Electrospun Nanofibers: Shaping the Future of Controlled and Responsive Drug Delivery
Abstract
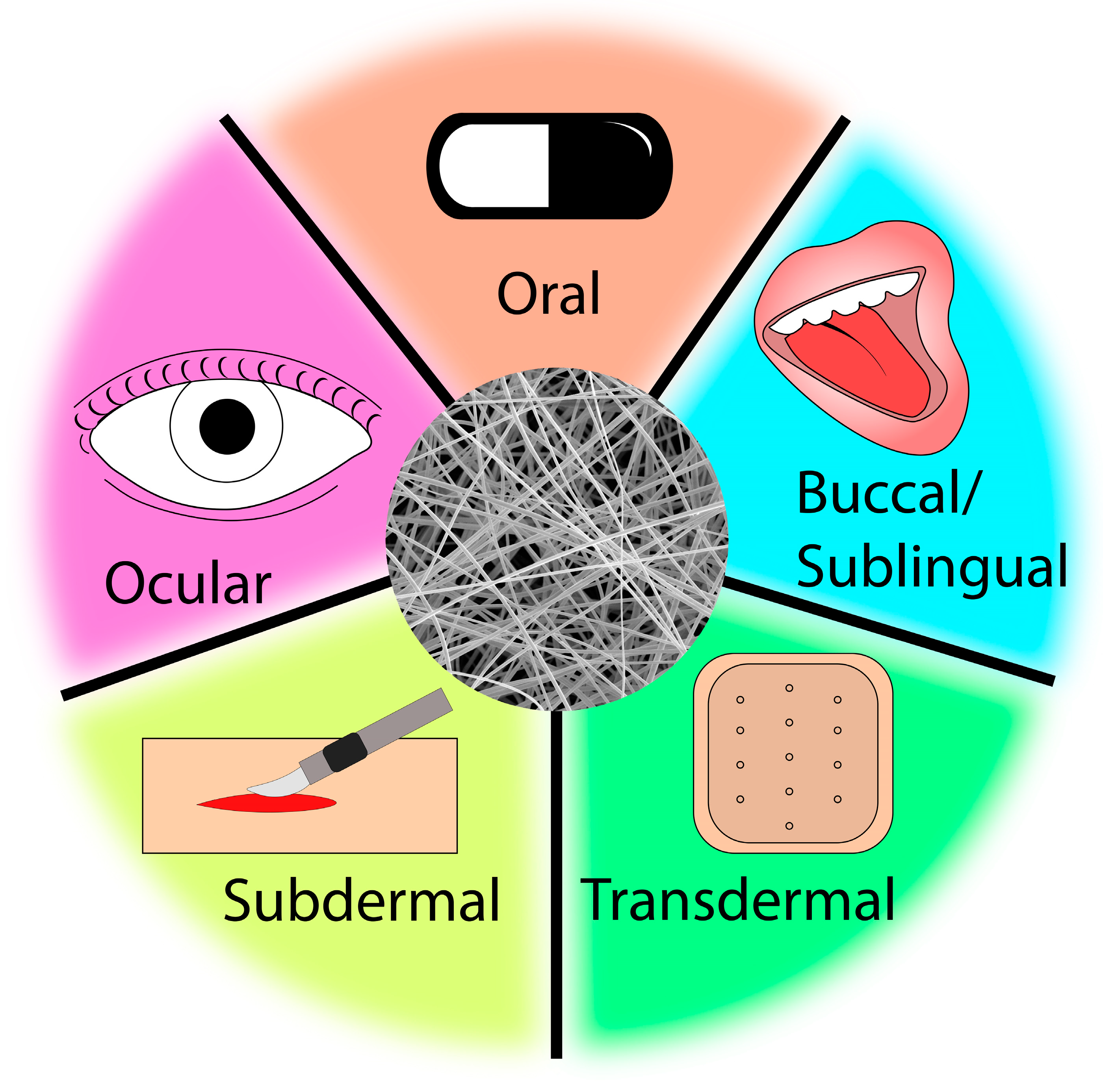
:1. Introduction
1.1. Electrospun Drug Delivery Systems
1.2. Pharmacology and Controlled Release
1.3. Electrospinning
1.4. Biopolymers
2. Controlled Release Electrospun Drug Delivery Systems
2.1. Electrospun Nanofibers for Drug Delivery
2.2. Chemotherapy Drugs
2.3. Biocompatibility
3. Advanced Controlled Release Drug Delivery Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled release of multiple epidermal induction factors through core–shell nanofibers for skin regeneration. Eur. J. Pharm. BioPharm. 2013, 85, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.B.; Punia, A.; Sellers, S.; Kadakia, P.; Ormes, J.D.; Khawaja, N.N.; Wylie, J.; Lamm, M.S. Effect of drug incorporation and polymer properties on the characteristics of electrospun nanofibers for drug delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103112. [Google Scholar] [CrossRef]
- Yu, D.-G.; Yu, J.-H.; Chen, L.; Williams, G.R.; Wang, X. Modified coaxial electrospinning for the preparation of high-quality ketoprofen-loaded cellulose acetate nanofibers. Carbohydr. Polym. 2012, 90, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, S.p.; Sinha-Ray, S.; Sinha-Ray, S.; Kristl, J.; Yarin, A.L. Controlled Release of Ciprofloxacin from Core–Shell Nanofibers with Monolithic or Blended Core. Mol. Pharm. 2016, 13, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Xia, Y. Maneuvering the Internal Porosity and Surface Morphology of Electrospun Polystyrene Yarns by Controlling the Solvent and Relative Humidity. Langmuir 2013, 29, 7070–7078. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Jin, J.; He, S.; Sun, W.; Zhong, C.; Dokmeci, M.R.; Khademhosseini, A.; et al. Chitin nanofiber micropatterned flexible substrates for tissue engineering. J. Mater. Chem. B 2013, 1, 4217–4224. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Feng, Y.; Huang, Z.-M.; Ramakrishna, S.; Lim, C.T. Fabrication of porous electrospun nanofibres. Nanotechnology 2006, 17, 901. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Gil, A.L.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef]
- Carson, D.; Jiang, Y.; Woodrow, K.A. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm. Res. 2016, 33, 125–136. [Google Scholar] [CrossRef]
- Rasekh, M.; Karavasili, C.; Soong, Y.L.; Bouropoulos, N.; Morris, M.; Armitage, D.; Li, X.; Fatouros, D.G.; Ahmad, Z. Electrospun PVP–indomethacin constituents for transdermal dressings and drug delivery devices. Int. J. Pharm. 2014, 473, 95–104. [Google Scholar] [CrossRef]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Dijck, A.V.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Godakanda, V.U.; Li, H.; Alquezara, L.; Zhao, L.; Zhu, L.-M.; Silva, R.d.; Silvab, K.M.N.d.; Williamsa, G.R. Tunable drug release from blend poly(vinyl pyrrolidone)-ethyl cellulose nanofibers. Int. J. Pharm. 2019, 562, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Gorain, M.; Inamdar, S.; Kundu, G.; Badiger, M. In Vivo Wound Healing Performance of Halloysite Clay and Gentamicin-Incorporated Cellulose Ether-PVA Electrospun Nanofiber Mats. ACS Appl. Bio Mater. 2019, 2, 4324–4334. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Z.; Liu, S.; Zhou, D.; Lu, X.; Jing, X.; Huang, Y. Biphasic drug release from electrospun polyblend nanofibers for optimized local cancer treatment. Biomater. Sci. 2018, 6, 324–331. [Google Scholar] [CrossRef]
- Doustgani, A. Doxorubicin release from optimized electrospun polylactic acid nanofibers. J. Ind. Text. 2017, 47, 71–88. [Google Scholar] [CrossRef]
- Qiu, K.; He, C.; Feng, W.; Wang, W.; Zhou, X.; Yin, Z.; Chen, L.; Wang, H.; Moabc, X. Doxorubicin-loaded electrospun poly(L-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J. Mater. Chem. B. 2013, 1, 4601–4611. [Google Scholar] [CrossRef]
