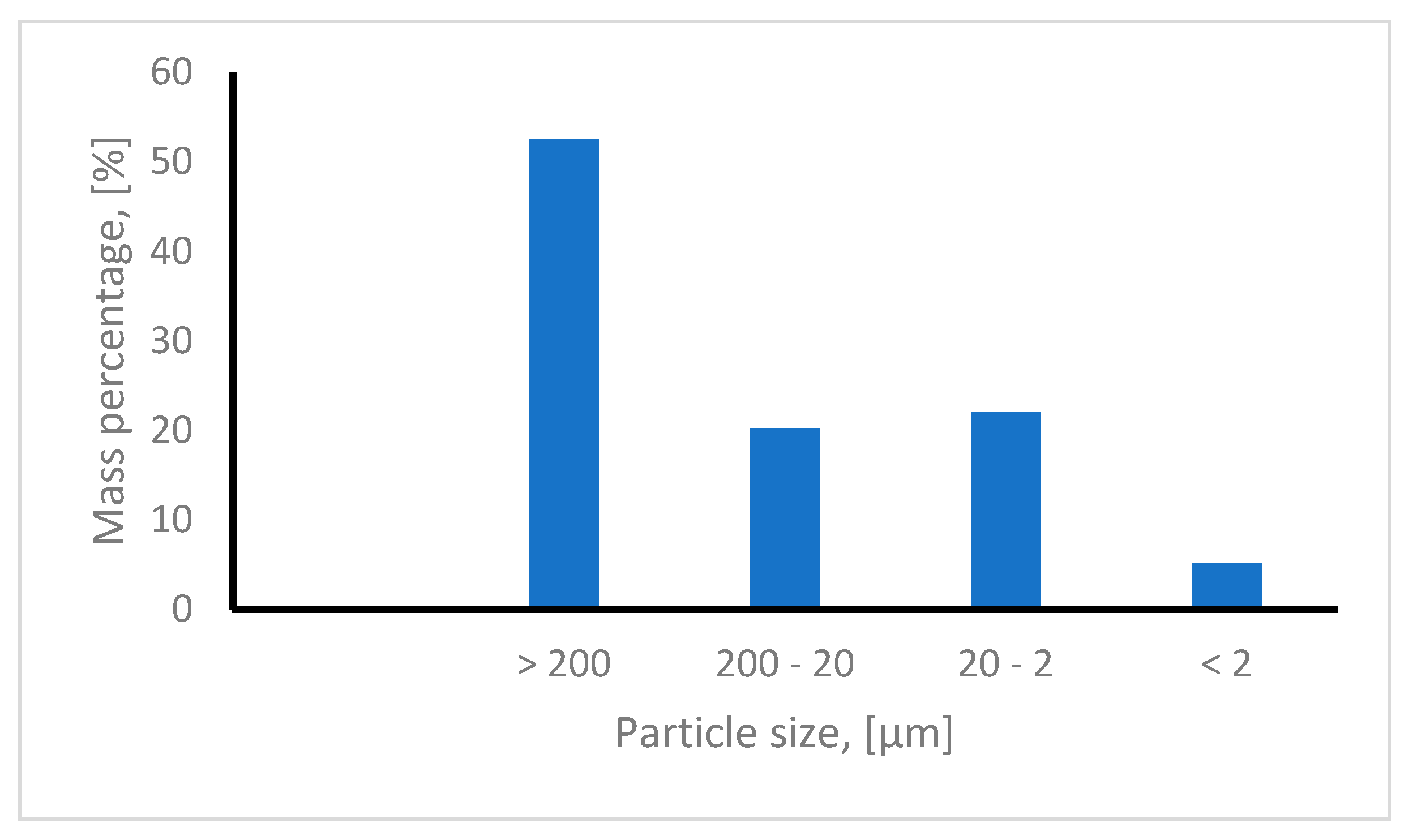
2.4. Red Mud Chemical and Granulometric Composition
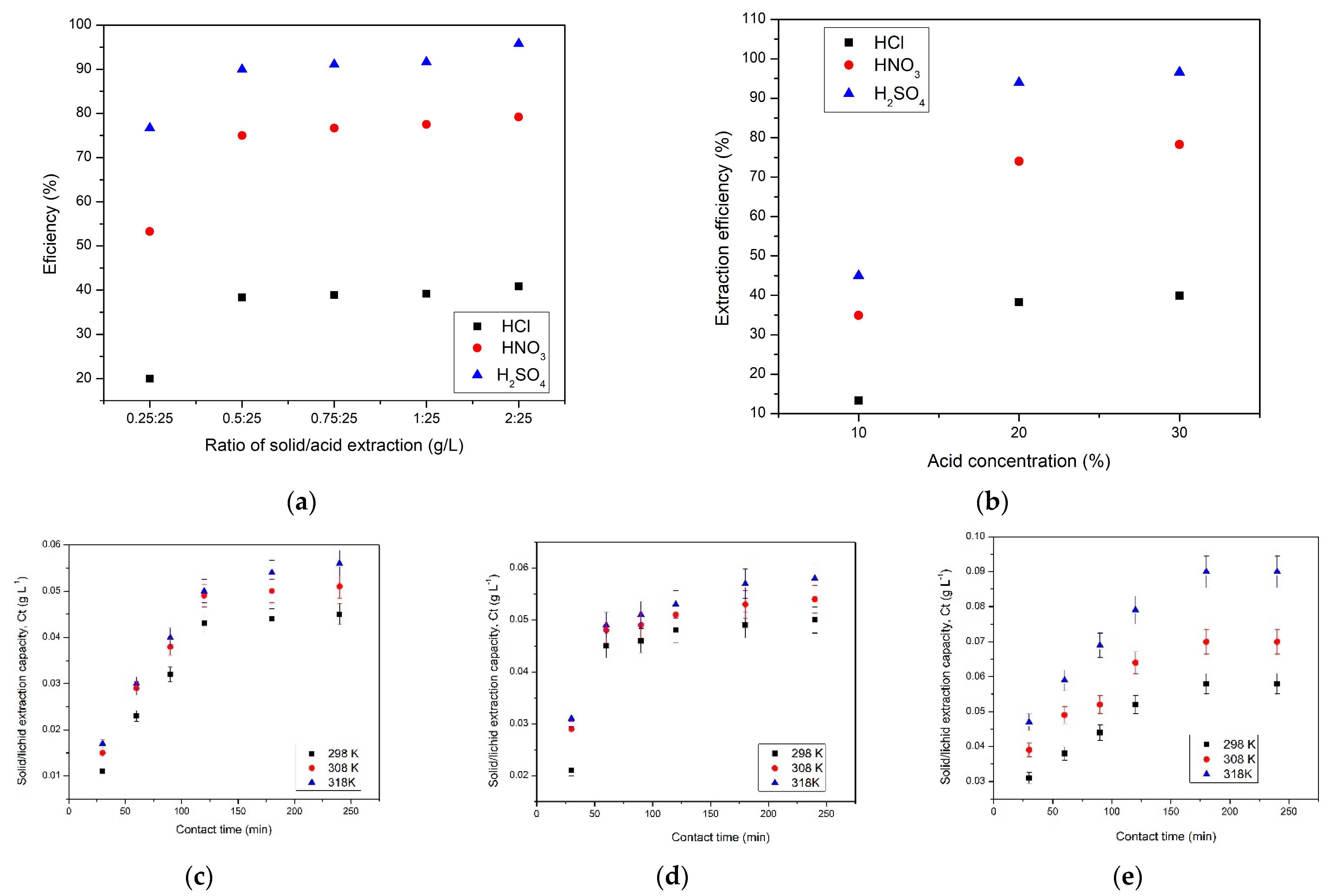
2.5. Acid Leaching of Sc(III) Studies
2.6. Effect of Solid (Red Mud)/Acid Extraction Ratio (S:L Ratio)
2.7. Effect of Acid Concentration
2.8. Effect of Stripping and Temperature
2.9. Kinetic Models of Solid-Liquid Extraction: Activation Energy
2.10. Error Analysis
2.11. Extraction Process Selectivity
3. Results and Discussions
3.1. Composition of Red Mud
3.2. Red Mud Characterization
- -
- one particle fraction with a dimension greater than 200 μm accounting for 52.5% of the sample;
- -
- one particle fraction with dimensions between 20 and 200 μm, accounting for 20.2% of the sample;
- -
- a dust-like particle fraction with particle dimensions between 2 and 20 μm, accounting for 22.1% of the sample;
- -
- one particle fraction consisting into very fine particles with dimensions less than 2 μm, accounting for 5.2% of the sample.
