Abstract
In the present work, cholesterol (Chol)-substituted zinc phthalocyanine (Chol-ZnPc) and its composite with graphene oxide (GO) were prepared for photodynamic therapy (PDT) applications. Briefly, Chol-substituted phthalonitrile (Chol-phthalonitrile) was synthesized first through the substitution of Chol to the phthalonitrile group over the oxygen bridge. Then, Chol-ZnPc was synthesized by a tetramerization reaction of Chol-phthalonitrile with ZnCl2 in a basic medium. Following this, GO was introduced to Chol-ZnPc, and the successful preparation of the samples was verified through FT-IR, UV–Vis, 1H-NMR, MALDI-TOF MS, SEM, and elemental analysis. Regarding PDT properties, we report that Chol-ZnPc exhibited a singlet oxygen quantum yield () of 0.54, which is slightly lower than unsubstituted ZnPc. Upon introduction of GO, the GO/Chol-ZnPc composite exhibited a higher , about 0.78, than that of unsubstituted ZnPc. Moreover, this enhancement was realized with a simultaneous improvement in fluorescence quantum yield () to 0.36. In addition, DPPH results suggest low antioxidant activity in the composite despite the presence of GO. Overall, GO/Chol-ZnPc might provide combined benefits for PDT, particularly in terms of image guidance and singlet oxygen generation.
1. Introduction
Photodynamic therapy (PDT) is a noninvasive cancer treatment method with fewer side effects and many more advantages than chemotherapy, radiotherapy, and surgery. In PDT, a light source, oxygen in the human tissue, and a photosensitizer are used in conjunction to destroy cancerous cells [1,2,3]. Once a photosensitizer is activated with a red light, it transfers energy to the dissolved oxygen in the vicinity of the cancer cell to produce reactive oxygen species (ROS) [2,3]. Singlet oxygen is one of the most well-known molecular oxygen derivatives among ROS with high toxicity that can directly kill cancer cells [3,4]. This means that an ideal photosensitizer material should exhibit a high singlet oxygen quantum yield in addition to a high absorption peak in the wavelengths corresponding to the therapeutic window. In this sense, phthalocyanine (Pc) and its derivatives are regarded as second-generation photosensitizer materials, since they not only meet the above requirements, but also could reduce skin phototoxicity as compared to photofrin-based first-generation photosensitizers [1,5,6]. Particularly, zinc Pc (ZnPc) and its derivatives are receiving considerable interest for PDT applications thanks to their relatively high efficiencies to yield singlet oxygen, low toxicity, easy synthesis, ease in modifying their structure, and high stability [7,8,9,10,11,12,13]. However, their main drawback is their poor solubility in aqueous solutions as a result of their extended flat hydrophobic aromatic surface, which makes it difficult to transport them to the target cell in the human body [14,15].
One way to resolve this involves the encapsulation of Pcs in liposome-based nanoparticles for their targeted delivery [16,17,18,19,20,21,22]. In that case, liposomes release Pcs during lipid exchange with lipoproteins, and then Pcs are bound to lipoproteins, which carry cholesterol (Chol) through the bloodstream [23,24,25]. The binding of Pcs to lipoproteins, particularly to Chol-rich, low-density lipoproteins (LDL), results in the selective targeting of cancer cells [23,24,25,26,27,28,29]. As is known, cancer is the uncontrollable and abnormal growth of cells. Thus, cancer cells need excessive Chol levels for proliferation; hence, they express higher LDL receptors than noncancer cells [23,24,25,26,27]. This, in turn, results in the systematic and selective accumulation of Pcs in tumor cells through receptor-mediated endocytosis [29,30]. In this sense, previous reports indicate that the encapsulation of ZnPcs in liposomes in the presence of cholesterol (Chol) significantly improves their photodynamic activity [19,20,21,22]. Chol is an organic molecule, and its influence on cancer has been studied since it was first reported in the early 1900s [31,32,33]. It is known that the restriction of Chol could prevent the growth of cancer cells, and cancer cells could attract Chol through LDL receptors, as mentioned previously [34,35,36].
Apart from this, it is known that the composites of Pc with low-dimensional carbon materials could enhance the charge transfer properties through π–π stacking. Particularly, graphene oxide (GO) is receiving considerable interest as a constituent material in such composite structures due to its oxygen-containing functional groups and high photothermal conversion efficiency [37,38,39,40,41]. In this context, various GO/Pc composites have been investigated for PDT, combined photothermal/PDT, combined sonodynamic/PDT, combined drug delivery/PDT, and image-guided photothermal/PDT applications, with promising results [41,42,43,44,45,46,47,48]. For instance, Rocha et al. reported that GO/ZnPc and GO/ZnPc(NO2)4 exhibited singlet oxygen quantum yields of about 0.71 and 0.32 in dimethylformamide (DMF), respectively, of which both values were higher than that of the singlet oxygen quantum yields of ZnPc and ZnPc(NO2)4 in DMF, which were only about 0.56 and 0.25, respectively [42]. Further, it was reported that a similar situation was persistent in acetonitrile (MeCN), since GO/ZnPc and GO/ZnPc(NO2)4 exhibited singlet oxygen quantum yields of about 0.71 and 0.29 in MeCN, while ZnPc and ZnPc(NO2)4 displayed much lower performances with singlet oxygen quantum yields of 0.50 and 0.27, respectively [42].
Based on the above considerations, we propose that Chol-substituted ZnPc and its composite with GO might provide interesting properties for PDT applications. Accordingly, the present work involves the preparation of Chol-ZnPc through a tetramerization reaction of Chol-substituted phthalonitrile (Chol-phthalonitrile) with zinc chloride (ZnCl2). Then, GO/Chol-ZnPc composites were prepared with the self-assembly method through π–π interactions. The samples were characterized using FT-IR, UV–Vis, 1H-NMR, MALDI-TOF MS, SEM, and elemental analysis, and both fluorescence quantum yield (ΦF) and singlet oxygen quantum yield (Φ∆) of Chol-ZnPc and GO/Chol-ZnPc were studied. Also, the antioxidant potency of the GO/Chol-ZnPc was determined considering the presence of GO in the composite, and the PDT potentials of the as-prepared materials were discussed.
2. Materials and Methods
2.1. Materials
Chol (≥99%), 4-nitrophthalonitrile (99%), ZnCl2 (≥98%), potassium carbonate (≥98%), dimethyl sulfoxide (DMSO) (≥99.5%), chloroform (anhydrous, ≥99%), hexane (anhydrous, 95%), 1-pentanol (≥99%), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (≥99.0%), and methanol (anhydrous, 99.8%) were purchased from Sigma Aldrich (Saint Louis, MO, USA) and used as received. GO was obtained from Nanografi Co., Ltd. (Ankara, Turkey), and used as received. Deionized (DI) water was used throughout the experiments.
2.2. Synthesis of Chol-ZnPc
First, we synthesized Chol-phthalonitrile, which was later used in the synthesis of Chol-ZnPc. For this, 1 g of 4-nitrophthalonitrile was mixed with 2.46 g of Chol in 25 mL DMSO. Then, 2.4 g of potassium carbonate was gradually introduced to the solution at room temperature over a 2 h period. The mixture was stirred at room temperature for 5 days. Following this, the solvent was removed in an evaporator, and the residue was dis-solved in chloroform and filtered. The reaction products were purified by column chromatography on silica gel using a mixture of chloroform and hexane (6:1). The yield for the synthesis of Chol-phthalonitrile was calculated as 26%, and the melting point of Chol-phthalonitrile was measured as 186 °C. For the synthesis of Chol-ZnPc, 100 mg of light yellow powder of as-synthesized Chol-phthalonitrile was mixed with 6.8 mg of ZnCl2 in 1 mL 1-pentanol in the presence of DBU. Then, the reaction was performed at 185 °C for 22 h. After cooling, the mixture was precipitated with methanol, filtered, and dried in a vacuum. The obtained dark green powders were then dissolved in chloroform and filtered. The residue was further purified by column chromatography over a silica gel eluted with a 9:2 mixture of chloroform and methane. The yield for the synthesis of Chol-ZnPc was calculated as 15%, and the melting point of Chol-ZnPc was found to be higher than 300 °C. The reactions are schematized in Figure 1.
