The Alphabet of Nanostructured Polypyrrole
Abstract
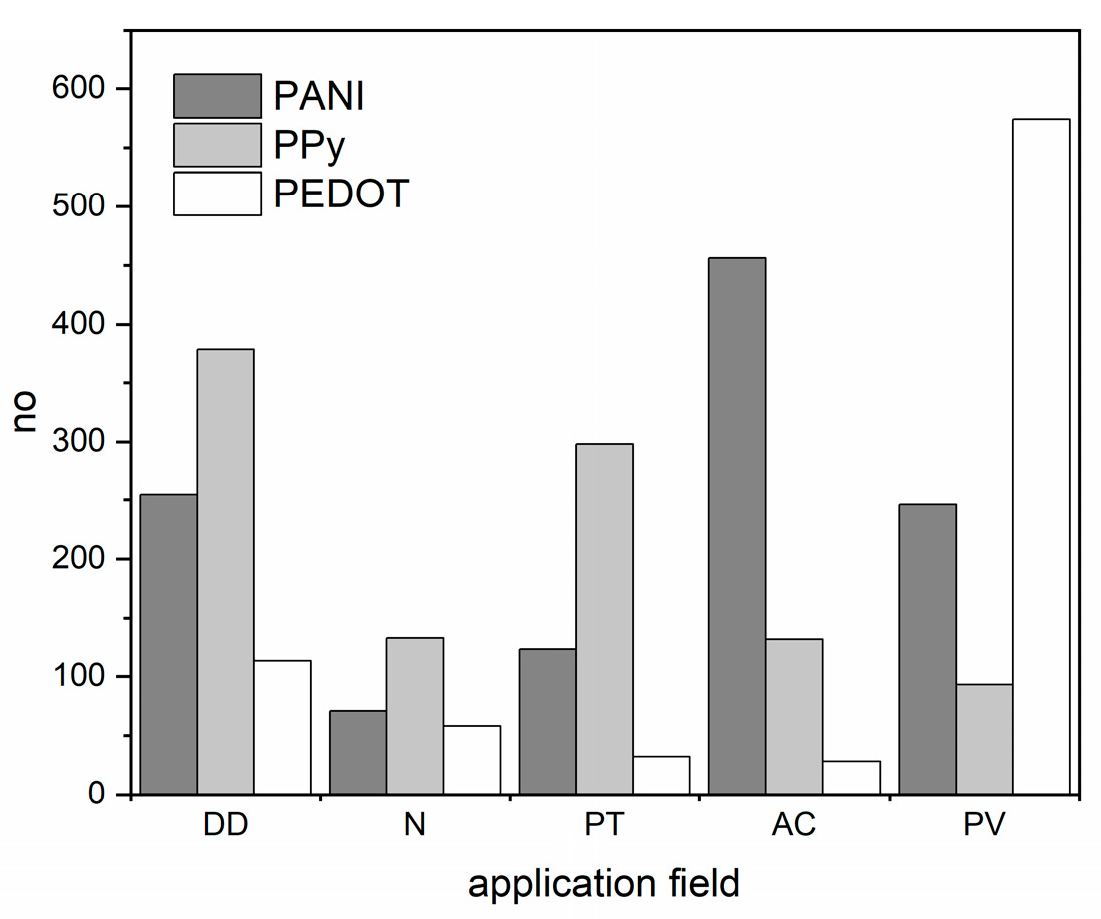
1. Introduction
2. Deposition of Electroactive Polypyrrole
3. Polypyrrole Doping and Conduction Path
4. Morphology of Polypyrroles
4.1. The Impact of Morphology on the Bio-Applicability of PPy
4.1.1. N as Neural Applications
4.1.2. A as Antibacterial and Implantable Applications
4.1.3. D as Drug-Delivery Platforms
4.1.4. S as Sensors and Sorbents
4.2. The Impact of Morphology on the Technological Applicability of PPy
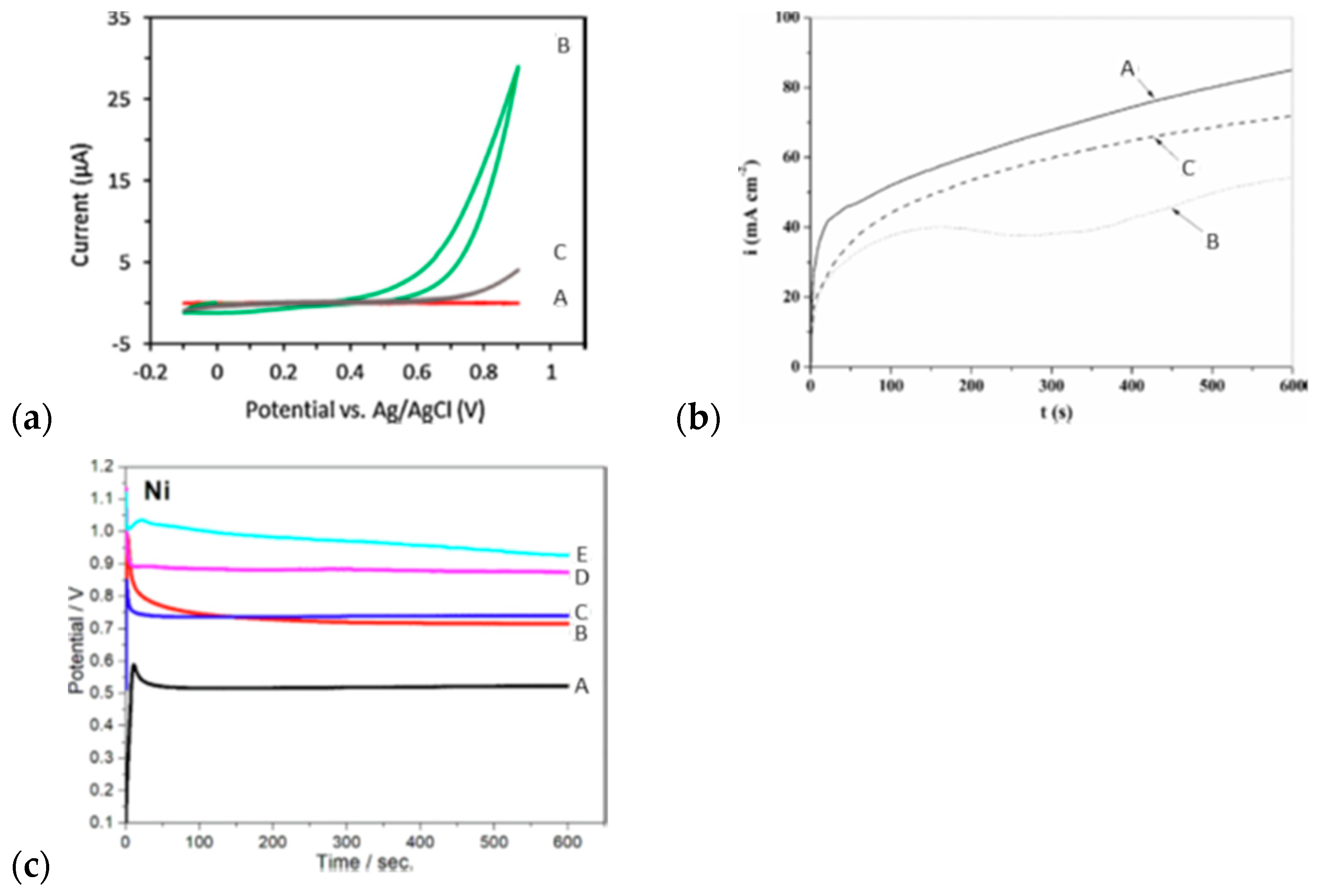
4.2.1. P as Corrosion Protection
4.2.2. M as Mechanical Aspects
4.2.3. B as Bubbles and Nanoporous Structures
4.2.4. C as Carbon-Based Materials
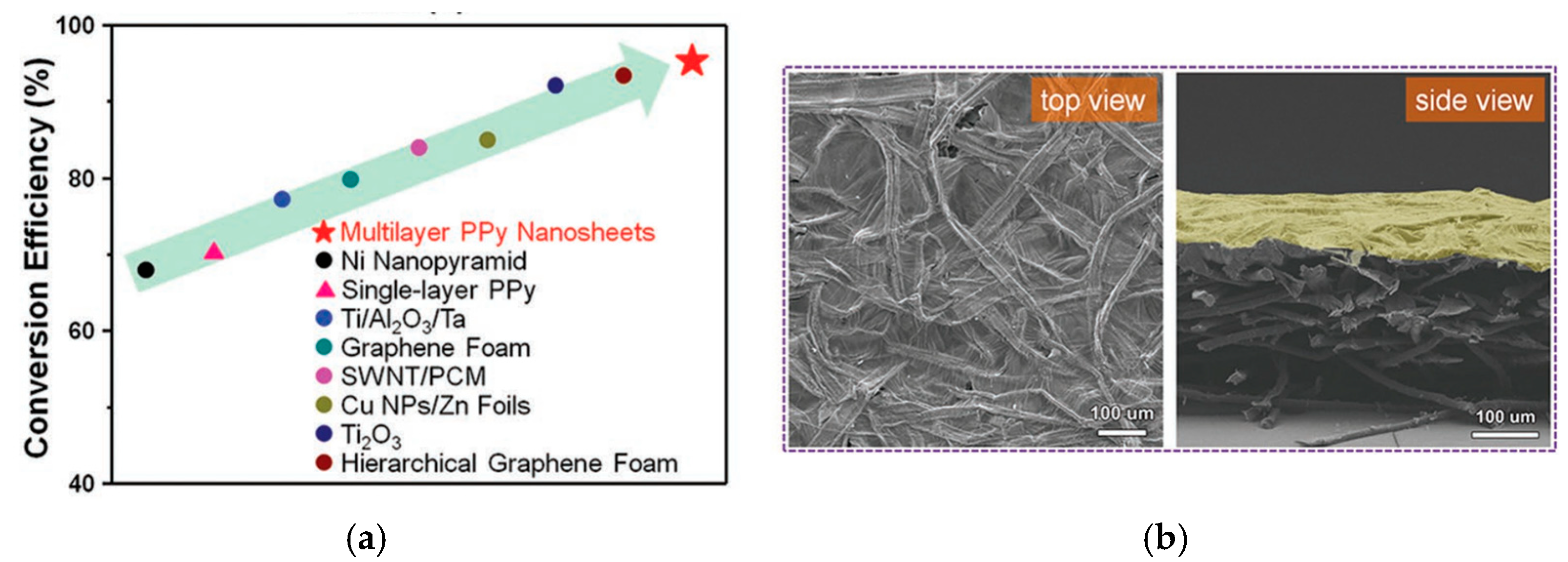
4.2.5. E as Energy Conversion Systems (Solar, Photothermal and Energy Storage Applications)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahvazi Zadeh, M.; Yeganeh, M.; Tavakoli Shoushtari, M.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synt. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; da Silva, C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739–135753. [Google Scholar] [CrossRef]
- El Guerraf, A.; Ben Jadi, S.; Karadas Bakirhan, N.; Eylul Kiymaci, M.; Bazzaoui, M.; Aysil Ozkan, S.; Arbi Bazzaoui, E. Antibacterial activity and volatile organic compounds sensing property of polypyrrole-coated cellulosic paper for food packaging purpose. Polym Bull. 2022, 79, 11543–11566. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Gniazdowska, B.; Jarosz, T.; Herman, A.P.; Boncel, S.; Turczyn, R. Effect of immobilization and release of ciprofloxacin and quercetin on electrochemical properties of poly(3,4-ethylenedioxypyrrole) matrix. Synt. Met. 2019, 249, 52–62. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, G.; Xu, H.; Kang, L. PPy doped with different metal sulphate as electrode materials for supercapacitors. Russ. J. Electrochem. 2017, 53, 359–365. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, J.; Miao, Z.; Qian, H.; Zha, Z. dl-Menthol Loaded Polypyrrole Nanoparticles as a Controlled Diclofenac Delivery Platform for Sensitizing Cancer Cells to Photothermal Therapy. ACS Appl. Bio. Mater. 2019, 2, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Ashu Tufa, R.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- da Silva, F.A.G., Jr.; Queiroz, J.C.; Macedo, E.R.; Fernandes, A.W.C.; Freire, N.B.; da Costa, M.M.; de Oliveira, H.P. Antibacterial behavior of polypyrrole: The influence of morphology and additives incorporation. Mater. Sci. Eng. C 2016, 62, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tracy Cui, X. Sponge-like nanostructured conducting polymers for electrically controlled drug release. Electrochem. Commun. 2009, 11, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, C.; Zhu, Z.; Liu, H.; Qu, J. Electrically Pore-Size-Tunable Polypyrrole Membrane for Antifouling and Selective Separation. Adv. Funct. Mater. 2019, 29, 1903081. [Google Scholar] [CrossRef]
- Riaz, U.; Singh, N.; Rashnas Srambikal, F.; Fatima, S. A review on synthesis and applications of polyaniline and polypyrrole hydrogels. Polym. Bull. 2022, 80, 1085–1116. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Li, X.; Zhu, L.; Xu, G.; Chen, Z.; Cheng, C.; Lu, Y.; Liu, Q. Wireless, battery-free and wearable device for electrically controlled drug delivery: Sodium salicylate released from bilayer polypyrrole by near-field communication on smartphone. Biomed. Microdev. 2020, 22, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq Ali Shah, S.; Firlak, M.; Ryan Berrow, S.; Ross Halcovitch, N.; Baldock, S.J.; Muhammad Yousafzai, B.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M.; Mahdavi, F. Application of the Electrochemical Quartz Crystal Microbalance for Electrochemically Controlled Binding and Release of Chlorpromazine from Conductive Polymer Matrix. Microchem. J. 1997, 56, 54–64. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Kinetic studies of electrochemically controlled release of salicylate from nanostructure conducting molecularly imprinted polymer. Electrochim. Acta 2013, 114, 409–415. [Google Scholar] [CrossRef]
- Kontturi, K.; Pentti, P.; Sundholm, G. Polypyrrole as a model membrane for drug delivery. J. Electroanal. Chem. 1998, 453, 231–238. [Google Scholar] [CrossRef]
- Wu, Y.; Ruan, Q.; Huang, C.; Liao, Q.; Liu, L.; Liu, P.; Mo, S.; Wang, G.; Wang, H.; Chu, P.K. Balancing the biocompatibility and bacterial resistance of polypyrrole by optimized silver incorporation. Biomat. Adv. 2022, 134, 112701–112715. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yu, M.; Chen, T.; Liu, Y.; Yi, Y.; Huang, C.; Tang, J.; Li, H.; Ou, M.; Wang, T.; et al. Polypyrrole Nanoenzymes as Tumor Microenvironment Modulators to Reprogram Macrophage and Potentiate Immunotherapy. Adv. Sci. 2022, 9, 2201703–2201720. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin Polypyrrole Nanosheets via Space-Confined Synthesis for Efficient Photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef]
- Sarkar, S.; Levi-Polyachenko, N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 40, 163–164. [Google Scholar] [CrossRef]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In Vitro and In Vivo Near-Infrared Photothermal Therapy of Cancer Using Polypyrrole Organic Nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef] [PubMed]
- Paúrova, M.; Taboubi, O.; Šeděnkova, I.; Hromădková, J.; Matouš, P.; Herynek, V.; Šefc, L.; Babič, M. Role of dextran in stabilization of polypyrrole nanoparticles for photoacoustic imaging. Eur. Polym. J. 2021, 157, 110634. [Google Scholar] [CrossRef]
- Liang, Y.; Mitriashkin, A.; Ting Lim, T.; Ting Lim, J. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. J. Biomat. 2021, 276, 121008–121022. [Google Scholar] [CrossRef]
- Lee, R.-J.; Temmer, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Renewable antioxidant properties of suspensible chitosan–polypyrrole composites. React. Funct. Polym. 2013, 73, 1072–1077. [Google Scholar] [CrossRef]
- Upadhyay, J.; Gogoi, B.; Kumar, A.; Buragohain, A.K. Diameter dependent antioxidant property of polypyrrole nanotubes for biomedical applications. Mat. Lett. 2013, 102–103, 33–35. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Mazzuchetti, G. Antibacterial efficacy of polypyrrole in textile applications. Fib. Polym. 2013, 14, 36–42. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A.; Gogoi, B.; Buragohain, A.K. Antibacterial and hemolysis activity of polypyrrole nanotubes decorated with silver nanoparticles by an in-situ reduction process. Mat. Sci. Eng. C 2015, 54, 8–13. [Google Scholar] [CrossRef]
- Soleimani, M.; Ghorbani, M.; Salahi, S. Antibacterial Activity of Polypyrrole-Chitosan Nanocomposite: Mechanism of Action. Int. J. Nanosci. Nanotechnol. 2016, 12, 191–197. [Google Scholar]
