Detection of Environmentally Harmful Malathion Pesticides Using a Bimetallic Oxide of CuO Nanoparticles Dispersed over a 3D ZnO Nanoflower
Abstract
:1. Introduction
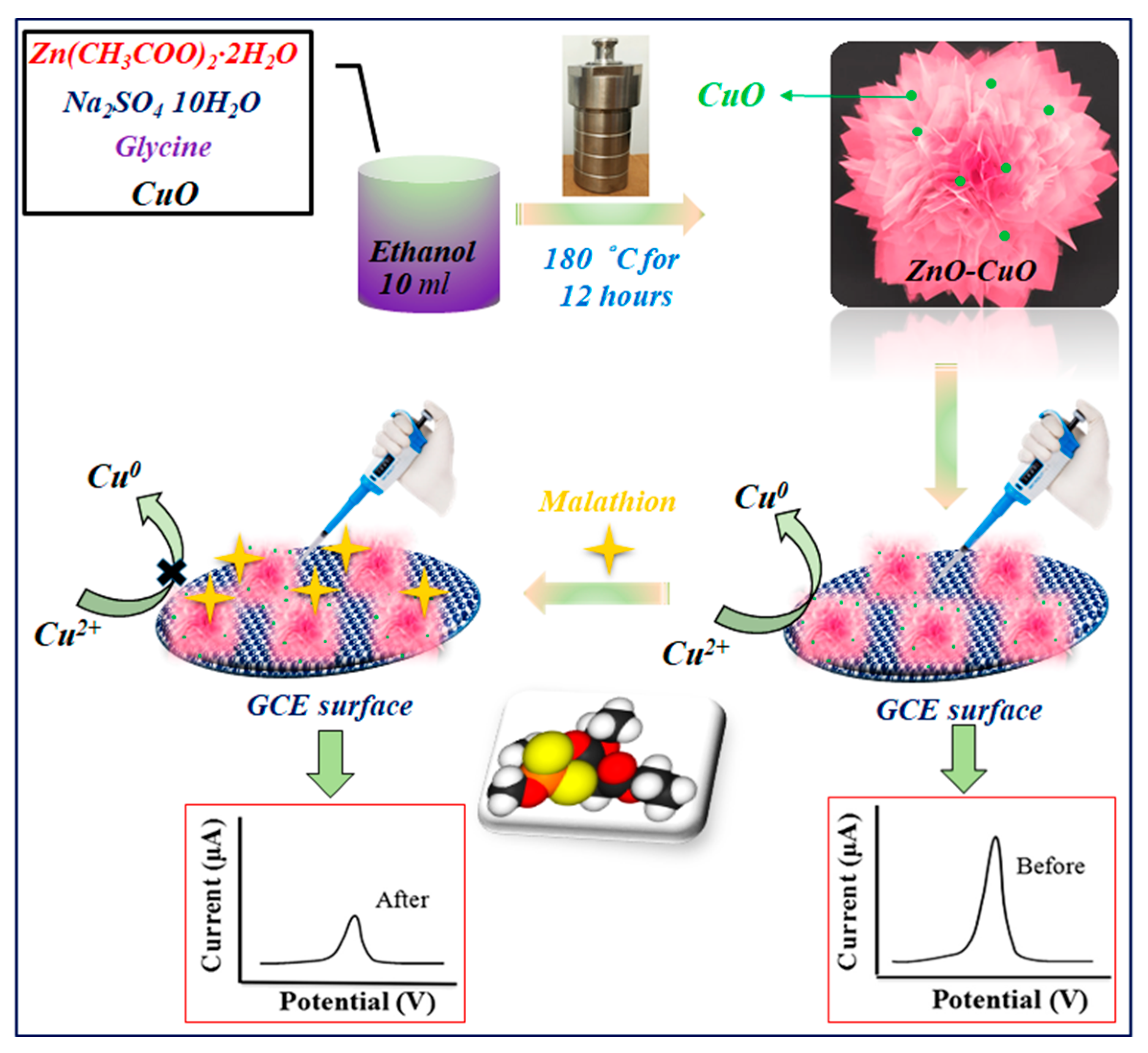
2. Materials and Methods
3. Results and Discussion
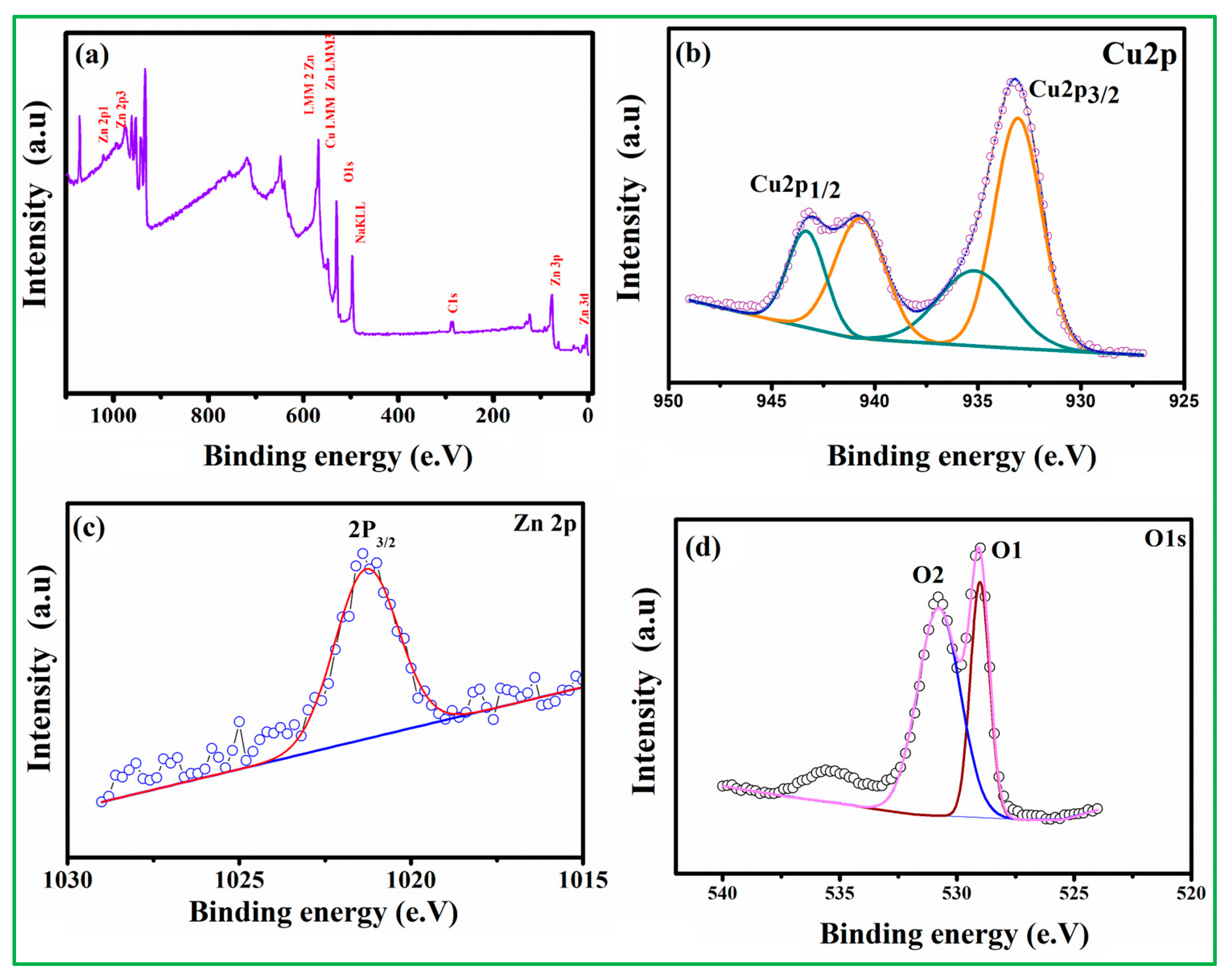
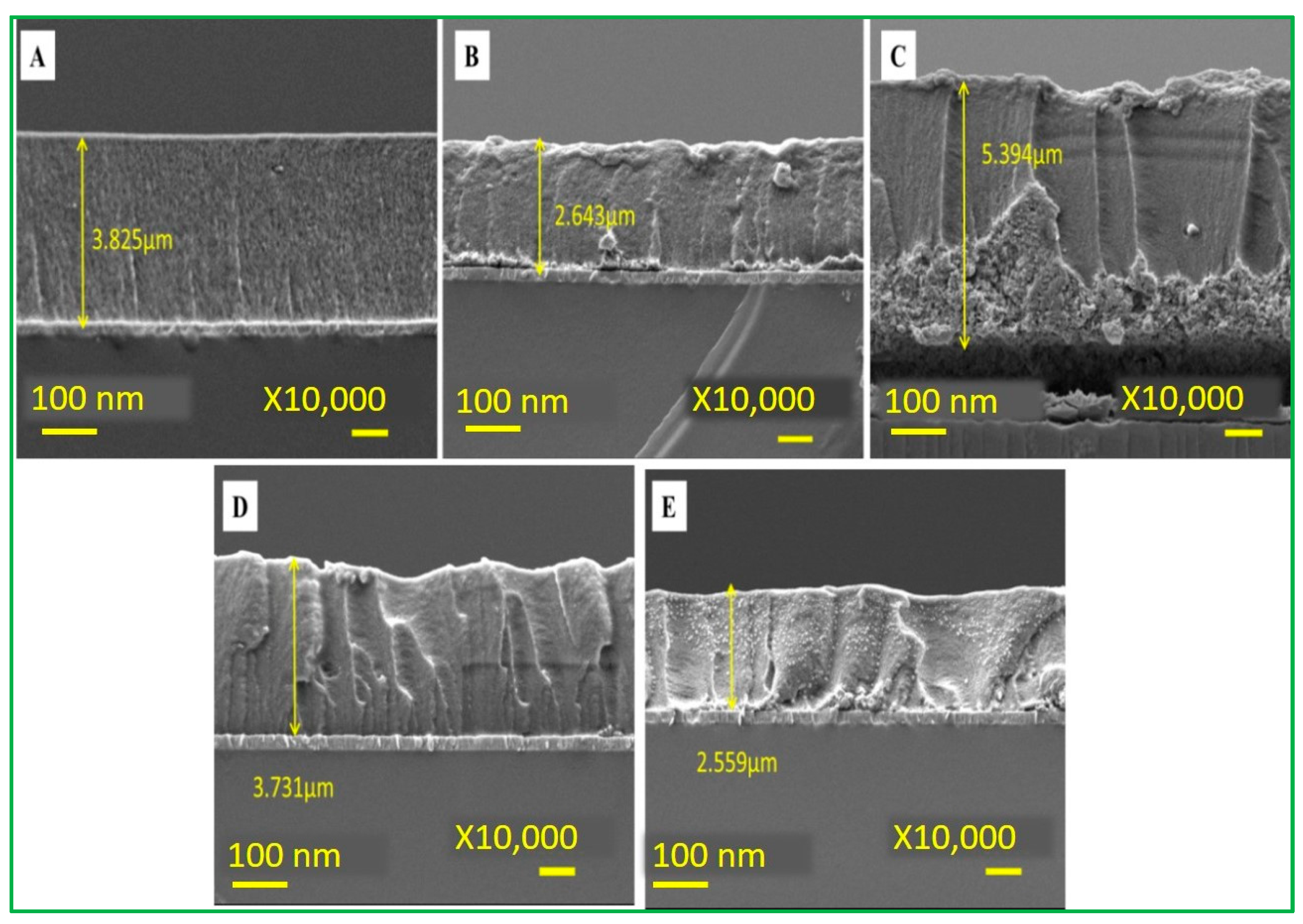
3.1. Characterization of Electrode Materials
3.2. Electrochemical Characteristics of Electrodes
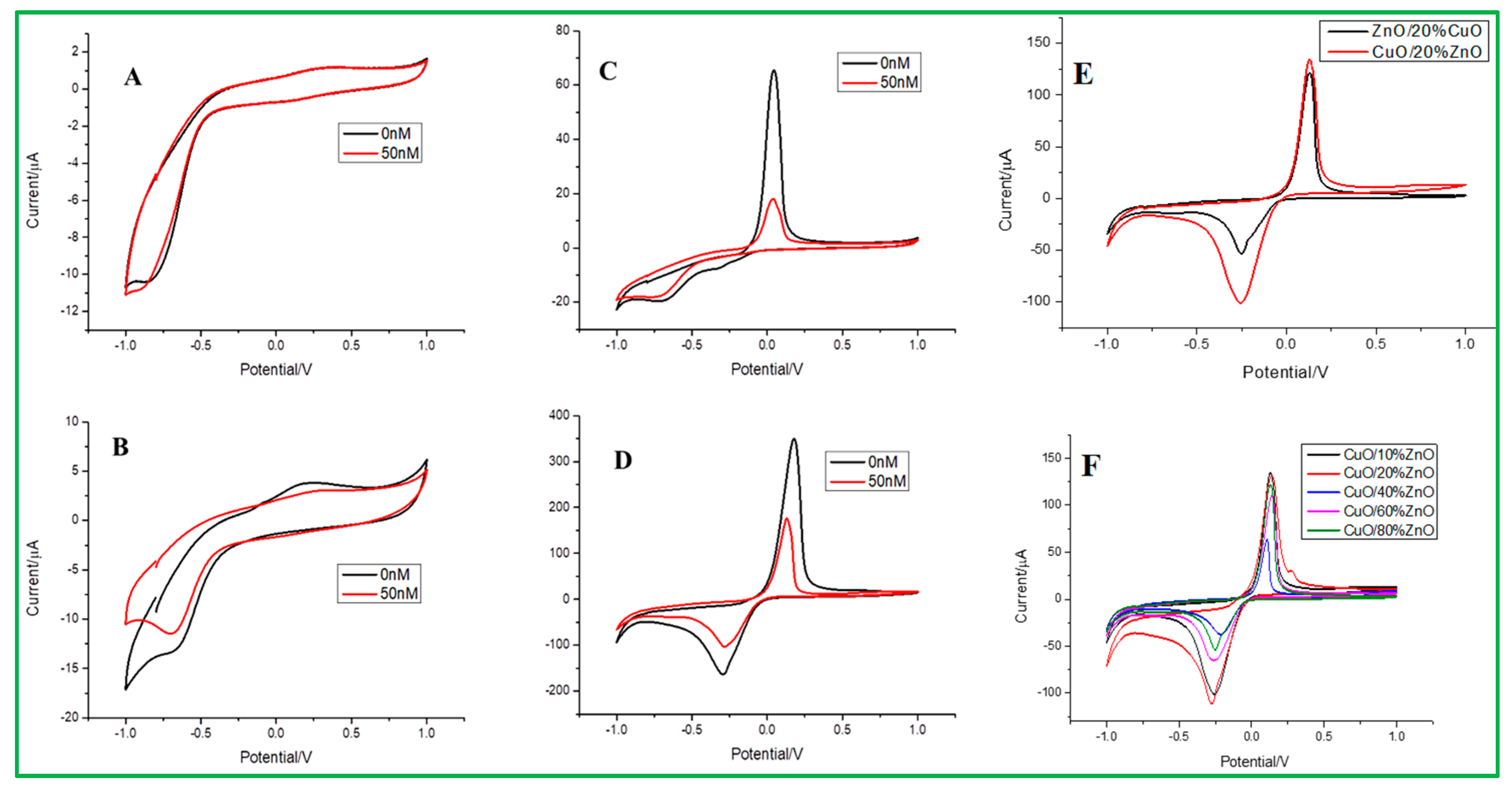
3.3. Cyclic Voltammetry (CV) Test for Malathion Detection by Electrodes
3.4. Different Pulse Voltammetry (DPV) for Malathion Detection
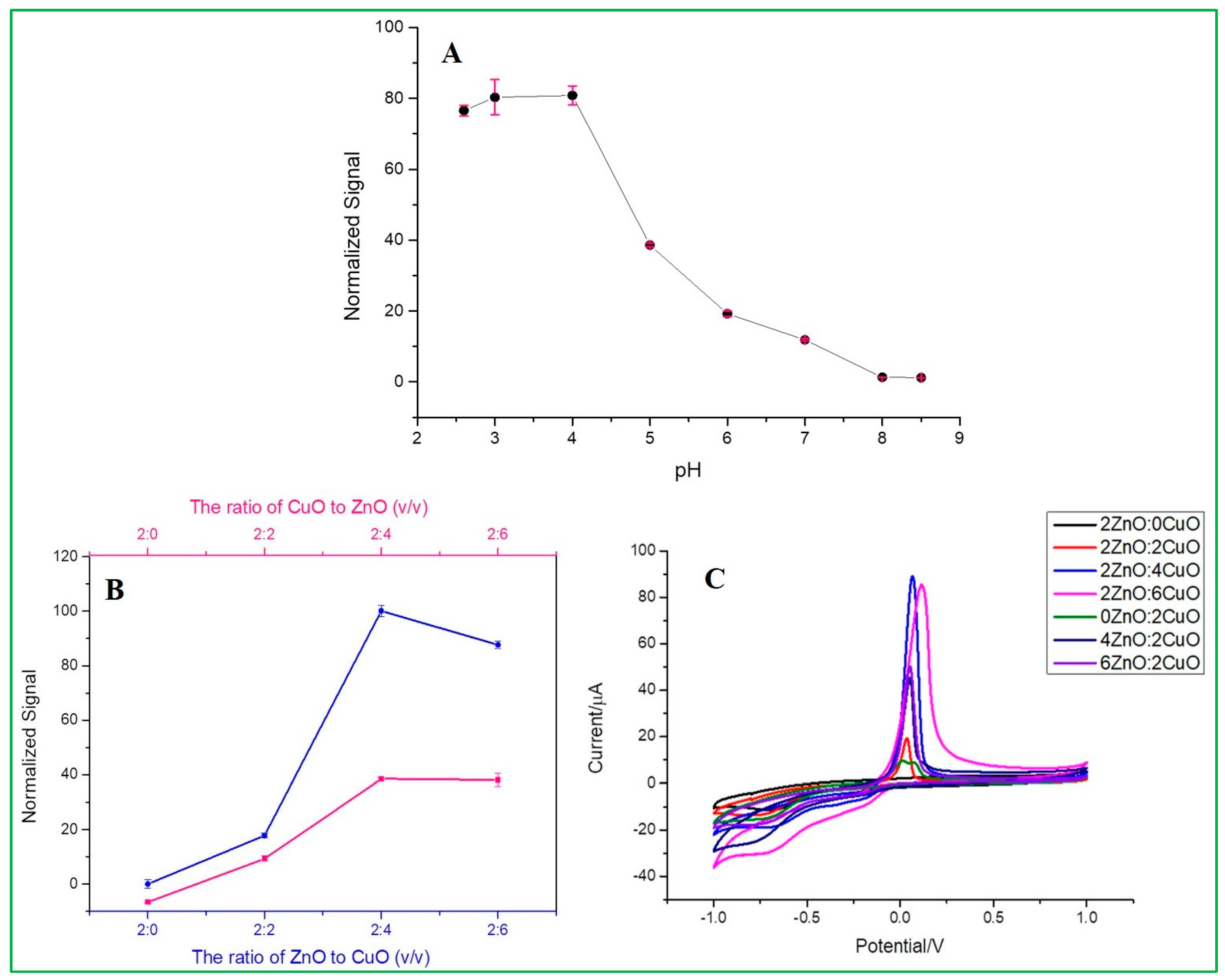
3.5. Optimization of the Experimental Conditions
3.5.1. Effect of pH
3.5.2. Effect of Volume Ratio
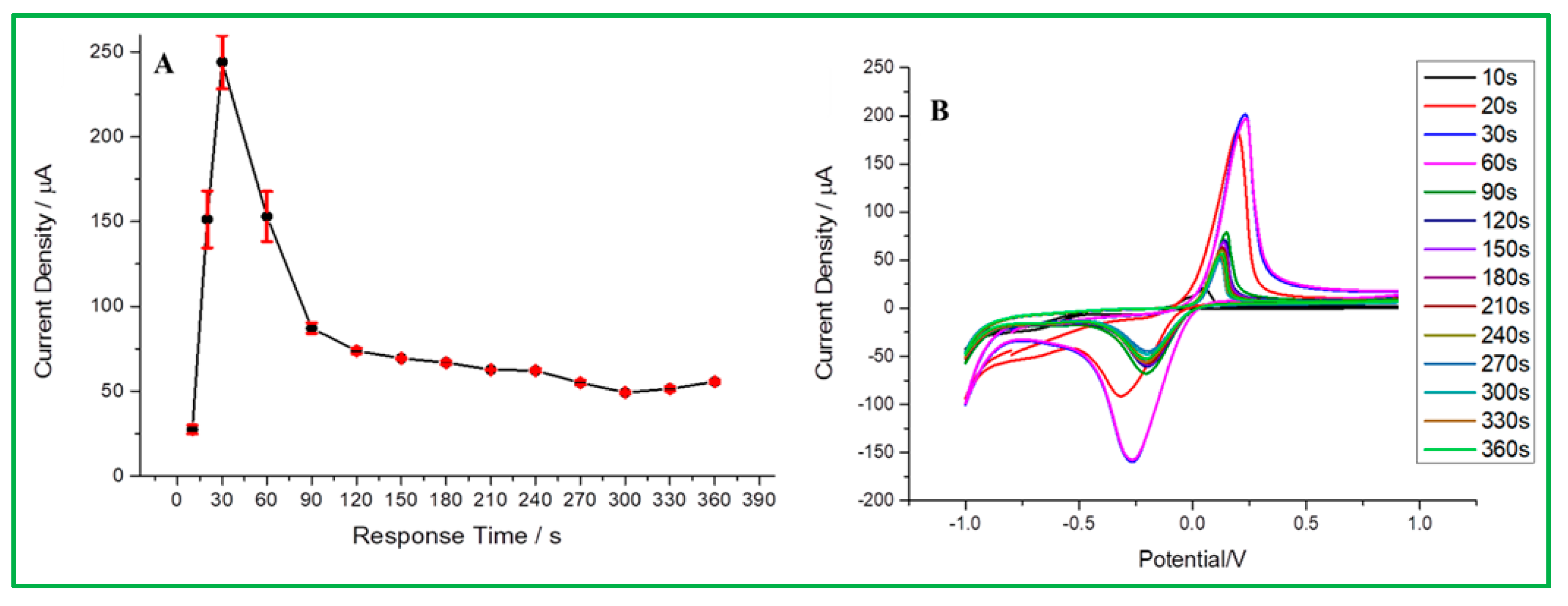
3.5.3. Effect of Different Response Time
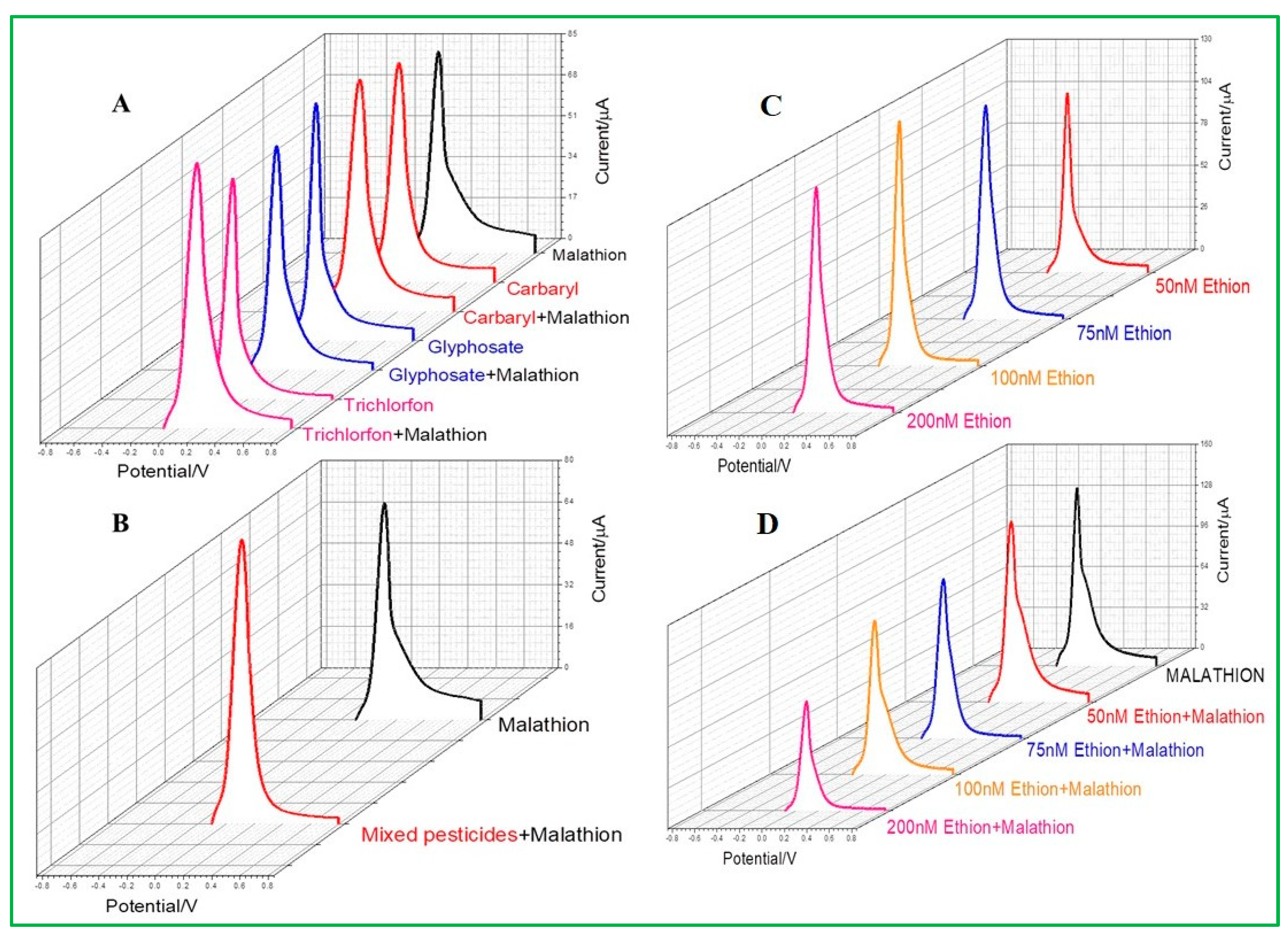
3.6. Selectivity Test
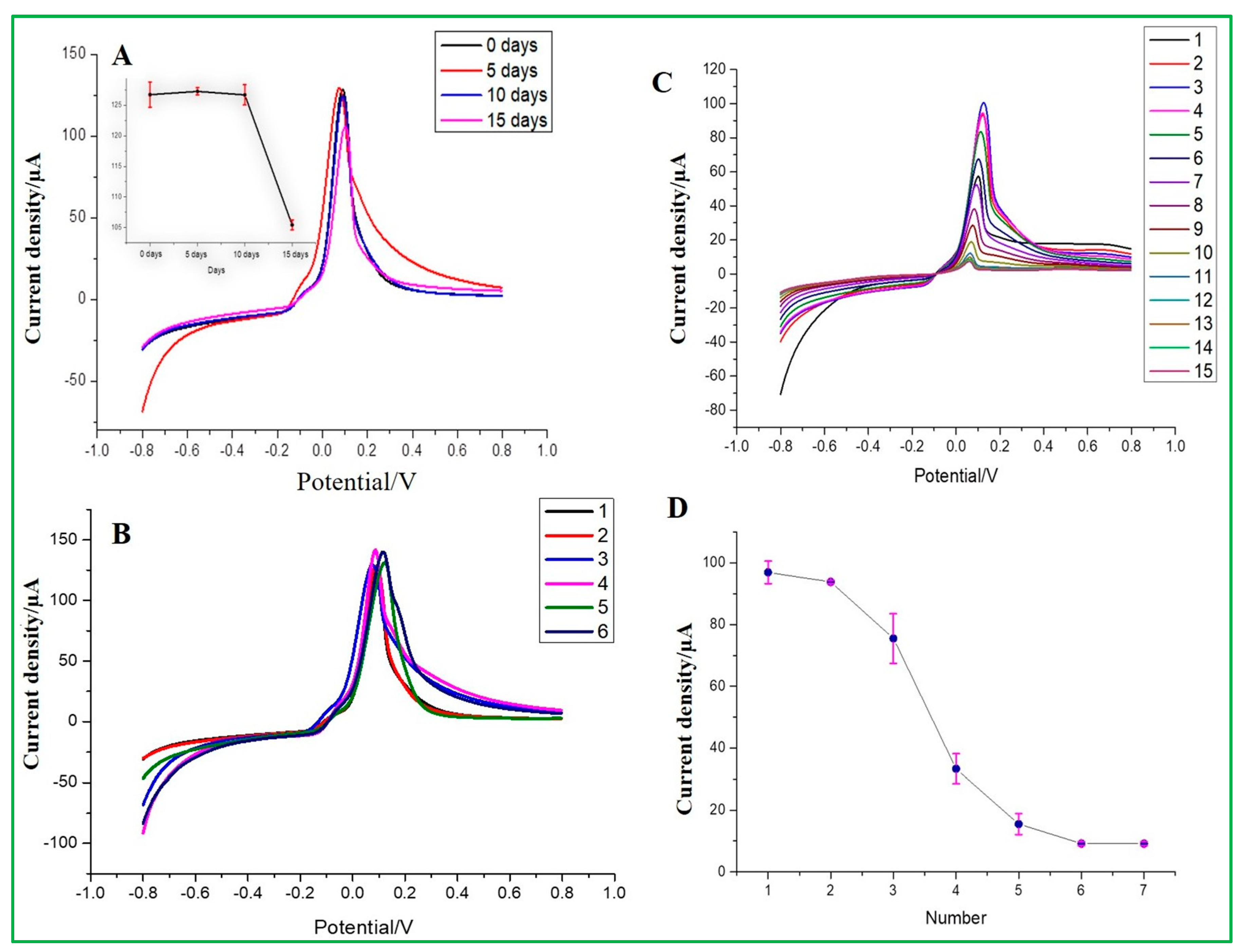
3.7. Reproducibility and Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzei, F.; Botre, F.; Montilla, S.; Pilloton, R.; Podestà, E.; Botrè, C. Alkaline Phosphatase Inhibition Based Electrochemical Sensors for the Detection of Pesticides. J. Electroanal. Chem. 2004, 574, 95–100. [Google Scholar] [CrossRef]
- Hossain, M.I.; Hasnat, M.A. Recent Advancements in Non-Enzymatic Electrochemical Sensor Development for the Detection of Organophosphorus Pesticides in Food and Environment. Heliyon 2023, 9, e19299. [Google Scholar] [CrossRef] [PubMed]
- Irfan Azizan, M.A.; Taufik, S.; Norizan, M.N.; Abdul Rashid, J.I. A Review on Surface Modification in the Development of Electrochemical Biosensor for Malathion. Biosens. Bioelectron. X 2023, 13, 100291. [Google Scholar] [CrossRef]