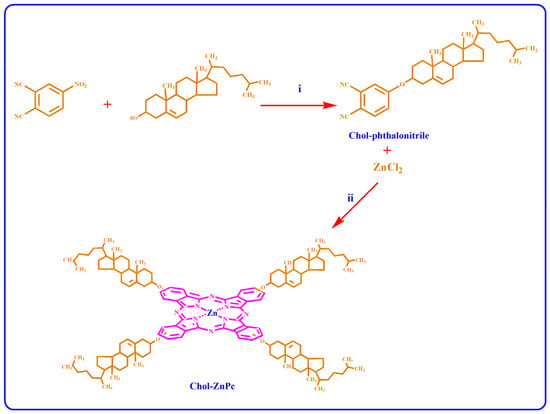
Figure 1.
Synthesis of Chol-substituted phthalonitrile (i) and Chol-ZnPc (ii).
Chol-phthalonitrile: Yield 770 mg (26%). Mp: 186 °C. 1H-NMR (400 MHz, CDCl3, 25 °C): δ = 8.70 (2H, Ar-H); 8.1 (1H, Ar-H); 5.3 (1H, Chol-H); 3.4 (1H, Chol-H); 2.2 (2H, Chol-H); 1.0 (3H, Chol-CH3); 0.9 (6H, Chol-CH3); 0.8 (3H, Chol-CH3); 0.7 (3H, Chol-CH3). FT-IR (KBr pellet) υ (cm−1) 3086; 3012; 2239; 1468; 731. Anal. Calc. for C35H48N2O: C, 81.98; H, 9.44; N, 5.46%, found: C, 81.05; H, 8.89; N, 5.70%.
Chol-ZnPc: Yield 41.0 mg (15%). Mp: >300 °C. 1H-NMR (400 MHz, CDCl3, 25 °C): δ = 8.1–7.1 (12H, Ar-H); 5.5 (1H, Chol-H); 3.6 (1H, Chol-H); 2.5–0.5 (43H, Chol-H). UV–Vis (DMSO) λmax/nm 724. 703, 639, 368. FT-IR (KBr pellet) υ (cm−1) 3056; 2926; 2860; 1645; 1608; 1522; 1491; 1444; 1398; 1315; 1261; 1144; 1101; 930; 768; 700. Anal. Calc. for C140H120N8O4Zn: C, 82.27; H, 5.92; N, 5.48%, found: C, 80.12; H, 5.27; N, 5.52%. MALDI-TOF MS m/z: 2045 [M+H]+.
2.3. Preparation of GO/Chol-ZnPc Composites
GO (2 µg/mL) was dispersed in DMSO at room temperature by using a Bandelin HD 2070 ultrasonic homogenizer (Berlin, Germany). At the same time, Chol-ZnPc was also prepared in DMSO (5 µg/mL), and then introduced to the GO-DMSO mixture. The resultant solution was further sonicated for 25 min at room temperature. Then, the GO/Chol-ZnPc composites were filtered, washed with DI water, and then dried in vacuum oven until a constant weight was reached. The weight percentage of GO (based on the feed composition) in the composite was ~29%. The process is schematized in Figure 2.
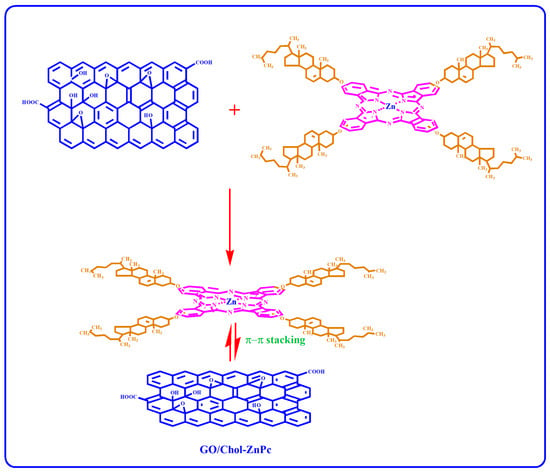
Figure 2.
Preparation of GO/Chol-ZnPc composites.
2.4. Characterization
Absorption and fluorescence spectra of the samples were measured on a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan) and Agilent G9800A fluorescence spectrophotometer (Santa Clara, CA, USA) in DMSO, respectively. As is known, concentration has an effect on such measurements [49,50], and it was kept at 5 μg/mL in the present work. Fourier-transform infrared (FT-IR) spectra were recorded using a Bruker Tensor II FT-IR spectrophotometer (Ettlingen, Germany). Elemental analysis was performed using an LECO elemental analyzer (Saint Joseph, MI, USA). The morphologies of the as-prepared samples were examined on a TESCAN MIRA 3 XMU scanning electron microscope (SEM) (Brno, Czech Republic). Proton nuclear magnetic resonance spectroscopy (1H-NMR) was performed at room temperature on a JEOL JNM-ECZ400S spectrometer (Tokyo, Japan) at 400 MHz by using chloroform-d as solvent. Melting points were determined using an Electrothermal 9100 apparatus (Staffordshire, UK). The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of GO/Chol-ZnPc was examined according to Blois method by using gallic acid as a standard [51]. In a regular measurement, DPPH was mixed with GO/Chol-ZnPc in DMSO for 2 h at room temperature, and the measurements were performed with an enzyme-linked immunosorbent assay (ELISA) reader.
3. Results and Discussion
It would be crucial to verify successful formation of Chol-phthalonitrile before the synthesis of Chol-ZnPc. Accordingly, we first studied the 1H-NMR spectrum of Chol-phthalonitrile, as shown in Figure 3. The aromatic protons belonging to phthalonitrile group were observed in the range of 8.7–8.1 ppm, as could be expected, while the protons belonging to the Chol group were observed between 5.3 and 0.7 ppm (Figure 3). Further, all relevant bonds are identified in Figure 3, and both the respective positions and integral ratios of the characteristic peaks were in good agreement with previous reports [52,53,54]. Regarding Chol-ZnPc, it was prepared by tetramerization reaction of Chol-phthalonitrile and ZnCl2. The 1H-NMR spectrum of Chol-ZnPc exhibited aromatic protons on the Pc ring in the range of 8.1–7.1 ppm, and the protons belonging to the Chol group were between 5.5 and 0.5 ppm. These chemical shifts were compatible with the structure of Chol-ZnPc, considering the 1H-NMR spectrum of the Chol-phthalonitrile. The chemical shifts in the 1H-NMR spectra of both Chol-phthalonitrile and Chol-ZnPc are listed in the experimental section. Overall, 1H-NMR results suggested that both Chol-phthalnotitrile and Chol-ZnPc were successfully prepared, and that the as-synthesized compounds were pure.
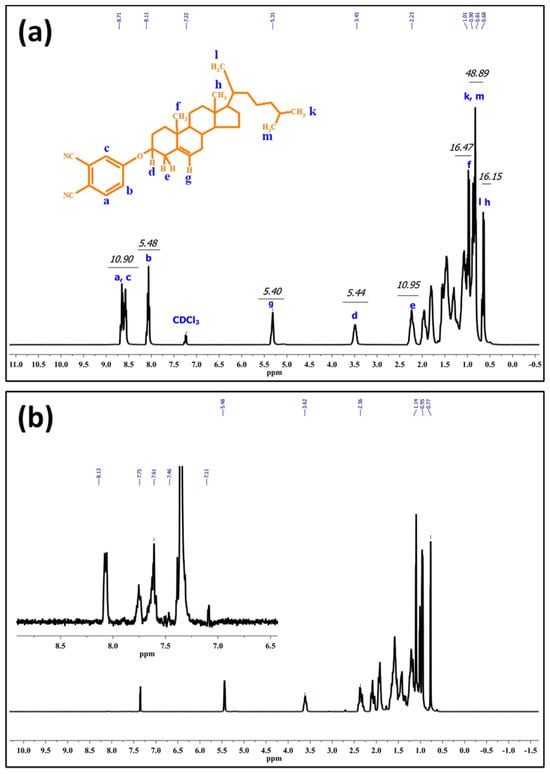
Figure 3.
1H-NMR spectrum of Chol-phthalonitrile (a) and Chol-ZnPc (b).