- Nautiyal, A.; Qiao, M.; Edwin Cook, J.; Zhang, X.; Huang, T.-S. High performance polypyrrole coating for corrosion protection and biocidal applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Z.; Liang, P.; Li, H.; Zhang, Y.; Fan, W.; Xu, G. Fabrication of polypyrrole coated superhydrophobic surfaces for effective oil/water separation. J. Mater. Res. Technol. 2022, 19, 4337–4349. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Progr. Org. Coat. 2020, 147, 105815–105825. [Google Scholar] [CrossRef]
- Morsi, S.M.M.; Abd El-Aziz, M.E.; Morsi, R.M.M.; Hussain, A.I. Polypyrrole-coated latex particles as core/shell composites for antistatic coatings and energy storage applications. J. Coat. Technol. Res. 2019, 16, 745–759. [Google Scholar] [CrossRef]
- Muro-Fraguas, I.; Sainz-García, A.; López, M.; Rojo-Bezares, B.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; González-Marcos, A.; Alba-Elías, F. Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments. Surf. Coat. Technol. 2020, 399, 126163–126173. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Kader, A.; Metwally, A.; El-Ahmady, S.H.; Metwally, E.S.; Ghonim, N.A.; Bayoumy, S.A.; Erfan, T.; Ashraf, R.; Fadel, M.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, X.; Wang, J.; Wang, Y.; Song, Y.; You, Q.; Sun, Q.; Liu, L.; Wang, S.; Tan, F.; et al. Polymer-based gadolinium oxide nanocomposites for FL/MR/PA imaging guided and photothermal/photodynamic combined antitumor therapy. J. Control. Release 2018, 277, 77–88. [Google Scholar] [CrossRef]
- Michalik, A.; Rohwerder, M. Conducting polymers for corrosion protection: A critical view. J. Phys. Chem. 2005, 219, 1547–1559. [Google Scholar] [CrossRef]
- Paliwoda-Porebska, G.; Stratmann, M.; Rohwerder, M.; Potje-Kamloth, K.; Lu, Y.; Pich, A.Z.; Adler, H.-J. On the development of polypyrrole coatings with self-healing properties for iron corrosion protection. Corros. Sci. 2005, 47, 3216–3233. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mat. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymers are not just conducting: A perspective for emerging technology. Polym. Int. 2020, 69, 662–664. [Google Scholar] [CrossRef]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-template rectangular nanotube molecularly imprinted polypyrrole for label-free impedimetric sensing of AFP and CEA as lung cancer biomarkers. Talanta 2022, 239, 123146–123156. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, T.; Han, Y.; Wang, Y.; Zhan, C.; Wang, Q. Optimizing the polymerization conditions of conductive polypyrrole. J. Photopolym. Sci. Technol. 2016, 29, 803–806. [Google Scholar] [CrossRef]
- Rath, A.; Theato, P. Advanced AAO Templating of Nanostructured Stimuli-Responsive Polymers: Hype or Hope? Adv. Funct. Mater. 2020, 30, 1902959–1902975. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Dazzi, A.; Lefrançois, P.; Gervais, M.; Néron, S.; Remita, S. Radiolytic Method as a Novel Approach for the Synthesis of Nanostructured Conducting Polypyrrole. Langmuir 2014, 30, 14086–14094. [Google Scholar] [CrossRef]
- Taouil, A.E.; Mourad Mahmoud, M.; Lallemand, F.; Lallemand, S.; Gigandet, M.-P.; Hihn, J.-Y. Corrosion protection by sonoelectrodeposited organic films on zinc coated steel. Ultras Sonochem. 2012, 19, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3 and Redox Cycling of [Fe(CN)6]4/[Fe(CN)6]3. Polymers 2018, 10, 749. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Grijalva-Bustamante, G.A.; Evans-Villegas, A.G.; del Castillo-Castro, T.; Castillo-Ortega, M.M.; Cruz-Silva, R.; Huerta, F.; Morallón, E. Enzyme mediated synthesis of polypyrrole in the presence of chondroitin sulfate and redox mediators of natural origin. Mater. Sci. Eng. C 2016, 63, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Fernandez, F.D.M.; Khadka, R.; Yim, J.-H. A comparative study between vapor phase polymerized PPy and PEDOT—Thermoplastic polyurethane composites for ammonia sensing. Polymer 2021, 217, 123463–123470. [Google Scholar] [CrossRef]
- Shafiqur Rahman, M.; Wasiu Adebayo, H.; Yahya, R.; Khairani Mohd Jamil, A.; Nabi Muhammad Ekramul Mahmud, H. One-step facile synthesis of poly(N-vinylcarbazole)-polypyrrole/graphene oxide nanocomposites: Enhanced solubility, thermal stability and good electrical conductivity. J. Macromol. Sci. Part. A 2019, 56, 384–391. [Google Scholar] [CrossRef]
- Kiefer, R.; Khadka, R.; Travas-Sejdic, J. Poly(ethylene oxide) in polypyrrole doped dodecylbenzenesulfonate: Characterisation and linear actuation. Int. J. Nanotechnol. 2018, 15, 689–694. [Google Scholar] [CrossRef]
- Brie, M.; Turcu, R.; Mihut, A. Stability study of conducting polypyrrole films and polyvinylchloride-polypyrrole composites doped with different counterions. Mater. Chem. Phys. 1997, 49, 174–178. [Google Scholar] [CrossRef]
- Kirsnytėa, M.; Jurkūnasa, M.; Kanclerisa, Ž.; Ragulisa, P.; Simniškisa, R.; Vareikisc, A.; Abraitienėa, A.; Požėlaa, K.; Whitesideb, B.; Tuinea-Bobeb, C.L.; et al. Investigation of in situ formed conductive polymer composite in adhesive matrix. Synt. Met. 2019, 258, 116181. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmad, S. A review on conducting polymer reinforced polyurethane composites. J. Ind. Eng. Chem. 2017, 53, 1–22. [Google Scholar] [CrossRef]
- Wampler, W.A.; Rajeshwara, K.; Pethe, R.G.; Hyer, R.C.; Sharma, S.C. Composites of polypyrrole and carbon black: Part III. Chemical synthesis and characterization. J. Mater. Res. 1995, 10, 1811–1822. [Google Scholar] [CrossRef]
- Hagler, J.R.; Peterson, B.N.; Murphy, A.R.; Leger, J.M. Performance of silk-polypyrrole bilayer actuators under biologically relevant conditions. J. Appl. Polym. Sci. 2019, 136, 46922–46932. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Ansari, R. Polypyrrole Conducting Electroactive Polymers: Synthesis and Stability Studies. J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef]
- Ansari Khalkhali, R. Electrochemical Synthesis and Characterization of Electroactive Conducting Polypyrrole Polymers. Russ. J. Electrochem. 2005, 41, 950–955. [Google Scholar] [CrossRef]
- Higgins, M.J.; McGovern, S.T.; Wallace, G.G. Visualizing Dynamic Actuation of Ultrathin Polypyrrole Films. Langmuir 2009, 25, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, D.; Boyd, B.J.; Garg, S.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers with defined micro- or nanostructures for drug delivery. Biomaterials 2016, 111, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Gribkovaa, O.L.; Kabanovaa, V.A.; Nekrasova, A.A. Electrochemical Polymerization of Pyrrole in the Presence of Sulfoacid Polyelectrolytes. Russ. J. Electrochem. 2019, 55, 1110–1117. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Jarosz, T.; Zak, J.K.; Lapkowski, M.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Bednarczyk-Cwynar, B. Advancing the delivery of anticancer drugs: Conjugated polymer/triterpenoid composite. Acta Biomater. 2015, 19, 158–165. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes. Environ. Sci. Pollut. Res. 2018, 25, 20012–20022. [Google Scholar] [CrossRef]
