Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of nHAPs
2.3. Formulation Design and Development of Toothpastes
2.4. Preparation of Toothpastes
2.5. Procedure for Obtaining Enamel Specimens
2.6. Enamel Treatment with Toothpaste
2.7. AFM Investigations
2.8. Evaluation of the Antibacterial Activity
2.9. Statistical Analysis
3. Results
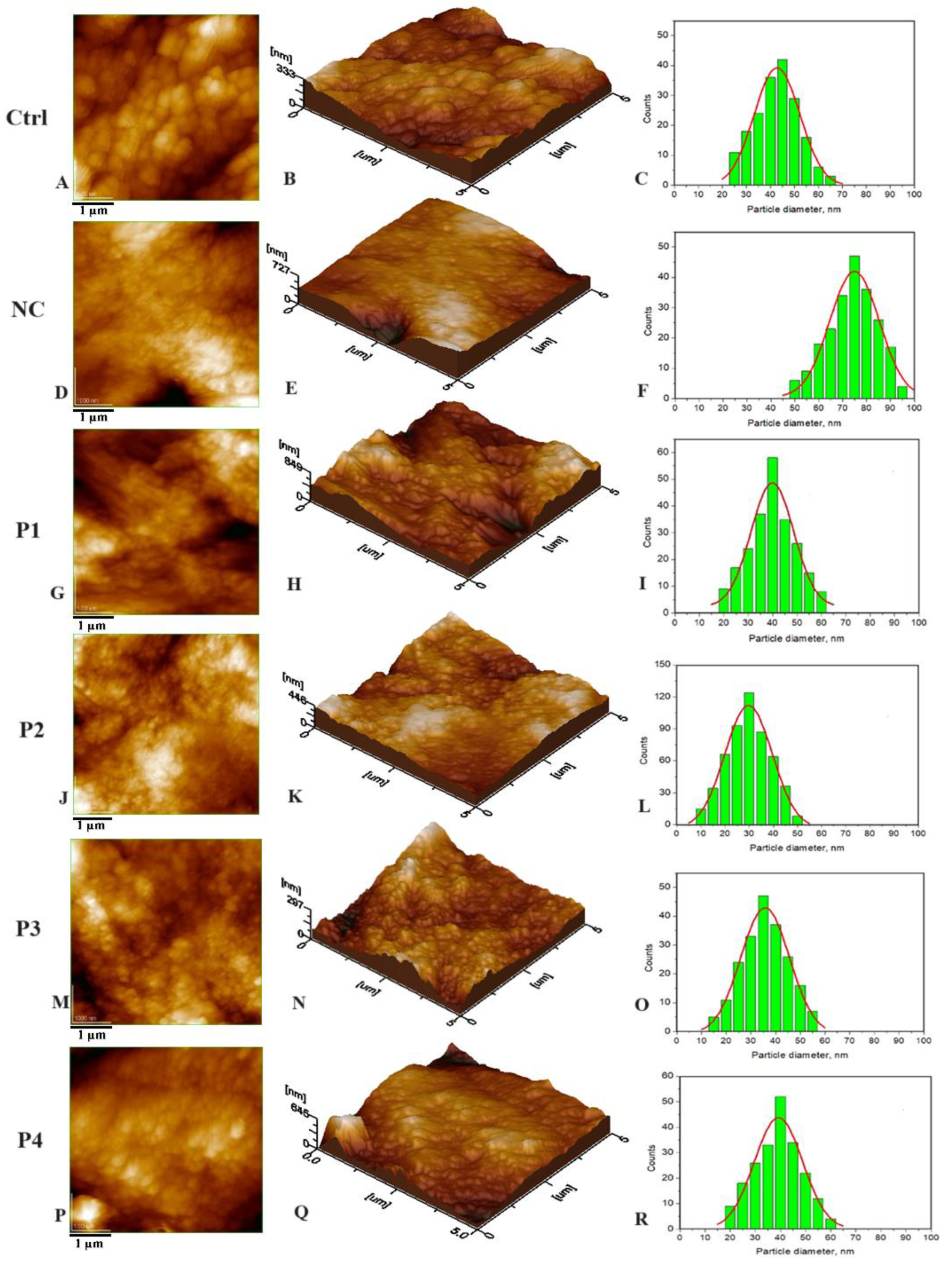
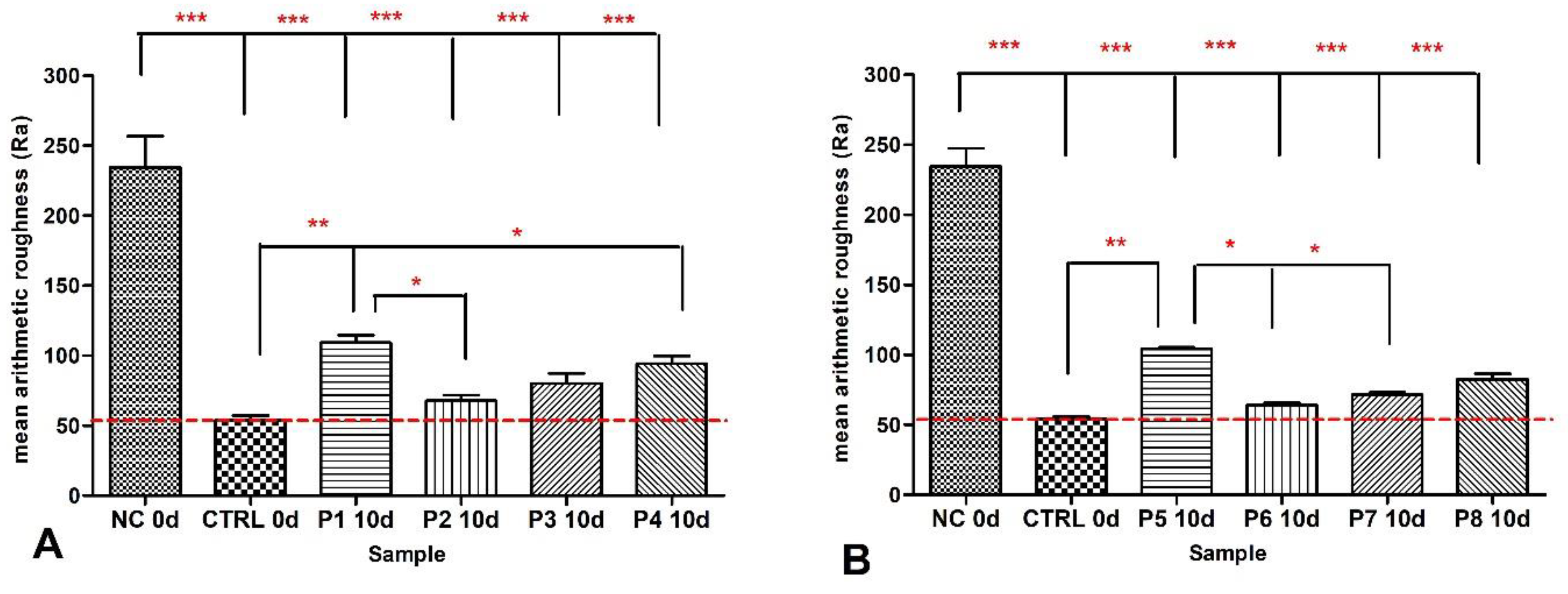
3.1. Nanostructure of the Tested Samples
