The Alphabet of Nanostructured Polypyrrole
Abstract
:1. Introduction
2. Deposition of Electroactive Polypyrrole
3. Polypyrrole Doping and Conduction Path
4. Morphology of Polypyrroles
4.1. The Impact of Morphology on the Bio-Applicability of PPy
4.1.1. N as Neural Applications
4.1.2. A as Antibacterial and Implantable Applications
4.1.3. D as Drug-Delivery Platforms
4.1.4. S as Sensors and Sorbents
4.2. The Impact of Morphology on the Technological Applicability of PPy
4.2.1. P as Corrosion Protection
4.2.2. M as Mechanical Aspects
4.2.3. B as Bubbles and Nanoporous Structures
4.2.4. C as Carbon-Based Materials
4.2.5. E as Energy Conversion Systems (Solar, Photothermal and Energy Storage Applications)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahvazi Zadeh, M.; Yeganeh, M.; Tavakoli Shoushtari, M.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synt. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; da Silva, C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739–135753. [Google Scholar] [CrossRef]
- El Guerraf, A.; Ben Jadi, S.; Karadas Bakirhan, N.; Eylul Kiymaci, M.; Bazzaoui, M.; Aysil Ozkan, S.; Arbi Bazzaoui, E. Antibacterial activity and volatile organic compounds sensing property of polypyrrole-coated cellulosic paper for food packaging purpose. Polym Bull. 2022, 79, 11543–11566. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Gniazdowska, B.; Jarosz, T.; Herman, A.P.; Boncel, S.; Turczyn, R. Effect of immobilization and release of ciprofloxacin and quercetin on electrochemical properties of poly(3,4-ethylenedioxypyrrole) matrix. Synt. Met. 2019, 249, 52–62. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, G.; Xu, H.; Kang, L. PPy doped with different metal sulphate as electrode materials for supercapacitors. Russ. J. Electrochem. 2017, 53, 359–365. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, J.; Miao, Z.; Qian, H.; Zha, Z. dl-Menthol Loaded Polypyrrole Nanoparticles as a Controlled Diclofenac Delivery Platform for Sensitizing Cancer Cells to Photothermal Therapy. ACS Appl. Bio. Mater. 2019, 2, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Ashu Tufa, R.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- da Silva, F.A.G., Jr.; Queiroz, J.C.; Macedo, E.R.; Fernandes, A.W.C.; Freire, N.B.; da Costa, M.M.; de Oliveira, H.P. Antibacterial behavior of polypyrrole: The influence of morphology and additives incorporation. Mater. Sci. Eng. C 2016, 62, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tracy Cui, X. Sponge-like nanostructured conducting polymers for electrically controlled drug release. Electrochem. Commun. 2009, 11, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, C.; Zhu, Z.; Liu, H.; Qu, J. Electrically Pore-Size-Tunable Polypyrrole Membrane for Antifouling and Selective Separation. Adv. Funct. Mater. 2019, 29, 1903081. [Google Scholar] [CrossRef]
- Riaz, U.; Singh, N.; Rashnas Srambikal, F.; Fatima, S. A review on synthesis and applications of polyaniline and polypyrrole hydrogels. Polym. Bull. 2022, 80, 1085–1116. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Li, X.; Zhu, L.; Xu, G.; Chen, Z.; Cheng, C.; Lu, Y.; Liu, Q. Wireless, battery-free and wearable device for electrically controlled drug delivery: Sodium salicylate released from bilayer polypyrrole by near-field communication on smartphone. Biomed. Microdev. 2020, 22, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq Ali Shah, S.; Firlak, M.; Ryan Berrow, S.; Ross Halcovitch, N.; Baldock, S.J.; Muhammad Yousafzai, B.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M.; Mahdavi, F. Application of the Electrochemical Quartz Crystal Microbalance for Electrochemically Controlled Binding and Release of Chlorpromazine from Conductive Polymer Matrix. Microchem. J. 1997, 56, 54–64. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Kinetic studies of electrochemically controlled release of salicylate from nanostructure conducting molecularly imprinted polymer. Electrochim. Acta 2013, 114, 409–415. [Google Scholar] [CrossRef]
- Kontturi, K.; Pentti, P.; Sundholm, G. Polypyrrole as a model membrane for drug delivery. J. Electroanal. Chem. 1998, 453, 231–238. [Google Scholar] [CrossRef]
- Wu, Y.; Ruan, Q.; Huang, C.; Liao, Q.; Liu, L.; Liu, P.; Mo, S.; Wang, G.; Wang, H.; Chu, P.K. Balancing the biocompatibility and bacterial resistance of polypyrrole by optimized silver incorporation. Biomat. Adv. 2022, 134, 112701–112715. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yu, M.; Chen, T.; Liu, Y.; Yi, Y.; Huang, C.; Tang, J.; Li, H.; Ou, M.; Wang, T.; et al. Polypyrrole Nanoenzymes as Tumor Microenvironment Modulators to Reprogram Macrophage and Potentiate Immunotherapy. Adv. Sci. 2022, 9, 2201703–2201720. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin Polypyrrole Nanosheets via Space-Confined Synthesis for Efficient Photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef]
- Sarkar, S.; Levi-Polyachenko, N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 40, 163–164. [Google Scholar] [CrossRef]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In Vitro and In Vivo Near-Infrared Photothermal Therapy of Cancer Using Polypyrrole Organic Nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef] [PubMed]
- Paúrova, M.; Taboubi, O.; Šeděnkova, I.; Hromădková, J.; Matouš, P.; Herynek, V.; Šefc, L.; Babič, M. Role of dextran in stabilization of polypyrrole nanoparticles for photoacoustic imaging. Eur. Polym. J. 2021, 157, 110634. [Google Scholar] [CrossRef]
- Liang, Y.; Mitriashkin, A.; Ting Lim, T.; Ting Lim, J. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. J. Biomat. 2021, 276, 121008–121022. [Google Scholar] [CrossRef]
- Lee, R.-J.; Temmer, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Renewable antioxidant properties of suspensible chitosan–polypyrrole composites. React. Funct. Polym. 2013, 73, 1072–1077. [Google Scholar] [CrossRef]
- Upadhyay, J.; Gogoi, B.; Kumar, A.; Buragohain, A.K. Diameter dependent antioxidant property of polypyrrole nanotubes for biomedical applications. Mat. Lett. 2013, 102–103, 33–35. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Mazzuchetti, G. Antibacterial efficacy of polypyrrole in textile applications. Fib. Polym. 2013, 14, 36–42. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A.; Gogoi, B.; Buragohain, A.K. Antibacterial and hemolysis activity of polypyrrole nanotubes decorated with silver nanoparticles by an in-situ reduction process. Mat. Sci. Eng. C 2015, 54, 8–13. [Google Scholar] [CrossRef]
- Soleimani, M.; Ghorbani, M.; Salahi, S. Antibacterial Activity of Polypyrrole-Chitosan Nanocomposite: Mechanism of Action. Int. J. Nanosci. Nanotechnol. 2016, 12, 191–197. [Google Scholar]