- Pardo-Figuerez, M.; Teno, J.; Lafraya, A.; Prieto, C.; Lagaron, J.M. Development of an Electrospun Patch Platform Technology for the Delivery of Carvedilol in the Oral Mucosa. Nanomaterials 2022, 12, 438. [Google Scholar] [CrossRef]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles—Important step towards effective mucosal vaccines. J. Control. Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- Nazari, K.; Kontogiannidou, E.; Ahmad, R.H.; Gratsani, A.; Rasekh, M.; Sunar, M.S.A.B.S.; Armitage, D.; Bouropoulos, N.; Chang, M.-W.; Li, X.; et al. Development and characterisation of cellulose based electrospun mats for buccal delivery of non steroidal anti-inflammatory drug (NSAID). Eur. J. Pharm. Sci. 2017, 102, 147–155. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Tort, S.; Acartürk, F. Electrospun metronidazole-loaded nanofibers for vaginal drug delivery. Drug Dev. Ind. Pharm. 2020, 46, 1015–1025. [Google Scholar] [CrossRef]
- Baskakova, A.; Awwad, S.; Jiménez, J.Q.; Gill, H.; Novikov, O.; Khaw, P.T.; Brocchini, S.; Zhilyakova, E.; Williams, G.R. Electrospun formulations of acyclovir, ciprofloxacin and cyanocobalamin for ocular drug delivery. Int. J. Pharm. 2016, 502, 208–218. [Google Scholar] [CrossRef]
- Göttel, B.; Silva, J.M.d.S.e.; Oliveira, C.S.d.; Syrowatka, F.; Fiorentzis, M.; Viestenz, A.; Viestenz, A.; Mäder, K. Electrospun nanofibers—A promising solid in-situ gelling alternative for ocular drug delivery. Eur. J. Pharm. BioPharm. 2020, 146, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Shen, X.-X.; Branford-White, C.; White, K.; Zhu, L.-M.; Bligh, S.W.A. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology 2009, 20, 055104. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Barker, S.A.; Craig, D.Q.M. Design and Characterization of Cyclosporine A-Loaded Nanofibers for Enhanced Drug Dissolution. ACS Omega 2020, 5, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Crowley, P.J. Controlled Release in Oral Drug Delivery; Rathbone, M.J., Ed.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.W.A.; Williams, G.R. Nanofibers Fabricated Using Triaxial Electrospinning as Zero Order Drug Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef]
- Ghafoor, B.; Aleem, A.; Ali, M.N.; Mir, M. Review of the fabrication techniques and applications of polymeric electrospun nanofibers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 48, 82–87. [Google Scholar] [CrossRef]
- Liao, D.; Liu, Z.; Wrasidlo, W.; Chen, T.; Luo, Y.; Xiang, R.; Reisfeld, R.A. Synthetic enzyme inhibitor: A novel targeting ligand for nanotherapeutic drug delivery inhibiting tumor growth without systemic toxicity. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 665–673. [Google Scholar] [CrossRef]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbljtts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H.N. On the Receiving End-Patient Perception of the Side-effects of Cancer Chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef]
- Lopez, F.L.; Shearman, G.C.; Gaisford, S.; Williams, G.R. Amorphous Formulations of Indomethacin and Griseofulvin Prepared by Electrospinning. Mol. Pharm. 2014, 4, 4327–4338. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Patel, D.; Wei, W.; Singh, H.; Xu, K.; Beck, C.; Wildy, M.; Schossig, J.; Hu, X.; Hyun, D.C.; Chen, W.; et al. Efficient and Secure Encapsulation of a Natural Phase Change Material in Nanofibers Using Coaxial Electrospinning for Sustainable Thermal Energy Storage. ACS Sustain. Chem. Eng. 2023, 11, 11570–11579. [Google Scholar] [CrossRef] [PubMed]
- Amarjargala, A.; Brunellia, M.; Fortunatoa, G.; Spanoa, F.; Kim, C.S.; Rossia, R.M. On-demand drug release from tailored blended electrospun nanofibers. J. Drug Deliv. Sci. Technol. 2019, 52, 8–14. [Google Scholar] [CrossRef]