- Gutiérrez-Pineda, E.; Alcaide, F.; José Rodríguez-Presa, M.; Bolzan, A.E.; Alfredo Gervasi, C. Electrochemical Preparation and Characterization of Polypyrrole/Stainless Steel Electrodes Decorated with Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 2677–2687. [Google Scholar] [CrossRef]
- Cysewska, K.; Karczewski, J.; Jasiński, P. The Influence of the Co-Dopant Dexamethasone Phosphate on the Electrodeposition Process and Drug-Release Properties of Polypyrrole-Salicylate on Iron. J. Electrochem. Soc. 2019, 166, G148. [Google Scholar] [CrossRef]
- Hua Lei, Y.; Seng, N.; Hyono, A.; Ueda, M.; Ohtsuka, T. Electrochemical synthesis of polypyrrole films on copper from phytic solution for corrosion protection. Corr. Sci. 2013, 76, 302–309. [Google Scholar] [CrossRef]
- Chebil, S.; Monod, M.O.; Fisicaro, P. Direct electrochemical synthesis and characterization of polypyrrole nano- and micro-snails. Electrochim. Acta 2014, 123, 527–534. [Google Scholar] [CrossRef]
- Borges, M.H.R.; Nagay, B.E.; Costa, R.C.; Sacramento, C.M.; Ruiz, K.G.; Landers, R.; van den Beucken, J.J.J.P.; Fortulan, C.A.; Rangel, E.C.; da Cruz, N.C.; et al. A tattoo-inspired electrosynthesized polypyrrole film: Crossing the line toward a highly adherent film for biomedical implant applications. Mater. Today Chem. 2022, 26, 101095–101099. [Google Scholar] [CrossRef]
- Saugo, M.; Flamini, D.O.; Brugnoni, L.I.; Saidman, S.B. Silver deposition on polypyrrole films electrosynthesised onto Nitinol alloy. Corrosion protection and antibacterial activity. Mat. Sci. Eng. C 2015, 56, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Yan, F.; Zhu, J.; Wang, J. Template-free prepared micro/nanostructured polypyrrole with ultrafast charging/discharging rate and long cycle life. J. Power Sources 2011, 196, 2373–2379. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Xia, D.; Zhang, L.; Hui, R.; Zhan, J. Whelk-like Helixes of Polypyrrole Synthesized by Electropolymerization. Adv. Funct. Mater. 2007, 17, 1844–1848. [Google Scholar] [CrossRef]
- Nezhadali, A.; Rouki, Z.; Nezhadali, M. Electrochemical preparation of a molecularly imprinted polypyrrole modified pencil graphite electrode for the determination of phenothiazine in model and real biological samples. Talanta 2015, 144, 456–465. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Pan, C.; Si, P.; Huang, P.; Zhou, J. Morphology-dependent electrochemical stability of electrodeposited polypyrrole/nano-ZnO composite coatings. Mater. Chem. Phys. 2022, 279, 125775–125787. [Google Scholar] [CrossRef]
- Bayat, M.; Izadan, H.; Molina, B.G.; Sánchez, M.; Santiago, S.; Semnani, D.; Dinari, M.; Guirado, G.; Estrany, F.; Alemán, C. Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers 2019, 11, 1757. [Google Scholar] [CrossRef]
- Sui, J.; Travas-Sejdic, J.; Chu, S.Y.; Li, K.C.; Kilmartin, P.A. The actuation behavior and stability of p-toluene sulfonate doped polypyrrole films formed at different deposition current densities. J. Appl. Polym. Sci. 2009, 111, 876–882. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Lyalina, N.V.; Maratkanova, A.N.; Smirnov, D.A. Molecular architecture of highly protective coatings of electrodeposited dodecyl sulfate-doped polypyrrole. Prog. Org. Coat. 2019, 131, 427–434. [Google Scholar] [CrossRef]
- Du, X.; Hao, X.; Wang, Z.; Ma, X.; Guan, G.; Abuliti, A.; Ma, G.; Liu, S. Highly stable polypyrrole film prepared by unipolar pulse electro-polymerization method as electrode for electrochemical supercapacitor. Synt. Met. 2013, 175, 138–145. [Google Scholar] [CrossRef]
- Zheng, W.; Razal, J.M.; Spinks, G.M.; Truong, V.-T.; Whitten, P.G.; Wallace, G.G. The Role of Unbound Oligomers in the Nucleation and Growth of Electrodeposited Polypyrrole and Method for Preparing High, Strength, High Conductivity Films. Langmuir 2012, 28, 10891–10897. [Google Scholar] [CrossRef]
- Reza Eslami, M.; Alizadeh, N. A dual usage smart sorbent/recognition element based on nanostructured conducting molecularly imprinted polypyrrole for simultaneous potential-induced nanoextraction/determination of ibuprofen in biomedical samples by quartz crystal microbalance sensor. Sens. Actuators B 2015, 220, 880–887. [Google Scholar] [CrossRef]
- Martinez, A.L.; Brugnoni, L.I.; Flamini, D.O.; Saidman, S.B. Immobilization of Zn species in a polypyrrole matrix to prevent corrosion and microbial growth on Ti-6Al-4V alloy for biomedical applications. Prog. Org. Coat. 2020, 144, 105650–105660. [Google Scholar] [CrossRef]
- Aouzal, Z.; Bouabdallaoui, M.; El Guerraf, A.; Ben Jadi, S.; Wang, R.; Bazzaoui, M.; Bazzaoui, E.A. Improvement of the anticorrosion resistance of nickel by polypyrrole coating electrosynthesized in salicylate medium. Mater. Today Proc. 2020, 31, S89–S95. [Google Scholar] [CrossRef]
- Joo, J.; Lee, J.K.; Lee, S.Y.; Jang, K.S.; Oh, E.J.; Epstein, A.J. Physical Characterization of Electrochemically and Chemically Synthesized Polypyrroles. Macromolecules 2000, 33, 5131–5136. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Yang, F.K.; Zhao, B. A Facile In Situ Approach to Polypyrrole Functionalization Through Bioinspired Catechols. Adv. Funct. Mater. 2015, 25, 1588–1597. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Yuan, S.J.; Yi, S.P.; Yin, E.Q. Synthesis and characterization of polypyrrole doped with anionic spherical polyelectrolyte brushes. eXPRESS Polym. Lett. 2012, 6, 697–705. [Google Scholar] [CrossRef]
- Ghilane, J.; Hapiot, P.; Bard, A.J. Metal/Polypyrrole Quasi-Reference Electrode for Voltammetry in Nonaqueous and Aqueous Solutions. Anal. Chem. 2006, 78, 6868–6872. [Google Scholar] [CrossRef]
- Sadat Eftekhari, B.; Eskandari, M.; Janmey, P.A.; Samadikuchaksaraei, A.; Gholipourmalekabadi, M. Surface Topography and Electrical Signaling: Single and Synergistic Effects on Neural Differentiation of Stem Cells. Adv. Funct. Mater. 2020, 30, 190792–190809. [Google Scholar] [CrossRef]
- Chandra Sekhar Rout, N.K. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5698. [Google Scholar] [CrossRef]
- Kong, H.; Yang, M.; Miao, Y.; Zhao, X. Polypyrrole as a Novel Chloride-Storage Electrode for Seawater Desalination. Energy Technol. 2019, 7, 1900835–1900842. [Google Scholar] [CrossRef]
- Mettai, B.; Mekki, A.F.; Bekkar Djelloul Sayah, Z.; Moustefai Soumia, K.; Safiddine, Z.; Mahmoud, R.; Mehdi Chehimi, M. In situ chemical deposition of PPy/NDSA and PPy/DBSA layers on QCM electrodes: Synthesis, structural, morphological and ammonia sensing performances study. J. Polym. Res. 2018, 25, 95–107. [Google Scholar] [CrossRef]
- Yousef Elahi, M.; Bathaie, S.Z.; Kazemi, S.H.; Mousavi, M.F. DNA immobilization on a polypyrrole nanofiber modified electrode and its interaction with salicylic acid/aspirin. Anal. Biochem. 2011, 411, 176–184. [Google Scholar] [CrossRef]