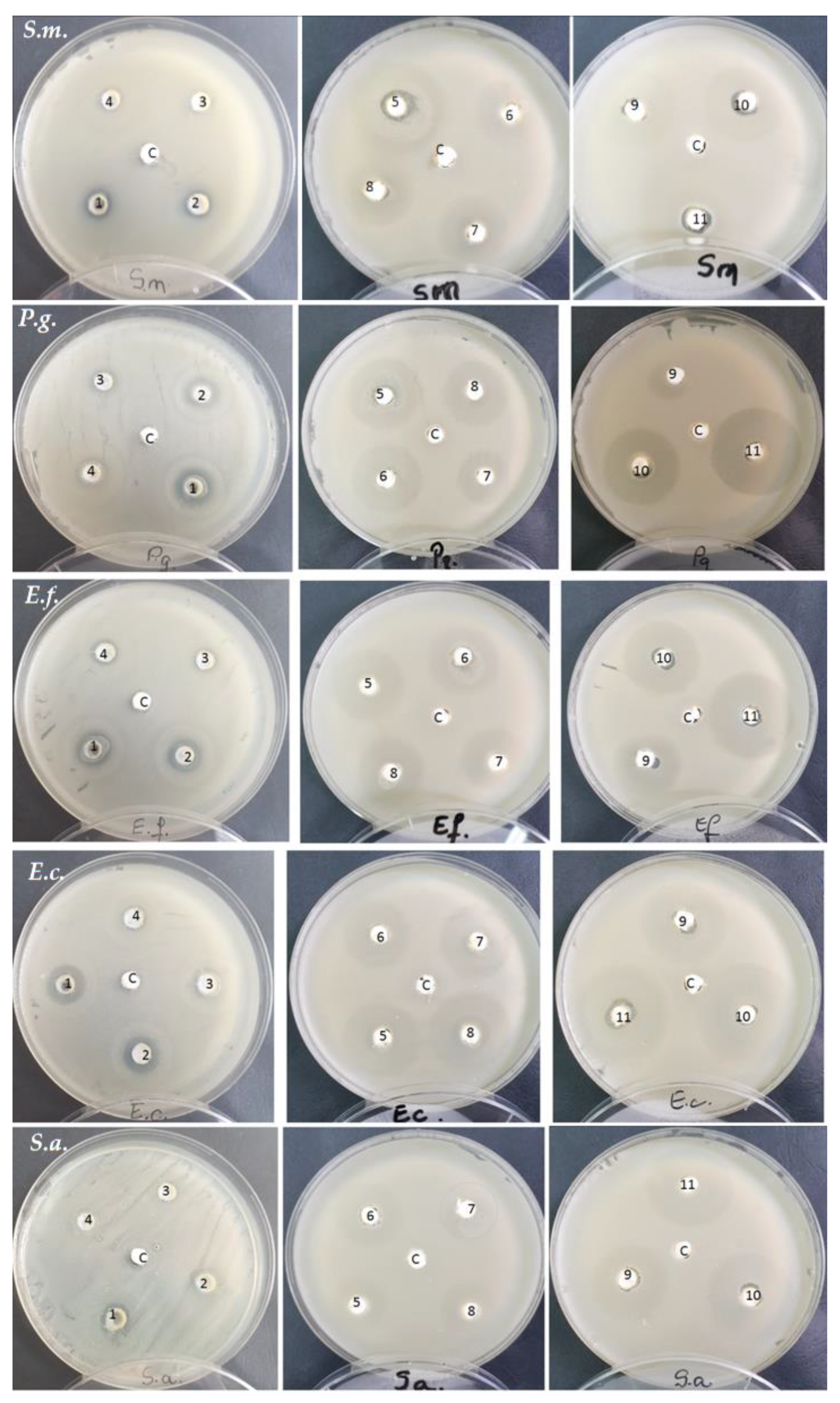
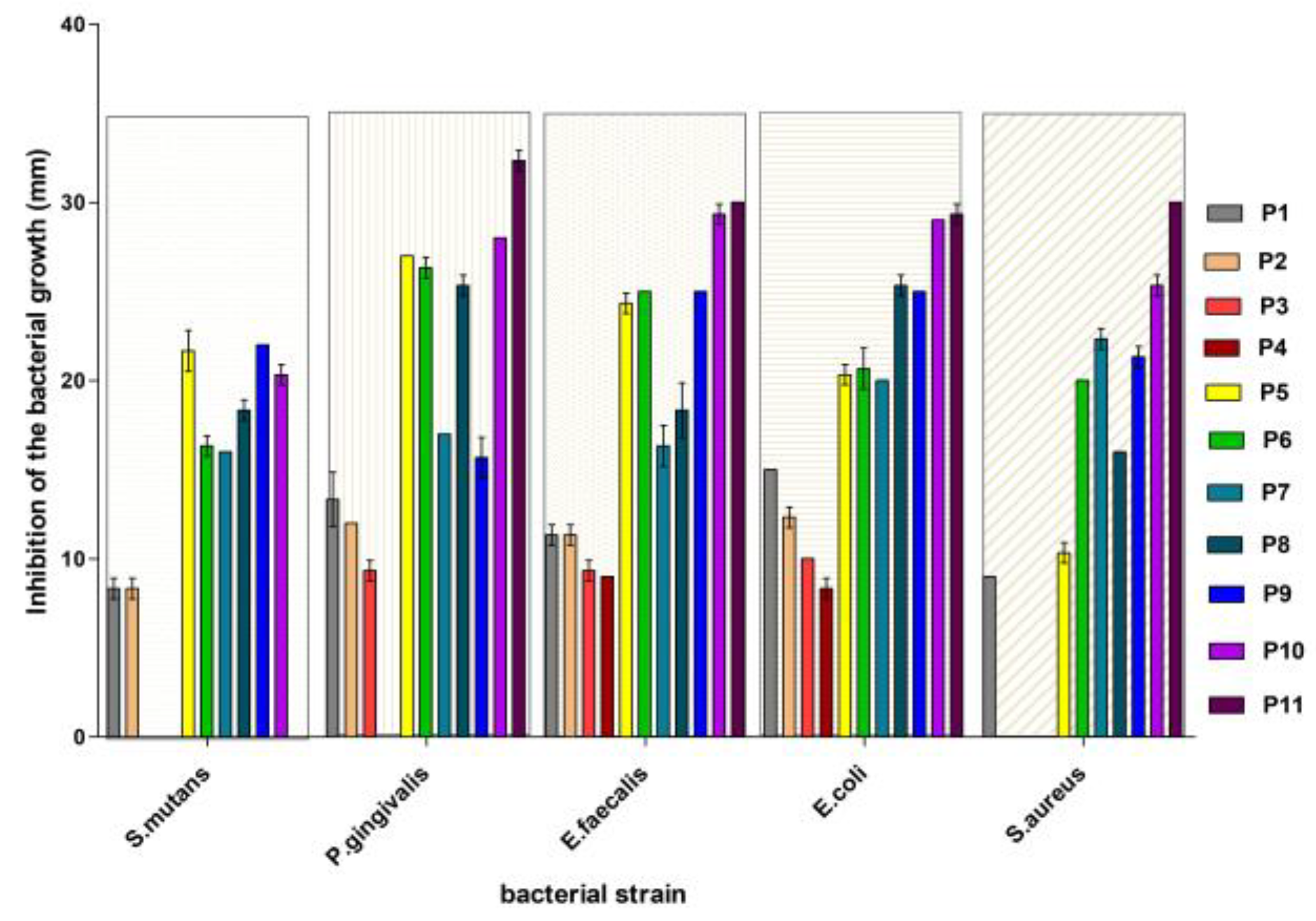
3.2. Antibacterial Activity Evaluation
4. Discussion
- For Streptococcus mutans: P9 > P5 > P10 > P8 ≈ P7 ≈ P6 > P1 ≈ P2 > P3 ≈ P4 ≈ P11.