Incorporation of GO to Chol-ZnPc was verified by FT-IR analysis, as shown in Figure 4. The characteristic C≡N peak of the phthalonitrile was recorded at about 2239 cm−1 in the FT-IR spectrum of Chol-phthalonitrile (Figure 4). This peak was not detected in the FT-IR spectrum of Chol-ZnPc, further indicating the successful tetramerization reaction. Also, the IR peaks at about 1522 cm−1, 1491 cm−1, 1444 cm−1, 1398 cm−1, 1315 cm−1, 1261 cm−1, 1144 cm−1, 1101 cm−1, 930 cm−1, 768 cm−1, and 700 cm−1 could be ascribed to the characteristic skeletal vibrations of ZnPc macrocycles, and were observed in Chol-ZnPc as expected [55,56,57,58,59,60,61,62]. Particularly, the characteristic Pc macrocycle bands at about 1491 cm−1, 1101 cm−1, and 768 cm−1 were red-shifted in the GO/Chol-ZnPc, indicating interactions between GO and Chol-ZnPc. Regarding the peaks at about 1766 cm−1 and 1716 cm−1, they could be ascribed to the C=O stretching vibrations of carbonyl and carboxyl groups of GO [63,64,65,66]. Moreover, the peak at about 1612 cm−1 could be ascribed to the C=C stretching vibrations of graphitic domains in GO [63,64,65]. These three peaks were only observed in the FT-IR spectrum of GO/Chol-ZnPc, and their presence indicates that the GO/Chol-ZnPc composites were successfully prepared.
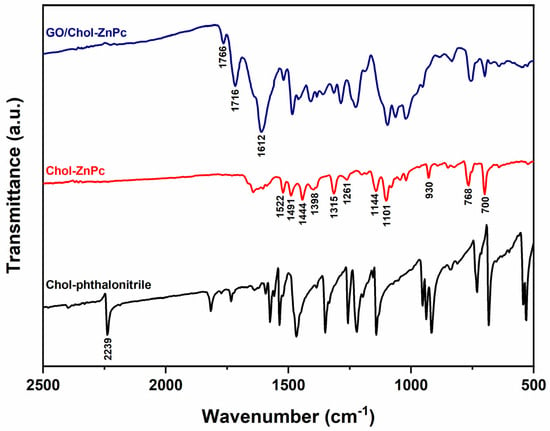
Figure 4.
FT-IR spectra of Chol-phthalonitrile, Chol-ZnPc, and GO/Chol-ZnPc.
Further, the elemental analysis and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) results are given in the experimental section and Figure 5, and both results verify the successful preparation of the samples.
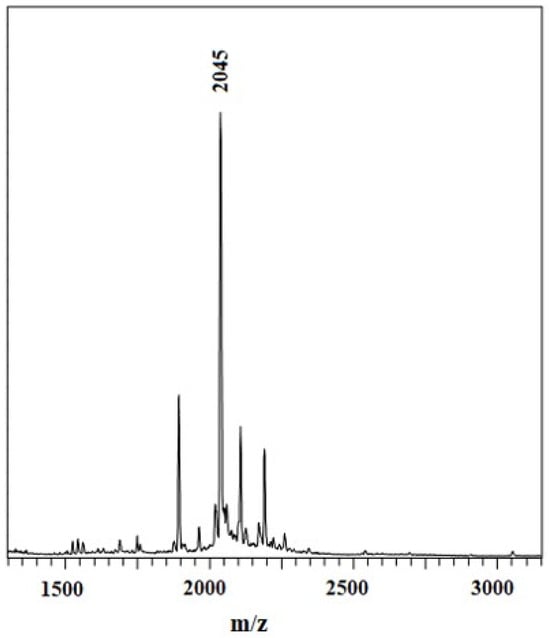
Figure 5.
MALDI-TOF MS spectra of Chol-ZnPc.
In addition, Chol-ZnPc and GO/Chol-ZnPc were studied using ultraviolet–visible (UV–Vis) spectroscopy in DMSO (Figure 6). Figure 6 shows that both the Chol-ZnPc and GO/Chol-ZnPc exhibited the typical Q bands of Pcs within the therapeutic window, in accordance with previous reports [67,68,69]. As is known, the characteristic Q band is due to the π−π* transition of the Pc ring from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) [70]. Interestingly, while GO/Chol-ZnPc showed monomeric Q bands, Chol-ZnPc exhibited a doublet with absorbance maxima at about 703 nm (Qx) and 724 nm (Qy), suggesting aggregation of Chol-ZnPc in the solvent [67]. Upon introduction of GO, the doublet disappeared and turned into a single peak, an observation in good agreement with previous reports [37]. Further, this peak was blue-shifted as compared to Qy. In fact, most previous studies reported a red shift in the Q band upon covalent attachment of GO to Pc [71,72,73]. However, the processing time was too limited in the present work for a covalent attachment. In addition, it could be realized that the functional groups in the present work cannot allow a hydrogen bonding, unlike the previous work of Yabas et al. [37]. In fact, both the red shift and the blue shift of the Q band were reported as depending on the substituted groups in previous works where various GO and Pc derivatives were coupled at relatively similar processing conditions [42,74,75], and the blue shift of the Qy in the present work might be ascribed to π−π and electrostatic interactions between GO and Chol-ZnPc.
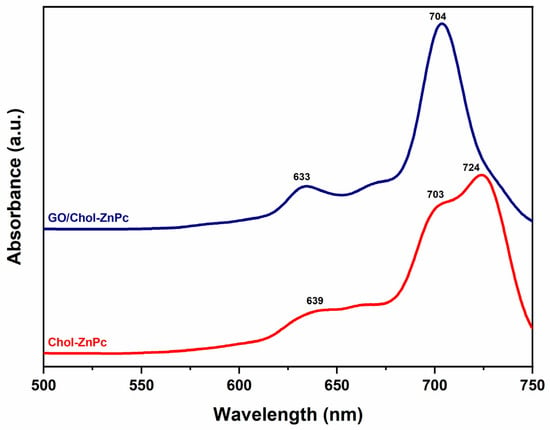
Figure 6.
UV–Vis spectra of Chol-ZnPc and GO/Chol-ZnPc.
The morphological features of the Chol-ZnPc and GO/Chol-ZnPc were studied using SEM analysis, as shown in Figure 7. Figure 7a–c show that the microstructure of GO exhibited layers of crumpled sheets. Then, upon preparation of GO/Chol-ZnPc, mostly spherical yet nonuniform particles and aggregates of Chol-ZnPc were found to be homogeneously distributed in the composite, as can be seen in Figure 7d–f. Also, the presence of Zn in the Chol-ZnPc was further confirmed by the SEM-EDX, which revealed that the atomic percentage of Zn in the compound was about 2% (Figure S1).
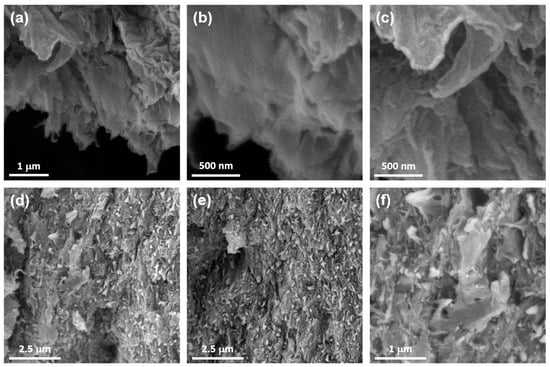
Figure 7.
SEM images of GO (a–c) and GO/Chol-ZnPc (d–f).
As is known, the efficiency of fluorescence emission is important in PDT applications, particularly for image guidance. In this sense, we studied the fluorescence spectra of Chol-ZnPc and GO/Chol-ZnPc in DMSO, as can be seen in Figure 7, and calculated the according to Equation (1) [76]:
where , , , and represent the fluorescence quantum yield, area under the fluorescence emission curve, absorbance at the excitation wavelength, and the refractive index of the solvent, respectively. The subscript in Equation (1) denotes that the corresponding data are for the standard sample, which was unsubstituted ZnPc ( in DMSO [77]) in the present work.