- Flamini, D.O.; González, M.B.; Saidman, S.B. ; Saidman, S.B. sis and Characterization of Heparin-Doped Polypyrrole Coatings Using an Electrochemical Quartz Crystal Microbalance (EQCM). Port. Electrochim. Acta 2022, 40, 47–57. [Google Scholar] [CrossRef]
- Baek, S.; Green, R.A.; Poole-Warren, L.A. Effects of dopants on the biomechanical properties of conducting polymer films on platinum electrodes. J. Biomed. Mater. Res. Part. A 2014, 102A, 2743–2754. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.F.; Chrisitne, J.; Robert, J.; Roger, L. Electrochemically synthesized polypyrrole nanotubules: Effects of different experimental conditions, Electrochemically synthesized polypyrrole nanotubules: Effects of different experimental conditions. Eur. Polym. J. 1998, 34, 1767–1774. [Google Scholar] [CrossRef]
- Otero, T.F.; Sansiñena, J.M. Influence of synthesis conditions on polypyrrole-poly(styrenesulphonate) composite electroactivity. J. Electroanal. Chem. 1996, 412, 109–116. [Google Scholar] [CrossRef]
- Wang, P.-C.; Yu, J.-Y. Dopant-dependent variation in the distribution of polarons and bipolarons as charge-carriers in polypyrrole thin films synthesized by oxidative chemical polymerization. React. Funct. Polym. 2012, 72, 311–316. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]
- Prokeš, J.; Varga, M.; Vrňata, M.; Valtera, S.; Stejskal, J.; Kopecký, D. Nanotubular polypyrrole: Reversibility of protonation/deprotonation cycles and long-term stability. Eur. Polym. J. 2019, 115, 290–297. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef] [PubMed]
- Debiemme-Chouvy, C.; Tuyet Mai Tran, T. An insight into the overoxidation of polypyrrole materials. Electrochem. Commun. 2008, 10, 947–950. [Google Scholar] [CrossRef]
- West, N.; Baker, P.G.L.; Arotiba, O.A.; Hendricks, N.R.; Baleg, A.A.; Waryo, T.T.; Ngece, R.F.; Iwuoha, E.I.; O’Sullivan, C. Overoxidized Polypyrrole Incorporated with Gold Nanoparticles as Platform for Impedimetric Anti-Transglutaminase Immunosensor. Anal. Lett. 2011, 44, 1956–1966. [Google Scholar] [CrossRef]
- Otero, T.F.; Padilla, J. Anodic shrinking and compaction of polypyrrole blend: Electrochemical reduction under conformational relaxation kinetic control. J. Electroanal. Chem. 2004, 561, 167–171. [Google Scholar] [CrossRef]
- Mashayekhi Mazar, F.; Martinez, J.G.; Tyagi, M.; Alijanianzadeh, M.; Turner, A.P.F.; Jager, E.W.H. Artificial Muscles Powered by Glucose. Adv. Mater. 2019, 31, 1901677–1901685. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Activation energy for pol Activation energy for polypyrrole oxidation pyrrole oxidation: Film thickness influence. J. Solid. State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, A. Dielectric spectroscopy for probing the relaxation and charge transport in polypyrrole nanofibers. J. Appl. Phys. 2011, 109, 114313–114323. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synt. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, T.; Huh, J.; Kang, M.; Eun Lee, J.; Yoon, H. Anisotropic Growth Control of Polyaniline Nanostructures and Their Morphology-Dependent Electrochemical Characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef]
- Bocchetta, P.; Frattini, D.; Tagliente, M.; Selleri, F. Electrochemical Deposition of Polypyrrole Nanostructures for Energy Applications: A Review. Curr. Nanosci. 2020, 16, 462–477. [Google Scholar] [CrossRef]
- Zang, J.; Bao, S.-J.; Li, C.M.; Bian, H.; Cui, X.; Bao, Q.; Sun, C.Q.; Guo, J.; Lian, K. Well-Aligned Cone-Shaped Nanostructure of Polypyrrole/RuO2 and Its Electrochemical Supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Cysewska, K.; Karczewski, J.; Jasiński, P. Electrochemical synthesis of 3D nano-/micro-structured porous polypyrrole. Mater. Lett. 2016, 183, 397–400. [Google Scholar] [CrossRef]
- Ramirez, A.M.R.; Gacitua, M.A.; Ortega, E.; Diaz, F.R.; del Valle, M.A. Electrochemical in situ synthesis of polypyrrole nanowires. Electrochem. Comm. 2019, 102, 94–98. [Google Scholar] [CrossRef]
- Mariano, A.; Lubrano, C.; Bruno, U.; Ausilio, C.; Bhupesh Dinger, N.; Santoro, F. Advances in Cell-Conductive Polymer Biointerfaces and Role of the Plasma Membrane. Chem. Rev. 2022, 122, 4–4552. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef]
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J. Biomater. Sci. Polym. Ed. 2010, 21, 1265–1282. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; Pu, X.; Yin, G.; Zhang, J. Fabrication of Chitosan/Polypyrrole-coated poly(L-lactic acid)/Polycaprolactone aligned fibre films for enhancement of neural cell compatibility and neurite growth. Cell Prolif. 2019, 52, 12588–12599. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wanga, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, L.; Sun, X.; Wang, X.; Jin, S.; Li, J.; Wu, Q. Neurogenic differentiation of human umbilical cord mesenchymal stem cells on aligned electrospun polypyrrole/polylactide composite nanofibers with electrical stimulation. Front. Mater. Sci. 2016, 10, 260–269. [Google Scholar] [CrossRef]
- Liang, Y.; Cho-Hong Goh, J. Polypyrrole-Incorporated Conducting Constructs for Tissue Engineering Applications: A Review. Bioelctricity 2020, 2, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Janas, D.; Vallejo-Giraldo, C.; Biggs, M.J.P. Self-supporting carbon nanotube films as flexible neural interfaces. Electrochim. Acta 2019, 295, 253–261. [Google Scholar] [CrossRef]
- Czerwinska-Główka, D.; Skonieczna, M.; Barylski, A.; Golba, S.; Przystaś, W.; Zabłocka-Godlewska, E.; Student, S.; Cwalina, B.; Krukiewicz, K. Bifunctional conducting polymer matrices with antibacterial and neuroprotective effect. Bioelectrochemistry 2022, 144, 10803–10817. [Google Scholar] [CrossRef]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible Neural Electrodes for Long-Lasting Intracortical Recording. Science 2020, 23, 101387. [Google Scholar] [CrossRef]
- Khorrami, M.; Antensteiner, M.; Fallahianbijan, F.; Borhan, A.; Reza, M.; Annu, A. Conducting polymer microcontainers for biomedical applications. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 23, 1869–1872. [Google Scholar] [CrossRef]
- Harjo, M.; Zondaka, Z.; Leemets, K.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole-coated fiber-scaffolds: Concurrent linear actuation and sensing. J. Appl. Polym. Sci. 2019, 136, 48533–48541. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Huang, Z.; Pu, X.; Shang, L.; Yin, G.; Xue, C. Preparation of carboxylic graphene oxide-composited polypyrrole conduits and their effect on sciatic nerve repair under electrical stimulation. J. Biomed. Mater. Res. 2019, 107, 2784–2795. [Google Scholar] [CrossRef]
- Zhang, Q.; Esrafilzadeh, D.; Crook, J.M.; Kapsa, R.; Stewart, E.M.; Tomaskovic-Crook, E.; Wallace, G.G.; Huang, X.-F. Electrical Stimulation Using Conductive Polymer Polypyrrole Counters Reduced Neurite Outgrowth of Primary Prefrontal Cortical Neurons from NRG1-KO and DISC1-LI Mice. Sci. Rep. 2017, 7, 42525–42533. [Google Scholar] [CrossRef]