- For Porphyromonas gingivalis: P5 > P6 > P11 > P10 > P8 > P7 ≈ P9 > P1 > P2 > P3 > P4.
- For Enterococcus faecalis: P11 > P10 > P9 = P6 ≈ P5 > P8 > P1 > P2 > P3 > P4.
- For Escherichia coli: P11> P10 > P8 ≈ P9 > P5 = P6 ≈ P7 > P1 > P2 > P3 > P4.
- For Staphylococcus aureus: P11 > P10 > P8 ≈ P9 > P5 = P6 ≈ P7 > P1 > P2 = P3 = P4.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedro, A.C.; Paniz, O.G.; Fernandes, I.d.A.A.; Bortolini, D.G.; Rubio, F.T.V.; Haminiuk, C.W.I.; Maciel, G.M.; Magalhães, W.L.E. The Importance of Antioxidant Biomaterials in Human Health and Technological Innovation: A Review. Antioxidants 2022, 11, 1644. [Google Scholar] [CrossRef]
- Insuasti-Cruz, E.; Suárez-Jaramillo, V.; Mena, K.A.; Pila-Varela, K.O.; Fiallos-Ayala, X.; Dahoumane, S.A.; Alexis, F. Natural biomaterials from biodiversity for healthcare applications. Adv. Healthc. Mater. 2022, 11, 2101389. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Nakahara, M.; Toyama, N.; Ekuni, D.; Takeuchi, N.; Maruyama, T.; Yokoi, A.; Morita, M. Trends in self-rated oral health and its associations with oral health status and oral health behaviors in Japanese university students: A cross-sectional study from 2011 to 2019. Int. J. Environ. Res. Public Health 2022, 19, 13580. [Google Scholar] [CrossRef]
- Sun, L.; Chow, L.C. Preparation and properties of nano-sized calcium fluoride for dental applications. Dent. Mater. 2008, 24, 111–116. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a toothpaste containing bioactive hydroxyapatites and In Vitro evaluation of its efficacy to remineralize enamel and to occlude dentinal tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted nano-hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef]
- Kranz, S.; Heyder, M.; Mueller, S.; Guellmar, A.; Krafft, C.; Nietzsche, S.; Tschirpke, C.; Herold, V.; Sigusch, B.; Reise, M. Remi-neralization of artificially demineralized human enamel and dentin samples by zinc-carbonate hydroxyapatite nanocrystals. Materials 2022, 15, 7173. [Google Scholar] [CrossRef]
- Florea, A.-D.; Pop, L.C.; Benea, H.-R.-C.; Tomoaia, G.; Racz, C.-P.; Mocanu, A.; Dobrota, C.-T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef]
- Silva, J.C.; Pereira, R.L.S.; de Freitas, T.S.; Rocha, J.E.; Macedo, N.S.; Nonato, C.d.F.A.; Linhares, M.L.; Tavares, D.S.A.; Cuhna, F.A.B.; Coutinho, H.D.M.; et al. Evaluation of antibacterial and toxicological activities of essential oil of Ocimum gratissimum L. and its major constituent eugenol. Food Biosci. 2022, 50, 102128. [Google Scholar] [CrossRef]
- Noshad, M.; Behbahani, B.A.; Nikfarjam, Z. Chemical composition, antibacterial activity and antioxidant activity of citrus bergamia essential oil: Molecular docking simulations. Food Biosci. 2022, 50, 102123. [Google Scholar] [CrossRef]
- Banday, J.A.; Rather, Z.U.; Yatoo, G.N.; Hajam, M.A.; Bhat, S.A.; Santhanakrishnan, V.P.; Farozi, A.; Rather, M.A.; Rasool, S. Gas chromatographic-mass spectrometric analysis, antioxidant, antiproliferative and antibacterial activities of the essential oil of prangos pabularia. Microb. Pathog. 2022, 166, 105540. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula—Traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Evaluation of Medicines for Human Use; 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK, Doc. Ref. EMEA/HMPC/244569/2006; European Medicines Agency: London, UK, 2008. [Google Scholar]
- Olmstead, M.J. Organic Toothpaste Containing Saponin. U.S. Patent 6,485,711, 26 November 2002. Available online: http://www.freepatentsonline.com/6485711.html (accessed on 30 March 2022).