- Batistaa, H.; Freitasb, J.P.; Abrunheiro, A.; Gonçalves, T.; Gil, M.H.; Figueiredo, M.; Coimbra, P. Electrospun composite fibers of PLA/PLGA blends and mesoporous silica nanoparticles for the controlled release of gentamicin sulfate. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 635–646. [Google Scholar] [CrossRef]
- Munj, H.R.; Lannutti, J.J.; Tomasko, D.L. Understanding drug release from PCL/gelatin electrospun blends. J. Biomater. Appl. 2017, 3, 933–949. [Google Scholar] [CrossRef]
- Schossig, J.; Gandotra, A.; Arizapana, K.; Weber, D.; Wildy, M.; Wei, W.; Xu, K.; Yu, L.; Chimenti, R.; Mantawy, I.; et al. CO2 to Value-Added Chemicals: Synthesis and Performance of Mono- and Bimetallic Nickel–Cobalt Nanofiber Catalysts. Catalysts 2023, 13, 1017. [Google Scholar] [CrossRef]
- Ghaban, R.; Duong, J.; Patel, D.; Singh, H.; Chen, W.; Lu, P. Solvent-Assisted Nanochannel Encapsulation of a Natural Phase Change Material in Polystyrene Hollow Fibers for High-Performance Thermal Energy Storage. ACS Appl. Energy Mater. 2020, 3, 10089–10096. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Focarete, M.L.; Gualandi, C.; Ramakrishna, S. (Eds.) Filtering Media by Electrospinning Next Generation Membranes for Separation Applications; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.-W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun Fibers for the Oral Delivery of Cannabinoids. AAPS PharmSciTech 2021, 22, 23. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef]
- Vass, P.; Démuth, B.; Farkas, A.; Hirsch, E.; Szabó, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous alternative to freeze drying: Manufacturing of cyclodextrinbased reconstitution powder from aqueous solution using scaled-up electrospinning. J. Control. Release 2019, 298, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Wilk, S.; Benko, A. Advances in Fabricating the Electrospun Biopolymer-Based Biomaterials. J. Funct. Biomater. 2021, 12, 26. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2018, 11, 107–117. [Google Scholar] [CrossRef]
- Liu, H.; Tang, C. Electrospinning of Cellulose Acetate in Solvent Mixture N,N-Dimethylacetamide (DMAc)/Acetone. Polym. J. 2007, 39, 65–72. [Google Scholar] [CrossRef]
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Production of a novel poly(ε-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. 2020, 109, 180–192. [Google Scholar] [CrossRef]
- Shukla, S.; Brinley, E.; Cho, H.J.; Seal, S. Electrospinning of hydroxypropyl cellulose fibers and their application in synthesis of nano and submicron tin oxide fibers. Polymer 2005, 46, 12130–12145. [Google Scholar] [CrossRef]
- Kerwald, J.; Junior, C.F.d.M.; Freitas, E.D.; Deus, J.o.d.; Segundo, P.d.M.; Vieira, R.S.; Beppu, M.M. Cellulose-based electrospun nanofibers: A review. Cellulose 2022, 29, 25–54. [Google Scholar] [CrossRef]
- Vueba, M.L.; Carvalho, L.A.E.B.d.; Veiga, F.; Sousa, J.J.; Pina, M.E. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. BioPharm. 2004, 58, 51–59. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Ford, J.L. Chapter 2 Design and Evaluation of Hydroxypropyl Methylcellulose Matrix Tablets for Oral Controlled Release: A Historical Perspective. In Hydrophilic Matrix Tablets for Oral Controlled Release; Timmins, P., Pygall, S.R., Melia, C.D., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhu, C.; Li, J.; Li, H.; Xia, Y. Integration of Phase-Change Materials with Electrospun Fibers for Promoting Neurite Outgrowth under Controlled Release. Adv. Funct. Mater. 2018, 28, 1705563. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, J.; Huang, W.; Jiang, H.; Zimba, B.L.; Chen, L.; Wan, J.; Wu, Q. Preparation of poly(lactic acid)/graphene oxide nanofiber membranes with different structures by electrospinning for drug delivery. RSC Adv. 2018, 8, 16619–16625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hummelga, M.; Lv, G.; Olin, H. Real time monitoring of the drug release of rhodamine B on graphene oxide. Carbon 2010, 49, 1126–1132. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast Dissolving Oral Drug Delivery System Based on Electrospun Nanofibrous Webs of Cyclodextrin/Ibuprofen Inclusion Complex Nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef]