- Sun, Y.; Quan, Q.; Meng, H.; Zheng, Y.; Peng, J.; Hu, Y.; Feng, Z.; Sang, X.; Qiao, K.; He, W.; et al. Enhanced Neurite Outgrowth on a Multiblock Conductive Nerve Scaffold with Self-Powered Electrical Stimulation. Adv. Healthcare Mater. 2019, 8, 1900127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, J.; Liu, X. Antibacterial Property and Biocompatibility of Polypyrrole Films Treated by Oxygen Plasma Immersion Ion Implantation. Adv. Mater. Interf. 2020, 7, 2000057. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.K.; Agarwal, K.; Singh, H.K.; Srivastava, P.; Singh, R. Effect of p-toluenesulfonate on inhibition of overoxidation of polypyrrole. J. Appl. Polym. Sci. 2013, 130, 434–442. [Google Scholar] [CrossRef]
- Lunkes Ely, V.; Matiuzzi da Costa, M.; Pequeno de Oliveira, H.; Antonio Gomes da Silva Júnior, F.; Brayer Pereira, D.I.; Pereira Soares, M.; De Vargas, A.C.; Sangioni, L.A.; Cargnelutti, J.F.; Garcia Ribeiro, M.; et al. In Vitro algicidal effect of polypyrrole on Prototheca species isolates from bovine mastitis Algicidal activity of polypyrrole on Prototheca spp. Med. Mycol. 2020, 58, 1114–1119. [Google Scholar] [CrossRef]
- Děkanovský, L.; Elashnikov, R.; Kubiková, M.; Vokatá, B.; Švorčík, V.; Lyutakov, O. Dual-Action Flexible Antimicrobial Material: Switchable Self-Cleaning, Antifouling, and Smart Drug Release. Adv. Funct. Mater. 2019, 29, 1901880–1901890. [Google Scholar] [CrossRef]
- Forero López, A.D.; Loperena, A.P.; Lehr, I.L.; Brugnoni, L.I.; Saidman, S.B. Corrosion protection of AZ91D magnesium alloy by a duplex coating. J. Serb. Chem. Soc. 2020, 85, 1317–1328. [Google Scholar] [CrossRef]
- Forero López, A.D.; Lehr, I.L.; Brugnoni, L.I.; Saidman, S.B. Improvement in the corrosion protection and bactericidal properties of AZ91D magnesium alloy coated with a microstructured polypyrrole film. J. Magn. Alloys 2018, 6, 15–22. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Gu, R.; Li, G.; Lian, J. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surf. B Biointerfaces 2020, 194, 111186. [Google Scholar] [CrossRef]
- Bhagya Mathi, D.; Gopi, D.; Kavith, L. Implication of lanthanum substituted hydroxyapatite/poly(n-methyl pyrrole) bilayer coating on titanium for orthopedic applications. Mater. Today Proc. 2020, 26, 3526–3530. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, L.; Chen, D.; Wang, Z.; Zhai, J.; Wang, R.; Tan, G.; Mao, J.; Yu, P.; Ning, C. Construction of high surface potential polypyrrole nanorods with enhanced antibacterial properties. J. Mater. Chem. B 2018, 6, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- González, M.B.; Quinzani, O.V.; Vela, M.E.; Rubert, A.A.; Benítez, G.; Saidman, S.B. Study of the electrosynthesis of hollow rectangular microtubes of polypyrrole. Synt. Met. 2012, 162, 1133–1139. [Google Scholar] [CrossRef]
- El Jaouhari, A.; El Asbahani, A.; Bouabdallaoui, M.; Aouzal, Z.; Filotás, D.; Bazzaoui, E.A.; Nagy, L.; Nagy, G.; Bazzaoui, M.; Albourine, A.; et al. Corrosion resistance and antibacterial activity of electrosynthesized polypyrrole. Synt. Met. 2017, 226, 15–24. [Google Scholar] [CrossRef]
- González, M.B.; Brugnoni, L.I.; Flamini, D.O.; Quinzani, L.M.; Saidman, S.B. Removal of Escherichia coli from well water using continuous laminar flow in a channel system containing PPy/Cu modified electrodes. J. Water Health 2018, 16, 921–929. [Google Scholar] [CrossRef]
- Hsu, C.F.; Zhang, L.; Peng, H.; Travas-Sejdic, J.; Kilmartin, P.A. Free radical scavenging properties of polypyrrole and poly(3,4-ethylenedioxythiophene). Curr. Appl. Phys. 2008, 8, 316–319. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Etminanfar, M.; Mahdavi, S.; Saman Safavi, M. Enhanced bioactivity of 316L stainless steel with deposition of polypyrrole/hydroxyapatite layered hybrid coating: Orthopedic applications. Surf. Interfaces 2022, 28, 101604–101615. [Google Scholar] [CrossRef]
- Du, H.; Parit, M.; Liu, K.; Zhang, M.; Jiang, Z.; Huang, T.-S.; Zhang, X.; Si, C. Multifunctional Cellulose Nanopaper with Superior Water-Resistant, Conductive, and Antibacterial Properties Functionalized with Chitosan and Polypyrrole. ACS Appl. Mater. Interf. 2021, 13, 32115–32125. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; del Valle, L.J.; Alemá, C. Drug delivery systems based on intrinsically conducting polymers. J. Control. Release 2019, 309, 244–264. [Google Scholar] [CrossRef]
- Michael Freedman, S.; Cui, X.T. Substrate Electrode Morphology Affects Electrically Controlled Drug Release from Electrodeposited Polypyrrole Films. Phys. Chem. Comm. 2014, 1, 15–25. [Google Scholar]
- Moloney, E.; Breslin, C.B. The formation and properties of polypyrrole doped with an immobile antibiotic. J. Sold. State Electrochem. 2019, 23, 2031–2042. [Google Scholar] [CrossRef]
- Wang, M.L.; Chamberlayne, C.F.; Xu, H.; Mofidfar, M.; Baltsavias, S.; Annes, J.P.; Zare, R.N.; Arbabian, A. On-demand electrochemically controlled compound release from an ultrasonically powered implant. RSC Adv. 2022, 12, 23337–23346. [Google Scholar] [CrossRef]
- Antensteiner, M.; Khorrami, M.; Fallahianbijan, F.; Borhan, A.; Reza Abidian, M. Conducting Polymer Microcups for Organic Bioelectronics and Drug Delivery Applications. Adv. Mater. 2017, 29, 1702576–1702587. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Nanostructured biocompatible thermal/electrical stimuli-responsive biopolymer-doped polypyrrole for controlled release of chlorpromazine: Kinetics studies. Int. J. Pharm. 2014, 472, 327–339. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Stokfisz, A.; Zak, J.K. Two approaches to the model drug immobilization into conjugated polymer matrix. Mater. Sci. Eng. C 2015, 54, 176–181. [Google Scholar] [CrossRef]
- Glosz, K.; Stolarczyk, A.; Jarosz, T. Electropolymerised Polypyrroles as Active Layers for Molecularly Imprinted Sensors: Fabrication and Applications. Materials 2021, 14, 1369. [Google Scholar] [CrossRef] [PubMed]
- Czaja, T.; Wójcik, K.; Grzeszczuk, M.; Szostak, R. Polypyrrole–Methyl Orange Raman pH Sensor. Polymers 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Boguzaite, R.; Ratautaite, V.; Mikoliunaite, L.; Pudzaitis, V.; Ramanaviciene, A.; Ramanavicius, A. Towards analytical application of electrochromic polypyrrole layers modified by phenothiazine derivatives. J. Electroanal. Chem. 2021, 886, 115132–115142. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duan, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004–045024. [Google Scholar] [CrossRef]
- Sharma Pushpendra, K.; Sharma, P.K.; Gupta, G.; Singh, V.V.; Tripathi, B.K.; Pandey, P.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Synthesis and characterization of polypyrrole by cyclic voltammetry at different scan rate and its use in electrochemical reduction of the simulant of nerve agents. Synt. Met. 2010, 160, 2631–2637. [Google Scholar] [CrossRef]