3.3. Acid Leaching of Sc(III) Studies
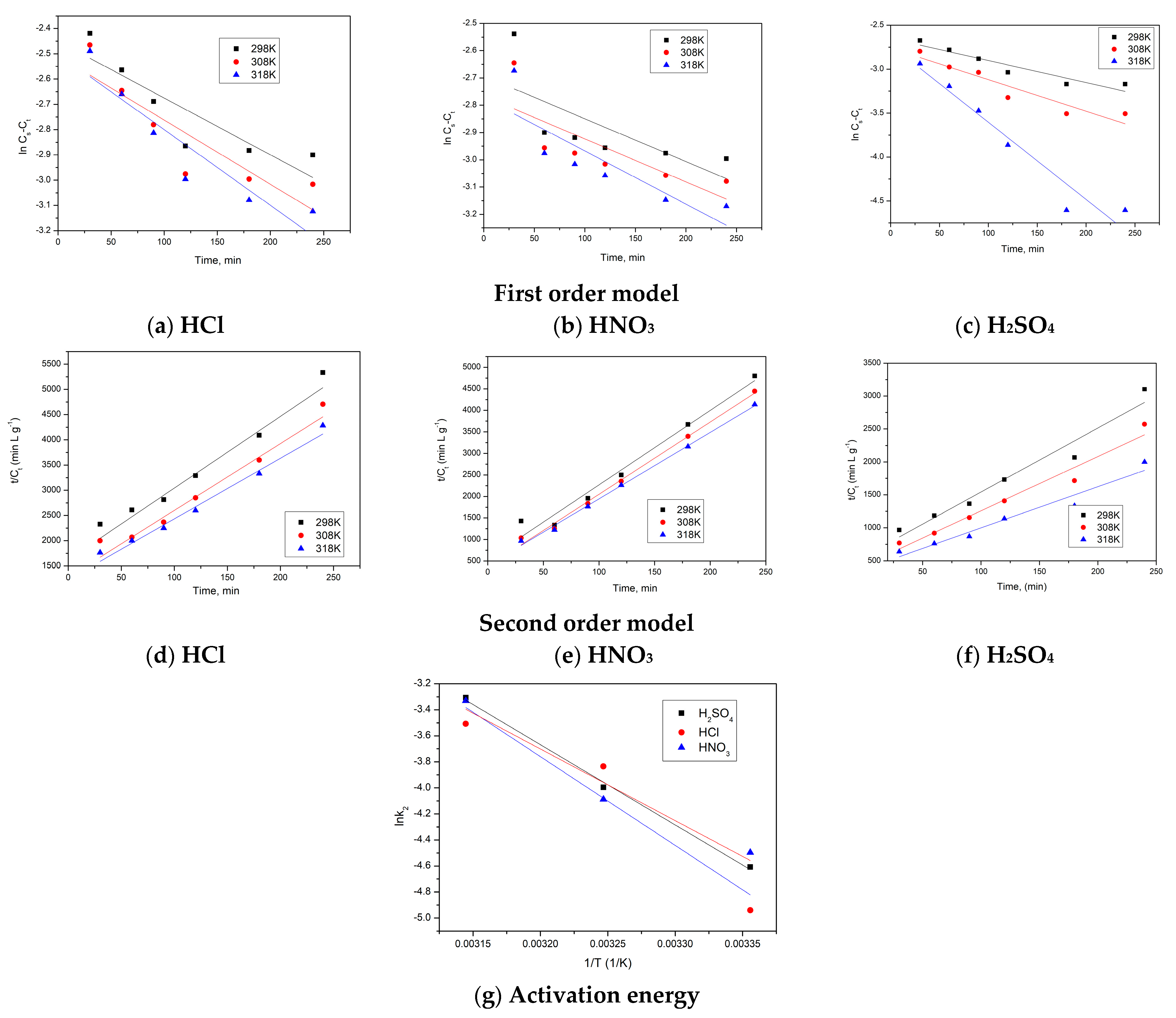
3.4. Kinetic Models of Solid–Liquid Extraction: Activation Energy
3.5. Extraction Process Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gräfe, M.; Power, G.; Klauber, C. Review of Bauxite Residue Alkalinity and Associated Chemistry; Clay Miner: Karawara, WA, Australia, 2009. [Google Scholar]
- Habibi, H.; Mokmeli, M.; Shakibania, S.; Pirouzan, D.; Pourkarimi, Z. Separation and recovery of titanium and scandium from the red mud. Sep. Purif. Technol. 2023, 317, 123882. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Selective Process Steps for the Recovery of Scandium from Jamaican Bauxite Residue (Red Mud). ACS Sustain. Chem. Eng. 2018, 6, 1478–1488. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview on Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Abhilash, S.S.; Meshram, P.; Pandey, B.D.; Behera, P.K.; Satpathy, B.K. RedMud: A Secondary Resource for Rare Earth Elements. IBAAS Bind 2014, 3, 148–162. [Google Scholar]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Grudinsky, P.; Pasechnik, L.; Yurtaeva, A.; Dyubanov, V.; Zinoveev, D. Recovery of Scandium, Aluminum, Titanium, and Silicon from Iron-Depleted Bauxite Residue into Valuable Products: A Case Study. Crystals 2022, 12, 1578. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in Red Mud Utilization—A Review. Miner. Process. Extr. Metall. Rev. 2004, 26, 1578. [Google Scholar] [CrossRef]
- Gladyshev, S.; Akcil, A.; Abdulvaliev, R.; Tastanov, Y.; Beisembekova, K.; Temirova, S. Kinetic study of gallium electrochemical reduction in alkaline solution. Hydrometallurgy 2013, 140, 95–101. [Google Scholar] [CrossRef]
- Gladyshev, S.; Akcil, A.; Abdulvaliyev, R.; Tastanov, E.; Beisembekova, K.; Temirova, S.; Deveci, H. Recovery of vanadium and gallium from solid waste by-products of Bayer process. Miner. Eng. 2015, 74, 91–98. [Google Scholar] [CrossRef]