- Nautiyal, A.; Qiao, M.; Edwin Cook, J.; Zhang, X.; Huang, T.-S. High performance polypyrrole coating for corrosion protection and biocidal applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Z.; Liang, P.; Li, H.; Zhang, Y.; Fan, W.; Xu, G. Fabrication of polypyrrole coated superhydrophobic surfaces for effective oil/water separation. J. Mater. Res. Technol. 2022, 19, 4337–4349. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Progr. Org. Coat. 2020, 147, 105815–105825. [Google Scholar] [CrossRef]
- Morsi, S.M.M.; Abd El-Aziz, M.E.; Morsi, R.M.M.; Hussain, A.I. Polypyrrole-coated latex particles as core/shell composites for antistatic coatings and energy storage applications. J. Coat. Technol. Res. 2019, 16, 745–759. [Google Scholar] [CrossRef]
- Muro-Fraguas, I.; Sainz-García, A.; López, M.; Rojo-Bezares, B.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; González-Marcos, A.; Alba-Elías, F. Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments. Surf. Coat. Technol. 2020, 399, 126163–126173. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Kader, A.; Metwally, A.; El-Ahmady, S.H.; Metwally, E.S.; Ghonim, N.A.; Bayoumy, S.A.; Erfan, T.; Ashraf, R.; Fadel, M.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, X.; Wang, J.; Wang, Y.; Song, Y.; You, Q.; Sun, Q.; Liu, L.; Wang, S.; Tan, F.; et al. Polymer-based gadolinium oxide nanocomposites for FL/MR/PA imaging guided and photothermal/photodynamic combined antitumor therapy. J. Control. Release 2018, 277, 77–88. [Google Scholar] [CrossRef]
- Michalik, A.; Rohwerder, M. Conducting polymers for corrosion protection: A critical view. J. Phys. Chem. 2005, 219, 1547–1559. [Google Scholar] [CrossRef]
- Paliwoda-Porebska, G.; Stratmann, M.; Rohwerder, M.; Potje-Kamloth, K.; Lu, Y.; Pich, A.Z.; Adler, H.-J. On the development of polypyrrole coatings with self-healing properties for iron corrosion protection. Corros. Sci. 2005, 47, 3216–3233. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mat. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymers are not just conducting: A perspective for emerging technology. Polym. Int. 2020, 69, 662–664. [Google Scholar] [CrossRef]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-template rectangular nanotube molecularly imprinted polypyrrole for label-free impedimetric sensing of AFP and CEA as lung cancer biomarkers. Talanta 2022, 239, 123146–123156. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, T.; Han, Y.; Wang, Y.; Zhan, C.; Wang, Q. Optimizing the polymerization conditions of conductive polypyrrole. J. Photopolym. Sci. Technol. 2016, 29, 803–806. [Google Scholar] [CrossRef]
- Rath, A.; Theato, P. Advanced AAO Templating of Nanostructured Stimuli-Responsive Polymers: Hype or Hope? Adv. Funct. Mater. 2020, 30, 1902959–1902975. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Dazzi, A.; Lefrançois, P.; Gervais, M.; Néron, S.; Remita, S. Radiolytic Method as a Novel Approach for the Synthesis of Nanostructured Conducting Polypyrrole. Langmuir 2014, 30, 14086–14094. [Google Scholar] [CrossRef]
- Taouil, A.E.; Mourad Mahmoud, M.; Lallemand, F.; Lallemand, S.; Gigandet, M.-P.; Hihn, J.-Y. Corrosion protection by sonoelectrodeposited organic films on zinc coated steel. Ultras Sonochem. 2012, 19, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3 and Redox Cycling of [Fe(CN)6]4/[Fe(CN)6]3. Polymers 2018, 10, 749. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Grijalva-Bustamante, G.A.; Evans-Villegas, A.G.; del Castillo-Castro, T.; Castillo-Ortega, M.M.; Cruz-Silva, R.; Huerta, F.; Morallón, E. Enzyme mediated synthesis of polypyrrole in the presence of chondroitin sulfate and redox mediators of natural origin. Mater. Sci. Eng. C 2016, 63, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Fernandez, F.D.M.; Khadka, R.; Yim, J.-H. A comparative study between vapor phase polymerized PPy and PEDOT—Thermoplastic polyurethane composites for ammonia sensing. Polymer 2021, 217, 123463–123470. [Google Scholar] [CrossRef]
- Shafiqur Rahman, M.; Wasiu Adebayo, H.; Yahya, R.; Khairani Mohd Jamil, A.; Nabi Muhammad Ekramul Mahmud, H. One-step facile synthesis of poly(N-vinylcarbazole)-polypyrrole/graphene oxide nanocomposites: Enhanced solubility, thermal stability and good electrical conductivity. J. Macromol. Sci. Part. A 2019, 56, 384–391. [Google Scholar] [CrossRef]
- Kiefer, R.; Khadka, R.; Travas-Sejdic, J. Poly(ethylene oxide) in polypyrrole doped dodecylbenzenesulfonate: Characterisation and linear actuation. Int. J. Nanotechnol. 2018, 15, 689–694. [Google Scholar] [CrossRef]
- Brie, M.; Turcu, R.; Mihut, A. Stability study of conducting polypyrrole films and polyvinylchloride-polypyrrole composites doped with different counterions. Mater. Chem. Phys. 1997, 49, 174–178. [Google Scholar] [CrossRef]
- Kirsnytėa, M.; Jurkūnasa, M.; Kanclerisa, Ž.; Ragulisa, P.; Simniškisa, R.; Vareikisc, A.; Abraitienėa, A.; Požėlaa, K.; Whitesideb, B.; Tuinea-Bobeb, C.L.; et al. Investigation of in situ formed conductive polymer composite in adhesive matrix. Synt. Met. 2019, 258, 116181. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmad, S. A review on conducting polymer reinforced polyurethane composites. J. Ind. Eng. Chem. 2017, 53, 1–22. [Google Scholar] [CrossRef]
- Wampler, W.A.; Rajeshwara, K.; Pethe, R.G.; Hyer, R.C.; Sharma, S.C. Composites of polypyrrole and carbon black: Part III. Chemical synthesis and characterization. J. Mater. Res. 1995, 10, 1811–1822. [Google Scholar] [CrossRef]
- Hagler, J.R.; Peterson, B.N.; Murphy, A.R.; Leger, J.M. Performance of silk-polypyrrole bilayer actuators under biologically relevant conditions. J. Appl. Polym. Sci. 2019, 136, 46922–46932. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Ansari, R. Polypyrrole Conducting Electroactive Polymers: Synthesis and Stability Studies. J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef]
- Ansari Khalkhali, R. Electrochemical Synthesis and Characterization of Electroactive Conducting Polypyrrole Polymers. Russ. J. Electrochem. 2005, 41, 950–955. [Google Scholar] [CrossRef]
- Higgins, M.J.; McGovern, S.T.; Wallace, G.G. Visualizing Dynamic Actuation of Ultrathin Polypyrrole Films. Langmuir 2009, 25, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, D.; Boyd, B.J.; Garg, S.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers with defined micro- or nanostructures for drug delivery. Biomaterials 2016, 111, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Gribkovaa, O.L.; Kabanovaa, V.A.; Nekrasova, A.A. Electrochemical Polymerization of Pyrrole in the Presence of Sulfoacid Polyelectrolytes. Russ. J. Electrochem. 2019, 55, 1110–1117. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Jarosz, T.; Zak, J.K.; Lapkowski, M.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Bednarczyk-Cwynar, B. Advancing the delivery of anticancer drugs: Conjugated polymer/triterpenoid composite. Acta Biomater. 2015, 19, 158–165. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes. Environ. Sci. Pollut. Res. 2018, 25, 20012–20022. [Google Scholar] [CrossRef]