- Guan, H.; Brewer, W.E.; Garris, S.T.; Morgan, S.L. Disposable Pipette Extraction for the Analysis of Pesticides in Fruit and Vegetables Using Gas Chromatography/Mass Spectrometry. J. Chromatogr. A 2010, 1217, 1867–1874. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, J.; Tian, Y.; Wu, Y.; He, Q.; Li, G.; Liu, J. Non-Enzymatic Electrochemical Sensors Based on Nanomaterials for Detection of Organophosphorus Pesticide Residues. Environ. Sci. Adv. 2023, 2, 933–956. [Google Scholar] [CrossRef]
- Heidarnia, Z.; Parvizi, R.; Khoshsima, H.; Heidari, H. Distinct Absorption Transducing Features of Silica Supported MoO3/PANI Hybrid Coated Optical Fiber towards Malathion Monitoring in Food Samples. Sens. Actuators B Chem. 2022, 371, 132501. [Google Scholar] [CrossRef]
- Ye, J.; Wu, J.; Liu, W. Enantioselective Separation and Analysis of Chiral Pesticides by High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2009, 28, 1148–1163. [Google Scholar] [CrossRef]
- Cai, J.; Du, D. A Disposable Sensor Based on Immobilization of Acetylcholinesterase to Multiwall Carbon Nanotube Modified Screen-printed Electrode for Determination of Carbaryl. J. Appl. Electrochem. 2008, 38, 1217–1222. [Google Scholar] [CrossRef]
- Mogha, N.K.; Sahu, V.; Sharma, M.; Sharma, R.K.; Masram, D.T. Biocompatible ZrO2- Reduced Graphene Oxide Immobilized AChE Biosensor for Chlorpyrifos Detection. Mater. Des. 2016, 111, 312–320. [Google Scholar] [CrossRef]
- He, C.; Yan, R.; Gao, X.; Xue, Q.; Wang, H. Non-Enzymatic Electrochemical Malathion Sensor Based on Bimetallic Cu-Co Metal-Organic Gels Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2023, 385, 133697. [Google Scholar] [CrossRef]
- Pathiraja, G.; Bonner, C.D.J.; Obare, S.O. Recent Advances of Enzyme-Free Electrochemical Sensors for Flexible Electronics in the Detection of Organophosphorus Compounds: A Review. Sensors 2023, 23, 1226. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, Y.; Lu, L.; Ma, X.; Gong, L.; Huang, X.; Liu, G.; Yu, Y. CuO Nanoparticles Decorated 3D Graphene Nanocomposite as Non-Enzymatic Electrochemical Sensing Platform for Malathion Detection. J. Electroanal. Chem. 2018, 812, 82–89. [Google Scholar] [CrossRef]