- Um-i-Zahra, S.; Zhu, L. Novel drug loaded duplicate nanofibers and their in-vitro drug release profiles. Am. Res. Throughts 2015, 1, 1683–1698. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-H.; Yu, D.-G. Higher quality quercetin sustained release ethyl cellulose nanofibersfabricated using a spinneret with a Teflon nozzle. Colloids Surf. B Biointerfaces 2014, 114, 404–409. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surf. 2013, 110, 148–155. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.g.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, L.; Gao, Y.; Zhu, Z.-T.; Fong, H. Preparation, characterization, and encapsulation/release studies of a composite nanofiber mat electrospun from an emulsion containing poly(lactic-co glycolic acid). Polymer 2008, 49, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Feng, W.; Lin, S.; He, C.; Gao, Y.; Wang, H. Antitumor efficacy of a PLGA composite nanofiber embedded with doxorubicin@MSNs and hydroxycamptothecin@HANPs. RSC Adv. 2014, 4, 53344. [Google Scholar] [CrossRef]
- Bil, M.; Kijeńska-Gawrońska, E.; Głodkowska-Mrówka, E.; Manda-Handzlik, A.; Mrówka, P. Design and in vitro evaluation of electrospun shape memory polyurethanes for self-fitting tissue engineering grafts and drug delivery systems. Mater. Sci. Eng. C 2020, 110, 110675. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.M.; Sridhar, K.; Jaison, D.; Gopinath, V.; Ibrahim, B.K.M.; Gupta, N.; Sundaram, A.; Sivaperumal, P.; Padmapriya, S.; Patil, S.S. Fabrication of tri-layered electrospun polycaprolactone mats with improved sustained drug release profile. Sci. Rep. 2020, 10, 18179. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Sua, X.; Yanga, Z.; Tana, K.B.; Chenb, J.; Huanga, J.; Li, Q. Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef]
- Jani, G.K.; Gohel, M.C. Effects of selected formulation parameters on the entrapment of diclofenac sodium in ethyl cellulose microspheres. J. Control. Release 1997, 43, 245–250. [Google Scholar] [CrossRef]
- Gunder, W.; Lippold, B.H.; Lippold, B.C. Release of drugs from ethyl cellulose microcapsules (diffusion pellets) with pore formers and pore fusion. Eur. J. Pharm. Sci. 1995, 3, 203–214. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, X.P.; Liu, X.F.; Guan, Y.L.; Yao, K.D. Study on antibacterial O-carboxymethylated chitosan/cellulose blend film from LiCl/NN-dimethylacetamide solution. Polymer 2002, 43, 1541–1547. [Google Scholar] [CrossRef]
- Gençtürk, A.; Kahraman, E.; Güngör, S.; Özsoy, Y.; Sarac, A.S. Effects of Polyvinylpyrrolidone and Ethyl Cellulose in Polyurethane Electrospun Nanofibers on Morphology and Drug Release Characteristics. Turk. J. Pharm. Sci. 2020, 6, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.-H.; Yu, D.-G.; Williams, G.R. Tunable biphasic drug release from ethyl cellulose nanofibers fabricated using a modified coaxial electrospinning process. Nanoscale Res. Lett. 2014, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, M.; Song, W.-L.; Yu, D.-G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biopolymers 2021, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.-Y.; Williams, G.R.; Yang, H.-H.; Tao, L.; Zhu, L.-M. Electrospun Poly(N-isopropylacrylamide)/Ethyl Cellulose Nanofibers as Thermoresponsive Drug Delivery Systems. J. Pharm. Sci. 2016, 105, 1104–1112. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef]