Figure 8 shows that the emission peak was shifted towards higher wavelengths in the GO/Chol-ZnPc composite as compared to the Chol-ZnPc. This could be ascribed to the π–π and electrostatic interactions between GO and Chol-ZnPc in the composite. Also, fluorescence emission was clearly enhanced in the composite (Figure 8), suggesting better charge transport in the GO/Chol-ZnPc. In line with this, of the GO/Chol-ZnPc composite () was, indeed, higher than that of both Chol-ZnPc () and unsubstituted ZnPc, as listed in Table 1.
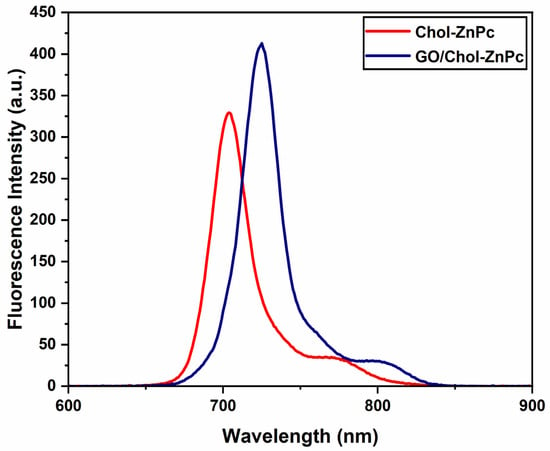
Figure 8.
Fluorescence emission spectra of Chol-ZnPc and GO/Chol-ZnPc.

Table 1.
PDT performance of Chol-ZnPc and GO/Chol-ZnPc in DMSO.
The singlet oxygen quantum yield () was determined in DMSO via a common method [77,78,79,80,81,82] by using 3-diphenylisobenzofuran (DPBF) as a singlet oxygen scavenger and unsubstituted ZnPc as a standard. As is known, DPBF does not decompose with singlet oxygen; instead, its reaction with singlet oxygen causes a decrease in the absorbance intensity at 417 nm, which could be used to measure the singlet oxygen generation ability [78]. Thus, DMSO solutions containing Chol-ZnPc and GO/Chol-ZnPc were prepared separately with DPBF in the dark, and irradiated for certain times. Then, the singlet oxygen generation-dependent degradation in the absorbance of DPBF was monitored for both Chol-ZnPc and GO/Chol-ZnPc at each time interval, as shown in Figure 9, and the singlet oxygen quantum yields were calculated by Equation (2) [77]:
where ΦΔ, , and Fabs represent the singlet oxygen quantum yield, photobleaching rate, and absorption correction coefficient, respectively. The ratio of is calculated using Equation (3) [78,79,80]:
where refers to the absorption at the wavelength of light source. The superscript in Equations (2) and (3) denotes that the corresponding data are for the standard sample, which was unsubstituted ZnPc ( in DMSO [77]) in the present work.
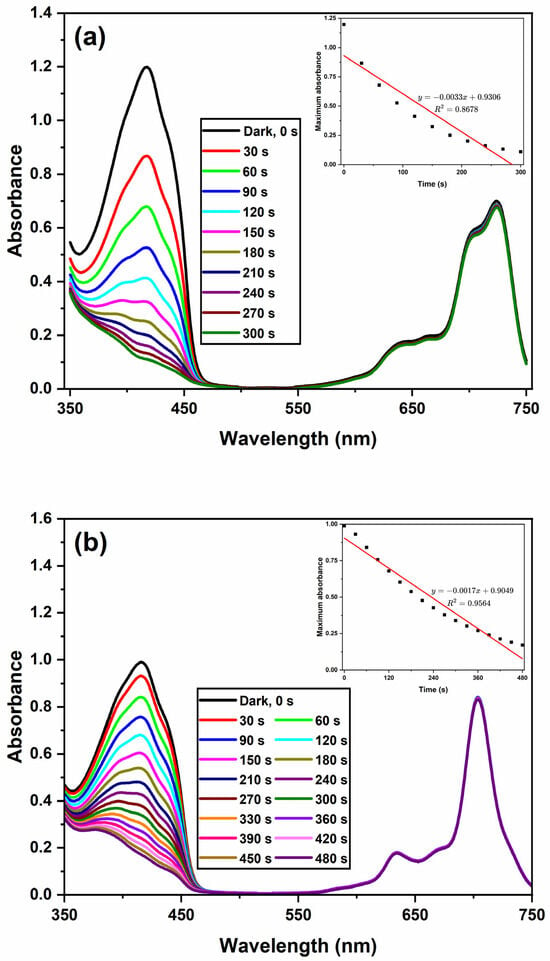
Figure 9.
Depletion in the absorbance of DBPF at 417 nm with respect to irradiation time for Chol-ZnPc (a) and GO/Chol-ZnPc (b). Inset shows the bleaching rate of DBPF at 417 nm upon illumination.
The results show that Chol-ZnPc exhibited a lower singlet oxygen generation ability (Φ∆ = 0.54) than unsubstituted ZnPc, as can be seen in Table 1. However, the extent of this decrease was relatively limited. In addition, the substitution of Chol might enhance targeted delivery of the photosensitizer to cancer cells, because it is known that cancer cells are keen to attract as much Chol as possible through expressing more LDL receptors [34,35,36]. In the field, the encapsulation of Pcs in liposome-based nanoparticles is among the most common approaches for the targeted delivery of photosensitizers [16,17,18,19,20,21,22]. In this approach, the photosensitizers were released during the lipid exchange with lipoproteins, they were attached to lipoproteins, and such attachment, particularly to LDLs, helps to the selective targeting of cancer cells [23,24,25,26,27,28,29]. In this sense, the presence of Chol might provide improved binding to LDL for better drug delivery, considering that Chol-modified structures could easily interact with Chol-rich molecules [36,83,84,85,86]. This means that the overall PDT performance might be enhanced in Chol-ZnPc via a possible improvement in the photosensitizer uptake of cancer cells, despite the slight decay in Φ∆. In addition, the decrease in Φ∆ could actually be neutralized by the introduction of GO to Chol-ZnPc, as can be seen in Table 1. In fact, the ΦΔ of GO/Chol-ZnPc (Φ∆ = 0.78) was actually higher than that of both Chol-ZnPc and unsubstituted ZnPc (Table 1). This means that a significant enhancement was realized upon introduction of GO, an improvement that might be ascribed to the better charge transport properties in the composite. Furthermore, this enhancement in ΦΔ was reached simultaneously with an increase in ΦF, as shown in Table 1. This is particularly important as it is not an easy task to concurrently realize a high ΦF and ΦΔ, since a high ΦΔ is often accompanied with a low ΦF, considering that intersystem crossing competes with fluorescence [42,67,82,87,88,89,90,91,92,93,94,95]. Strikingly, as compared to the ΦF and ΦΔ results of previous similar studies in DMSO, the as-prepared GO/Chol-ZnPc composite in the present work seems to be a promising candidate, considering that Gunsel et al. [45] reported a ΦF of 0.092 and ΦΔ of 0.628 for a 10% GO containing ZnPc derivative, Gunsel et al. [44] reported a ΦF of 0.186 and ΦΔ of 0.638 for a 10% GO containing ZnPc derivative, Matshitse and Nyokong [96] reported a ΦF of 0.09 and ΦΔ of 0.11 for a 50% graphene quantum dot containing ZnPc derivative, Nwahara et al. [97] reported a ΦF of 0.23 and ΦΔ of 0.52 for a 23% glutathione-capped graphene quantum dot containing aluminum Pc derivative, and Nwahara et al. [98] reported a ΦF of 0.16 and ΦΔ of 0.65 for a 33% L-glutathione functionalized graphene quantum dot containing ZnPc derivative.