- Liu, J.; Ye, L.Y.; Zhang, Y.; Yang, H.; Zhou, L.; Luo, E.; Lei, J. Nonenzymatic Target-Driven DNA Nanomachine for Monitoring Malathion Contamination in Living Cells and Bioaccumulation in Foods. Anal. Chem. 2022, 94, 5667–5673. [Google Scholar] [CrossRef] [PubMed]
- Toboonsung, B.; Singjai, P. A Flexible Angle Sensor Made from MWNT/CuO/Cu2O Nanocomposite Films Deposited by an Electrophoretic Co-Deposition Process. J. Alloys Compd. 2012, 533, 62–66. [Google Scholar] [CrossRef]
- Li, T.; Ai, X.P.; Yang, H.X. Reversible Electrochemical Conversion Reaction of Li2O/CuO Nanocomposites and Their Application as High-Capacity Cathode Materials for Li-Ion Batteries. J. Phys. Chem. C 2011, 115, 6167–6174. [Google Scholar] [CrossRef]
- Prasannakumaran Nair Chandrika Kumari, P.; Asadevi, H.; Thekku Veedu, S.; Raghunandan, R. Hydrogen Bond Mediated Turn-on Sensor: Ultra-Sensitive and Label Free Barium-MOF for Probing Malathion an Organophosphate Pesticide. J. Mol. Struct. 2023, 1286, 135542. [Google Scholar] [CrossRef]
- Vidyasagar, C.C.; Naik, Y.A.; Venkatesh, T.G.; Viswanatha, R. Solid-State Synthesis and Effect of Temperature on Optical Properties of Cu–ZnO, Cu–CdO and CuO Nanoparticles. Powder Technol. 2011, 214, 337–343. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.-C.; Zhang, W.-D.; Gunasekaran, S. A Highly Sensitive Non-Enzymatic Glucose Sensor Based on a Simple Two-Step Electrodeposition of Cupric Oxide (CuO) Nanoparticles onto Multi-Walled Carbon Nanotube Arrays. Talanta 2010, 82, 25–33. [Google Scholar] [CrossRef]