- Gutiérrez-Pineda, E.; Alcaide, F.; José Rodríguez-Presa, M.; Bolzan, A.E.; Alfredo Gervasi, C. Electrochemical Preparation and Characterization of Polypyrrole/Stainless Steel Electrodes Decorated with Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 2677–2687. [Google Scholar] [CrossRef]
- Cysewska, K.; Karczewski, J.; Jasiński, P. The Influence of the Co-Dopant Dexamethasone Phosphate on the Electrodeposition Process and Drug-Release Properties of Polypyrrole-Salicylate on Iron. J. Electrochem. Soc. 2019, 166, G148. [Google Scholar] [CrossRef]
- Hua Lei, Y.; Seng, N.; Hyono, A.; Ueda, M.; Ohtsuka, T. Electrochemical synthesis of polypyrrole films on copper from phytic solution for corrosion protection. Corr. Sci. 2013, 76, 302–309. [Google Scholar] [CrossRef]
- Chebil, S.; Monod, M.O.; Fisicaro, P. Direct electrochemical synthesis and characterization of polypyrrole nano- and micro-snails. Electrochim. Acta 2014, 123, 527–534. [Google Scholar] [CrossRef]
- Borges, M.H.R.; Nagay, B.E.; Costa, R.C.; Sacramento, C.M.; Ruiz, K.G.; Landers, R.; van den Beucken, J.J.J.P.; Fortulan, C.A.; Rangel, E.C.; da Cruz, N.C.; et al. A tattoo-inspired electrosynthesized polypyrrole film: Crossing the line toward a highly adherent film for biomedical implant applications. Mater. Today Chem. 2022, 26, 101095–101099. [Google Scholar] [CrossRef]
- Saugo, M.; Flamini, D.O.; Brugnoni, L.I.; Saidman, S.B. Silver deposition on polypyrrole films electrosynthesised onto Nitinol alloy. Corrosion protection and antibacterial activity. Mat. Sci. Eng. C 2015, 56, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Yan, F.; Zhu, J.; Wang, J. Template-free prepared micro/nanostructured polypyrrole with ultrafast charging/discharging rate and long cycle life. J. Power Sources 2011, 196, 2373–2379. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Xia, D.; Zhang, L.; Hui, R.; Zhan, J. Whelk-like Helixes of Polypyrrole Synthesized by Electropolymerization. Adv. Funct. Mater. 2007, 17, 1844–1848. [Google Scholar] [CrossRef]
- Nezhadali, A.; Rouki, Z.; Nezhadali, M. Electrochemical preparation of a molecularly imprinted polypyrrole modified pencil graphite electrode for the determination of phenothiazine in model and real biological samples. Talanta 2015, 144, 456–465. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Pan, C.; Si, P.; Huang, P.; Zhou, J. Morphology-dependent electrochemical stability of electrodeposited polypyrrole/nano-ZnO composite coatings. Mater. Chem. Phys. 2022, 279, 125775–125787. [Google Scholar] [CrossRef]
- Bayat, M.; Izadan, H.; Molina, B.G.; Sánchez, M.; Santiago, S.; Semnani, D.; Dinari, M.; Guirado, G.; Estrany, F.; Alemán, C. Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers 2019, 11, 1757. [Google Scholar] [CrossRef]
- Sui, J.; Travas-Sejdic, J.; Chu, S.Y.; Li, K.C.; Kilmartin, P.A. The actuation behavior and stability of p-toluene sulfonate doped polypyrrole films formed at different deposition current densities. J. Appl. Polym. Sci. 2009, 111, 876–882. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Lyalina, N.V.; Maratkanova, A.N.; Smirnov, D.A. Molecular architecture of highly protective coatings of electrodeposited dodecyl sulfate-doped polypyrrole. Prog. Org. Coat. 2019, 131, 427–434. [Google Scholar] [CrossRef]
- Du, X.; Hao, X.; Wang, Z.; Ma, X.; Guan, G.; Abuliti, A.; Ma, G.; Liu, S. Highly stable polypyrrole film prepared by unipolar pulse electro-polymerization method as electrode for electrochemical supercapacitor. Synt. Met. 2013, 175, 138–145. [Google Scholar] [CrossRef]
- Zheng, W.; Razal, J.M.; Spinks, G.M.; Truong, V.-T.; Whitten, P.G.; Wallace, G.G. The Role of Unbound Oligomers in the Nucleation and Growth of Electrodeposited Polypyrrole and Method for Preparing High, Strength, High Conductivity Films. Langmuir 2012, 28, 10891–10897. [Google Scholar] [CrossRef]
- Reza Eslami, M.; Alizadeh, N. A dual usage smart sorbent/recognition element based on nanostructured conducting molecularly imprinted polypyrrole for simultaneous potential-induced nanoextraction/determination of ibuprofen in biomedical samples by quartz crystal microbalance sensor. Sens. Actuators B 2015, 220, 880–887. [Google Scholar] [CrossRef]
- Martinez, A.L.; Brugnoni, L.I.; Flamini, D.O.; Saidman, S.B. Immobilization of Zn species in a polypyrrole matrix to prevent corrosion and microbial growth on Ti-6Al-4V alloy for biomedical applications. Prog. Org. Coat. 2020, 144, 105650–105660. [Google Scholar] [CrossRef]
- Aouzal, Z.; Bouabdallaoui, M.; El Guerraf, A.; Ben Jadi, S.; Wang, R.; Bazzaoui, M.; Bazzaoui, E.A. Improvement of the anticorrosion resistance of nickel by polypyrrole coating electrosynthesized in salicylate medium. Mater. Today Proc. 2020, 31, S89–S95. [Google Scholar] [CrossRef]
- Joo, J.; Lee, J.K.; Lee, S.Y.; Jang, K.S.; Oh, E.J.; Epstein, A.J. Physical Characterization of Electrochemically and Chemically Synthesized Polypyrroles. Macromolecules 2000, 33, 5131–5136. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Yang, F.K.; Zhao, B. A Facile In Situ Approach to Polypyrrole Functionalization Through Bioinspired Catechols. Adv. Funct. Mater. 2015, 25, 1588–1597. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Yuan, S.J.; Yi, S.P.; Yin, E.Q. Synthesis and characterization of polypyrrole doped with anionic spherical polyelectrolyte brushes. eXPRESS Polym. Lett. 2012, 6, 697–705. [Google Scholar] [CrossRef]
- Ghilane, J.; Hapiot, P.; Bard, A.J. Metal/Polypyrrole Quasi-Reference Electrode for Voltammetry in Nonaqueous and Aqueous Solutions. Anal. Chem. 2006, 78, 6868–6872. [Google Scholar] [CrossRef]
- Sadat Eftekhari, B.; Eskandari, M.; Janmey, P.A.; Samadikuchaksaraei, A.; Gholipourmalekabadi, M. Surface Topography and Electrical Signaling: Single and Synergistic Effects on Neural Differentiation of Stem Cells. Adv. Funct. Mater. 2020, 30, 190792–190809. [Google Scholar] [CrossRef]
- Chandra Sekhar Rout, N.K. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5698. [Google Scholar] [CrossRef]
- Kong, H.; Yang, M.; Miao, Y.; Zhao, X. Polypyrrole as a Novel Chloride-Storage Electrode for Seawater Desalination. Energy Technol. 2019, 7, 1900835–1900842. [Google Scholar] [CrossRef]
- Mettai, B.; Mekki, A.F.; Bekkar Djelloul Sayah, Z.; Moustefai Soumia, K.; Safiddine, Z.; Mahmoud, R.; Mehdi Chehimi, M. In situ chemical deposition of PPy/NDSA and PPy/DBSA layers on QCM electrodes: Synthesis, structural, morphological and ammonia sensing performances study. J. Polym. Res. 2018, 25, 95–107. [Google Scholar] [CrossRef]
- Yousef Elahi, M.; Bathaie, S.Z.; Kazemi, S.H.; Mousavi, M.F. DNA immobilization on a polypyrrole nanofiber modified electrode and its interaction with salicylic acid/aspirin. Anal. Biochem. 2011, 411, 176–184. [Google Scholar] [CrossRef]
- Flamini, D.O.; González, M.B.; Saidman, S.B. ; Saidman, S.B. sis and Characterization of Heparin-Doped Polypyrrole Coatings Using an Electrochemical Quartz Crystal Microbalance (EQCM). Port. Electrochim. Acta 2022, 40, 47–57. [Google Scholar] [CrossRef]