On the other hand, it is worth mentioning that the actual PDT performance of a photosensitizer on a living cell is a much more complex case, and it necessitates information on pharmacodynamics and pharmacokinetics to draw more precise conclusions. Particularly, although some previous reports indicated positive effects of using GO in terms of improving PDT properties [41,42,43,44,45,46,47,48,99], there exists diverse information regarding the cytotoxicity of GO depending on the synthesis conditions, functional groups, aggregation, contamination, cell type, and so forth [100,101,102,103,104,105,106,107]. While we did not provide the cytotoxicity assay of the GO/Chol-ZnPc in the present work, the antioxidant activity of the composite was evaluated using the Blois method, as shown in Figure 10. This method is based on the reduction of DPPH, a stable free radical, by the antioxidant and its color change. As is known, DPPH provides the maximum absorption at 517 nm (violet color) [108,109]. When the single electron in the DPPH free radical pairs with hydrogen from the antioxidant, the intensity of the strong absorbance band at 517 nm decreases, and the color of DPPH changes from violet to yellow. We determined the color intensity of the component using an ELISA reader, which helps to observe the antioxidant property of the material [110,111]. Also, the antioxidant activity of gallic acid, a typical antioxidant, was determined at the same conditions, and is plotted in Figure 10 for comparison. For GO/Chol-ZnPc, only a slight change in the violet color of DPPH was observed after 2 h. Thus, as can be seen in Figure 10, the antioxidant activity of the GO/Chol-ZnPc composite was much lower than that of the gallic acid standard, and the percentage antioxidant activity of the composite was less than 50% at all concentrations tested. Hence, it can be said that GO/Chol-ZnPc does not show a significant antioxidant activity on the DPPH radical. Overall, the GO/Chol-ZnPc composite exhibited relatively high ΦF and ΦΔ simultaneously, did not exhibit a significant antioxidant activity, and might be considered as a promising material for PDT applications.
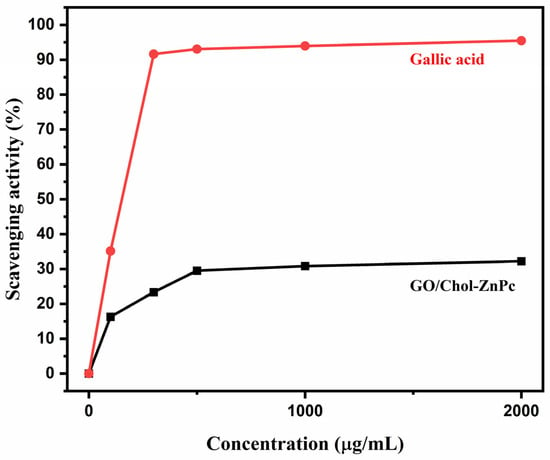
Figure 10.
DPPH free radical scavenging activity of GO/Chol-ZnPc and gallic acid standard.
4. Conclusions
The present work involves preparation of Chol-ZnPc and GO/Chol-ZnPc, and investigation of their PDT properties. Briefly, Chol-phthalonitrile was synthesized first, and then Chol-ZnPc was prepared through the tetramerization reaction of Chol-phthalonitrile and ZnCl2. Following this, GO/Chol-ZnPc composites were prepared using the self-assembly method. The samples were characterized by 1H-NMR, FT-IR, UV–Vis, SEM, DPPH, MALDI-TOF MS, and elemental analysis. Further, fluorescence quantum yield and singlet oxygen quantum yield of the Chol-ZnPc and GO/Chol-ZnPc were also determined for PDT applications. The results show that Chol-ZnPc exhibited a slightly lower singlet oxygen generation ability (Φ∆ = 0.54) than unsubstituted ZnPc. However, it is likely that Chol substitution might improve the photosensitizer uptake of the cancer cells, considering that the encapsulation of photosensitizers to liposome-based nanoparticles is a common drug targeting strategy in the field. This is because it is known that cancer cells attract more Chol than normal for proliferation by expressing high LDL receptors, and the Chol substitution might provide a better binding between the photosensitizer and LDL following the release of the Pcs during lipid exchange of liposomes with lipoproteins in the targeted delivery of photosensitizers by using liposome-based nanoparticles. Also, we report that preparation of GO/Chol-ZnPc resulted in the simultaneous enhancement of ΦF and ΦΔ to 0.36 and 0.78, respectively. This is particularly important as a combination of high ΦF and ΦΔ is desired for image-guided PDT applications, despite a high ΦF often being an indicator of low ΦΔ. In addition, the antioxidant activity of the GO/Chol-ZnPc composite was also determined using DPPH radical scavenging assay, considering the diverse information in the literature regarding the cytotoxicity of GO, and the results of the DPPH tests suggest that GO/Chol-ZnPc did not exhibit a significant antioxidant activity. Overall, it could be thought that GO/Chol-ZnPc composites might have the potential to provide combined advantages for PDT applications, particularly in terms of image guidance and singlet oxygen generation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16227060/s1, Figure S1. SEM-EDX spectrum of Chol-ZnPc.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The author is grateful to Ebru Yabaş for insightful suggestions, and for her help during the synthesis and NMR analysis.
Conflicts of Interest
The author declares no conflict of interest.
References
- Borzecka, W.; Dominski, A.; Kowalczuk, M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials 2021, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Yabaş, E.; Erden, F. Water-Soluble Quaternized Serotonin Substituted Zinc-Phthalocyanine for Photodynamic Therapy Applications. Cumhur. Sci. J. 2023, 44, 99–105. [Google Scholar] [CrossRef]
- Lin, J.; Li, D.; Li, C.; Zhuang, Z.; Chu, C.; Ken Ostrikov, K.; Thompson, E.W.; Liu, G.; Wang, P. A review on reactive oxygen species (ROS)-inducing nanoparticles activated by uni- or multi-modal dynamic treatment for oncotherapy. Nanoscale 2023, 15, 11813–11833. [Google Scholar] [CrossRef] [PubMed]
- Bonacin, J.A.; Engelmann, F.M.; Severino, D.; Toma, H.E.; Baptista, M.S. Singlet Oxygen Quantum Yields (φd) in Water using Beetroot Extract and an Array of LEDs. J. Braz. Chem. Soc. 2009, 20, 31–36. [Google Scholar] [CrossRef]
- Ben-Hur, E.; Rosenthal, I. The phthalocyanines: A new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 47, 145–147. [Google Scholar] [CrossRef]
- Yabaş, E.; Şahin-Bölükbaşı, S.; Şahin-İnan, Z.D. New water soluble magnesium phthalocyanine as a potential anticancer drug: Cytotoxic and apoptotic effect on different cancer cell lines. J. Porphyr. Phthalocyanines 2021, 26, 65–77. [Google Scholar] [CrossRef]
- Sekkat, N.; van den Bergh, H.; Nyokong, T.; Lange, N. Like a bolt from the blue: Phthalocyanines in biomedical optics. Molecules 2011, 17, 98–144. [Google Scholar] [CrossRef]
- Jiang, Z.; Shao, J.; Yang, T.; Wang, J.; Jia, L. Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy. J. Pharm. Biomed. Anal. 2014, 87, 98–104. [Google Scholar] [CrossRef]
- Makhseed, S.; Machacek, M.; Alfadly, W.; Tuhl, A.; Vinodh, M.; Simunek, T.; Novakova, V.; Kubat, P.; Rudolf, E.; Zimcik, P. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem. Commun. 2013, 49, 11149–11151. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Nitzsche, B.; Ma, A.; Atilla, D.; Gürek, A.G.; Höpfner, M. Photodynamic therapy of hepatocellular carcinoma using tetra-triethyleneoxysulfonyl zinc phthalocyanine as photosensitizer. J. Photochem. Photobiol. B Biol. 2020, 208, 111915. [Google Scholar] [CrossRef]