- Li, P.; Zhan, H.; Tao, S.; Xie, Z.; Huang, J. Bio-Inspired Aptamers Decorated Gold Nanoparticles Enable Visualized Detection of Malathion. Front. Bioeng. Biotechnol. 2023, 11, 1165724. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Diouf, A.; Österlund, L.; Bouchikhi, B.; ElBari, N. Development of a Molecularly Imprinted Polymer Electrochemical Sensor and Its Application for Sensitive Detection and Determination of Malathion in Olive Fruits and Oils. Bioelectrochemistry 2020, 132, 107404. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A Disposable Electrochemical Sensor for Simultaneous Determination of Norepinephrine and Serotonin in Rat Cerebrospinal Fluid Based on MWNTs-ZnO/Chitosan Composites Modified Screen-Printed Electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Beitollai, H.; Garkani Nejad, F.; Tajik, S.; Jahani, S.; Biparva, P. Voltammetric Determination of Amitriptyline Based on Graphite Screen Printed Electrode Modified with a Copper Oxide Nanoparticles. Inter. J. Nano Dimens. 2017, 8, 197–205. [Google Scholar]
- Chandegara, V.R.; Joshi, P.; Chavda, S.; Oza, H.M.; Parmar, M.; Dhruv, D.; Solanki, P.S.; Pandya, D.D.; Joshi, A.D.; Shah, N.A.; et al. Studies on Properties of Green Synthesised CuO/ZnO Nano Particle/Nano Rod Composites in PVA Matrix. Opt. Mater. 2023, 145, 114369. [Google Scholar] [CrossRef]
- Alsulmi, A.; Mohammed, N.N.; Soltan, A.; Messih, M.F.A.; Ahmed, M.A. Engineering S-Scheme CuO/ZnO Heterojunctions Sonochemically for Eradicating RhB Dye from Wastewater under Solar Radiation. RSC Adv. 2023, 13, 13269–13281. [Google Scholar] [CrossRef]
- George, A.; Magimai Antoni Raj, D.; Venci, X.; Dhayal Raj, A.; Albert Irudayaraj, A.; Josephine, R.L.; John Sundaram, S.; Al-Mohaimeed, A.M.; AlFarraj, D.A.; Chen, T.-W.; et al. Photocatalytic Effect of CuO Nanoparticles Flower-like 3D Nanostructures under Visible Light Irradiation with the Degradation of Methylene Blue (MB) Dye for Environmental Application. Environ. Res. 2022, 203, 111880. [Google Scholar] [CrossRef]