- Baek, S.; Green, R.A.; Poole-Warren, L.A. Effects of dopants on the biomechanical properties of conducting polymer films on platinum electrodes. J. Biomed. Mater. Res. Part. A 2014, 102A, 2743–2754. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.F.; Chrisitne, J.; Robert, J.; Roger, L. Electrochemically synthesized polypyrrole nanotubules: Effects of different experimental conditions, Electrochemically synthesized polypyrrole nanotubules: Effects of different experimental conditions. Eur. Polym. J. 1998, 34, 1767–1774. [Google Scholar] [CrossRef]
- Otero, T.F.; Sansiñena, J.M. Influence of synthesis conditions on polypyrrole-poly(styrenesulphonate) composite electroactivity. J. Electroanal. Chem. 1996, 412, 109–116. [Google Scholar] [CrossRef]
- Wang, P.-C.; Yu, J.-Y. Dopant-dependent variation in the distribution of polarons and bipolarons as charge-carriers in polypyrrole thin films synthesized by oxidative chemical polymerization. React. Funct. Polym. 2012, 72, 311–316. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]
- Prokeš, J.; Varga, M.; Vrňata, M.; Valtera, S.; Stejskal, J.; Kopecký, D. Nanotubular polypyrrole: Reversibility of protonation/deprotonation cycles and long-term stability. Eur. Polym. J. 2019, 115, 290–297. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef] [PubMed]
- Debiemme-Chouvy, C.; Tuyet Mai Tran, T. An insight into the overoxidation of polypyrrole materials. Electrochem. Commun. 2008, 10, 947–950. [Google Scholar] [CrossRef]
- West, N.; Baker, P.G.L.; Arotiba, O.A.; Hendricks, N.R.; Baleg, A.A.; Waryo, T.T.; Ngece, R.F.; Iwuoha, E.I.; O’Sullivan, C. Overoxidized Polypyrrole Incorporated with Gold Nanoparticles as Platform for Impedimetric Anti-Transglutaminase Immunosensor. Anal. Lett. 2011, 44, 1956–1966. [Google Scholar] [CrossRef]
- Otero, T.F.; Padilla, J. Anodic shrinking and compaction of polypyrrole blend: Electrochemical reduction under conformational relaxation kinetic control. J. Electroanal. Chem. 2004, 561, 167–171. [Google Scholar] [CrossRef]
- Mashayekhi Mazar, F.; Martinez, J.G.; Tyagi, M.; Alijanianzadeh, M.; Turner, A.P.F.; Jager, E.W.H. Artificial Muscles Powered by Glucose. Adv. Mater. 2019, 31, 1901677–1901685. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Activation energy for pol Activation energy for polypyrrole oxidation pyrrole oxidation: Film thickness influence. J. Solid. State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, A. Dielectric spectroscopy for probing the relaxation and charge transport in polypyrrole nanofibers. J. Appl. Phys. 2011, 109, 114313–114323. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synt. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, T.; Huh, J.; Kang, M.; Eun Lee, J.; Yoon, H. Anisotropic Growth Control of Polyaniline Nanostructures and Their Morphology-Dependent Electrochemical Characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef]
- Bocchetta, P.; Frattini, D.; Tagliente, M.; Selleri, F. Electrochemical Deposition of Polypyrrole Nanostructures for Energy Applications: A Review. Curr. Nanosci. 2020, 16, 462–477. [Google Scholar] [CrossRef]
- Zang, J.; Bao, S.-J.; Li, C.M.; Bian, H.; Cui, X.; Bao, Q.; Sun, C.Q.; Guo, J.; Lian, K. Well-Aligned Cone-Shaped Nanostructure of Polypyrrole/RuO2 and Its Electrochemical Supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Cysewska, K.; Karczewski, J.; Jasiński, P. Electrochemical synthesis of 3D nano-/micro-structured porous polypyrrole. Mater. Lett. 2016, 183, 397–400. [Google Scholar] [CrossRef]
- Ramirez, A.M.R.; Gacitua, M.A.; Ortega, E.; Diaz, F.R.; del Valle, M.A. Electrochemical in situ synthesis of polypyrrole nanowires. Electrochem. Comm. 2019, 102, 94–98. [Google Scholar] [CrossRef]
- Mariano, A.; Lubrano, C.; Bruno, U.; Ausilio, C.; Bhupesh Dinger, N.; Santoro, F. Advances in Cell-Conductive Polymer Biointerfaces and Role of the Plasma Membrane. Chem. Rev. 2022, 122, 4–4552. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef]
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J. Biomater. Sci. Polym. Ed. 2010, 21, 1265–1282. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; Pu, X.; Yin, G.; Zhang, J. Fabrication of Chitosan/Polypyrrole-coated poly(L-lactic acid)/Polycaprolactone aligned fibre films for enhancement of neural cell compatibility and neurite growth. Cell Prolif. 2019, 52, 12588–12599. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wanga, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, L.; Sun, X.; Wang, X.; Jin, S.; Li, J.; Wu, Q. Neurogenic differentiation of human umbilical cord mesenchymal stem cells on aligned electrospun polypyrrole/polylactide composite nanofibers with electrical stimulation. Front. Mater. Sci. 2016, 10, 260–269. [Google Scholar] [CrossRef]
- Liang, Y.; Cho-Hong Goh, J. Polypyrrole-Incorporated Conducting Constructs for Tissue Engineering Applications: A Review. Bioelctricity 2020, 2, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Janas, D.; Vallejo-Giraldo, C.; Biggs, M.J.P. Self-supporting carbon nanotube films as flexible neural interfaces. Electrochim. Acta 2019, 295, 253–261. [Google Scholar] [CrossRef]
- Czerwinska-Główka, D.; Skonieczna, M.; Barylski, A.; Golba, S.; Przystaś, W.; Zabłocka-Godlewska, E.; Student, S.; Cwalina, B.; Krukiewicz, K. Bifunctional conducting polymer matrices with antibacterial and neuroprotective effect. Bioelectrochemistry 2022, 144, 10803–10817. [Google Scholar] [CrossRef]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible Neural Electrodes for Long-Lasting Intracortical Recording. Science 2020, 23, 101387. [Google Scholar] [CrossRef]
- Khorrami, M.; Antensteiner, M.; Fallahianbijan, F.; Borhan, A.; Reza, M.; Annu, A. Conducting polymer microcontainers for biomedical applications. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 23, 1869–1872. [Google Scholar] [CrossRef]
- Harjo, M.; Zondaka, Z.; Leemets, K.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole-coated fiber-scaffolds: Concurrent linear actuation and sensing. J. Appl. Polym. Sci. 2019, 136, 48533–48541. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Huang, Z.; Pu, X.; Shang, L.; Yin, G.; Xue, C. Preparation of carboxylic graphene oxide-composited polypyrrole conduits and their effect on sciatic nerve repair under electrical stimulation. J. Biomed. Mater. Res. 2019, 107, 2784–2795. [Google Scholar] [CrossRef]
- Zhang, Q.; Esrafilzadeh, D.; Crook, J.M.; Kapsa, R.; Stewart, E.M.; Tomaskovic-Crook, E.; Wallace, G.G.; Huang, X.-F. Electrical Stimulation Using Conductive Polymer Polypyrrole Counters Reduced Neurite Outgrowth of Primary Prefrontal Cortical Neurons from NRG1-KO and DISC1-LI Mice. Sci. Rep. 2017, 7, 42525–42533. [Google Scholar] [CrossRef]