- Okudan, M.D.; Akcil, A.; Tuncuk, A.; Deveci, H. Recovery of Gallium and Aluminum from Electrofilter Dust of Alumina Calcination Plant in Bayer Process. Sep. Sci. Technol. 2015, 50, 2596–2605. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Sovová, H.; Planinić, M.; Tomas, S. Temperature-dependent kinetics of grape seed phenolic compounds extraction: Experiment and model. Food Chem. 2013, 136, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, S. Kinetic modelling of the solid–liquid extraction process of polyphenolic compounds from apple pomace: Influence of solvent composition and temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C. Kinetics and model building of leaching of water-soluble compounds of Tilia sapwood. Sep. Purif. Technol. 2005, 45, 169–173. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from coirpith. Sep. Purif. Technol. 2001, 24, 497–505. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Amacher, M.; Kotuby-Amacher, J.; Selim, H.; Iskandar, I. Retention and release of metals by soils—Evaluation of several models. Geoderma 1986, 38, 131–154. [Google Scholar] [CrossRef]
- Varshney, K.; Khan, A.; Gupta, U.; Maheshwari, S. Kinetics of adsorption of phosphamidon on antimony (V) phosphate cation exchanger: Evaluation of the order of reaction and some physical parameters. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 19–23. [Google Scholar] [CrossRef]
- Comber, S.D.W.; Gardner, M.; Gunn, A.; Whalley, C. Kinetics of trace metal sorption to estuarine suspended particulate matter. Chemosphere 1996, 33, 1027–1040. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Kaczynski, J.; Kraft, M. Numerical investigation of particle separation in a centrifugal air separator. Trans. Inst. Fluid-Flow Mach. 2017, 135, 57–71. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substabces. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Armas, A.F.; Canchanya-Huaman, Y.