- Nagarani, S.; Sasikala, G.; Yuvaraj, M.; Kumar, R.D.; Balachandran, S.; Kumar, M. ZnO-CuO Nanoparticles Enameled on Reduced Graphene Nanosheets as Electrode Materials for Supercapacitors Applications. J. Energy Storage 2022, 52, 104969. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Jayadevappa, H. CuO Nanoparticle Sensor for the Electrochemical Determination of Dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, M.S. A Novel Structural Specific Creatinine Sensing Scheme for the Determination of the Urine Creatinine. Biosens. Bioelectron. 2012, 31, 90–94. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Žagar, K.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Electrochemical Sensors for the Simultaneous Determination of Zinc, Cadmium and Lead Using a Nafion/Ionic Liquid/Graphene Composite Modified Screen-Printed Carbon Electrode. Anal. Chim. Acta 2016, 918, 26–34. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, H.-Y.; Zeng, Q.-X.; Jia, L.; Wang, Y.; Wang, S.-F. Direct Electrochemistry and Electrocatalysis of Heme Proteins Immobilized in Single-Wall Carbon Nanotubes-Surfactant Films in Room Temperature Ionic Liquids. Electrochem. Commun. 2009, 11, 286–289. [Google Scholar] [CrossRef]
- Basnet, P.; Samanta, D.; Inakhunbi Chanu, T.; Mukherjee, J.; Chatterjee, S. Assessment of Synthesis Approaches for Tuning the Photocatalytic Property of ZnO Nanoparticles. SN Appl. Sci. 2019, 1, 633. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Qureshi, M.S.; Malik, M.M.; Haque, F.Z. Studies of Structural, Optical, and Electrical Properties Associated with Defects in Sodium-Doped Copper Oxide (CuO/Na) Nanostructures. J. Mater. Sci. 2018, 53, 8826–8843. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S. Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors 2018, 18, 773. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Sun, H.; Guo, X.; Xu, J.; Zhang, H.; Zhang, X. In Situ Growth of Cu2O/CuO Nanosheets on Cu Coating Carbon Cloths as a Binder-Free Electrode for Asymmetric Supercapacitors. Front. Chem. 2019, 7, 420. [Google Scholar] [CrossRef]