- Sun, Y.; Quan, Q.; Meng, H.; Zheng, Y.; Peng, J.; Hu, Y.; Feng, Z.; Sang, X.; Qiao, K.; He, W.; et al. Enhanced Neurite Outgrowth on a Multiblock Conductive Nerve Scaffold with Self-Powered Electrical Stimulation. Adv. Healthcare Mater. 2019, 8, 1900127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, J.; Liu, X. Antibacterial Property and Biocompatibility of Polypyrrole Films Treated by Oxygen Plasma Immersion Ion Implantation. Adv. Mater. Interf. 2020, 7, 2000057. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.K.; Agarwal, K.; Singh, H.K.; Srivastava, P.; Singh, R. Effect of p-toluenesulfonate on inhibition of overoxidation of polypyrrole. J. Appl. Polym. Sci. 2013, 130, 434–442. [Google Scholar] [CrossRef]
- Lunkes Ely, V.; Matiuzzi da Costa, M.; Pequeno de Oliveira, H.; Antonio Gomes da Silva Júnior, F.; Brayer Pereira, D.I.; Pereira Soares, M.; De Vargas, A.C.; Sangioni, L.A.; Cargnelutti, J.F.; Garcia Ribeiro, M.; et al. In Vitro algicidal effect of polypyrrole on Prototheca species isolates from bovine mastitis Algicidal activity of polypyrrole on Prototheca spp. Med. Mycol. 2020, 58, 1114–1119. [Google Scholar] [CrossRef]
- Děkanovský, L.; Elashnikov, R.; Kubiková, M.; Vokatá, B.; Švorčík, V.; Lyutakov, O. Dual-Action Flexible Antimicrobial Material: Switchable Self-Cleaning, Antifouling, and Smart Drug Release. Adv. Funct. Mater. 2019, 29, 1901880–1901890. [Google Scholar] [CrossRef]
- Forero López, A.D.; Loperena, A.P.; Lehr, I.L.; Brugnoni, L.I.; Saidman, S.B. Corrosion protection of AZ91D magnesium alloy by a duplex coating. J. Serb. Chem. Soc. 2020, 85, 1317–1328. [Google Scholar] [CrossRef]
- Forero López, A.D.; Lehr, I.L.; Brugnoni, L.I.; Saidman, S.B. Improvement in the corrosion protection and bactericidal properties of AZ91D magnesium alloy coated with a microstructured polypyrrole film. J. Magn. Alloys 2018, 6, 15–22. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Gu, R.; Li, G.; Lian, J. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surf. B Biointerfaces 2020, 194, 111186. [Google Scholar] [CrossRef]
- Bhagya Mathi, D.; Gopi, D.; Kavith, L. Implication of lanthanum substituted hydroxyapatite/poly(n-methyl pyrrole) bilayer coating on titanium for orthopedic applications. Mater. Today Proc. 2020, 26, 3526–3530. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, L.; Chen, D.; Wang, Z.; Zhai, J.; Wang, R.; Tan, G.; Mao, J.; Yu, P.; Ning, C. Construction of high surface potential polypyrrole nanorods with enhanced antibacterial properties. J. Mater. Chem. B 2018, 6, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- González, M.B.; Quinzani, O.V.; Vela, M.E.; Rubert, A.A.; Benítez, G.; Saidman, S.B. Study of the electrosynthesis of hollow rectangular microtubes of polypyrrole. Synt. Met. 2012, 162, 1133–1139. [Google Scholar] [CrossRef]
- El Jaouhari, A.; El Asbahani, A.; Bouabdallaoui, M.; Aouzal, Z.; Filotás, D.; Bazzaoui, E.A.; Nagy, L.; Nagy, G.; Bazzaoui, M.; Albourine, A.; et al. Corrosion resistance and antibacterial activity of electrosynthesized polypyrrole. Synt. Met. 2017, 226, 15–24. [Google Scholar] [CrossRef]
- González, M.B.; Brugnoni, L.I.; Flamini, D.O.; Quinzani, L.M.; Saidman, S.B. Removal of Escherichia coli from well water using continuous laminar flow in a channel system containing PPy/Cu modified electrodes. J. Water Health 2018, 16, 921–929. [Google Scholar] [CrossRef]
- Hsu, C.F.; Zhang, L.; Peng, H.; Travas-Sejdic, J.; Kilmartin, P.A. Free radical scavenging properties of polypyrrole and poly(3,4-ethylenedioxythiophene). Curr. Appl. Phys. 2008, 8, 316–319. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Etminanfar, M.; Mahdavi, S.; Saman Safavi, M. Enhanced bioactivity of 316L stainless steel with deposition of polypyrrole/hydroxyapatite layered hybrid coating: Orthopedic applications. Surf. Interfaces 2022, 28, 101604–101615. [Google Scholar] [CrossRef]
- Du, H.; Parit, M.; Liu, K.; Zhang, M.; Jiang, Z.; Huang, T.-S.; Zhang, X.; Si, C. Multifunctional Cellulose Nanopaper with Superior Water-Resistant, Conductive, and Antibacterial Properties Functionalized with Chitosan and Polypyrrole. ACS Appl. Mater. Interf. 2021, 13, 32115–32125. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; del Valle, L.J.; Alemá, C. Drug delivery systems based on intrinsically conducting polymers. J. Control. Release 2019, 309, 244–264. [Google Scholar] [CrossRef]
- Michael Freedman, S.; Cui, X.T. Substrate Electrode Morphology Affects Electrically Controlled Drug Release from Electrodeposited Polypyrrole Films. Phys. Chem. Comm. 2014, 1, 15–25. [Google Scholar]
- Moloney, E.; Breslin, C.B. The formation and properties of polypyrrole doped with an immobile antibiotic. J. Sold. State Electrochem. 2019, 23, 2031–2042. [Google Scholar] [CrossRef]
- Wang, M.L.; Chamberlayne, C.F.; Xu, H.; Mofidfar, M.; Baltsavias, S.; Annes, J.P.; Zare, R.N.; Arbabian, A. On-demand electrochemically controlled compound release from an ultrasonically powered implant. RSC Adv. 2022, 12, 23337–23346. [Google Scholar] [CrossRef]
- Antensteiner, M.; Khorrami, M.; Fallahianbijan, F.; Borhan, A.; Reza Abidian, M. Conducting Polymer Microcups for Organic Bioelectronics and Drug Delivery Applications. Adv. Mater. 2017, 29, 1702576–1702587. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Nanostructured biocompatible thermal/electrical stimuli-responsive biopolymer-doped polypyrrole for controlled release of chlorpromazine: Kinetics studies. Int. J. Pharm. 2014, 472, 327–339. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Stokfisz, A.; Zak, J.K. Two approaches to the model drug immobilization into conjugated polymer matrix. Mater. Sci. Eng. C 2015, 54, 176–181. [Google Scholar] [CrossRef]
- Glosz, K.; Stolarczyk, A.; Jarosz, T. Electropolymerised Polypyrroles as Active Layers for Molecularly Imprinted Sensors: Fabrication and Applications. Materials 2021, 14, 1369. [Google Scholar] [CrossRef] [PubMed]
- Czaja, T.; Wójcik, K.; Grzeszczuk, M.; Szostak, R. Polypyrrole–Methyl Orange Raman pH Sensor. Polymers 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Boguzaite, R.; Ratautaite, V.; Mikoliunaite, L.; Pudzaitis, V.; Ramanaviciene, A.; Ramanavicius, A. Towards analytical application of electrochromic polypyrrole layers modified by phenothiazine derivatives. J. Electroanal. Chem. 2021, 886, 115132–115142. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duan, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004–045024. [Google Scholar] [CrossRef]
- Sharma Pushpendra, K.; Sharma, P.K.; Gupta, G.; Singh, V.V.; Tripathi, B.K.; Pandey, P.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Synthesis and characterization of polypyrrole by cyclic voltammetry at different scan rate and its use in electrochemical reduction of the simulant of nerve agents. Synt. Met. 2010, 160, 2631–2637. [Google Scholar] [CrossRef]
- West, N.; Baker, P.; Waryo, T.; Ngece, F.R.; Iwuoha, E.I.; O’Sullivan, C.; Katakis, I. Highly sensitive gold-overoxidized polypyrrole nanocomposite immunosensor for antitransglutaminase antibody. J. Bioact. Compat. Polym. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, P.; Turner, A.P.F. Interference-Free Electrochemical Detection of Nanomolar Dopamine Using Doped Polypyrrole and Silver Nanoparticles. Electroanalysis 2014, 26, 2197–2206. [Google Scholar] [CrossRef]