- West, N.; Baker, P.; Waryo, T.; Ngece, F.R.; Iwuoha, E.I.; O’Sullivan, C.; Katakis, I. Highly sensitive gold-overoxidized polypyrrole nanocomposite immunosensor for antitransglutaminase antibody. J. Bioact. Compat. Polym. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, P.; Turner, A.P.F. Interference-Free Electrochemical Detection of Nanomolar Dopamine Using Doped Polypyrrole and Silver Nanoparticles. Electroanalysis 2014, 26, 2197–2206. [Google Scholar] [CrossRef]
- Nguyen, V.-A.; Nguyen, H.L.; Nguyen, D.T.; Do, Q.P.; Tran, L.D. Electrosynthesized poly(1,5-diaminonaphthalene)/polypyrrole nanowires bilayer as an immunosensor platform for breast cancer biomarker CA 15-3. Curr. Appl. Phys. 2017, 17, 1422–1429. [Google Scholar] [CrossRef]
- Fakhry, A.; Cachet, H.; Debiemme-Chouvy, C. Mechanism of formation of templateless electrogenerated polypyrrole nanostructures. Electrochim. Acta 2015, 179, 297–303. [Google Scholar] [CrossRef]
- Fakhry, A.; Pilliera, F.; Debiemme-Chouvy, C. Templateless electrogeneration of polypyrrole nanostructures: Impact of the anionic composition and pH of the monomer solution. J. Mater. Chem. A 2014, 2, 9859–9865. [Google Scholar] [CrossRef][Green Version]
- Landim, V.P.A.; Foguel, M.V.; Prado, C.M.; Sotomayor, M.P.T.; Vieira, I.C.; Silva, B.V.M.; Dutran, R.F. A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors 2022, 12, 545. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. [Google Scholar] [CrossRef]
- Taheri, N.; Alizadeh, N. Vertically grown nanosheets conductive polypyrrole as a sorbent for nanomolar detection of salicylic acid. J. Pharm. Biomed. Anal. 2020, 188, 113365–113373. [Google Scholar] [CrossRef]
- Olszowy, P.; Szultka, M.; Ligor, T.; Nowaczyk, J.; Buszewski, B. Fibers with polypyrrole and polythiophene phases for isolation and determination of adrenolytic drugs from human plasma by SPME-HPLC. J. Chromatogr. B 2010, 878, 2226–2234. [Google Scholar] [CrossRef]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Efremova Aaron, S.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater. 2020, 3, 815–839. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Janaki, V.; Senthilkumar, P.; Kamala-Kannan, S. Polypyrrole/zeolite composite—A nanoadsorbent for reactive dyes removal from synthetic solution. Chemosphere 2022, 287, 132164–132172. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Nawaz Bhatti, H.; Iqbal, M.; Hussain, F.; Malik Sarim, F. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Chandra Lohani, P.; Prasad Tiwari, A.; Muthurasu, A.; Pathak, I.; Babu Poudel, M.; Chhetri, K.; Dahal, B.; Acharya, D.; Hoon Ko, T.; Yong Kim, H. Phytic acid empowered two nanos “Polypyrrole tunnels and transition Metal-(Oxy)hydroxide Sheets” in a single platform for unmitigated redox water splitting. Chem. Eng. J. 2023, 463, 142280. [Google Scholar] [CrossRef]
- Su, D.; Zhou, J.; Ahmed, K.S.; Ma, Q.; Lv, G.; Chen, J. Fabrication and characterization of collagen-heparin-polypyrrole composite conductive film for neural scaffold. Int. J. Biol. Macromol. 2019, 129, 895–903. [Google Scholar] [CrossRef]
- Biallozor, S.; Kupniewska, A. Conducting polymers electrodeposited on active metals. Synt. Met. 2005, 155, 443–449. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of bilayered polypyrrole on 316 L stainless steel prevention. Prog. Org. Coat. 2015, 78, 21–27. [Google Scholar] [CrossRef]
- Zeybek, B.; Özçiçek Pekmez, N.; Kılıç, E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances. Electrochim. Acta 2011, 56, 9277–9286. [Google Scholar] [CrossRef]
- Takamatsu, T.; Sijie, Y.; Shujie, F.; Xiaohan, L.; Miyake, T. Multifunctional High-Power Sources for Smart Contact Lenses. Adv. Funct. Mater. 2019, 30, 1906225–1906233. [Google Scholar] [CrossRef]
- El Jaouhari, A.; Ben Jadi, S.; Aouzal, Z.; Bouabdallaoui, M.; Bazzaoui, E.A.; Wang, R.; Bazzaoui, M. Comparison study between corrosion protection of polypyrrole synthesized on stainless steel from phthalate and saccharinate aqueous medium. Polym. Test. 2018, 67, 302–308. [Google Scholar] [CrossRef]
- Yan, Q.; Pan, W.; Zhong, S.; Zhu, R.; Li, G. Effect of solvents on the preparation and corrosion protection of polypyrrole. Prog. Org. Coat. 2019, 132, 298–304. [Google Scholar] [CrossRef]
- Raman, S.; Ravi Sankar, A. Intrinsically conducting polymers in flexible and stretchable resistive strain sensors: A review. J. Mater. Sci. 2022, 57, 13152–13178. [Google Scholar] [CrossRef]
- Brito de Morais, V.; Crispilho Corrêa, C.; Martin Lanzoni, E.; Rodrigues Costa, C.A.; César Bof Bufon, C.; Santhiago, M. Wearable binary cooperative polypyrrole nanofilms for chemical mapping on skin. Mater. Chem. A 2019, 7, 5227–5234. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Huang, W.-H. Stretchable Electrochemical Sensors for Cell and Tissue Detection. Angew. Chem. 2021, 60, 2757–2767. [Google Scholar] [CrossRef]
- Petty, A.J.; Keate, R.L.; Jiang, B.; Ameer, G.A.; Rivnay, J. Conducting Polymers for Tissue Regeneration in vivo. Chem. Mater. 2020, 32, 4095–4115. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef]
- Chen, A.X.; Kleinschmidt, A.T.; Choudhary, K.; Lipomi, D.J. Beyond Stretchability: Strength, Toughness, and Elastic Range in Semiconducting Polymers. Chem. Mater. 2020, 32, 7582–7601. [Google Scholar] [CrossRef]
- Uzun, O.; Başman, N.; Alkan, C.; Kölemen, U.; Yılmaz, F. Investigation of mechanical and creep properties of polypyrrole by depth-sensing indentation. Polym. Bull. 2011, 66, 649–660. [Google Scholar] [CrossRef]
- Mazur, M. Preparation of three-dimensional polymeric structures using gas bubbles as templates. J. Phys. Chem. C 2008, 112, 13528–13534. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G.; Chen, F.; Zhang, J. Electrochemical Growth of Polypyrrole Microcontainers. Macromolecules 2003, 36, 1063–1067. [Google Scholar] [CrossRef]
- Qu, L.; Shi, G. Hollow microstructures of polypyrrole doped by poly(styrene sulfonic acid). J. Polym. Sci. A Polym. Chem. 2004, 42, 3170–3177. [Google Scholar] [CrossRef]
- Turco, A.; Mazzotta, E.; Di Franco, C.; Santacroce, M.V.; Scamarcio, G.; Grazia Monteduro, A.; Primiceri, E.; Malitesta, C. Templateless synthesis of polypyrrole nanowires by non-static solution-surface electropolymerization. J. Solid. State Electrochem. 2016, 20, 2143–2151. [Google Scholar] [CrossRef]
- Chagas, G.R.; Darmanin, T.; Guittard, F. One-Step and Templateless Electropolymerization Process Using Thienothiophene Derivatives to Develop Arrays of Nanotubes and Tree-like Structures with High Water Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 22732–22743. [Google Scholar] [CrossRef]
- McCarthy, C.P.; McGuinness, N.B.; Carolan, P.B.; Fox, C.M.; Alcock-Earley, B.E.; Breslin, C.B.; Rooney, A.D. Electrochemical Deposition of Hollow N-Substituted Polypyrrole Microtubes from an Acoustically Formed Emulsion. Macromolecules 2013, 46, 1008–1016. [Google Scholar] [CrossRef]