- Ball, C.; Chou, S.-F.; Jiang, Y.; Woodrow, K.A. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mater. Sci. Eng. C 2016, 63, 117–124. [Google Scholar] [CrossRef]
- Um-i-Zahra, S.; Shen, X.X.; Li, H.; Zhu, L. Study of sustained release drug-loaded nanofibers of cellulose acetate and ethyl cellulose polymer blends prepared by electrospinning and their in-vitro drug release profiles. J. Polym. Res. 2014, 21, 602. [Google Scholar] [CrossRef]
- Zaitoon, A.; Lim, L.-T. Effect of poly(ethylene oxide) on the electrospinning behavior and characteristics of ethyl cellulose composite fibers. Materialia 2020, 10, 100649. [Google Scholar] [CrossRef]
- Ahmadian, S.; Ghorbani, M.; Mahmoodzadeh, F. Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int. J. Biol. Macromol. 2020, 162, 1555–1565. [Google Scholar] [CrossRef]
- Li, S.-F.; Wu, J.-H.; Hu, T.-G.; Wu, H. Encapsulation of quercetin into zein-ethyl cellulose coaxial nanofibers: Preparation, characterization and its anticancer activity. Int. J. Biol. Macromol. 2023, 248, 125797. [Google Scholar] [CrossRef]
- Lia, H.; Zhanga, Z.; Godakanda, V.U.; Chiu, Y.-J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; Silva, R.M.d.; Silvac, K.M.N.d.; Zhu, L.-M.; et al. The effect of collection substrate on electrospun ciprofloxacin-loaded poly(vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109917. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.C.; Burnett, E.; Chou, S.-F. Physicomechanical properties and in vitro release behaviors of electrospun ibuprofen-loaded blend PEO/EC fibers. Mater. Today Commun. 2022, 30, 103205. [Google Scholar] [CrossRef]
- Lu, H.; Wanga, Q.; Li, G.; Qiua, Y.; Wei, Q. Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mater. Sci. Eng. C 2017, 74, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Hu, M.-H.; Zhou, W.; Chen, B.-Y.; Wang, X. Electrospun ketoprofen sustained release nanofibers prepared using coaxial electrospinning. Appl. Mech. Mater. 2013, 395–396, 138–143. [Google Scholar] [CrossRef]
- Nouri, A.; Dizaji, B.F.; Kianinejad, N.; Rad, A.J.; Rahimi, S.; Irani, M.; Jazi, F.S. Simultaneous linear release of folic acid and doxorubicin from ethyl cellulose/chitosan/g-C3N4/MoS2 core-shell nanofibers and its anticancer properties. J. Biomed. Mater. Res. 2021, 109, 903–914. [Google Scholar] [CrossRef]
- Tipduangta, P.; Belton, P.; Fábián, L.s.; Wang, L.Y.; Tang, H.; Eddleston, M.; Qi, S. Electrospun Polymer Blend Nanofibers for Tunable Drug Delivery: The Role of Transformative Phase Separation on Controlling the Release Rate. Mol. Pharm. 2015, 13, 25–39. [Google Scholar] [CrossRef]
- Kima, K.; Luu, Y.K.; Changa, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Meredith, A.-M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2015, 68, 729–741. [Google Scholar] [CrossRef]
- Zhao, H.; Chi, H. Electrospun Bead-on-String Fibers: Useless or Something of Value? In Novel Aspects of Nanofibers; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabricating porous poly(lactic acid) fibres via electrospinning. Eur. Polym. J. 2018, 99, 464–476. [Google Scholar] [CrossRef]
- Wang, X.; He, G.; Liu, H.; Zheng, G.; Sun, D. Fabrication and Morphological Control of Electrospun Ethyl Cellulose Nanofibers. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 324–327. [Google Scholar]
- Park, J.Y.; Han, S.W.; Lee, I.H. Preparation of Electrospun Porous Ethyl Cellulose Fiber by THF/DMAc Binary Solvent System. J. Ind. Eng. Chem. 2007, 13, 1002–1008. [Google Scholar]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Chanunpanich, N.; Park, J.S. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012, 439, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, B.; Ahmad, Z.; Huang, J.; Chang, M.-W.; Li, J.-S. Surface modified electrospun porous magnetic hollow fibers using secondary downstream collection solvent contouring. Mater. Lett. 2017, 204, 73–76. [Google Scholar] [CrossRef]