- Nguyen, V.-A.; Nguyen, H.L.; Nguyen, D.T.; Do, Q.P.; Tran, L.D. Electrosynthesized poly(1,5-diaminonaphthalene)/polypyrrole nanowires bilayer as an immunosensor platform for breast cancer biomarker CA 15-3. Curr. Appl. Phys. 2017, 17, 1422–1429. [Google Scholar] [CrossRef]
- Fakhry, A.; Cachet, H.; Debiemme-Chouvy, C. Mechanism of formation of templateless electrogenerated polypyrrole nanostructures. Electrochim. Acta 2015, 179, 297–303. [Google Scholar] [CrossRef]
- Fakhry, A.; Pilliera, F.; Debiemme-Chouvy, C. Templateless electrogeneration of polypyrrole nanostructures: Impact of the anionic composition and pH of the monomer solution. J. Mater. Chem. A 2014, 2, 9859–9865. [Google Scholar] [CrossRef]
- Landim, V.P.A.; Foguel, M.V.; Prado, C.M.; Sotomayor, M.P.T.; Vieira, I.C.; Silva, B.V.M.; Dutran, R.F. A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors 2022, 12, 545. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. [Google Scholar] [CrossRef]
- Taheri, N.; Alizadeh, N. Vertically grown nanosheets conductive polypyrrole as a sorbent for nanomolar detection of salicylic acid. J. Pharm. Biomed. Anal. 2020, 188, 113365–113373. [Google Scholar] [CrossRef]
- Olszowy, P.; Szultka, M.; Ligor, T.; Nowaczyk, J.; Buszewski, B. Fibers with polypyrrole and polythiophene phases for isolation and determination of adrenolytic drugs from human plasma by SPME-HPLC. J. Chromatogr. B 2010, 878, 2226–2234. [Google Scholar] [CrossRef]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Efremova Aaron, S.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater. 2020, 3, 815–839. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Janaki, V.; Senthilkumar, P.; Kamala-Kannan, S. Polypyrrole/zeolite composite—A nanoadsorbent for reactive dyes removal from synthetic solution. Chemosphere 2022, 287, 132164–132172. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Nawaz Bhatti, H.; Iqbal, M.; Hussain, F.; Malik Sarim, F. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Chandra Lohani, P.; Prasad Tiwari, A.; Muthurasu, A.; Pathak, I.; Babu Poudel, M.; Chhetri, K.; Dahal, B.; Acharya, D.; Hoon Ko, T.; Yong Kim, H. Phytic acid empowered two nanos “Polypyrrole tunnels and transition Metal-(Oxy)hydroxide Sheets” in a single platform for unmitigated redox water splitting. Chem. Eng. J. 2023, 463, 142280. [Google Scholar] [CrossRef]
- Su, D.; Zhou, J.; Ahmed, K.S.; Ma, Q.; Lv, G.; Chen, J. Fabrication and characterization of collagen-heparin-polypyrrole composite conductive film for neural scaffold. Int. J. Biol. Macromol. 2019, 129, 895–903. [Google Scholar] [CrossRef]
- Biallozor, S.; Kupniewska, A. Conducting polymers electrodeposited on active metals. Synt. Met. 2005, 155, 443–449. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of bilayered polypyrrole on 316 L stainless steel prevention. Prog. Org. Coat. 2015, 78, 21–27. [Google Scholar] [CrossRef]
- Zeybek, B.; Özçiçek Pekmez, N.; Kılıç, E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances. Electrochim. Acta 2011, 56, 9277–9286. [Google Scholar] [CrossRef]
- Takamatsu, T.; Sijie, Y.; Shujie, F.; Xiaohan, L.; Miyake, T. Multifunctional High-Power Sources for Smart Contact Lenses. Adv. Funct. Mater. 2019, 30, 1906225–1906233. [Google Scholar] [CrossRef]
- El Jaouhari, A.; Ben Jadi, S.; Aouzal, Z.; Bouabdallaoui, M.; Bazzaoui, E.A.; Wang, R.; Bazzaoui, M. Comparison study between corrosion protection of polypyrrole synthesized on stainless steel from phthalate and saccharinate aqueous medium. Polym. Test. 2018, 67, 302–308. [Google Scholar] [CrossRef]
- Yan, Q.; Pan, W.; Zhong, S.; Zhu, R.; Li, G. Effect of solvents on the preparation and corrosion protection of polypyrrole. Prog. Org. Coat. 2019, 132, 298–304. [Google Scholar] [CrossRef]
- Raman, S.; Ravi Sankar, A. Intrinsically conducting polymers in flexible and stretchable resistive strain sensors: A review. J. Mater. Sci. 2022, 57, 13152–13178. [Google Scholar] [CrossRef]
- Brito de Morais, V.; Crispilho Corrêa, C.; Martin Lanzoni, E.; Rodrigues Costa, C.A.; César Bof Bufon, C.; Santhiago, M. Wearable binary cooperative polypyrrole nanofilms for chemical mapping on skin. Mater. Chem. A 2019, 7, 5227–5234. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Huang, W.-H. Stretchable Electrochemical Sensors for Cell and Tissue Detection. Angew. Chem. 2021, 60, 2757–2767. [Google Scholar] [CrossRef]
- Petty, A.J.; Keate, R.L.; Jiang, B.; Ameer, G.A.; Rivnay, J. Conducting Polymers for Tissue Regeneration in vivo. Chem. Mater. 2020, 32, 4095–4115. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef]
- Chen, A.X.; Kleinschmidt, A.T.; Choudhary, K.; Lipomi, D.J. Beyond Stretchability: Strength, Toughness, and Elastic Range in Semiconducting Polymers. Chem. Mater. 2020, 32, 7582–7601. [Google Scholar] [CrossRef]
- Uzun, O.; Başman, N.; Alkan, C.; Kölemen, U.; Yılmaz, F. Investigation of mechanical and creep properties of polypyrrole by depth-sensing indentation. Polym. Bull. 2011, 66, 649–660. [Google Scholar] [CrossRef]
- Mazur, M. Preparation of three-dimensional polymeric structures using gas bubbles as templates. J. Phys. Chem. C 2008, 112, 13528–13534. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G.; Chen, F.; Zhang, J. Electrochemical Growth of Polypyrrole Microcontainers. Macromolecules 2003, 36, 1063–1067. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G. Hollow microstructures of polypyrrole doped by poly(styrene sulfonic acid). J. Polym. Sci. A Polym. Chem. 2004, 42, 3170–3177. [Google Scholar] [CrossRef]
- Turco, A.; Mazzotta, E.; Di Franco, C.; Santacroce, M.V.; Scamarcio, G.; Grazia Monteduro, A.; Primiceri, E.; Malitesta, C. Templateless synthesis of polypyrrole nanowires by non-static solution-surface electropolymerization. J. Solid. State Electrochem. 2016, 20, 2143–2151. [Google Scholar] [CrossRef]
- Chagas, G.R.; Darmanin, T.; Guittard, F. One-Step and Templateless Electropolymerization Process Using Thienothiophene Derivatives to Develop Arrays of Nanotubes and Tree-like Structures with High Water Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 22732–22743. [Google Scholar] [CrossRef]
- McCarthy, C.P.; McGuinness, N.B.; Carolan, P.B.; Fox, C.M.; Alcock-Earley, B.E.; Breslin, C.B.; Rooney, A.D. Electrochemical Deposition of Hollow N-Substituted Polypyrrole Microtubes from an Acoustically Formed Emulsion. Macromolecules 2013, 46, 1008–1016. [Google Scholar] [CrossRef]
- Liu, D.; Uda, M.; Seike, M.; Fukui, S.; Hirai, T.; Nakamura, Y.; Fujii, S. Polypyrrole-coated Pickering-type droplet as light-responsive carrier of oily material. Colloids Polym. Sci. 2022, 300, 255–265. [Google Scholar] [CrossRef]
- Hui, F.; Li, B.; He, P.; Hu, J.; Fang, Y. Electrochemical fabrication of nanoporous polypyrrole film on HOPG using nanobubbles as templates. Electrochem. Comm. 2009, 11, 639–642. [Google Scholar] [CrossRef]