- Liu, D.; Uda, M.; Seike, M.; Fukui, S.; Hirai, T.; Nakamura, Y.; Fujii, S. Polypyrrole-coated Pickering-type droplet as light-responsive carrier of oily material. Colloids Polym. Sci. 2022, 300, 255–265. [Google Scholar] [CrossRef]
- Hui, F.; Li, B.; He, P.; Hu, J.; Fang, Y. Electrochemical fabrication of nanoporous polypyrrole film on HOPG using nanobubbles as templates. Electrochem. Comm. 2009, 11, 639–642. [Google Scholar] [CrossRef]
- Lee, J.I.; Hyo Cho, S.; Park, S.-M.; Kon Kim, J.; Kyeong Kim, J.; Woong Yu, J.; Kim, C.Y.; Russell, T.P. Highly Aligned Ultrahigh Density Arrays of Conducting Polymer Nanorods using Block Copolymer Templates. Nano Lett. 2008, 8, 2315–2320. [Google Scholar] [CrossRef]
- Li, X.; Malardier-Jugroot, C. Confinement Effect in the Synthesis of Polypyrrole within Polymeric Templates in Aqueous Environments. Macromolecules 2013, 46, 2258–2266. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.; Stavaux, P.-Y. Effect of Electrolyte Concentration and Nature on the Morphology and the Electrical Properties of Electropolymerized Polypyrrole Nanotubules. Chem. Mater. 1999, 11, 829–834. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Mikoliunaite, L.; Plausinaitiene, V.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Polypyrrole Formation from Pyrrole ‘Adlayer’. Phys. Chem. Chem. Phys. 2017, 19, 1029–1038. [Google Scholar] [CrossRef]
- Páramo-Garcia, U.; Avalos-Perez, A.; Guzman-Pantoja, J.; Díaz-Zavala, N.P.; Melo-Banda, J.A.; Gallardo-Rivas, N.V.; Reyes-Gómez, J.; Pozas-Zepeda, D.; Ibanez, J.G.; Batina, N. Polypyrrole microcontainer structures and doughnuts designed by electrochemical oxidation: An electrochemical and scanning electron microscopy study. e-Polymers 2014, 14, 75–84. [Google Scholar] [CrossRef]
- Mosch, H.L.K.S.; Akintola, O.; Plass, W.; Hoeppener, S.; Schubert, U.S.; Ignaszak, A. The specific surface versus electrochemically active area of the carbon/polypyrrole capacitor: The correlation of ion dynamics studied by an electrochemical quartz crystal microbalance with BET surface. Langmuir 2016, 32, 4440–4449. [Google Scholar] [CrossRef] [PubMed]
- Iordoc, M.; Bara, A.; Prioteasa, P.; Teisanu, A.; Marinescu, V. Electropolymerization of Conducting Polypyrrole on Carbon Nanotubes/Silicon Composite for Supercapacitor Applications. Rev. Chim. 2015, 66, 196–201. [Google Scholar]
- Krukiewicz, K.; Herman, A.P.; Turczyn, R.; Szymańska, K.; Koziol, K.K.; Boncel, S.; Zak, J.K. A role of nanotube dangling pyrrole and oxygen functions in the electrochemical synthesis of polypyrrole/MWCNTs hybrid materials. Appl. Surf. Sci. 2014, 317, 794–802. [Google Scholar] [CrossRef]
- Carragher, U.; Branagan, D.; Breslin, C.B. The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper. Materials 2019, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, B.; Abu-Zurayk, R.; Waleed, H.; Alqader Ibrahim, A. Fabrication of a Novel (PVDF/MWCNT/Polypyrrole) Antifouling High Flux Ultrafiltration Membrane for Crude Oil Wastewater Treatment. Membranes 2022, 12, 751. [Google Scholar] [CrossRef]
- Jara-Cornejo, E.; Khan, S.; Vega-Chacón, J.; Wong, A.; Chagas da Silva Neres, L.; Picasso, G.; Sotomayor, M.D.P.T. Biomimetic Material for Quantification of Methotrexate Using Sensor Based on Molecularly Imprinted Polypyrrole Film and MWCNT/GCE. Biomimetics 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Rohanifar, A.; Rodriguez, L.B.; Devasurendra, A.M.; Alipourasiabi, N.; Anderson, J.L.; Kirchhoff, J.R. Solid-Phase Microextraction of Heavy Metals in Natural Water with a Polypyrrole/Carbon Nanotube/1,10–Phenanthroline Composite Sorbent Material. Talanta 2018, 108, 570–577. [Google Scholar] [CrossRef]
- Amiri, A.; Baghayeri, M.; Shahabizadeh, M. Polypyrrole/carbon nanotube coated stainless steel mesh as a novel sorbent. New J. Chem. 2023, 47, 4402–4408. [Google Scholar] [CrossRef]
- Arjunan, T.V.; Senthil, T.S. Review: Dye sensitised solar cells. Mater. Technol. 2013, 28, 9–14. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Saberi Motlagh, M.; Mottaghita, V.; Rismanchi, A.; Rafieepoor Chirani, M.; Hasanzadeh, M. Performance modelling of textile solar cell developed by carbon fabric/polypyrrole flexible counter electrode. Int. J. Sustain. Energy 2022, 41, 1106–1126. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Sun, W. Electropolymerization and application of polyoxometalate doped polypyrrole film electrodes in dye-sensitized solar cells. Electrochem. Comm. 2020, 122, 106879–106896. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer Polypyrrole Nanosheets with Self-Organized Surface Structures for Flexible and Efficient Solar–Thermal Energy Conversion. Adv. Mater. 2019, 31, 1807716–1807725. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Song, X.; Huang, M.; Jiang, H. A Facile and General Strategy to Deposit Polypyrrole on Various Substrates for Efficient Solar-Driven Evaporation. Adv. Sustain. Syst. 2019, 3, 1800108–1800116. [Google Scholar] [CrossRef]
- Lee, S.; Bayarkhuu, B.; Han, Y.; Kim, H.-W.; Jeong, S.; Boo, C.; Byun, J. Multifunctional photo-Fenton-active membrane for solar-driven water purification. J. Membr. Sci. 2022, 32, 4440. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Bertelsmann, K.; Fan, D.E. Portable Low-Pressure Solar Steaming-Collection Unisystem with Polypyrrole Origamis. Adv. Mater. 2019, 31, 1900720–1900728. [Google Scholar] [CrossRef]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311–312, 147–161. [Google Scholar] [CrossRef]
- Zhang Haiyan Pan, C.; Wang, X.; Sun, S.-K. Microwave-assisted ultrafast fabrication of high-performance polypyrrole nanoparticles for photothermal therapy of tumors in vivo. Biomater. Sci. 2018, 6, 2750–2756. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef]
- Maity, K.; Mandal, D. All-Organic High-Performance Piezoelectric Nanogenerator with Multilayer Assembled Electrospun Nanofiber Mats for Self-Powered Multifunctional Sensors. ACS Appl. Mater. Interfaces 2018, 10, 18257–18269. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Pan, Z.; Cheng, Y.; Zhai, Y.; Zhang, Y.; Ding, X.; Liu, I.; Zhai, J.; Xu, J. Three-dimensional polypyrrole induced high-performance flexible piezoelectric nanogenerators for mechanical energy harvesting. Compos. Sci. Technol. 2022, 219, 109260–109268. [Google Scholar] [CrossRef]
- Maity, K.; Garain, S.; Henkel, K.; Schmeißer, D.; Mandal, D. Self-Powered Human-Health Monitoring through Aligned PVDF Nanofibers Interfaced Skin-Interactive Piezoelectric Sensor. ACS Appl. Polym. Mater. 2020, 2, 862–878. [Google Scholar] [CrossRef]
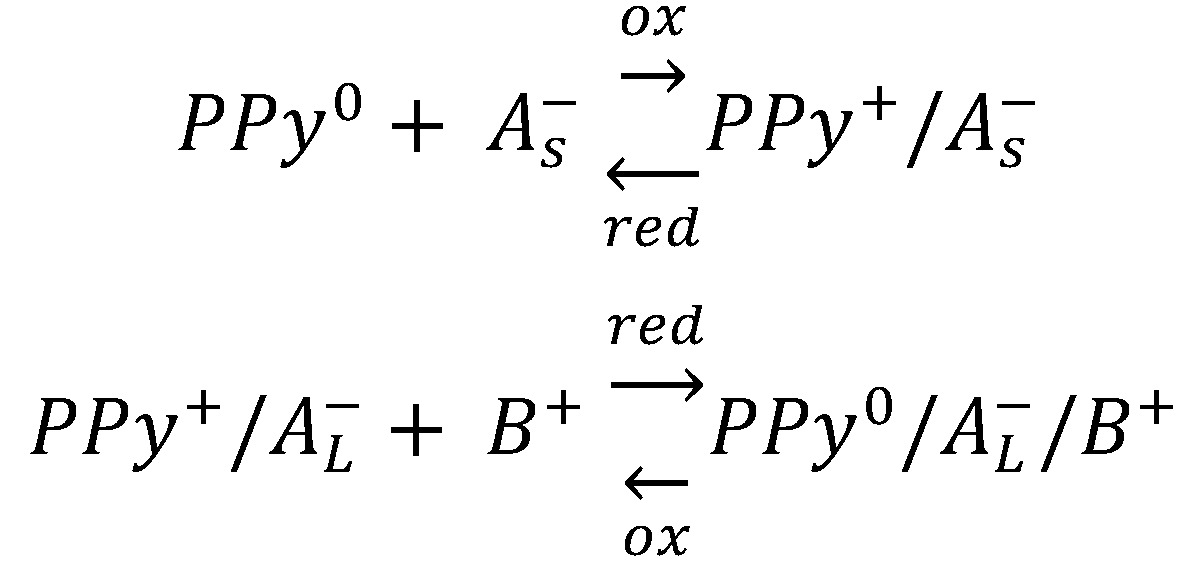

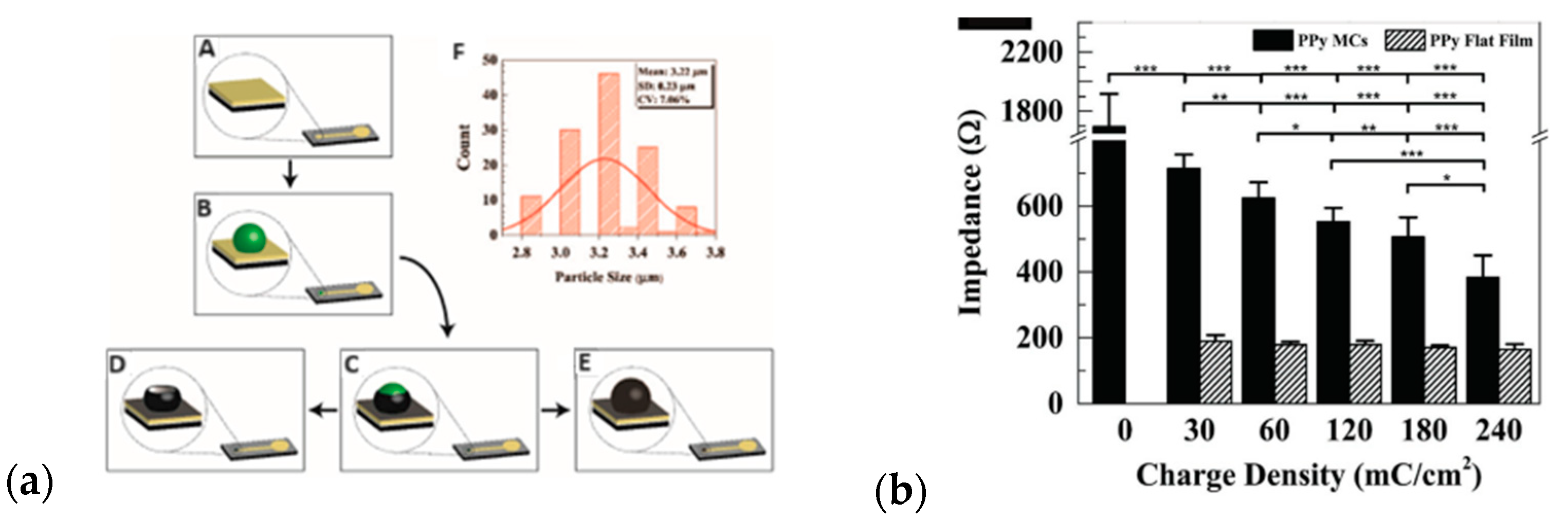

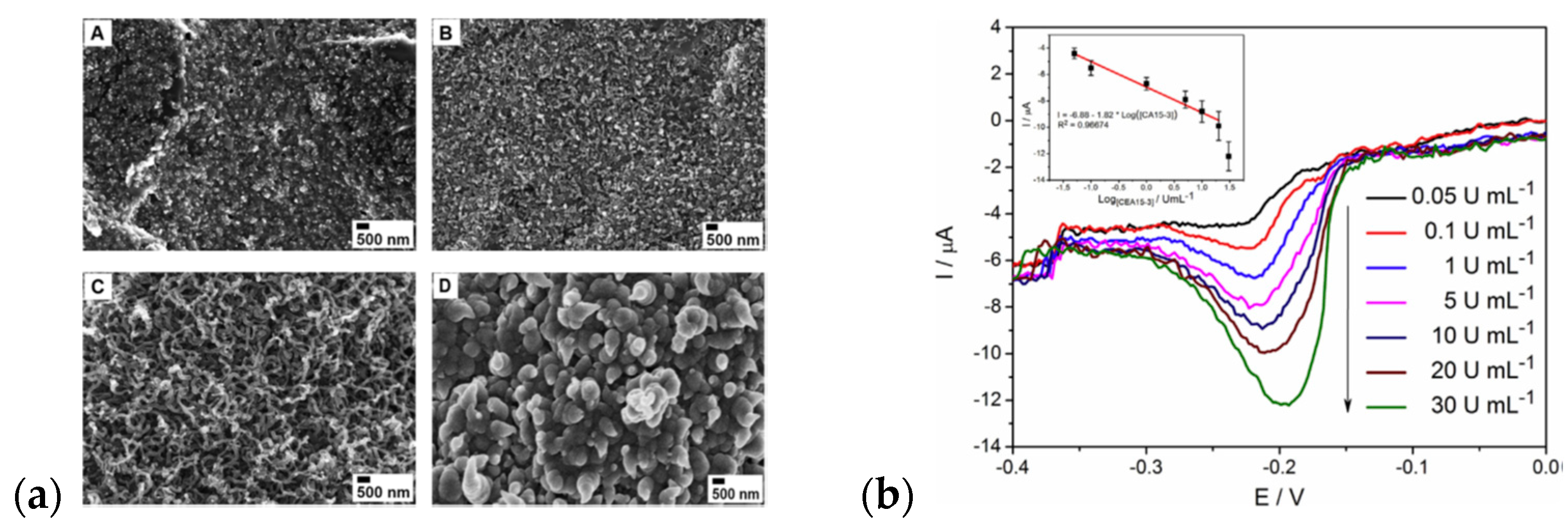
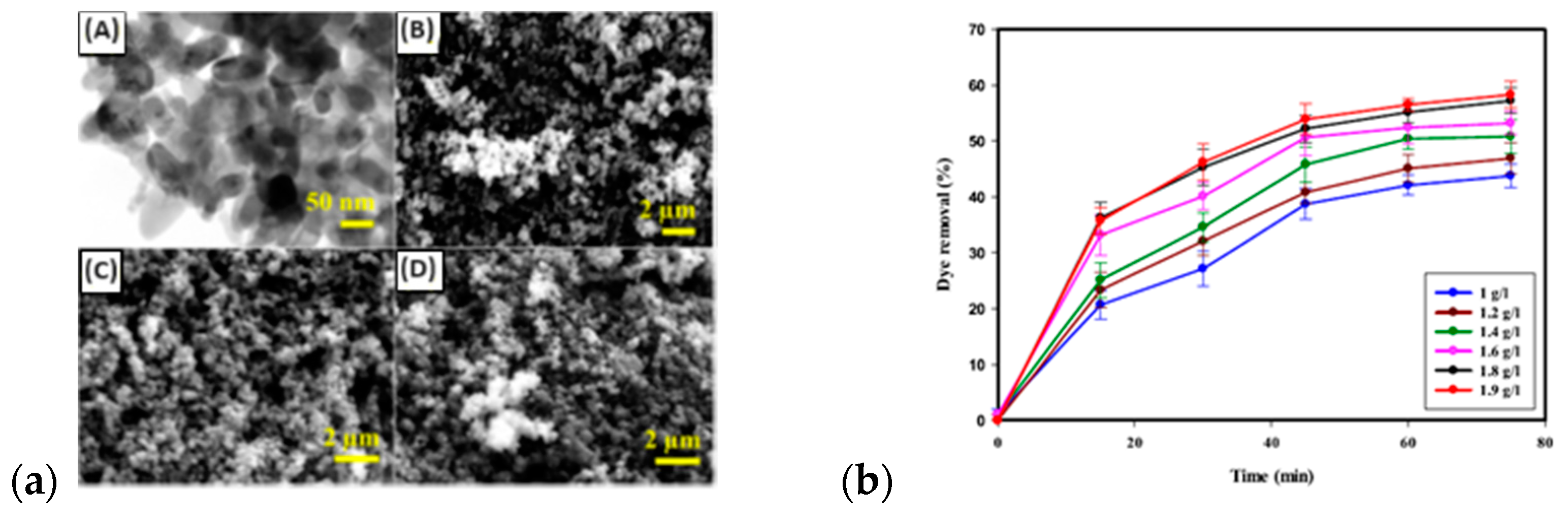
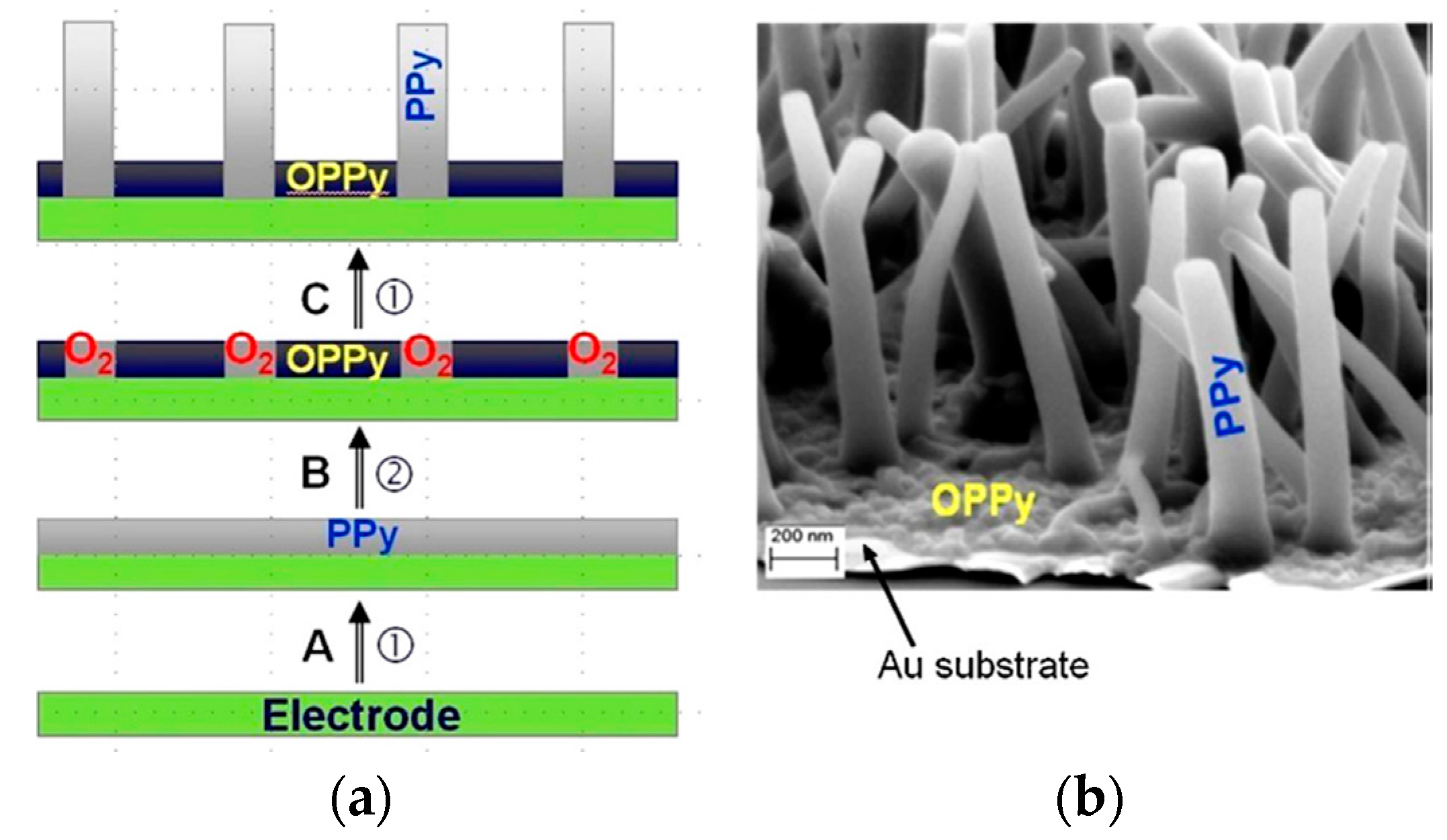
| Material | Synthesis Mode | Technique | Dopant/Initiator | Conductivity S/cm | Morphology | Application Filed | Source |
|---|---|---|---|---|---|---|---|