- Yu, X.; Xuan, Y. Investigation on Thermo-Optical Properties of CuO/Ag Plasmonic Nanofluids. Sol. Energy 2018, 160, 200–207. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Gao, Y.; Xu, X.; Yan, Z.; Ren, N. CuO/ZnO Core/Shell Nanowire Arrays and Their Photovoltaics Application. Mater. Lett. 2014, 132, 409–412. [Google Scholar] [CrossRef]
- Wu, L.Q.; Li, Y.C.; Li, S.Q.; Li, Z.Z.; Tang, G.D.; Qi, W.H.; Xue, L.C.; Ge, X.S.; Ding, L.L. Method for Estimating Ionicities of Oxides Using O1s Photoelectron Spectra. AIP Adv. 2015, 5, 97210. [Google Scholar] [CrossRef]
- Nannan, L.; Fang, Z.; Qiang, X.; Pridgeon, J.W.; Xiwu, G. Behavioral Change, Physiological Modification, and Metabolic Detoxification: Mechanisms of Insecticide Resistance. Kun Chong Xue Bao Acta Entomol. Sin. 2006, 49, 671–679. [Google Scholar]
- Tak, M.; Gupta, V.; Tomar, M. Flower-like ZnO Nanostructure Based Electrochemical DNA Biosensor for Bacterial Meningitis Detection. Biosens. Bioelectron. 2014, 59, 200–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, T.F.; Wan, Y.W.; Chen, S.Y. Gold Nanoparticles-carbon Nanotubes Modified Sensor for Electrochemical Determination of Organophosphate Pesticides. Microchim. Acta 2009, 165, 307–311. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R.; Satpati, B. Nanocrystalline Titanosilicate–Acetylcholinesterase Electrochemical Biosensor for the Ultra-Trace Detection of Toxic Organophosphate Pesticides. ChemElectroChem 2015, 2, 1164–1173. [Google Scholar] [CrossRef]