- Lee, J.I.; Hyo Cho, S.; Park, S.-M.; Kon Kim, J.; Kyeong Kim, J.; Woong Yu, J.; Kim, C.Y.; Russell, T.P. Highly Aligned Ultrahigh Density Arrays of Conducting Polymer Nanorods using Block Copolymer Templates. Nano Lett. 2008, 8, 2315–2320. [Google Scholar] [CrossRef]
- Li, X.; Malardier-Jugroot, C. Confinement Effect in the Synthesis of Polypyrrole within Polymeric Templates in Aqueous Environments. Macromolecules 2013, 46, 2258–2266. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.; Stavaux, P.-Y. Effect of Electrolyte Concentration and Nature on the Morphology and the Electrical Properties of Electropolymerized Polypyrrole Nanotubules. Chem. Mater. 1999, 11, 829–834. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Mikoliunaite, L.; Plausinaitiene, V.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Polypyrrole Formation from Pyrrole ‘Adlayer’. Phys. Chem. Chem. Phys. 2017, 19, 1029–1038. [Google Scholar] [CrossRef]
- Páramo-Garcia, U.; Avalos-Perez, A.; Guzman-Pantoja, J.; Díaz-Zavala, N.P.; Melo-Banda, J.A.; Gallardo-Rivas, N.V.; Reyes-Gómez, J.; Pozas-Zepeda, D.; Ibanez, J.G.; Batina, N. Polypyrrole microcontainer structures and doughnuts designed by electrochemical oxidation: An electrochemical and scanning electron microscopy study. e-Polymers 2014, 14, 75–84. [Google Scholar] [CrossRef]
- Mosch, H.L.K.S.; Akintola, O.; Plass, W.; Hoeppener, S.; Schubert, U.S.; Ignaszak, A. The specific surface versus electrochemically active area of the carbon/polypyrrole capacitor: The correlation of ion dynamics studied by an electrochemical quartz crystal microbalance with BET surface. Langmuir 2016, 32, 4440–4449. [Google Scholar] [CrossRef] [PubMed]
- Iordoc, M.; Bara, A.; Prioteasa, P.; Teisanu, A.; Marinescu, V. Electropolymerization of Conducting Polypyrrole on Carbon Nanotubes/Silicon Composite for Supercapacitor Applications. Rev. Chim. 2015, 66, 196–201. [Google Scholar]
- Krukiewicz, K.; Herman, A.P.; Turczyn, R.; Szymańska, K.; Koziol, K.K.; Boncel, S.; Zak, J.K. A role of nanotube dangling pyrrole and oxygen functions in the electrochemical synthesis of polypyrrole/MWCNTs hybrid materials. Appl. Surf. Sci. 2014, 317, 794–802. [Google Scholar] [CrossRef]
- Carragher, U.; Branagan, D.; Breslin, C.B. The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper. Materials 2019, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, B.; Abu-Zurayk, R.; Waleed, H.; Alqader Ibrahim, A. Fabrication of a Novel (PVDF/MWCNT/Polypyrrole) Antifouling High Flux Ultrafiltration Membrane for Crude Oil Wastewater Treatment. Membranes 2022, 12, 751. [Google Scholar] [CrossRef]
- Jara-Cornejo, E.; Khan, S.; Vega-Chacón, J.; Wong, A.; Chagas da Silva Neres, L.; Picasso, G.; Sotomayor, M.D.P.T. Biomimetic Material for Quantification of Methotrexate Using Sensor Based on Molecularly Imprinted Polypyrrole Film and MWCNT/GCE. Biomimetics 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Rohanifar, A.; Rodriguez, L.B.; Devasurendra, A.M.; Alipourasiabi, N.; Anderson, J.L.; Kirchhoff, J.R. Solid-Phase Microextraction of Heavy Metals in Natural Water with a Polypyrrole/Carbon Nanotube/1,10–Phenanthroline Composite Sorbent Material. Talanta 2018, 108, 570–577. [Google Scholar] [CrossRef]
- Amiri, A.; Baghayeri, M.; Shahabizadeh, M. Polypyrrole/carbon nanotube coated stainless steel mesh as a novel sorbent. New J. Chem. 2023, 47, 4402–4408. [Google Scholar] [CrossRef]
- Arjunan, T.V.; Senthil, T.S. Review: Dye sensitised solar cells. Mater. Technol. 2013, 28, 9–14. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Saberi Motlagh, M.; Mottaghita, V.; Rismanchi, A.; Rafieepoor Chirani, M.; Hasanzadeh, M. Performance modelling of textile solar cell developed by carbon fabric/polypyrrole flexible counter electrode. Int. J. Sustain. Energy 2022, 41, 1106–1126. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Sun, W. Electropolymerization and application of polyoxometalate doped polypyrrole film electrodes in dye-sensitized solar cells. Electrochem. Comm. 2020, 122, 106879–106896. [Google Scholar] [CrossRef]
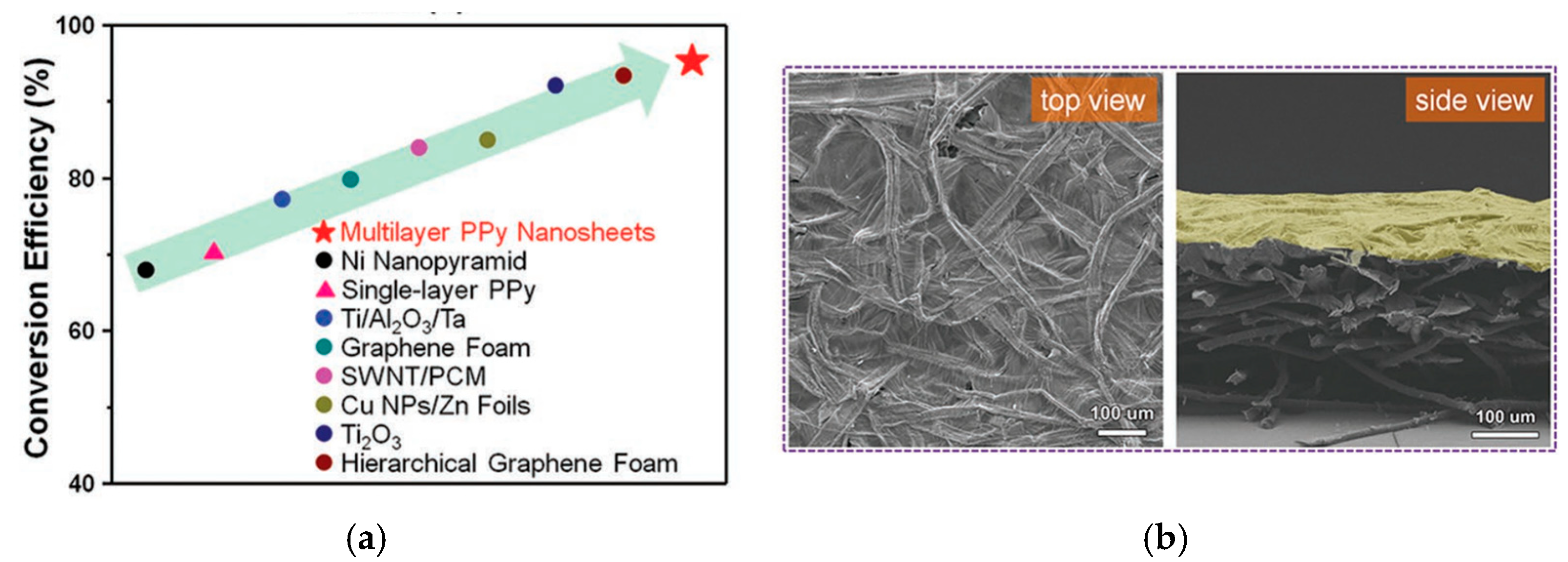
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer Polypyrrole Nanosheets with Self-Organized Surface Structures for Flexible and Efficient Solar–Thermal Energy Conversion. Adv. Mater. 2019, 31, 1807716–1807725. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Song, X.; Huang, M.; Jiang, H. A Facile and General Strategy to Deposit Polypyrrole on Various Substrates for Efficient Solar-Driven Evaporation. Adv. Sustain. Syst. 2019, 3, 1800108–1800116. [Google Scholar] [CrossRef]
- Lee, S.; Bayarkhuu, B.; Han, Y.; Kim, H.-W.; Jeong, S.; Boo, C.; Byun, J. Multifunctional photo-Fenton-active membrane for solar-driven water purification. J. Membr. Sci. 2022, 32, 4440. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Bertelsmann, K.; Fan, D.E. Portable Low-Pressure Solar Steaming-Collection Unisystem with Polypyrrole Origamis. Adv. Mater. 2019, 31, 1900720–1900728. [Google Scholar] [CrossRef]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311–312, 147–161. [Google Scholar] [CrossRef]
- Zhang Haiyan Pan, C.; Wang, X.; Sun, S.-K. Microwave-assisted ultrafast fabrication of high-performance polypyrrole nanoparticles for photothermal therapy of tumors in vivo. Biomater. Sci. 2018, 6, 2750–2756. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef]
- Maity, K.; Mandal, D. All-Organic High-Performance Piezoelectric Nanogenerator with Multilayer Assembled Electrospun Nanofiber Mats for Self-Powered Multifunctional Sensors. ACS Appl. Mater. Interfaces 2018, 10, 18257–18269. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Pan, Z.; Cheng, Y.; Zhai, Y.; Zhang, Y.; Ding, X.; Liu, I.; Zhai, J.; Xu, J. Three-dimensional polypyrrole induced high-performance flexible piezoelectric nanogenerators for mechanical energy harvesting. Compos. Sci. Technol. 2022, 219, 109260–109268. [Google Scholar] [CrossRef]
- Maity, K.; Garain, S.; Henkel, K.; Schmeißer, D.; Mandal, D. Self-Powered Human-Health Monitoring through Aligned PVDF Nanofibers Interfaced Skin-Interactive Piezoelectric Sensor. ACS Appl. Polym. Mater. 2020, 2, 862–878. [Google Scholar] [CrossRef]
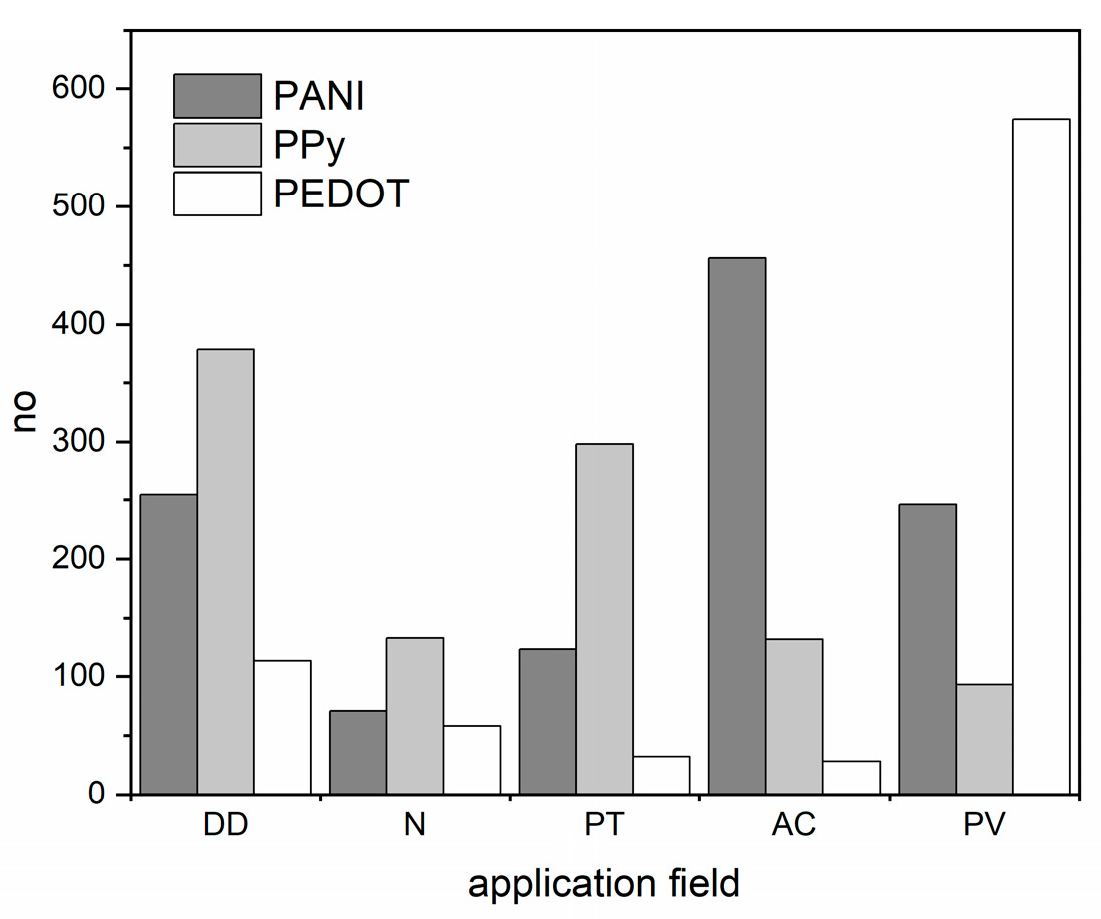
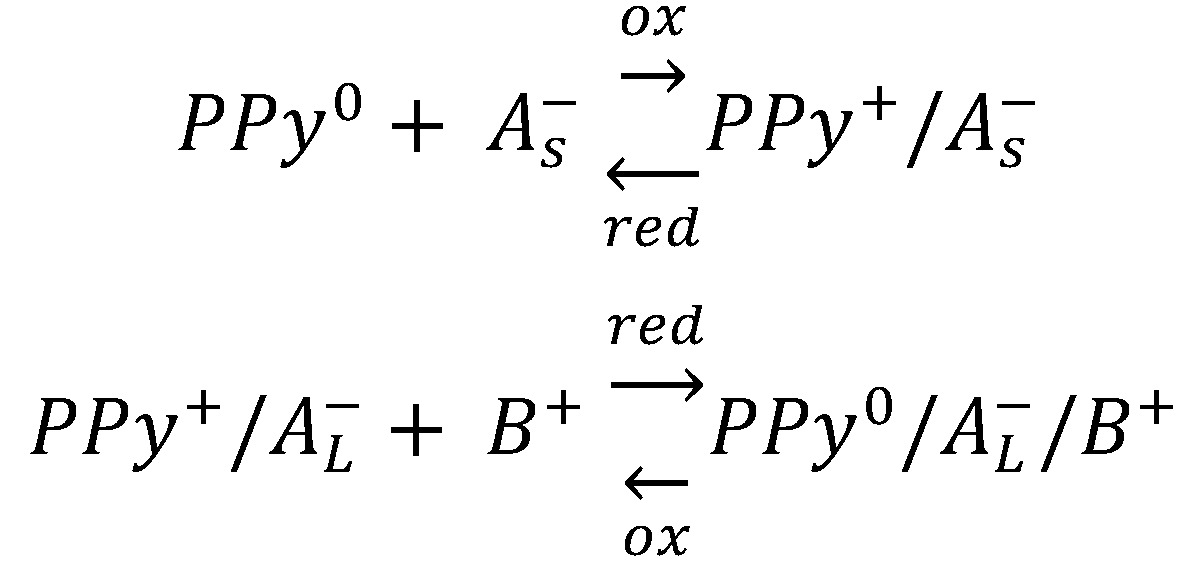
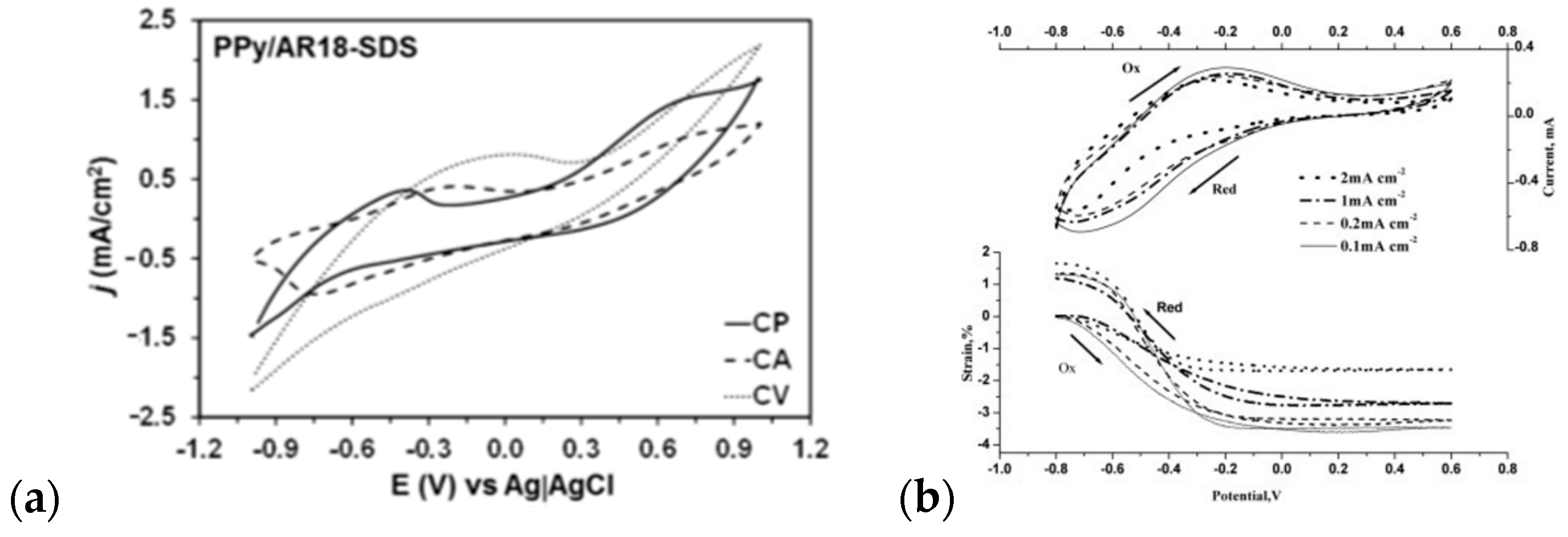

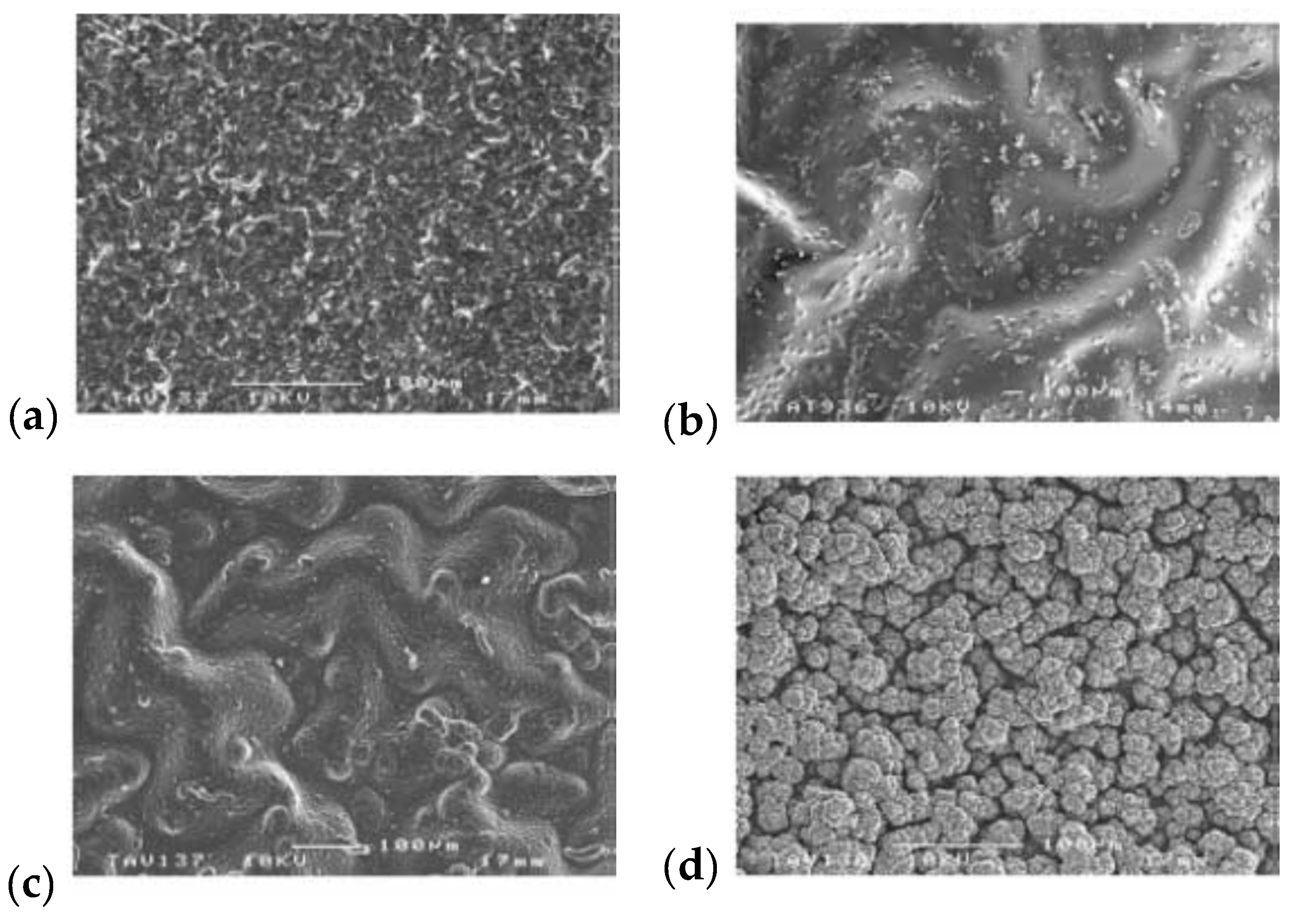
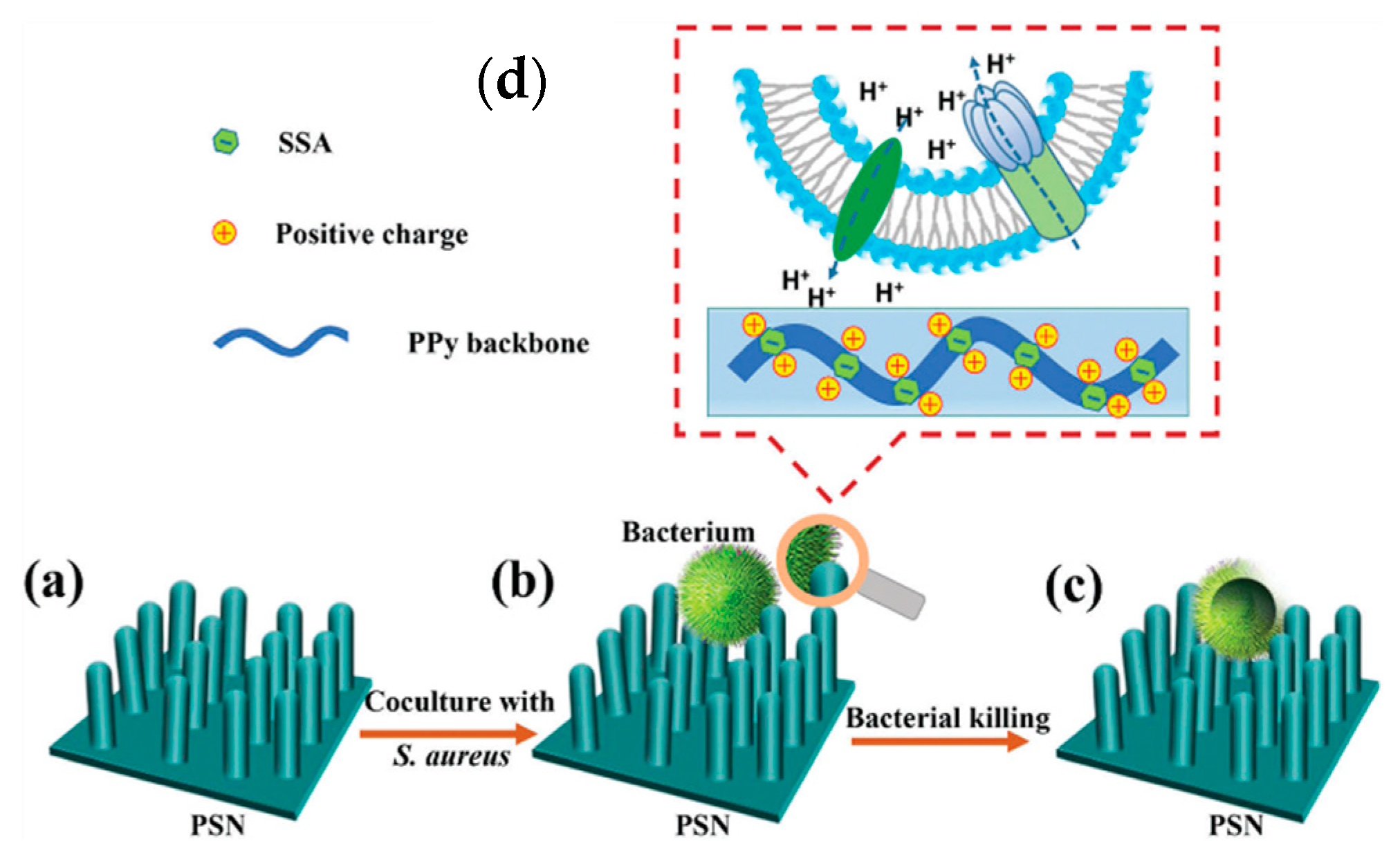
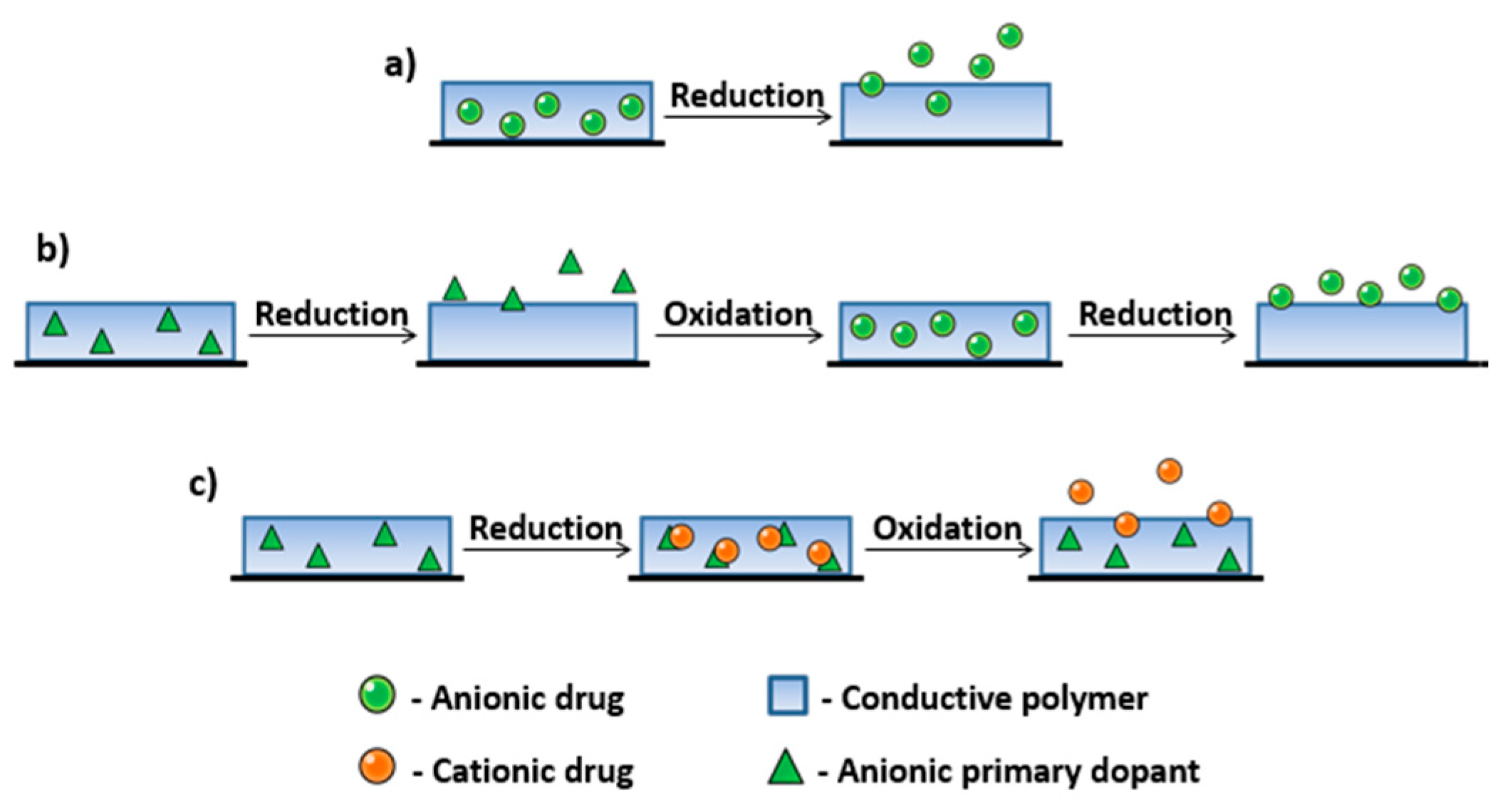
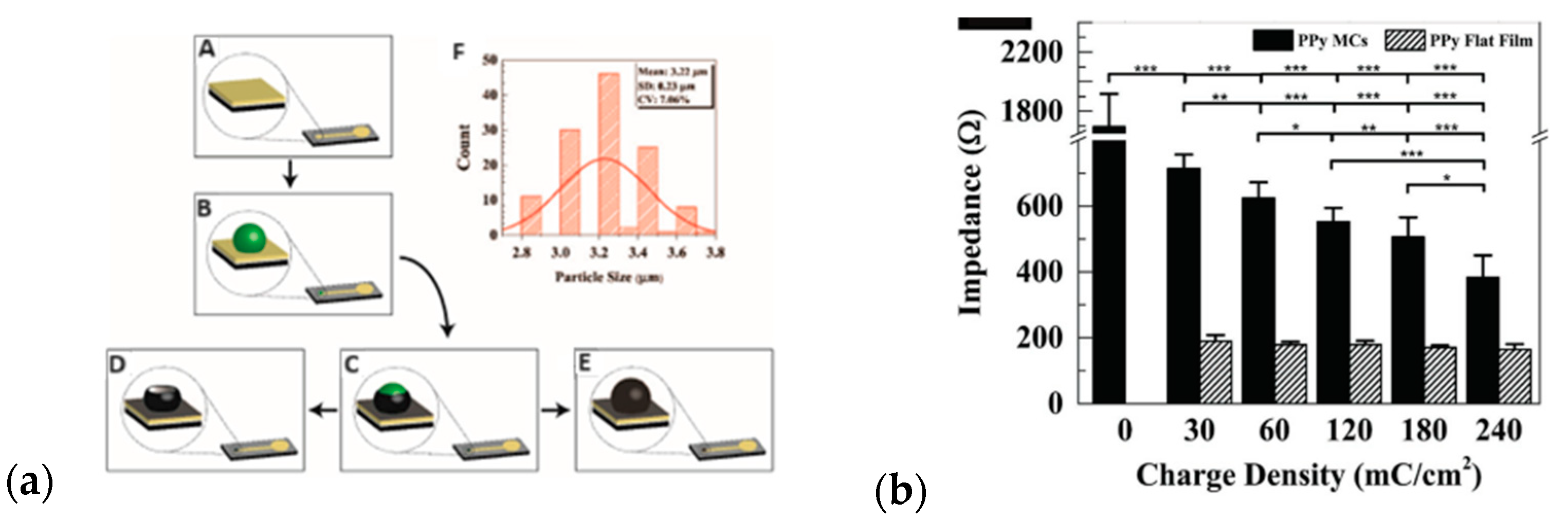

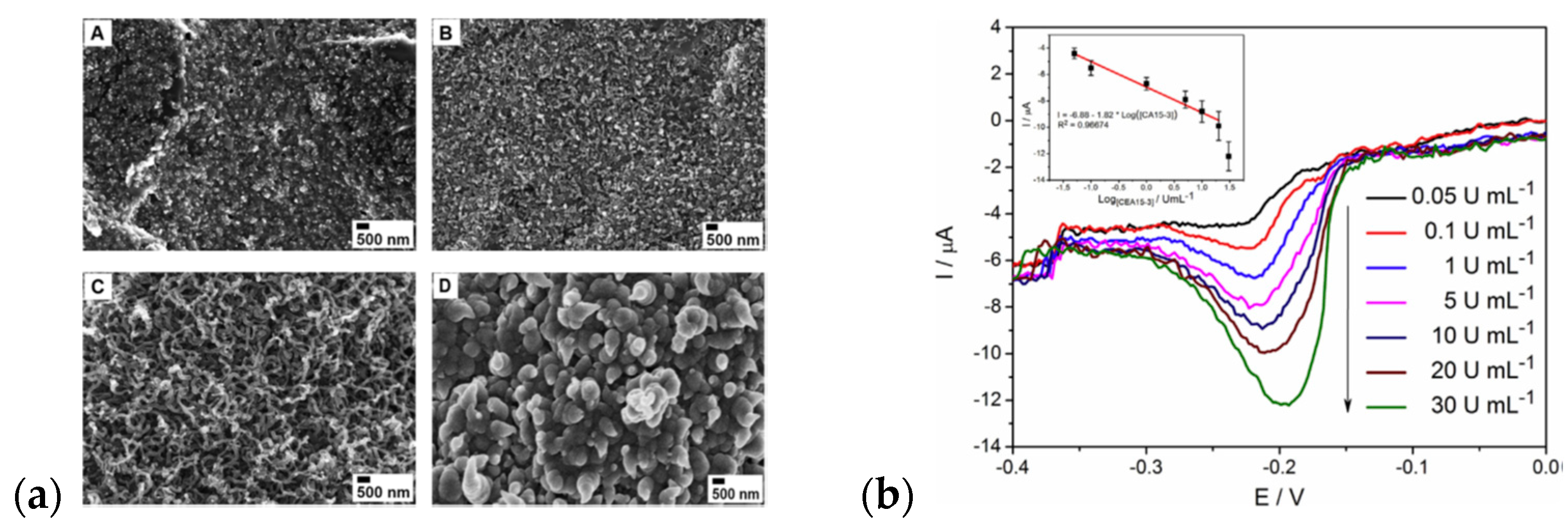
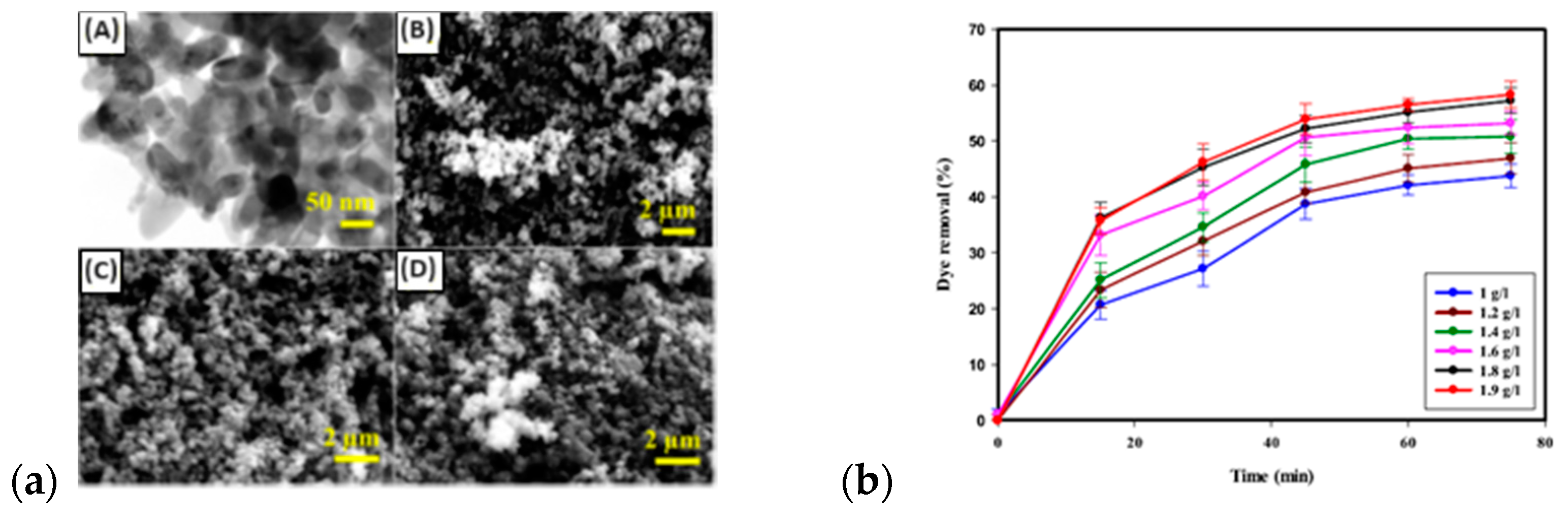
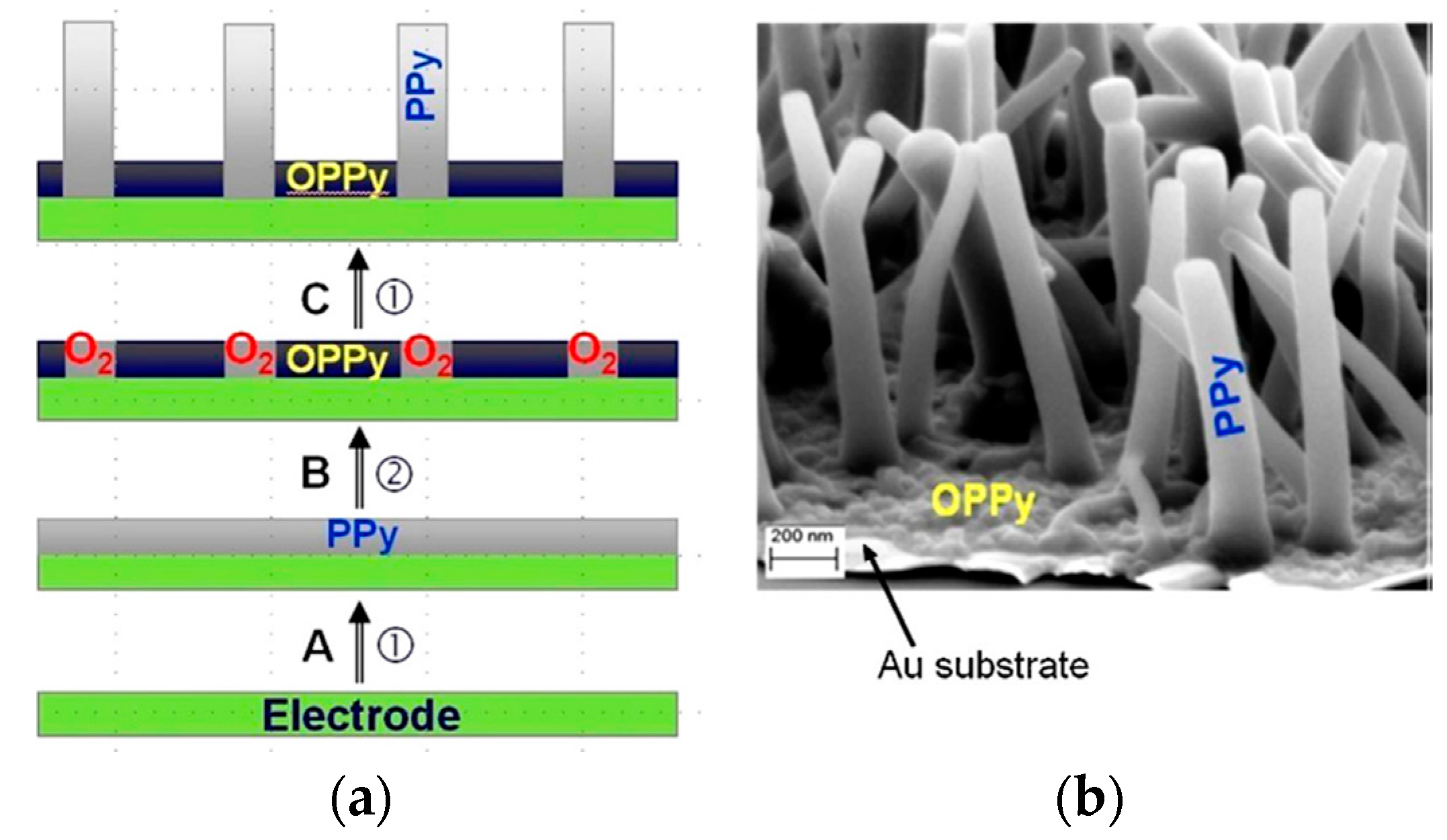
| Material | Synthesis Mode | Technique | Dopant/Initiator | Conductivity S/cm | Morphology | Application Filed | Source |
|---|---|---|---|---|---|---|---|