- Al-Ghutaimel, H.; Riba, H.; Al-Kahtani, S.; Al-Duhaimi, S. Common periodontal diseases of children and adolescents. Int. J. Dent. 2014, 2014, 850674. [Google Scholar] [CrossRef]
- Nishida, N.; Grossi, S.G.; Dunford, R.G.; Ho, A.W.; Trevisan, M.; Genco, R.J. Dietary vitamin C and the risk for periodontal disease. J. Periodontol. 2000, 71, 1215–1223. [Google Scholar] [CrossRef]
- Rao, P.; Rao, R.; Langade, D.; Rathod, V. Rare Case of Scorbutic Gingivitis. Ind. Med. Gaz. 2014, 4, 81–84. [Google Scholar]
- Pussinen, P.J.; Laatikainen, T.; Alfthan, G.; Asikainen, S.; Jousilahti, P. Periodontitis is associated with a low concentration of vitamin C in plasma. Clin. Diagn. Lab. Immunol. 2013, 10, 897–902. [Google Scholar] [CrossRef]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Szuster-Ciesielska, A.; Rzeski, W.; Kanderfer-Szerszen, M. Therapeutic properties of betulin and betulinic acid, components of birch bark extract. Farm. Prz. Nauk. 2010, 7, 33–39. [Google Scholar]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Koca, I.; Tekgüler, B.; Türkyilmaz, B. Bioactive terpenic acids: Their properties, natural sources and effects on health. Int. Congr. Med. Aromat. Plants 2017, 1, 429–439. [Google Scholar]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and characterisation of antimicrobial properties of semisynthetic Betulin derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef]
- Viszwapriya, D.; Subramenium, G.A.; Radhika, S.; Pandian, S.K. Betulin inhibits cariogenic properties of Streptococcus mutans by targeting vicRK and gtf genes. Antonie Van Leeuwenhoek 2017, 110, 153–165. [Google Scholar] [CrossRef]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef]
- Gallo, M.B.P.; Bullet, M.; Sarachine, J. Biological activities of Lupeol. Int. J. Res. Pharm. Biomed. Sci. 2009, 3, 46–66. [Google Scholar] [CrossRef]
- Lee, T.K.; Poon, R.T.P.; Wo, J.Y.; Ma, S.; Guan, X.Y.; Myers, J.N.; Altevogt, P.; Yuen, A.P. Lupeol suppresses cisplatin-induced nuclear factor-B activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in osteophatic nude mouse model. Cancer Res. 2007, 67, 8800–8809. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent developments in the functionalization of betulinic acid and its natural analogues: A route to new bioactive compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef]
- Florea, A.D.; Tomoaia-Cotisel, M.; Dobrota, C.T. Use of Betula species extracts in therapeutic and preventive oral health care. In Betula: Ecology and Uses, 1st ed.; Bertelsen, C.T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2020; pp. 137–162. [Google Scholar]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. Microbiol. Open 2019, 9, e00944. [Google Scholar] [CrossRef]
- Kocaçalişkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Für Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-Y.; Jeon, J.-H.; Lee, C.-H.; Lee, H.-S. Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chem. 2009, 115, 1006–1010. [Google Scholar] [CrossRef]
- Berberian, V.; Allen, C.C.R.; Sharma, N.D.; Boyd, D.R.; Hardacre, C. A Comparative Study of the Synthesis of 3-Substituted Catechols using an Enzymatic and a Chemoenzymatic Method. Adv. Synth. Catal. 2007, 349, 727–739. [Google Scholar] [CrossRef]
- Demirci, B.; Paper, D.H.; Demirci, F.; Can Başer, K.H.; Franz, G. Essential oil of Betula pendula roth. Buds. Evid. Based Complement. Altern. Med. 2004, 1, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, M.S.; Nikolic, V.D.; Stanojevic, L.P.; Stanojevic, J.S.; Nikolic, L.B.; Danilovic, R.B.; Marinkovic, V.D. Chemical composition, antimicrobial and antioxidant activity of birch (Betula pendula Roth.) Buds essential oil. J. Essent. Oil-Bear. Plants 2019, 22, 120–130. [Google Scholar] [CrossRef]
- Markert, B.; Steinbeck, R. Some aspects of element distribution in Betula alba, a contribution to representative sampling of terrestrial plants for multi-element analysis. Fresenius’ Z. Für Anal. Chem. 1988, 331, 616–619. [Google Scholar] [CrossRef]
- Raal, A.; Boikova, T.; Püssa, T. Content and dynamics of polyphenols in Betula spp. Leaves naturally growing in Estonia. Rec. Nat. Prod. 2015, 9, 41–48. [Google Scholar]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive–dentin interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Lingström, P.; Örtengren, U.; Niwano, Y. Photo-irradiated caffeic acid exhibits antimicrobial activity against Streptococcus mutans biofilms via hydroxyl radical formation. Sci. Rep. 2017, 7, 6353. Available online: https://www.nature.com/articles/s41598-017-07007-z (accessed on 30 March 2023). [CrossRef]
- Jumanca, D.; Galuscan, A.; Podariu, A.C.; Borcan, F.; Earar, K. Anti-inflammatory action of toothpastes containing betulin nanocapsules. Rev. Chim. 2014, 65, 1472–1476. [Google Scholar]
- Zyryanova, O.A.; Terazawa, M.; Takayoshi, K.; Zyryanov, V.I. White birch trees as resource species of Russia: Their distribution, ecophysiological features, multiple utilizations. Eurasian J. For. Res. 2010, 13, 25–40. [Google Scholar]