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Checca-Huaman, N.R.; Ramos-Guivar, J.A. Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A. Water 2023, 15, 1892. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Dai, Z.; Zhu, L.; Xiao, T.; Zhang, X.; Yin, S.; Soltanian, M.R. Adsorption model identification for chromium (VI) transport in unconsolidated sediments. J. Hydrol. 2021, 598, 126228. [Google Scholar] [CrossRef]
- Claeskens, G.; Jansen, M. Model Selection and Model Averaging. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Wright, J.D., Ed.; Elsevier: Oxford, UK, 2015; pp. 647–652. [Google Scholar]
- Nath, H.; Sahoo, A. A study on the characterization of red mud. Int. J. Appl. Bioeng. 2014, 8. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Kazak, O.; Eker, Y.R.; Akin, I.; Bingol, H.; Tor, A. Green preparation of a novel red mud@carbon composite and its application for adsorption of 2,4-dichlorophenoxyacetic acid from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 23057–23068. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Characterization of alkali activated slag–fly ash blends containing nano-silica. Constr. Build. Mater. 2015, 98, 397–406. [Google Scholar] [CrossRef]
- Landreau, X.; Lanfant, B.; Merle, T.; Dublanche-Tixier, C.; Tristant, P. A thorough FT-IR spectroscopy study on micrometric silicon oxide films deposited by atmospheric pressure microwave plasma torch. Eur. Phys. J. D 2012, 66, 160. [Google Scholar] [CrossRef]
- Lacatusu, R.; Kiselev, A.; Stroe, V.M.; Rizea, N.; Lungu, M.; Lazar, R.; Stanciu-Burileanu, M.M.; Calciu, I.; Popa, R.G.; Filipescu, L. Plant Growth Suitable Nutritive Red Mud Composite Materials from the Romanian Dry Landfilled Red Mud I. Red Mud Chemical and Agrochemical Characterization. Rev. Chim. 2014, 65, 1008–1014. [Google Scholar]