- Kuzyniak, W.; Ermilov, E.A.; Atilla, D.; Gurek, A.G.; Nitzsche, B.; Derkow, K.; Hoffmann, B.; Steinemann, G.; Ahsen, V.; Hopfner, M. Tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine for photodynamic cancer therapy. Photodiagn. Photodyn. Ther. 2016, 13, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhou, J.; Huang, D.; Wang, X.; Zhang, B.; Shen, J. Synthesis and Type I/Type II photosensitizing properties of a novel amphiphilic zinc phthalocyanine. Dye. Pigment. 2006, 71, 61–67. [Google Scholar] [CrossRef]
- Idowu, M.; Nyokong, T. Photophysical and photochemical properties of zinc and aluminum phthalocyanines in the presence of magnetic fluid. J. Photochem. Photobiol. A Chem. 2007, 188, 200–206. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, J.; Xu, S.; Zhou, Y.; Zhu, L.; Xiang, Y.; Xia, D. The effect of a hydrogen bond on the supramolecular self-aggregation mode and the extent of metal-free benzoxazole-substituted phthalocyanines. New J. Chem. 2015, 39, 5750–5758. [Google Scholar] [CrossRef]
- de la Escosura, A.; Martínez-Díaz, M.V.; Thordarson, P.; Rowan, A.E.; Nolte, R.J.M.; Torres, T. Donor−Acceptor Phthalocyanine Nanoaggregates. J. Am. Chem. Soc. 2003, 125, 12300–12308. [Google Scholar] [CrossRef]
- Ginevra, F.; Biffanti, S.; Pagnan, A.; Biolo, R.; Reddi, E.; Jori, G. Delivery of the tumour photosensitizer zinc(II)-phthalocyanine to serum proteins by different liposomes: Studies in vitro and in vivo. Cancer Lett. 1990, 49, 59–65. [Google Scholar] [CrossRef]
- Luo, T.; Nash, G.T.; Xu, Z.; Jiang, X.; Liu, J.; Lin, W. Nanoscale Metal-Organic Framework Confines Zinc-Phthalocyanine Photosensitizers for Enhanced Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 13519–13524. [Google Scholar] [CrossRef]
- Ceron Jayme, C.; Rezende, N.; Fernandes, D.S.; B de Paula, L.; Gimenes de Castro, B.; Takahashi, L.A.U.; Tedesco, A.C. Target selectivity of cholesterol-phosphatidylcholine liposome loaded with phthalocyanine for breast cancer diagnosis and treatment by photodynamic therapy. Photodiagn. Photodyn. Ther. 2022, 39, 102992. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Huang, Y.; He, Y.; Sun, X.; Fu, X.; Xu, X.; Wei, G.; Chen, D.; Zhao, C. Phthalocyanine-based coordination polymer nanoparticles for enhanced photodynamic therapy. Nanoscale 2017, 9, 15883–15894. [Google Scholar] [CrossRef]
- Nunes, S.M.T.; Sguilla, F.S.; Tedesco, A.C. Photophysical studies of zinc phthalocyanine and chloroaluminum phthalocyanine incorporated into liposomes in the presence of additives. Braz. J. Med. Biol. Res. 2004, 37, 273–284. [Google Scholar] [CrossRef]
- Reddi, E.; Cernuschi, S.; Biolo, R.; Jori, G. Liposome- or LDL-administered Zn(ll)-phthalocyanine as a Photodynamic Agent for Tumours III, Effect of Cholesterol on Pharmacokinetic and Phototherapeutic Properties. Lasers Med. Sci. 1990, 5, 339–343. [Google Scholar] [CrossRef]
- de Oliveira, C.A.; Kohn, L.K.; Antonio, M.A.; Carvalho, J.E.; Moreira, M.R.; Machado, A.E.; Pessine, F.B. Photoinactivation of different human tumor cell lines and sheep red blood cells in vitro by liposome-bound Zn(II) Phthalocyanine: Effects of cholesterol. J. Photochem. Photobiol. B 2010, 100, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Kabesova, M.; Benes, J.; Pouckova, P.; Vetvicka, D. Advances in Liposome-Encapsulated Phthalocyanines for Photodynamic Therapy. Life 2023, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Derycke, A.S.; de Witte, P.A. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef]
- Versluis, A.J.; Rensen, P.C.N.; Kuipers, M.E.; Love, W.G.; Taylor, P.W. Interaction between zinc(II)-phthalocyanine-containing and human low density lipoprotein. J. Photochem. Photobiol. B Biol. 1994, 23, 141–148. [Google Scholar] [CrossRef]
- Polo, L.; Valduga, G.; Jori, G.; Reddi, E. Low-density lipoprotein receptors in the uptake of tumour photosensitizers by human and rat transformed fibroblasts. Int. J. Biochem. Cell Biol. 2002, 34, 10–23. [Google Scholar] [CrossRef]
- Renno, R.Z.; Miller, J.W. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv. Drug Deliv. Rev. 2001, 52, 63–78. [Google Scholar] [CrossRef]
- Allison, B.A.; Crespo, M.T.; Jain, A.K.; Richter, A.M.; Hsiang, Y.N.; Levy, J.G. Delivery of benzoporphyrin derivative, a photosensitizer, into atherosclerotic plaque of Watanabe heritable hyperlipidemic rabbits and balloon-injured New Zealand rabbits. Photochem. Photobiol. 1997, 65, 877–883. [Google Scholar] [CrossRef]
- Segallal, A.; Milanesil, C.; Joril, G.; Capraro, H.-G.; Isele, U.; Schieweck, K. CGP 55398, a liposomal Ge(IV) phthalocyanine bearing two axially ligated cholesterol moieties: A new potential agent for photodynamic therapy of tumours. Br. J. Cancer 1994, 69, 817–825. [Google Scholar] [CrossRef]
- Pucelik, B.; Gurol, I.; Ahsen, V.; Dumoulin, F.; Dabrowski, J.M. Fluorination of phthalocyanine substituents: Improved photoproperties and enhanced photodynamic efficacy after optimal micellar formulations. Eur. J. Med. Chem. 2016, 124, 284–298. [Google Scholar] [CrossRef]
- Mayengbam, S.S.; Singh, A.; Pillai, A.D.; Bhat, M.K. Influence of cholesterol on cancer progression and therapy. Transl. Oncol. 2021, 14, 101043. [Google Scholar] [CrossRef] [PubMed]
- Halimi, H.; Farjadian, S. Cholesterol: An important actor on the cancer immune scene. Front. Immunol. 2022, 13, 1057546. [Google Scholar] [CrossRef]
- Yan, A.; Jia, Z.; Qiao, C.; Wang, M.; Ding, X. Cholesterol metabolism in drug-resistant cancer (Review). Int. J. Oncol. 2020, 57, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Axmann, M.; Sezgin, E.; Karner, A.; Novacek, J.; Brodesser, M.D.; Rohrl, C.; Preiner, J.; Stangl, H.; Plochberger, B. Receptor-Independent Transfer of Low Density Lipoprotein Cargo to Biomembranes. Nano Lett. 2019, 19, 2562–2567. [Google Scholar] [CrossRef] [PubMed]
- Bennis, F.; Favre, G.; Gaillard, F.L.; Soula, G. Importance of mevalonate-derived products in the control of HMG-CoA reductase activity and growth of human lung adenocarcinoma cell line a549. Int. J. Cancer 1993, 55, 640–645. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Dong, S.; Chen, J.; Xie, S.; Ma, D.; Chen, L.; Yang, H.; Huang, Y.; Peng, Y. Distribution, Trafficking, and in Vitro Photodynamic Therapy Efficacy of Cholesterol Silicon(IV) Phthalocyanine and Its Nanoparticles in Breast Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 5976–5984. [Google Scholar] [CrossRef]
- Yabaş, E.; Şenadım-Tüzemen, E.; Kaya, S.; Maslov, M.M.; Erden, F. Incorporation of graphene oxide to metal-free phthalocyanine through hydrogen bonding for optoelectronic applications: An experimental and computational study. J. Phys. Org. Chem. 2023, e4496. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, P.; Gao, Y.; Yu, S.; Yang, Y. Preparation and third order nonlinear optical properties of corrole functionalized GO nanohybrids. Opt. Laser Technol. 2022, 149, 107813. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagn. Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hu, Q.; Wang, F.; Xu, Z.-X. Synthesis of N-CuMe2Pc nanorods/graphene oxide nanocomposite for symmetric supercapacitor electrode with excellent cyclic stability. Electrochim. Acta 2019, 298, 770–777. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, Y.; Shi, D. Graphene-based nanovehicles for photodynamic medical therapy. Int. J. Nanomed. 2015, 10, 2451–2459. [Google Scholar]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N. Graphene oxide–metallophthalocyanine hybrids with enhanced singlet oxygen generation. Mater. Res. Bull. 2019, 114, 45–51. [Google Scholar] [CrossRef]