- Chufamo Jikamo, S.; Haile Habtemariam, T.; Heliso Dolla, T. Polyaniline-ZnO−NiO Nanocomposite Based Non-Enzymatic Electrochemical Sensor for Malathion Detection. Electroanalysis 2023, 35, e202200317. [Google Scholar] [CrossRef]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical Sensor Based on Polyaniline Nanofibers/Single Wall Carbon Nanotubes Composite for Detection of Malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Serag, E.; El-Maghraby, A.; Hassan, N.; El Nemra, A. CuO@MWCNTs Nanocomposite as Non-enzyme Electrochemical Sensor for the Detection of Malathion in Seawater. Desalin. Water Treat. 2021, 236, 240–249. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Sanfelice, R.C.; Mercante, L.A.; Facure, M.H.; Correa, D.S. Electrochemical Sensor Based on Polyamide 6/polypyrrole Electrospun Nanofibers Coated with Reduced Graphene Oxide for Malathion Pesticide Detection. Mater. Res. Express 2019, 7, 015601. [Google Scholar] [CrossRef]
- Al’Abri, A.M.; Abdul Halim, S.N.; Abu Bakar, N.K.; Saharin, S.M.; Sherino, B.; Rashidi Nodeh, H.; Mohamad, S. Highly Sensitive and Selective Determination of Malathion in Vegetable Extracts by an Electrochemical Sensor Based on Cu-Metal Organic Framework. J. Environ. Sci. Health Part B 2019, 54, 930–941. [Google Scholar] [CrossRef]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A highly efficient organophosphorus pesticides sensor based on CuO nanowires–SWCNTs hybrid nanocomposite. Sensor Actua. B–Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Memon, S.S.; Willander, M. Amino Acid Assisted Growth of CuO Nanostructures and Their Potential Application in Electrochemical Sensing of Organophosphate Pesticide. Electrochim. Acta 2016, 190, 972–979. [Google Scholar] [CrossRef]

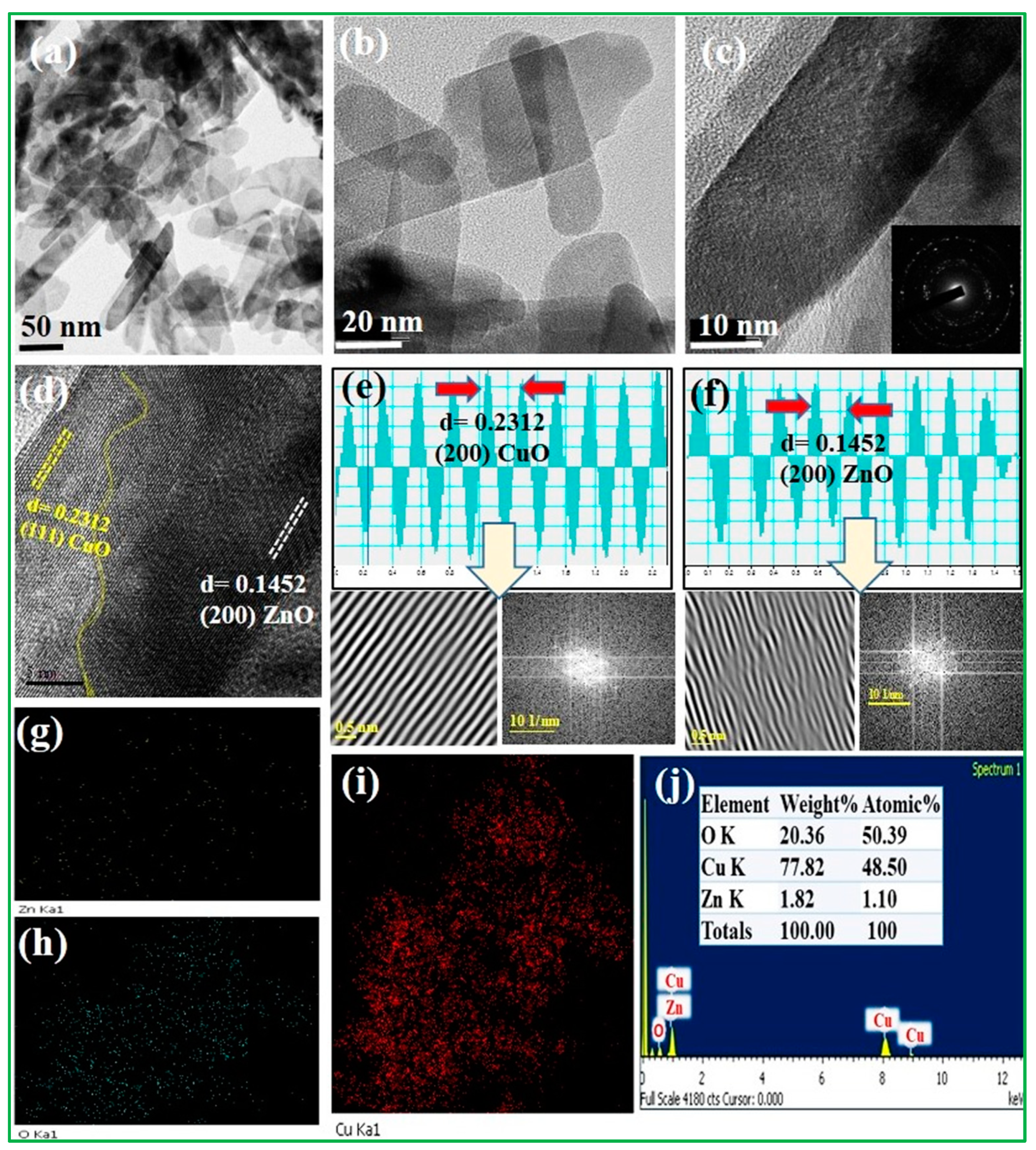
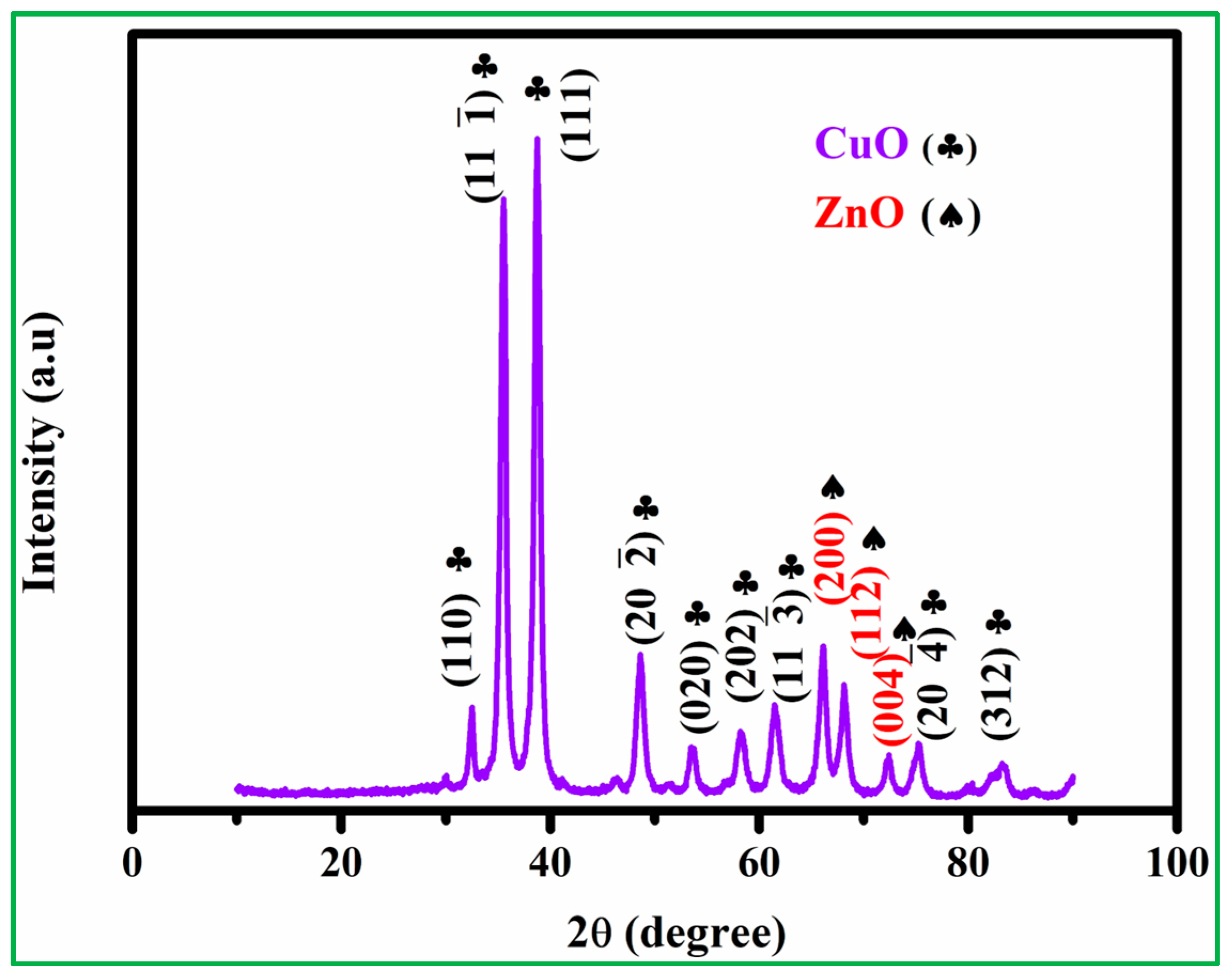

| Electrode | Crystalline Size | Lattice Parameter | Macrostrain |
|---|---|---|---|
| CuO | 12.4 nm (11) 11 nm (111) | a = 4.685 A° b = 3.423 A° c = 5.132 A° | 0.05 × 10−3 (11) 1.15 × 10−3 (111) |