- Zhang, Y.; Zhao, H.; Sun, M.; Zhang, Y.; Meng, X.; Zhang, L.; Lv, X.; Davaasambuu, S.; Qiu, G. Scandium extraction from silicates by hydrometallurgical process at normal pressure and temperature. J. Mater. Res. Technol. 2020, 9, 709–717. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Ning, S.; Zhong, Y.; Wang, X.; Fujita, T.; Wei, Y. Highly efficient recovery and purification of scandium from the waste sulfuric acid solution from titanium dioxide production by solvent extraction. J. Environ. Chem. Eng. 2021, 9, 106226. [Google Scholar] [CrossRef]
- Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.; Ho, Y.S. Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood in a continuous stirred tank reactor. Chem. Eng. Commun. 2007, 194, 537–552. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Reza Saeb, M.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is one performing the treatment data of adsorption kinetics correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
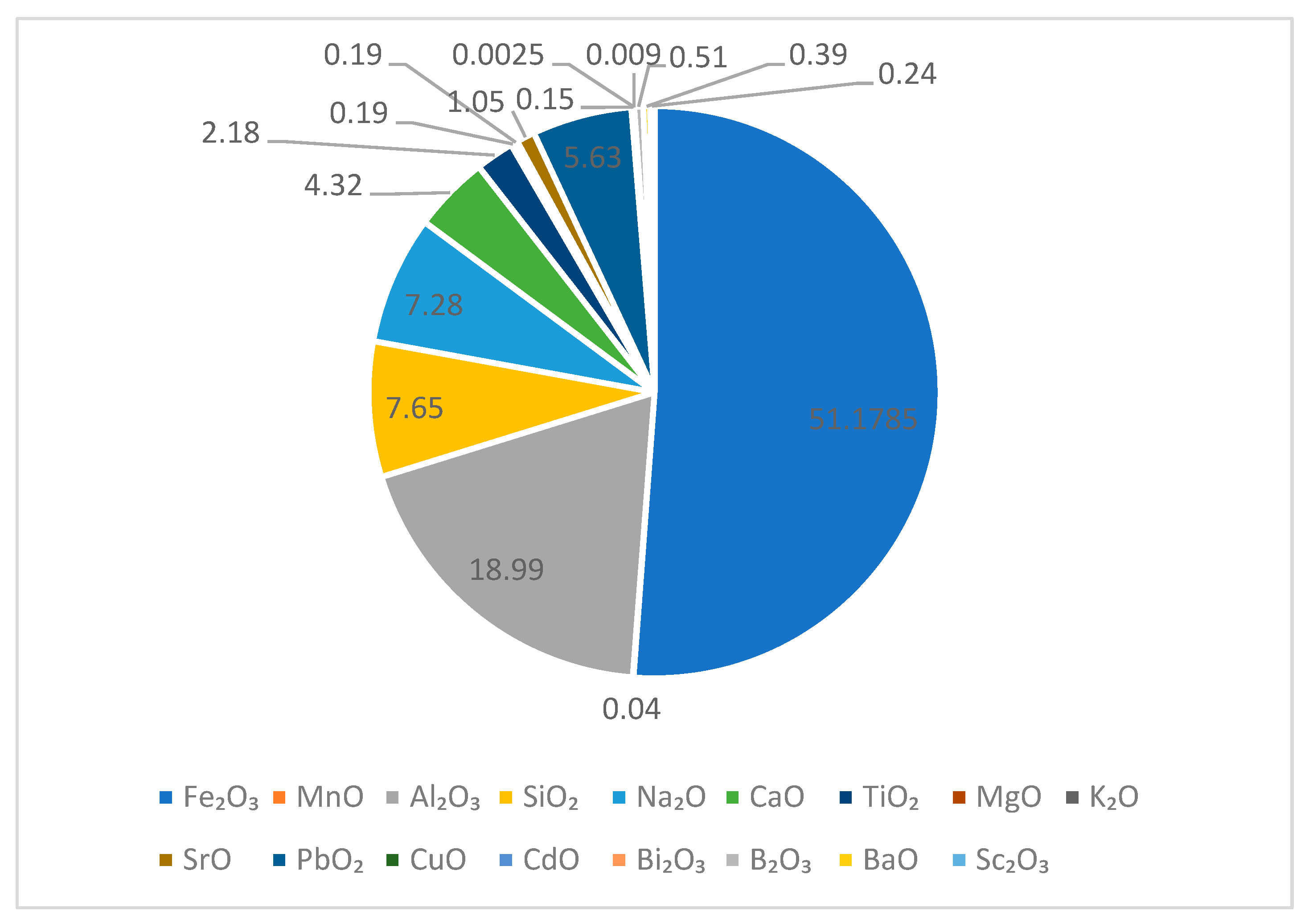
| Na | Mg | Ca | Cr | Mn | Fe | Zn | Sr | Pb | Cu | Cd | Bi | B | Ba | Sc | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.41 ± 0.27 | 3.82 ± 0.19 | 12.7 ± 0.64 | 0.06 ± 0.003 | 3.18 ± 0.16 | 63.7 ± 3.18 | 0.05 ± 0.0025 | 1.18 ± 0.06 | 7.01 ± 0.35 | 0.18 ± 0.009 | 0.03 ± 0.0015 | 0.05 ± 0.0025 | 0.67 ± 0.033 | 0.64 ± 0.032 | 0.32 ± 0.016 | 0.97 ± 0.048 |
| Pseudo First-Order Model | ||||||
| Acid Nature | Temperature, K | k1, min−1 (×10−3) | Saturation Concentration, Cs, g L−1 | R2 | RSS | BIC |
| H2SO4 | 298 | 2.5 | 0.063 | 0.898 | 4.000 | −2.773 |
| 308 | 3.6 | 0.065 | 0.8908 | 9.000 | −4.395 | |
| 318 | 8.8 | 0.070 | 0.9379 | 16.000 | −5.545 | |
| HCl | 298 | 2.2 | 0.059 | 0.8097 | 4.000 | −2.773 |
| 308 | 3.4 | 0.069 | 0.891 | 6.000 | −3.665 | |
| 318 | 8.5 | 0.080 | 0.9382 | 7.000 | −3.973 | |
| HNO3 | 298 | 1.5 | 0.065 | 0.896 | 4.000 | −2.773 |
| 308 | 3.4 | 0.069 | 0.8909 | 6.000 | −3.665 | |
| 318 | 8.7 | 0.077 | 0.9376 | 32.000 | 6.962 | |
| Pseudo Second-Order Model | ||||||
| Acid Nature | Temperature, K | k2 g L−1∙min−1 (×10−3) | Saturation Concentration, Cs, g L−1 | R2 | RSS | BIC |