- Tahershamsi, L.; Gerasymchuk, Y.; Wedzynska, A.; Ptak, M.; Tretyakova, I.; Lukowiak, A. Synthesis, Spectroscopic Characterization and Photoactivity of Zr(IV) Phthalocyanines Functionalized with Aminobenzoic Acids and Their GO-Based Composites. C 2020, 6, 1. [Google Scholar] [CrossRef]
- Günsel, A.; Mutlu, N.; Yaşa Atmaca, G.; Günsel, H.; Bilgiçli, A.T.; Erdoğmuş, A.; Nilüfer Yarasir, M. Novel Graphene Oxide/Zinc Phthalocyanine Composites Bearing 3-Chloro-4-Fluorophenoxy: Potential Usage for Sono/Photochemical Applications. ChemistrySelect 2023, 8, e202204546. [Google Scholar] [CrossRef]
- Günsel, A.; Küçük, M.C.; Günsel, H.; Yaşa Atmaca, G.; Bilgiçli, A.T.; Erdoğmuş, A.; Yarasir, M.N. 3-Chloro-4-fluorophenoxy substituted zinc phthalocyanine/graphene oxide composites: Exploring of their sono-photochemical properties. Chem. Pap. 2023, 77, 5721–5731. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Fang, W.; Liu, W.; Le, K.; Xu, D.; Li, X. Fluorescent Phthalocyanine–Graphene Conjugate with Enhanced NIR Absorbance for Imaging and Multi-Modality Therapy. ACS Appl. Nano Mater. 2018, 1, 2785–2795. [Google Scholar] [CrossRef]
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef]
- Popanda, B.; Grolik, J.; Gieszczyk, W.; Środa, M. Effect of the sol-gel condition on photostability and optical properties of alkoxy-substituted zinc phthalocyanine in the hybrid glass matrix. Dye. Pigment. 2023, 213, 111142. [Google Scholar] [CrossRef]
- Yüzeroğlu, M.; Keser Karaoğlan, G.; Gümrükçü Köse, G.; Erdoğmuş, A. Synthesis of new zinc phthalocyanines including schiff base and halogen; photophysical, photochemical, and fluorescence quenching studies. J. Mol. Struct. 2021, 1238, 130423. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ahmetali, E.; Galstyan, A.; Süer, N.C.; Eren, T.; Şener, M.K. Poly(oxanorbornene)s bearing triphenylphosphonium and PEGylated zinc(ii) phthalocyanine with boosted photobiological activity and singlet oxygen generation. Polym. Chem. 2023, 14, 259–267. [Google Scholar] [CrossRef]
- Duncan, D.C.; Whitten, D.G. 1H NMR Investigation of the Composition, Structure, and Dynamics of Cholesterol−Stilbene Tethered Dyad Organogels. Langmuir 2000, 16, 6445–6452. [Google Scholar] [CrossRef]
- Yabaş, E.; Sülü, M.; Saydam, S.; Dumludağ, F.; Salih, B.; Bekaroğlu, Ö. Synthesis, characterization and investigation of electrical and electrochemical properties of imidazole substituted phthalocyanines. Inorganica Chim. Acta 2011, 365, 340–348. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Wan, Y.; Cong, T.; Liang, Q.; Li, Z.; Xu, S.; Peng, Y.; Lu, D. Facile in-situ solvothermal method to synthesize ZnPc–MWCNTs composites with enhanced visible light photocatalytic activity. Ceram. Int. 2016, 42, 2425–2430. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Liang, Q.; Zhou, M.; Xu, S.; Li, Z.; Sun, D. Novel tetrasubstituted zinc phthalocyanine-attapulgite composites for efficient catalytic oxidation of styrene with tert-butyl hydroperoxide as oxidant. Solid State Sci. 2019, 97, 106010. [Google Scholar] [CrossRef]
- Wang, F.; Cao, S.; Men, J.; Lei, N.; Wang, R. Phthalocyanine-modified surfactant-encapsulated polyoxometalate and its self-assembly in solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125056. [Google Scholar] [CrossRef]
- Karakılıç, E.; Alım, Z.; Günel, A.; Baran, A. A versatile study of novel A3B-type unsymmetric zinc(II) phthalocyanines containing thiazolidin-4-one: Their, carbonic anhydrase I, II isoenzymes, and xanthine oxidase inhibitors evaluation. J. Mol. Struct. 2022, 1257, 132630. [Google Scholar] [CrossRef]
- Erdoğmuş, A.; Durmuş, M.; Uğur, A.L.; Avciata, O.; Avciata, U.; Nyokong, T. Synthesis, photophysics, photochemistry and fluorescence quenching studies on highly soluble substituted oxo-titanium(IV) phthalocyanine complexes. Synth. Met. 2010, 160, 1868–1876. [Google Scholar] [CrossRef]
- Hernández, A.; Hemasiri, N.H.; Kazim, S.; Ortiz, J.; Ahmad, S.; Sastre-Santos, Á. Fluorinated- and non-fluorinated-diarylamine-Zn(ii) and Cu(ii) phthalocyanines as symmetrical vs. asymmetrical hole selective materials. J. Mater. Chem. C 2023, 11, 8243–8253. [Google Scholar] [CrossRef]
- Tackley, D.R.; Dent, G.; Ewen Smith, W. IR and Raman assignments for zinc phthalocyanine from DFT calculations. Phys. Chem. Chem. Phys. 2000, 2, 3949–3955. [Google Scholar] [CrossRef]
- Ren, P.G.; Yan, D.X.; Ji, X.; Chen, T.; Li, Z.M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Xing, W.; Wang, G.; Cui, H.; Zhuo, S.; Xue, Q.; Yan, Z.; Qiao, S.Z. High-rate capacitive performance of graphene aerogel with a superhigh C/O molar ratio. J. Mater. Chem. 2012, 22, 23186–23193. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Fan, W.; Peng, G.; Qu, M. Effects of functional groups of graphene oxide on the electrochemical performance of lithium-ion batteries. RSC Adv. 2015, 5, 90041–90048. [Google Scholar] [CrossRef]
- Oluwole, D.O.; Sari, F.A.; Prinsloo, E.; Dube, E.; Yuzer, A.; Nyokong, T.; Ince, M. Photophysicochemical properties and photodynamic therapy activity of highly water-soluble Zn(II) phthalocyanines. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 236–243. [Google Scholar] [CrossRef]
- Guleryuz, B.; Unal, U.; Gulsoy, M. Near infrared light activated upconversion nanoparticles (UCNP) based photodynamic therapy of prostate cancers: An in vitro study. Photodiagn. Photodyn. Ther. 2021, 36, 102616. [Google Scholar] [CrossRef]
- Yabaş, E.; Bağda, E.; Bağda, E. The water soluble ball-type phthalocyanine as new potential anticancer drugs. Dye. Pigment. 2015, 120, 220–227. [Google Scholar] [CrossRef]
- Günsel, A.; Kırbaç, E.; Tüzün, B.; Erdoğmuş, A.; Bilgiçli, A.T.; Yarasir, M.N. Selective chemosensor phthalocyanines for Pd2+ ions; synthesis, characterization, quantum chemical calculation, photochemical and photophysical properties. J. Mol. Struct. 2019, 1180, 127–138. [Google Scholar] [CrossRef]
- Zu, Y.; He, C.; Liu, D.; Chen, L. Synthesis of hybrid structures based on metal phthalocyanines/graphene oxide towards nonlinear optical applications. Dye. Pigment. 2020, 173, 107841. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Song, W.; Gao, Y.; Chen, Z.; Dong, Y.; Zhao, C.; Li, Z.; Wu, Y. The effect of peripheral substituents attached to phthalocyanines on the third order nonlinear optical properties of graphene oxide–zinc(ii)phthalocyanine hybrids. RSC Adv. 2015, 5, 94144–94154. [Google Scholar] [CrossRef]
- Li, Z.; He, C.; Wang, Z.; Gao, Y.; Dong, Y.; Zhao, C.; Chen, Z.; Wu, Y.; Song, W. Ethylenediamine-modified graphene oxide covalently functionalized with a tetracarboxylic Zn(ii) phthalocyanine hybrid for enhanced nonlinear optical properties. Photochem. Photobiol. Sci. 2016, 15, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Markad, G.B.; Padma, N.; Chadha, R.; Gupta, K.C.; Rajarajan, A.K.; Deb, P.; Kapoor, S. Mutual influence on aggregation and magnetic properties of graphene oxide and copper phthalocyanine through non-covalent, charge transfer interaction. Appl. Surf. Sci. 2020, 505, 144624. [Google Scholar] [CrossRef]