| block co-polymers of PPy with poly(ε-caprolactone) and poly(ethyl cyanoacrylate) | ChOP | two-step procedure: macromonomer formation and Py co-polymerization | para-toluene sulfonate (pTS−) | (18–32) | a flat compacted surface | cell proliferation platform (rat PC12 cells) | [120] |
| PLLA/PCL fibers coated with PPy and chitosan (CS) | EChP | WE: ITO with electrospun PLLA/PCL fibers galvanostatic (8 min/2 mA) | chloride (Cl−) | (1–1.1)·10−2 | cell differentiation platform and neurite growth (PC12 cells) | [122] | |
| hydrogel based on sodium alginate, gelatin and polypyrrole | ChOP | rapid mixing/−20 °C | ammonium persulfate (I) | (1.2–1.6)·10−2 | network structure with well-dispersed polypyrrole particles | self-healing conductive hydrogels | [123] |
| aligned PPy/PLA composite electrospun films | ChOP | P123 used as a template dropwise method (18 °C/6 h) | aqueous FeCl3 | 4.6 | spherical PPy particles | platform for differentiation of human cord mesenchymal stem cells | [124] |
| collagen-heparin-polypyrrole composite | ChOP | vigorous stirring for 30 min followed by standing at r.t | FeCl3 | 0.11–0.336 | compact structure with partial orientation | neural scaffold in the application of peripheral nerve regeneration (PC12 cells cultured) | [174] |
| Material | Synthesis Mode | Technique | Dopant/Initiator | Tested Strain | Morphology | Application Filed | Source |
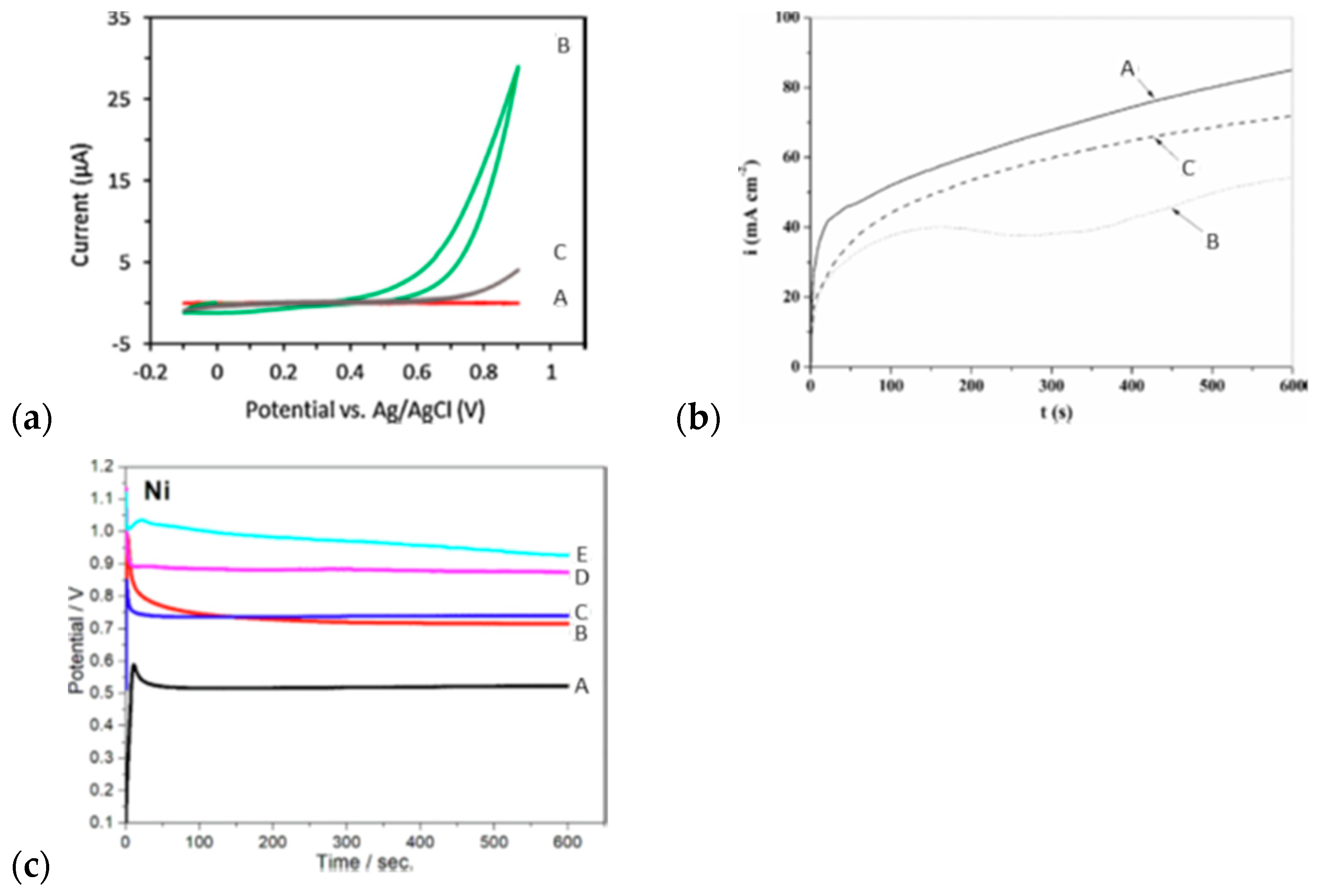
| branched polypyrrole | EChP | WE: anode (metal wire) potentiostatic (9 V/10 min) | sodium dodecylbenzenesulfonate (DBSA), cetyl trimethylammonium bromid (CTAB) | S. aureus, E. coli, K. pneumoniae | fractal structure | antibacterial material | [8] |
| polydimethylsiloxane (PDMS) gradient doped with PPy | ChOP | dropwise technique under continuous mixing/ 2.5 h at 150 rpm | FeCl3 | E. coli | increased surface roughness with typical granular forms | switchable superhydrophobic and self-cleaning material with drug releasing ability | [137] |
| a duplex coating based on PPy and molybdate—originated layer loaded with silver | EChP | WE: AZ91D (magnesium alloy) potentiostatic (1.15 V/600 s for 0.50 M NaSa and 0.80 V/1800 s for 0.10 M NaSa) | sodium salicylate (NaSa) | E. coli | globular morphology for lower NaSa concentrations, rectangular microtubes for higher | antibacterial activity with anticorrosive performance | [138] |
| PPy with oxygen plasma immersion ion implantation (O-PIIi) | EChP | WE: Ti/galvanostatic (5 mA/cm2, 10 min), r.t. | TsONa (sodium p-toluenesulfonate) | E. coli, S. aureus | Cauliflower morphology, after O-PIII treatment—pit-like structure occurs | antibacterial material | [134] |
| nanostructured PPy | template-free EChP | WE: Ti/galvanostatic (0.9 mA/cm2, 5 min), r.t. | sulfosalicylic acid in PBS | S. aureus | oriented nanorods with large specific surface area | antibacterial material | [142] |
| Material | Synthesis Mode | Technique | Dopant | Release Mode | Morphology | Application Filed | Source |
| polypyrrole | EChP | WE: Pt-black coated glass/potentiostatic (0.7 V, 200 s) | fluorescein | 10 s pulses/−2.0 V into PBS | typical globular morphology | drug-delivery module | [150] |
| polypyrrole nanowire | EChP | WE: Au electrode/ galvanostatic (0.477 mA/cm2, 1600 s) pTS− in PBS (pH 7.40) | adenosine triphosphate (ATP) dexamethasone (Dex) | CV stimulation (−0.9:0.6) V | nanowire network with porous interwoven structures | drug-delivery module | [111] |
| oxacillin-doped PPy (PPyOx) PPyOx modified with chitosan | EChP | WE: gold, platinum titanium/potentiostatic (−0.80 V vs. SCE, 500 s) | oxacillin | constant potential at 0.30 V or 0.60 V | smooth polymer films with roughness induced by the oxacillin presence | drug-delivery module | [151] |
| nanostructu olysaccharideride-doped polypyrrole | EChP | WE: Pt Two-step procedure: pre-electropolymerization in hep presence, potentiostatic (+0.9 V vs. Ag/AgCl/100 s) and electropolymerization in the CPZ presence potentiostatic (+0.7 V vs. Ag/AgCl/900 s) | heparin sodium salt (50,000 units) chlorpromazine hydrochloride | OCP and constant potential conditions (0.1:0.4) V | homogeneous, porous nanostructure with spherical morphology | drug-delivery module | [154] |
| doped polypyrrole | ECHP | WE: platinum foil in-situ drug immobilization mode—cyclic voltammetry (CV) ex-situ drug immobilization—CV for polymerization of Py followed by oxidative immobilization of drugs | quercetin and ciprofloxacin | constant a reduction potential (−0.5 V vs. Ag/AgCl) in PBS | matrix obtained by ex-situ mode less uniform with larger PPy grains and rougher surface | drug-delivery module | [155] |
| Material | Synthesis Mode | Technique | Detected Analyte | Detection Mode | Morphology | Application Filed | Source |
| Doped polypyrrole | EChP | WE: gold electrode/sodium perchlorate CV with different scan rate (5:50) mV/s | dimethyl methyl phosphate (DMMP) | EIS | globular and rod (for slow sr) structures and packed globular system for high sr | sensor | [160] |
| Molecularly imprinted polypyrrole | template assisted EChP | WE: fluorine-tin oxide FTO/CV (10 cycles, (0.0:0.7) V, 50 mV/s) in PBS, pH = 7.2 | carcinoembryonic antigen (CEA) alpha-fetoprotein (AFP) | EiS | PPy-MO NIP: hollow rectangular nanotubes PPy-MO DMIP: rougher tubular structure | sensor | [42] |
| Gold—overoxidized polypyrrole nanocomposite | ECHP | WE: glassy carbon electrode (GCE), LiClO4, potentiostatic (800 mV (vs. Ag/AgCl)/120 s, overoxidized at 1.0 V/420 s), AuNP- CV (0.2:−1.0) V, 50 mV/s, 15 cycles | tissue transglutaminase (tTG)-specific antigen | EIS | OPPy: cauliflower-like structure with good surface coverage of the AuNP | sensor | [161] |
| Poly(1,5-diaminonaphthalene)/polypyrrole bilayer | EChP guided with oxygen nanobubbles | WE: screen-printed electrodes (SPEs) Two-step procedure: electropolymerization of Py potentiostatically (0.75 V/500 s in 0.2 M Na2HPO4, LiClO4 (1:−15) mM) followed by P(1,5DAN) deposition, CV (50 mV/s, (−0.02:0.75 V) | CA 15-3 antigen | DPV | nanowires (for optimized dopant concentration), for high concentration cauliflower-like structure | sensor | [163] |
| Polypyrrole/Nanoclay Hybrid Film | EChP | WE: glassy carbon electrode (GCE) CV ((−0.10:1.0) V, 200 mV/s, 20 cycles)I ACN, 0.1 M LiClO4 Anti-cTnT antibodies immobilized with glutaraldehyde (GA) | cardiac troponins (T and I) | SWV | heterogenous film formed by agglomerates of two-dimensional laminar shapes | sensor | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golba, S.; Loskot, J. The Alphabet of Nanostructured Polypyrrole. Materials 2023, 16, 7069. https://doi.org/10.3390/ma16227069
Golba S, Loskot J. The Alphabet of Nanostructured Polypyrrole. Materials. 2023; 16(22):7069. https://doi.org/10.3390/ma16227069
Chicago/Turabian StyleGolba, Sylwia, and Jan Loskot. 2023. "The Alphabet of Nanostructured Polypyrrole" Materials 16, no. 22: 7069. https://doi.org/10.3390/ma16227069
APA StyleGolba, S., & Loskot, J. (2023). The Alphabet of Nanostructured Polypyrrole. Materials, 16(22), 7069. https://doi.org/10.3390/ma16227069