| H2SO4 | 298 | 9.96 | 0.07 | 0.9917 | 400.00 | −11.983 |
| 308 | 18.40 | 0.08 | 0.9919 | 625.00 | −12.875 | |
| 318 | 36.64 | 0.11 | 0.9917 | 3025.0 | −16.029 | |
| HCl | 298 | 7.15 | 0.06 | 0.9918 | 100.00 | −9.210 |
| 308 | 21.60 | 0.09 | 0.9921 | 625.00 | −12.875 | |
| 318 | 29.95 | 0.10 | 0.9918 | 900.00 | 13.605 | |
| HNO3 | 298 | 11.15 | 0.07 | 0.9917 | 400.00 | −11.983 |
| 308 | 16.78 | 0.08 | 0.9923 | 225.00 | −10.832 | |
| 318 | 35.79 | 0.11 | 0.9921 | 1600.0 | −14.755 | |
| Activation Energy | ||
|---|---|---|
| Acid Nature | Ea, KJ/mol | R2 |
| H2SO4 | 5.50 | 0.9973 |
| HCl | 5.25 | 0.9982 |
| HNO3 | 5.43 | 0.9993 |
| Ion | Na | Mg | Ca | Cr | Mn | Fe | Zn | Sr | Pb | Cu | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction efficiency (%) | 93.67 | 7.50 | 17.29 | 1.22 | 0.08 | 0.21 | 9.97 | 0.15 | 0.02 | 1.57 | 3.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daminescu, D.; Duteanu, N.; Ciopec, M.; Negrea, A.; Negrea, P.; Nemeş, N.S.; Pascu, B.; Lazău, R.; Berbecea, A. Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature. Materials 2023, 16, 6998. https://doi.org/10.3390/ma16216998
Daminescu D, Duteanu N, Ciopec M, Negrea A, Negrea P, Nemeş NS, Pascu B, Lazău R, Berbecea A. Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature. Materials. 2023; 16(21):6998. https://doi.org/10.3390/ma16216998
Chicago/Turabian StyleDaminescu, Diana, Narcis Duteanu, Mihaela Ciopec, Adina Negrea, Petru Negrea, Nicoleta Sorina Nemeş, Bogdan Pascu, Radu Lazău, and Adina Berbecea. 2023. "Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature" Materials 16, no. 21: 6998. https://doi.org/10.3390/ma16216998
APA StyleDaminescu, D., Duteanu, N., Ciopec, M., Negrea, A., Negrea, P., Nemeş, N. S., Pascu, B., Lazău, R., & Berbecea, A. (2023). Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature. Materials, 16(21), 6998. https://doi.org/10.3390/ma16216998