- Yabaş, E. New cobalt phthalocyanine–graphene oxide hybrid nanomaterial prepared by strong π–π interactions. J. Aust. Ceram. Soc. 2021, 58, 63–70. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Lavabre, D. Are Fluorescence Quantum Yields So Tricky to Measure? A Demonstration Using Familiar Stationery Products. J. Chem. Educ. 1999, 76, 1260. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Chen, J.-Y.; Nyokong, T. Photophysical and photochemical studies of zinc(ii) phthalocyanine derivatives—Effects of substituents and solvents. New J. Chem. 2004, 28, 822–827. [Google Scholar] [CrossRef]
- Yang, W.; Yang, G.; Hu, W.; Li, M.-Y.; Liu, Z.-Y.; Yu, D.-J.; Liao, Y.-H.; Liu, H.-Y. Photodynamic antitumor activity of halogenated gallium(III) and phosphorus(V) corroles. J. Photochem. Photobiol. A Chem. 2023, 438, 114580. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Yu, X.; Zhang, T.; Ye, Q.; Xiao, W.; Chen, L.; Huang, B.; Peng, Y. Copper-cysteamine nanoparticles encapsulating fluorocoumarin silicon(IV) phthalocyanines: Synthesis, characterization, and photophysical properties. J. Coord. Chem. 2019, 72, 3589–3601. [Google Scholar] [CrossRef]
- Ozturk Gunduz, E.; Tasasiz, B.; Gedik, M.E.; Gunaydin, G.; Okutan, E. NI-BODIPY-GO Nanocomposites for Targeted PDT. ACS Omega 2023, 8, 8320–8331. [Google Scholar] [CrossRef]
- Lutkus, L.V.; Rickenbach, S.S.; McCormick, T.M. Singlet oxygen quantum yields determined by oxygen consumption. J. Photochem. Photobiol. A Chem. 2019, 378, 131–135. [Google Scholar] [CrossRef]
- Mai, D.K.; Kim, C.; Lee, J.; Vales, T.P.; Badon, I.W.; De, K.; Cho, S.; Yang, J.; Kim, H.J. BODIPY nanoparticles functionalized with lactose for cancer-targeted and fluorescence imaging-guided photodynamic therapy. Sci. Rep. 2022, 12, 2541. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Wang, H.Y.; Chen, Z.; Wu, F.G. Subcellular Fate of a Fluorescent Cholesterol-Poly(ethylene glycol) Conjugate: An Excellent Plasma Membrane Imaging Reagent. Langmuir 2016, 32, 10126–10135. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.B.; Ishii, K.; Makino, A.; Iwabuchi, K.; Yamaji-Hasegawa, A.; Senoh, Y.; Nagaoka, I.; Sakuraba, H.; Kobayashi, T. Distribution and transport of cholesterol-rich membrane domains monitored by a membrane-impermeant fluorescent polyethylene glycol-derivatized cholesterol. J. Biol. Chem. 2004, 279, 23790–23796. [Google Scholar] [CrossRef]
- Ishiwata, H.; Sato, S.B.; Vertut-Doi, A.; Miyajima, K. Cholesterol derivative of poly(ethylene glycol) inhibits clathrin-independent, but not clathrin-dependent endocytosis. Biochim. Biophys. Acta 1997, 1359, 123–135. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Chen, Y.; Song, M.; Huang, M.; Xue, J.; Liu, L.; Li, J. Probing the interactions of phthalocyanine-based photosensitizers with model phospholipid bilayer by molecular dynamics simulations. J. Porphyr. Phthalocyanines 2018, 22, 764–770. [Google Scholar] [CrossRef]
- Galstyan, A. Turning Photons into Drugs: Phthalocyanine-Based Photosensitizers as Efficient Photoantimicrobials. Chemistry 2021, 27, 1903–1920. [Google Scholar] [CrossRef]
- Liang, S.; Shephard, K.; Pierce, D.T.; Zhao, J.X. Effects of a nanoscale silica matrix on the fluorescence quantum yield of encapsulated dye molecules. Nanoscale 2013, 5, 9365–9373. [Google Scholar] [CrossRef]
- Khoza, P.; Antunes, E.; Chen, J.-Y.; Nyokong, T. Synthesis and photophysicochemical studies of a water soluble conjugate between folic acid and zinc tetraaminophthalocyanine. J. Lumin. 2013, 134, 784–790. [Google Scholar] [CrossRef]
- Zou, J.; Yin, Z.; Wang, P.; Chen, D.; Shao, J.; Zhang, Q.; Sun, L.; Huang, W.; Dong, X. Photosensitizer synergistic effects: D–A–D structured organic molecule with enhanced fluorescence and singlet oxygen quantum yield for photodynamic therapy. Chem. Sci. 2018, 9, 2188–2194. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Jue Bae, Y.; Krzyaniak, M.D.; Majewski, M.B.; Desroches, M.; Morin, J.F.; Wu, Y.L.; Wasielewski, M.R. Competition between Singlet Fission and Spin-Orbit-Induced Intersystem Crossing in Anthanthrene and Anthanthrone Derivatives. Chempluschem 2019, 84, 1432–1438. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Pan, G.; Han, W.; Wang, B.; Cui, L.; Ma, H.; An, Z.; Xie, Z.; Xu, B.; et al. Exploiting radical-pair intersystem crossing for maximizing singlet oxygen quantum yields in pure organic fluorescent photosensitizers. Chem. Sci. 2020, 11, 10921–10927. [Google Scholar] [CrossRef] [PubMed]
- Ossola, R.; Jonsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen Quantum Yields in Environmental Waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef]
- Matshitse, R.; Nyokong, T. Singlet Oxygen Generating Properties of Different Sizes of Charged Graphene Quantum Dot Nanoconjugates with a Positively Charged Phthalocyanine. J. Fluoresc. 2018, 28, 827–838. [Google Scholar] [CrossRef]
- Nwahara, N.; Britton, J.; Nyokong, T. Improving singlet oxygen generating abilities of phthalocyanines: Aluminum tetrasulfonated phthalocyanine in the presence of graphene quantum dots and folic acid. J. Coord. Chem. 2017, 70, 1601–1616. [Google Scholar] [CrossRef]
- Nwahara, N.; Achadu, O.J.; Nyokong, T. In-situ synthesis of gold nanoparticles on graphene quantum dots-phthalocyanine nanoplatforms: First description of the photophysical and surface enhanced Raman scattering behaviour. J. Photochem. Photobiol. A Chem. 2018, 359, 131–144. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Wei, Y.; Tao, L.; Li, J.; Hu, W.; Zhang, X. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelos, K. Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Gies, V.; Lopinski, G.; Augustine, J.; Cheung, T.; Kodra, O.; Zou, S. The impact of processing on the cytotoxicity of graphene oxide. Nanoscale Adv. 2019, 1, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Ioannides, N.; Trzebicka, B.; Hampel, S.; Rümmeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 1–26. [Google Scholar] [CrossRef]
- Fiorillo, M.; Verre, A.F.; Iliut, M.; Peiris-Pagés, M.; Ozsvari, B.; Gandara, R.; Cappello, A.R.; Sotgia, F.; Vijayaraghavan, A.; Lisanti, M.P. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget 2015, 6, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Casais-Molina, M.L.; Cab, C.; Canto, G.; Medina, J.; Tapia, A. Carbon Nanomaterials for Breast Cancer Treatment. J. Nanomater. 2018, 2018, 2058613. [Google Scholar] [CrossRef]
- Patnala, K.; Vishwas, S.; Malla, R.R. Chapter 17—Nanotechnology advances in breast cancer. In A Theranostic and Precision Medicine Approach for Female-Specific Cancers; Malla, R.R., Nagaraju, G.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 271–287. [Google Scholar]
- Kumar, D.; Rawat, D.S. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett. 2013, 23, 641–645. [Google Scholar]
- Formagio, A.S.; Volobuff, C.R.; Santiago, M.; Cardoso, C.A.; Vieira Mdo, C.; Valdevina Pereira, Z. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Hossain, S.; Rahaman, A.; Nahar, T.; Basunia, M.A.; Mowsumi, F.R.; Uddin, B.; Shahriar, M.; Mahmud, I. Syzygium cumini (L.) skeels seed extract ameliorates in vitro and in vivo oxidative potentials of the brain cerebral cortex of alcohol-treated rats. Orient. Pharm. Exp. Med. 2012, 12, 59–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).