- Wadhwa, J.; Nair, A.; Kumria, R. Emulsion forming drug delivery system for lipophilic drugs. Acta Pol. Pharm. Drug Res. 2012, 69, 179–191. [Google Scholar]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability. Colloids Surf. B Biointerfaces 2018, 167, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xiaoqiang, L.; Shuiping, L.; Xiumei, M.; Ramakrishna, S. Controlled release of dual drugs from emulsion electrospun nanofibrous mats. Colloids Surf. B Biointerfaces 2009, 73, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Zech, J.; Mader, M.; Gündel, D.; Metz, H.; Odparlik, A.; Agarwal, S.; Mäder, K.; Greiner, A. Noninvasive characterization (EPR, μCT, NMR) of 3D PLA electrospun fiber sponges for controlled drug delivery. Int. J. Pharm. X 2020, 2, 100055. [Google Scholar] [CrossRef]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 257, 117631. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, G.; Liu, Z.; Liu, J.; Tang, J.; Xiao, Y.; Xu, W.; Liu, Y.; Chen, C. Direct site-specific treatment of skin cancer using doxorubicin loaded nanofibrous membranes. Sci. Bull. 2018, 63, 92–100. [Google Scholar] [CrossRef]
- Li, J.-J.; Yang, Y.-Y.; Yu, D.-G.; Du, Q.; Yang, X.-L. Fast dissolving drug delivery membrane based on the ultra-thin shell of electrospun core-shell nanofibers. Eur. J. Pharm. Sci. 2018, 122, 195–204. [Google Scholar] [CrossRef]
- Yan, E.; Jiang, J.; Yang, X.; Fan, L.; Wang, Y.; An, Q.; Zhang, Z.; Lu, B.; Wang, D.; Zhang, D. pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101455. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.-s.; Shao, Y.-w.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Coaxial electrospun membranes with thermal energy storage and shape memory functions for simultaneous thermal/moisture management in personal cooling textiles. Eur. Polym. J. 2021, 145, 110245. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Utilising Co-Axial Electrospinning as a Taste-Masking Technology for Paediatric Drug Delivery. Pharmaceutics 2021, 13, 1665. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-K.; Zhang, K.; Gong, Q.; Yu, D.-G.; Wang, J.; Tan, X.; Quana, H. Ethylcellulose-based drug nano depots fabricated using a modified triaxial electrospinning. Int. J. Biol. Macromol. 2020, 152, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Schiffman, J.D. Beyond the Single-Nozzle: Coaxial Electrospinning Enables Innovative Nanofiber Chemistries, Geometries, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Darbasizadeh, B.; Mortazavi, S.A.; Kobarfard, F.; Jaafari, M.R.; Hashemi, A.; Farhadnejad, H.; Feyzi-barnaji, B. Electrospun Doxorubicin-loaded PEO/PCL core/sheath nanofibers for chemopreventive action against breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 64, 102576. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Xin, B.; Li, T. Doxorubicin hydrochloride-loaded electrospun poly(L-lactide-co-ε-caprolactone)/gelatin core–shell nanofibers for controlled drug release. Polym. Int. 2021, 70, 1717–1724. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef]
- Gohary, M.I.E.; Hady, B.M.A.E.; Saeed, A.A.A.; Tolba, E.; Rashedi, A.M.I.E.; Saleh, S. Electrospinning of doxorubicin loaded silica/poly(ε-caprolactone) hybrid fiber mats for sustained drug release. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025002. [Google Scholar] [CrossRef]
- Dai, J.; Jin, J.; Yang, S.; Li, G. Doxorubicin-loaded PLA/pearl electrospun nanofibrous scaffold for drug delivery and tumor cell treatment. Mater. Res. Express 2017, 4, 075403. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, Y.; Cheng, R.; Sheng, L.; Wu, W.; Pan, G.; Feng, Q.; Cu, W. Doxorubicin-loaded mesoporous silica nanoparticle composite nanofibers for longterm adjustments of tumor apoptosis. Nanotechnology 2016, 27, 245101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Hou, J.; Yang, G.; Zhou, S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small 2020, 16, 1902262. [Google Scholar] [CrossRef]