| block co-polymers of PPy with poly(ε-caprolactone) and poly(ethyl cyanoacrylate) | ChOP | two-step procedure: macromonomer formation and Py co-polymerization | para-toluene sulfonate (pTS−) | (18–32) | a flat compacted surface | cell proliferation platform (rat PC12 cells) | [120] |
| PLLA/PCL fibers coated with PPy and chitosan (CS) | EChP | WE: ITO with electrospun PLLA/PCL fibers galvanostatic (8 min/2 mA) | chloride (Cl−) | (1–1.1)·10−2 | cell differentiation platform and neurite growth (PC12 cells) | [122] | |
| hydrogel based on sodium alginate, gelatin and polypyrrole | ChOP | rapid mixing/−20 °C | ammonium persulfate (I) | (1.2–1.6)·10−2 | network structure with well-dispersed polypyrrole particles | self-healing conductive hydrogels | [123] |
| aligned PPy/PLA composite electrospun films | ChOP | P123 used as a template dropwise method (18 °C/6 h) | aqueous FeCl3 | 4.6 | spherical PPy particles | platform for differentiation of human cord mesenchymal stem cells | [124] |
| collagen-heparin-polypyrrole composite | ChOP | vigorous stirring for 30 min followed by standing at r.t | FeCl3 | 0.11–0.336 | compact structure with partial orientation | neural scaffold in the application of peripheral nerve regeneration (PC12 cells cultured) | [174] |
| Material | Synthesis Mode | Technique | Dopant/Initiator | Tested Strain | Morphology | Application Filed | Source |
| branched polypyrrole | EChP | WE: anode (metal wire) potentiostatic (9 V/10 min) | sodium dodecylbenzenesulfonate (DBSA), cetyl trimethylammonium bromid (CTAB) | S. aureus, E. coli, K. pneumoniae | fractal structure | antibacterial material | [8] |
| polydimethylsiloxane (PDMS) gradient doped with PPy | ChOP | dropwise technique under continuous mixing/ 2.5 h at 150 rpm | FeCl3 | E. coli | increased surface roughness with typical granular forms | switchable superhydrophobic and self-cleaning material with drug releasing ability | [137] |
| a duplex coating based on PPy and molybdate—originated layer loaded with silver | EChP | WE: AZ91D (magnesium alloy) potentiostatic (1.15 V/600 s for 0.50 M NaSa and 0.80 V/1800 s for 0.10 M NaSa) | sodium salicylate (NaSa) | E. coli | globular morphology for lower NaSa concentrations, rectangular microtubes for higher | antibacterial activity with anticorrosive performance | [138] |
| PPy with oxygen plasma immersion ion implantation (O-PIIi) | EChP | WE: Ti/galvanostatic (5 mA/cm2, 10 min), r.t. | TsONa (sodium p-toluenesulfonate) | E. coli, S. aureus | Cauliflower morphology, after O-PIII treatment—pit-like structure occurs | antibacterial material | [134] |
| nanostructured PPy | template-free EChP | WE: Ti/galvanostatic (0.9 mA/cm2, 5 min), r.t. | sulfosalicylic acid in PBS | S. aureus | oriented nanorods with large specific surface area | antibacterial material | [142] |
| Material | Synthesis Mode | Technique | Dopant | Release Mode | Morphology | Application Filed | Source |
| polypyrrole | EChP | WE: Pt-black coated glass/potentiostatic (0.7 V, 200 s) | fluorescein | 10 s pulses/−2.0 V into PBS | typical globular morphology | drug-delivery module | [150] |
| polypyrrole nanowire | EChP | WE: Au electrode/ galvanostatic (0.477 mA/cm2, 1600 s) pTS− in PBS (pH 7.40) | adenosine triphosphate (ATP) dexamethasone (Dex) | CV stimulation (−0.9:0.6) V | nanowire network with porous interwoven structures | drug-delivery module | [111] |
| oxacillin-doped PPy (PPyOx) PPyOx modified with chitosan | EChP | WE: gold, platinum titanium/potentiostatic (−0.80 V vs. SCE, 500 s) | oxacillin | constant potential at 0.30 V or 0.60 V | smooth polymer films with roughness induced by the oxacillin presence | drug-delivery module | [151] |
| nanostructu olysaccharideride-doped polypyrrole | EChP | WE: Pt Two-step procedure: pre-electropolymerization in hep presence, potentiostatic (+0.9 V vs. Ag/AgCl/100 s) and electropolymerization in the CPZ presence potentiostatic (+0.7 V vs. Ag/AgCl/900 s) | heparin sodium salt (50,000 units) chlorpromazine hydrochloride | OCP and constant potential conditions (0.1:0.4) V | homogeneous, porous nanostructure with spherical morphology | drug-delivery module | [154] |
| doped polypyrrole | ECHP | WE: platinum foil in-situ drug immobilization mode—cyclic voltammetry (CV) ex-situ drug immobilization—CV for polymerization of Py followed by oxidative immobilization of drugs | quercetin and ciprofloxacin | constant a reduction potential (−0.5 V vs. Ag/AgCl) in PBS | matrix obtained by ex-situ mode less uniform with larger PPy grains and rougher surface | drug-delivery module | [155] |
| Material | Synthesis Mode | Technique | Detected Analyte | Detection Mode | Morphology | Application Filed | Source |
| Doped polypyrrole | EChP | WE: gold electrode/sodium perchlorate CV with different scan rate (5:50) mV/s | dimethyl methyl phosphate (DMMP) | EIS | globular and rod (for slow sr) structures and packed globular system for high sr | sensor | [160] |
| Molecularly imprinted polypyrrole | template assisted EChP | WE: fluorine-tin oxide FTO/CV (10 cycles, (0.0:0.7) V, 50 mV/s) in PBS, pH = 7.2 | carcinoembryonic antigen (CEA) alpha-fetoprotein (AFP) | EiS | PPy-MO NIP: hollow rectangular nanotubes PPy-MO DMIP: rougher tubular structure | sensor | [42] |
| Gold—overoxidized polypyrrole nanocomposite | ECHP | WE: glassy carbon electrode (GCE), LiClO4, potentiostatic (800 mV (vs. Ag/AgCl)/120 s, overoxidized at 1.0 V/420 s), AuNP- CV (0.2:−1.0) V, 50 mV/s, 15 cycles | tissue transglutaminase (tTG)-specific antigen | EIS | OPPy: cauliflower-like structure with good surface coverage of the AuNP | sensor | [161] |
| Poly(1,5-diaminonaphthalene)/polypyrrole bilayer | EChP guided with oxygen nanobubbles | WE: screen-printed electrodes (SPEs) Two-step procedure: electropolymerization of Py potentiostatically (0.75 V/500 s in 0.2 M Na2HPO4, LiClO4 (1:−15) mM) followed by P(1,5DAN) deposition, CV (50 mV/s, (−0.02:0.75 V) | CA 15-3 antigen | DPV | nanowires (for optimized dopant concentration), for high concentration cauliflower-like structure | sensor | [163] |
| Polypyrrole/Nanoclay Hybrid Film | EChP | WE: glassy carbon electrode (GCE) CV ((−0.10:1.0) V, 200 mV/s, 20 cycles)I ACN, 0.1 M LiClO4 Anti-cTnT antibodies immobilized with glutaraldehyde (GA) | cardiac troponins (T and I) | SWV | heterogenous film formed by agglomerates of two-dimensional laminar shapes | sensor | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golba, S.; Loskot, J. The Alphabet of Nanostructured Polypyrrole. Materials 2023, 16, 7069. https://doi.org/10.3390/ma16227069
Golba S, Loskot J. The Alphabet of Nanostructured Polypyrrole. Materials. 2023; 16(22):7069. https://doi.org/10.3390/ma16227069
Chicago/Turabian StyleGolba, Sylwia, and Jan Loskot. 2023. "The Alphabet of Nanostructured Polypyrrole" Materials 16, no. 22: 7069. https://doi.org/10.3390/ma16227069
APA StyleGolba, S., & Loskot, J. (2023). The Alphabet of Nanostructured Polypyrrole. Materials, 16(22), 7069. https://doi.org/10.3390/ma16227069