- Shu, X.; Zhao, S.; Huo, W.; Tang, Y.; Zou, L.; Li, Z.; Li, L.; Wang, X. Clinical study of a spray containing birch juice for repairing sensitive skin. Arch. Dermatol. Res. 2023, 315, 2271–2281. [Google Scholar] [CrossRef]
- Imran, E.; Cooper, P.R.; Ratnayake, J.; Ekambaram, M.; Mei, M.L. Potential Beneficial Effects of Hydroxyapatite Nanoparticles on Caries Lesions In Vitro—A Review of the Literature. Dent. J. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Maha, D.; Habib, A.; Badawy, R. Occlusion of Dentinal Tubules Using a Novel Dentifrice with Fluoro-Hydroxyapatite Nanoparticles Derived from a Biologic Source. (an in vitro study). Dent. Sci. Updates 2022, 3, 39–44. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Gafurov, M.R.; Goldberg, M.A. The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study. Materials 2022, 15, 9046. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rahmani, A.; Tahmasebi, E.; Tebyanian, H.; Yazdanian, A.; Mosaddad, S.A. Current and advanced nanomaterials in dentistry as regeneration agents: An update. Mini Rev. Med. Chem. 2021, 21, 899–918. [Google Scholar] [CrossRef]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si multi-substituted hydroxyapatites for bone regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef] [PubMed]
- Oltean-Dan, D.; Dogaru, G.-B.; Tomoaia-Cotisel, M.; Apostu, D.; Mester, A.; Benea, H.R.C.; Paiusan, M.G.; Jianu, E.-M.; Mocanu, A.; Balint, R.; et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic short-waves and titanium implants coated with biomimetic composite embedded into PLA matrix: In vivo evaluation. Int. J. Nanomed. 2019, 14, 5799–5816. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Str. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Peng, X.; Han, S.; Wang, K.; Ding, L.; Liu, Z.; Zhang, L. The Amelogenin-Derived Peptide TVH-19 Promotes Dentinal Tubule Occlusion and Mineralization. Polymers 2021, 13, 2473. [Google Scholar] [CrossRef] [PubMed]
- Frangopol, P.T.; Mocanu, A.; Almasan, V.; Garbo, C.; Balint, R.; Borodi, G.; Bratu, I.; Horovitz, O.; Tomoaia-Cotisel, M. Synthesis and structural characterization of strontium substituted hydroxyapatites. Rev. Roum. Chim. 2016, 61, 337–344. [Google Scholar]
- Dotta, T.C.; Hayann, L.; de Padua Andrade Almeida, L.; Nogueira, L.F.B.; Arnez, M.M.; Castelo, R.; Cassiano, A.F.B.; Faria, G.; Martelli-Tosi, M.; Bottini, M.; et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. J. Funct. Biomater. 2022, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, F.V.; Lonni, A.A.S.G.; D’Alpino, P. Silicon-enriched hydroxyapatite formed induced by REFIX-based toothpaste on the enamel surface. Braz. Dent. Sci. 2021, 24, 4. [Google Scholar] [CrossRef]
- Khonina, T.G.; Chupakhin, O.N.; Shur, V.Y.; Turygin, A.P.; Sadovsky, V.V.; Mandra, Y.V.; Sementsova, E.A.; Kotikova, A.Y.; Legkikh, A.V.; Nikitina, E.Y.; et al. Silicon-hydroxyapatite—Glycerohydrogel as a promising biomaterial for dental applications. Colloids Surf. B 2020, 189, 110851. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.L.S.; Silva, J.G.V.C.; de Sousa, E.B.G.; D’Alpino, P.H.P.; de Oliveira, A.F.B.; de Jong, E.D.J.; Sampaio, F.C. Effectiveness of fluoride-containing toothpastes associated with different technologies to remineralize enamel after pH cycling: An in vitro study. BMC Oral Health 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Horovitz, O.; Tomoaia, G.; Mocanu, A.; Yupsanis, T.; Tomoaia-Cotisel, M. Protein binding to gold autoassembled films. Gold Bull. 2007, 40, 295–304. [Google Scholar] [CrossRef]
- Zdrenghea, U.V.; Tomoaia, G.; Pop-Toader, D.-V.; Mocanu, A.; Horovitz, O.; Tomoaia-Cotisel, M. Procaine effect on human erythrocyte membrane explored by atomic force microscopy. Comb. Chem. High Throughput Screen. 2011, 14, 237–247. [Google Scholar] [CrossRef]
- Machoy, M.; Wilczyński, S.; Szyszka-Sommerfeld, L.; Woźniak, K.; Deda, A.; Kulesza, S. Mapping of Nanomechanical Properties of Enamel Surfaces Due to Orthodontic Treatment by AFM Method. Appl. Sci. 2021, 11, 3918. [Google Scholar] [CrossRef]
- Teutle-Coyotecatl, B.; Contreras-Bulnes, R.; Rodríguez-Vilchis, L.E.; Scougall-Vilchis, R.J.; Velazquez-Enriquez, U.; Almaguer-Flores, A.; Arenas-Alatorre, J.A. Effect of Surface Roughness of Deciduous and Permanent Tooth Enamel on Bacterial Adhesion. Microorganisms 2022, 10, 1701. [Google Scholar] [CrossRef]
- Besnard, C.; Marie, A.; Sasidharan, S.; Harper, R.A.; Marathe, S.; Moffat, J.; Shelton, R.M.; Landini, G.; Korsunsky, A.M. Time-Lapse In Situ 3D Imaging Analysis of Human Enamel Demineralisation Using X-ray Synchrotron Tomography. Dent. J. 2023, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Antimicrobial Susceptibility Testing: EUCAST Disk Diffusion Method; EUCAST: Basel, Switzerland, 2021; p. 16. Available online: www.eucast.org (accessed on 1 March 2023).