- Jaworsk, J.; Smolarczyk, R.; Musiał-Kulik, M.; Cichoń, T.; Karpeta-Jarząbek, P.; Włodarczyk, J.; Stojko, M.; Janeczek, H.; Kordyka, A.; Kaczmarczyk, B.Z.; et al. Electrospun paclitaxel delivery system based on PGCL/PLGA in local therapy combined with brachytherapy. Int. J. Pharm. 2021, 602, 120596. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Yuan, C.; Long, X.; Li, J.; Cai, Q. Coaxially electrospun 5-fluorouracil-loaded PLGA/PVP fibrous membrane for skin tumor treatment. Biomed. Mater. 2021, 16, 065014. [Google Scholar] [CrossRef]
- Poursharifi, N.; Semnani, D.; Soltani, P.; Amanpour, S. Designing a novel and versatile multi-layered nanofibrous structure loaded with MTX and 5-FU for the targeted delivery of anticancer drugs. Polym. Degrad. Stab. 2020, 179, 109275. [Google Scholar] [CrossRef]
- Jun, E.; Kim, S.C.; Lee, C.M.; Oh, J.; Lee, S.; Shim, I.K. Synergistic effect of a drug loaded electrospun patch and systemic chemotherapy in pancreatic cancer xenograft. Sci. Rep. 2017, 7, 12381. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Gunasekaran, S. Electrospun plant mucilage nanofibers as biocompatible scaffolds for cell proliferation. Int. J. Biol. Macromol. 2018, 115, 1218–1224. [Google Scholar] [CrossRef]
- Mohajer, F.; Ziarani, G.M.; Badiei, A.; Iravani, S.; Varma, R.S. Advanced MXene-Based Micro- and Nanosystems for Targeted Drug Delivery in Cancer Therapy. Micromachines 2022, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, i.; An, J.; Wang, T.; Li, L.; Si, X.; He, L.; Wu, X.; Wang, C.; Su, Z. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014, 50, 100–1002. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Radmansouri, M.; Bahmani, E.; Sarikhani, E.; Rahmani, K.; Sharifianjazi, F.; Irani, M. Doxorubicin hydrochloride—Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol. 2018, 116, 378–384. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Zhao, P.; Yue, Q.; He, H.; Gao, B.; Wang, Y.; Li, Q. Study on phase diagram of fatty acids mixtures to determine eutectic temperatures and the corresponding mixing proportions. Appl. Energy 2014, 115, 483–490. [Google Scholar] [CrossRef]
- Zhu, C.; Huo, D.; Chen, Q.; Xue, J.; Shen, S.; Xia, Y. A Eutectic Mixture of Natural Fatty Acids Can Serve as the Gating Material for Near-Infrared-Triggered Drug Release. Adv. Mater. 2017, 29, 1703702. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Liu, S.-Q.; Heng, P.W.-S.; Yang, Y.-Y. Evaluating proteins release from, and their interactions with, thermosensitive poly (N-isopropylacrylamide) hydrogels. J. Control. Release 2005, 102, 361–372. [Google Scholar] [CrossRef]
- Choi, S.-W.; Zhang, Y.; Xia, Y. A Temperature-Sensitive Drug Release System Based on Phase-Change Materials. Angew. Chem. Int. Ed. 2010, 49, 7904–7908. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Shekaari, H.; Zafarani-Moattar, M.T. Synthesis of nanoencapsulated vitamin E in phase changematerial (PCM) shell as thermo-sensitive drug delivery purpose. J. Mol. Liq. 2020, 320, 114429. [Google Scholar] [CrossRef]
- Pan, F.; Amarjargal, A.; Altenried, S.; Liu, M.; Zuber, F.; Zeng, Z.; Rossi, R.M.; Maniura-Weber, K.; Ren, Q. Bioresponsive Hybrid Nanofibers Enable Controlled Drug Delivery Through Glass Transition Switching. ACS Appl. Bio Mater. 2021, 4, 4271–4279. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xue, J.; Zhu, G.; Liu, H.; Xia, Y. Encapsulation of a Phase-Change Material in Nanocapsules with a Well-Defined Hole in the Wall for the Controlled Release of Drugs. Angew. Chem. 2019, 131, 10716–10721. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Yuan, K.; Zhang, Z.; Zhang, X.; Fang, X. A multi-controlled drug delivery system based on magnetic mesoporous Fe3O4 nanopaticles and a phase change material for cancer thermo-chemotherapy. Nanotechnology 2017, 28, 405101. [Google Scholar] [CrossRef]