| ZnO | 14.5 nm (200) 12.5 nm (112) | a = 3.25 A° c = 5.21 A° | 0.302 × 10−3 (200) 0.203 × 10−3 (112) |
| Electrode | Linear Range | Method | LOD | References |
|---|---|---|---|---|
| PANI-ZnO−NiO/GCE | 0.01–0.07 µM | DPV | 0.01 µM | [42] |
| PANI-ES/SWCNTs | 0.2–1.4 µM | DPV | 0.2 µM | [43] |
| c-MWCNT-CuO | 20–300 nM | CV | 0.143 nM | [44] |
| PA6/PPy/CRGO/FTO | 1.7–67 µM | DPV | 12.7 × 10−3 µM | [45] |
| BTCA-P-CuCP/CPE | 0.6 × 10−3–24.0 × 10−3 | CV | 0.2 × 10−3 | [46] |
| CuO NWs–SWCNTs/GCE | 0.3–1.4 nM | DPV | 0.3 nM | [47] |
| Gly-CuO/GCE/Nafion | 1–12 nM | DPV | 0.1 nM | [48] |
| ZnO–CuO/GCE | 0 to 200 nM | DPV | 1.367 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurusamy, L.; Cheng, R.-W.; Anandan, S.; Liu, C.-H.; Wu, J.J. Detection of Environmentally Harmful Malathion Pesticides Using a Bimetallic Oxide of CuO Nanoparticles Dispersed over a 3D ZnO Nanoflower. Materials 2023, 16, 7065. https://doi.org/10.3390/ma16227065
Gurusamy L, Cheng R-W, Anandan S, Liu C-H, Wu JJ. Detection of Environmentally Harmful Malathion Pesticides Using a Bimetallic Oxide of CuO Nanoparticles Dispersed over a 3D ZnO Nanoflower. Materials. 2023; 16(22):7065. https://doi.org/10.3390/ma16227065
Chicago/Turabian StyleGurusamy, Lakshmanan, Ru-Wen Cheng, Sambandam Anandan, Cheng-Hua Liu, and Jerry J. Wu. 2023. "Detection of Environmentally Harmful Malathion Pesticides Using a Bimetallic Oxide of CuO Nanoparticles Dispersed over a 3D ZnO Nanoflower" Materials 16, no. 22: 7065. https://doi.org/10.3390/ma16227065
APA StyleGurusamy, L., Cheng, R.-W., Anandan, S., Liu, C.-H., & Wu, J. J. (2023). Detection of Environmentally Harmful Malathion Pesticides Using a Bimetallic Oxide of CuO Nanoparticles Dispersed over a 3D ZnO Nanoflower. Materials, 16(22), 7065. https://doi.org/10.3390/ma16227065