- Wang, S.; Zhang, L.; Chen, W.; Jin, H.; Zhang, Y.; Wu, L.; Li, Q. Rapid regeneration of enamel-like-oriented inorganic crystals by using rotary evaporation. Mater. Sci. Eng. 2020, 115, 111141. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Rohanizadeh, R. A review of chemical surface modification of bioceramics: Effects on protein adsorption and cellular response. Colloids Surf. B Biointerfaces 2014, 122, 823–834. [Google Scholar] [CrossRef]
- Zhang, X.; Ramirez, B.E.; Liao, S.; Diekwisch, T.G.H. Amelogenin supramolecular assembly in nanospheres defined by a complex helix-coil-PPII helix 3D-structure. PLoS ONE 2011, 6, e24952. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.-C.; Hu, Y.; Lu, Y.; Smith, C.E.; Lertlam, R.; Wright, J.T.; Suggs, C.; MacKee, M.D.; Beniah, E.; Kabir, M.E.; et al. Enamelin Is critical for ameloblast integrity and enamel ultrastructure formation. PLoS ONE 2014, 9, e89303. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Stanic, V.; Dimitrijevic, S.; Stankovic, J.A.; Mitrić, M.; Jokic, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
| Plant Part | Chemical Constituents | Pharmacological Effects | References |
|---|---|---|---|
| Roots | Essential oils, ascorbic acid, coumarins, sterols, saponins, tannins, potassium, sodium | Antiscorbutic, antiarthritic, anticancer, antidiabetic, anti-inflammatory, antimicrobial, antioxidant, antiviral, gastroprotective, hepatoprotective, immunomodulatory | [15,16,17,18,19,20,21,22] |
| Outer bark | Pentacyclic triterpenes (mainly betulin up to 34%) terpenes, methylsalicylate, creosol, guaiacol | Antibacterial effect against Streptococcus, Porphyromonas gingivalis, Streptococcus pyogenes, Escherichia coli, Staphylococcus aureus, and Enterococcus faecalis; and antifungal, anticarcinogenic, antiperiodontic, anticancer, anti-inflammatory, antiviral, antibiofilm activities. | [17,18,23,24,25,26,27,28,29,30,31,32,33] |
| Bark extracts | Phenols, terpenoids, alkaloids, glycosylated molecules, organic acids, cathecol, oleuro-pein-aglycone, acacetin, kaempferide, dimethylquercetin, pentacosyl, resorcinol | Anti-inflammatory, antimicrobial, antioxidant, antiviral, immunomodulatory, antiarthritic, anticancer, antidiabetic, gastroprotective, hepatoprotective, prevention of degenerative diseases. | [16,23,34,35,36] |
| Birch buds | Essential oil (up to 3.8%) triterpenoids, diarylheptanoids, phenylbutanoids, lignans, phenolics and flavonoids Sesquiterpene | Antiarthritic, anticancer, antidiabetic, anti-inflammatory, antimicrobial, antioxidant, antiviral, gastroprotective, hepatoprotective, and immunomodulatory activities. | [23,37,38,39] |
| Leaves | Flavones glycosides (1–3%), quercetin, glycosides, kaempferol glycosides myricetin glycoside, betulorentic acid, caffeic acid, saponins, tannins, sesquiterpenes, chlorogenic acid, triterpene alcohol, malonyl esters of dimarene type, polymeric proanthocyanidins, macro- and micronutrients up to 38% | Oral health as preventive and therapeutic agent, anti-inflammatory antioxidant, bactericidal effect, natural surfactants. | [17,18,40,41,42,43] |
| Birch sap | Al, Ca, Mg, Zn, and Ni, ascorbic malic, citric, phosphoric, and succinic acids, botulin, betulic acid | Antiscorbutic, anticancer, bacteriostatic, anti-inflammatory. | [19,20,21,22,31,32,44,45] |
| Variant | nHAP 3.40% for Each Formulation | Birch Extract (%) | Basic Chemical Composition of the Group (%) | |