- Moon, G.D.; Choi, S.-W.; Cai, X.; Li, W.; Cho, E.C.; Jeong, U.; Wang, L.V.; Xia, Y. A New Theranostic System Based on Gold Nanocages and Phase-Change Materials with Unique Features for Photoacoustic Imaging and Controlled Release. J. Am. Chem. Soc. 2011, 133, 4762–4766. [Google Scholar] [CrossRef]
- Gavila´n, H.; Avugadda, S.K.; Ferna´ndez-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Gopi, C.V.V.M. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Yan, Z.; Yu, Z.J.; Yang, T.; Li, S.; Zhang, G. Impact of ultrasound on the melting process and heat transfer of phase change material. Energy Procedia 2019, 158, 5014–5019. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, H.; Zheng, Y.; Yao, Y.; Li, X.; Yu, J.; Ding, B. Nanofibrous hydrogels embedded with phase-change materials: Temperature-responsive dressings for accelerating skin wound healing. Compos. Commun. 2021, 25, 100752. [Google Scholar] [CrossRef]






| Polymer/Materials | Drug | Release Profile | Ref. |
|---|---|---|---|
| EC | IMC | Sustained | [86] |
| EC | Quercetin | Sustained | [61] |
| EC/CA | IMC | Sustained | [80] |
| EC/PVP | Naproxen (Nap) | Sustained/tunable | [14] |
| EC, PVP | CIF | Sustained | [84] |
| EC/HPMC/Tween 80 | IMC | Rapid | [20] |
| CA | Thymol (THY) | Sustained | [13] |
| PLA | DOX | Sustained | [16] |
| PLA/MSN | DOX | Sustained | [17] |
| PLA/GO | RhB | Controlled | [56] |
| PLGA | CIF | Biphasic | [63] |
| PLA/PLGA/Si nanoparticles | Gentamicin sulfate (GS) | Controlled | [35] |
| PLA/PEO | DOX | Biphasic | [15] |
| PCL | IBU | Sustained | [67] |
| PCL | Carvedilol (CVD) | Controlled/tunable | [18] |
| PCL/PEO/Si nanoparticles | DOX | Sustained | [91] |
| PVP | Cyclosporine A (CA) | Rapid | [25] |
| PVP | Metronidazole (MET) | Rapid | [21] |
| PVP/Eudragit RS100 | CBD, CBG | Rapid | [41] |
| PVA, PVAc | CIF | Rapid | [2] |
| Core | Sheath | Drug | Release Profile | Ref. |
|---|---|---|---|---|
| PVP | EC | Maraviroc | Controlled/tunable | [79] |
| EC | PVP | Quercetin | Biphasic | [75] |
| Zein | EC | Quercetin | Sustained | [83] |
| EC | Ethanol | KET | Sustained | [87] |
| Chitosan | EC/nanosheets | DOX/FA | Sustained | [88] |
| PCL, PVA | PVP | Quercetin, Tamoxifen citrate (TC) | Rapid | [104] |
| PEO | PCL | DOX | Sustained | [110] |
| Carboxymethyl chitosan (CMC) | PCL | DOX | Controlled/Sustained | [102] |
| PLGA/PCL | Gelaton/Genipin | DOX | Controlled/Sustained | [103] |
| DOX | PLCL/Gelatin | DOX | Sustained | [111] |
| Polyethylene Gylcol (PEG) | PLA | Salicylic acid (SA) | Sustained | [96] |
| PEG | PU | |||
| PVA | PMMA | CIP | Sustained | [4] |
| PVA | PCL | DOX | Controlled | [105] |
| Kollicoat® Smartseal | Eudragit® EPO | Chloropheniramine maleate | Rapid | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wildy, M.; Lu, P. Electrospun Nanofibers: Shaping the Future of Controlled and Responsive Drug Delivery. Materials 2023, 16, 7062. https://doi.org/10.3390/ma16227062
Wildy M, Lu P. Electrospun Nanofibers: Shaping the Future of Controlled and Responsive Drug Delivery. Materials. 2023; 16(22):7062. https://doi.org/10.3390/ma16227062
Chicago/Turabian StyleWildy, Michael, and Ping Lu. 2023. "Electrospun Nanofibers: Shaping the Future of Controlled and Responsive Drug Delivery" Materials 16, no. 22: 7062. https://doi.org/10.3390/ma16227062
APA StyleWildy, M., & Lu, P. (2023). Electrospun Nanofibers: Shaping the Future of Controlled and Responsive Drug Delivery. Materials, 16(22), 7062. https://doi.org/10.3390/ma16227062