|---|---|---|---|---|
| P1 | HAP-5%Zn | - | Distillated water | 58.58 |
| Glycerol | 25.18 | |||
| P2 | Pure HAP | - | Sorbitol | 2.99 |
| Silicon dioxide | 3.73 | |||
| P3 | HAP-0.23%Mg-3.09%Zn-2%Si-10%Sr | - | Xanthan gum | 0.17 |
| Na lauryl sulphate | 0.17 | |||
| P4 | HAP-2.5%Mg-2.9%Si-1.34%Zn | - | ||
| P5 | HAP-5%Zn | 1.30 | Distillated water | 58.49 |
| Glycerol | 25.15 | |||
| P6 | Pure HAP | 1.30 | Sorbitol | 2.99 |
| Silicon dioxide | 3.73 | |||
| P7 | HAP-0.23%Mg-3.09%Zn-2%Si-10%Sr | 1.30 | HAP | 3.42 |
| Xanthan gum | 0.17 | |||
| P8 | HAP-2.5%Mg-2.9%Si-1.34%Zn | 1.30 | Na lauryl sulphate Ethyl alcohol | 0.17 3.7 |
| Distillated water | 83.32 | |||
| Glycerol | 5.9 | |||
| Silicon dioxide | 5.32 | |||
| P9 | - | 0.25 | Xanthan gum | 0.25 |
| Na lauryl sulphate | 0.25 | |||
| Sorbitol | 4.27 | |||
| Ethyl alcohol | 0.44 | |||
| Distillated water | 79.41 | |||
| Glycerol | 9.06 | |||
| Sorbitol | 4.06 | |||
| P10 | - | 0.70 | Silicon dioxide | 5.06 |
| Xanthan gum | 0.23 | |||
| Na lauryl sulphate | 0.23 | |||
| Ethyl alcohol | 1.25 | |||
| Distillated water | 74.2 | |||
| Glycerol | 13.15 | |||
| Sorbitol | 3.8 | |||
| P11 | - | 1.30 | Silicon dioxide | 4.72 |
| Xanthan gum | 0.21 | |||
| Na lauryl sulphate | 0.21 | |||
| Ethyl alcohol | 2.41 |
| Diameter of the Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| Sample | Streptococcus mutans | Porphyromonas gingivalis | Enterococcus faecalis | Escherichia coli | Staphylococcus aureus |
| P1 | 8.3 ± 0.57 | 13.3 ± 1.52 | 11.3 ± 0.57 | 15.0 ± 0 | 9.0 ± 0 |
| P2 | 8.3 ± 1.15 | 12.0 ± 0 | 11.3 ± 1.15 | 12.3 ± 0.57 | - |
| P3 | - | 9.3 ± 0.57 | 9.3 ± 0.57 | 10.0 ± 0 | - |
| P4 | - | 0 | 9.0 ± 0 | 8.3 ± 1.52 | - |
| P5 | 21.6 ± 1.15 | 27.0 ± 0 | 24.3 ± 0.57 | 20.3 ± 0.57 | 10.3 ± 0.57 |
| P6 | 16.3 ± 0.57 | 26.6 ± 1.15 | 25.0 ± 0 | 20.3 ± 1.15 | 20.0 ± 0 |
| P7 | 16.0 ± 0 | 17.0 ± 0 | 15.3 ± 1.15 | 20.0 ± 0 | 22.3 ± 1.15 |
| P8 | 18.3 ± 1.52 | 25.3 ± 0.57 | 18.3 ± 1.52 | 25.3 ± 1.15 | 16.0 ± 0 |
| P9 | 22.0 ± 0 | 15.3 ± 1.15 | 25.0 ± 0 | 25.0 ± 0 | 21.3 ± 1.52 |
| P10 | 20.3 ± 0.57 | 28.0 ± 0 | 29.3 ± 0.57 | 29.0 ± 0 | 25.3 ± 0.57 |
| P11 | - | 32.3 ± 0.57 | 30.0 ± 0 | 29.3 ± 0.57 | 30.0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.-D.; Dobrota, C.T.; Carpa, R.; Racz, C.-P.; Tomoaia, G.; Mocanu, A.; Avram, A.; Soritau, O.; Pop, L.C.; Tomoaia-Cotisel, M. Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care. Materials 2023, 16, 7143. https://doi.org/10.3390/ma16227143
Florea A-D, Dobrota CT, Carpa R, Racz C-P, Tomoaia G, Mocanu A, Avram A, Soritau O, Pop LC, Tomoaia-Cotisel M. Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care. Materials. 2023; 16(22):7143. https://doi.org/10.3390/ma16227143
Chicago/Turabian StyleFlorea, Alexandra-Diana, Cristina Teodora Dobrota, Rahela Carpa, Csaba-Pal Racz, Gheorghe Tomoaia, Aurora Mocanu, Alexandra Avram, Olga Soritau, Lucian Cristian Pop, and Maria Tomoaia-Cotisel. 2023. "Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care" Materials 16, no. 22: 7143. https://doi.org/10.3390/ma16227143
APA StyleFlorea, A.-D., Dobrota, C. T., Carpa, R., Racz, C.-P., Tomoaia, G., Mocanu, A., Avram, A., Soritau, O., Pop, L. C., & Tomoaia-Cotisel, M. (2023). Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care. Materials, 16(22), 7143. https://doi.org/10.3390/ma16227143








