Assessment of Water Transport and Chemical Attack of Meta-Illite Calcined Clay Blended Cement in High-Performance Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Specimen Testing
2.3.1. Water Absorption
2.3.2. Sorptivity
2.3.3. Chemical Resistance
3. Results and Discussion
3.1. Water Absorption
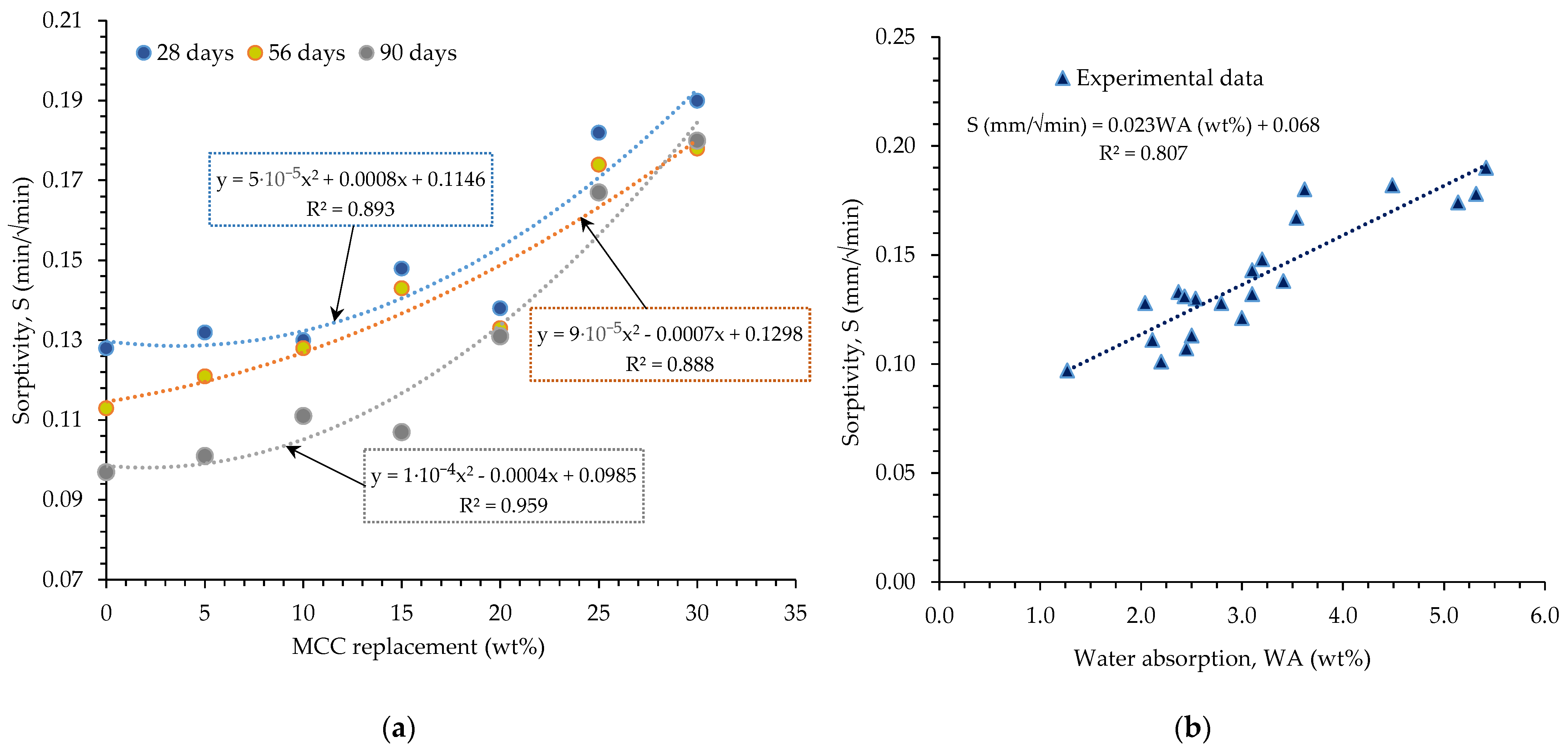
3.2. Sorptivity
3.3. Chemical Durability: Acid, Sulphate and Chlorine Attacks on HPC
3.3.1. Weight Changes
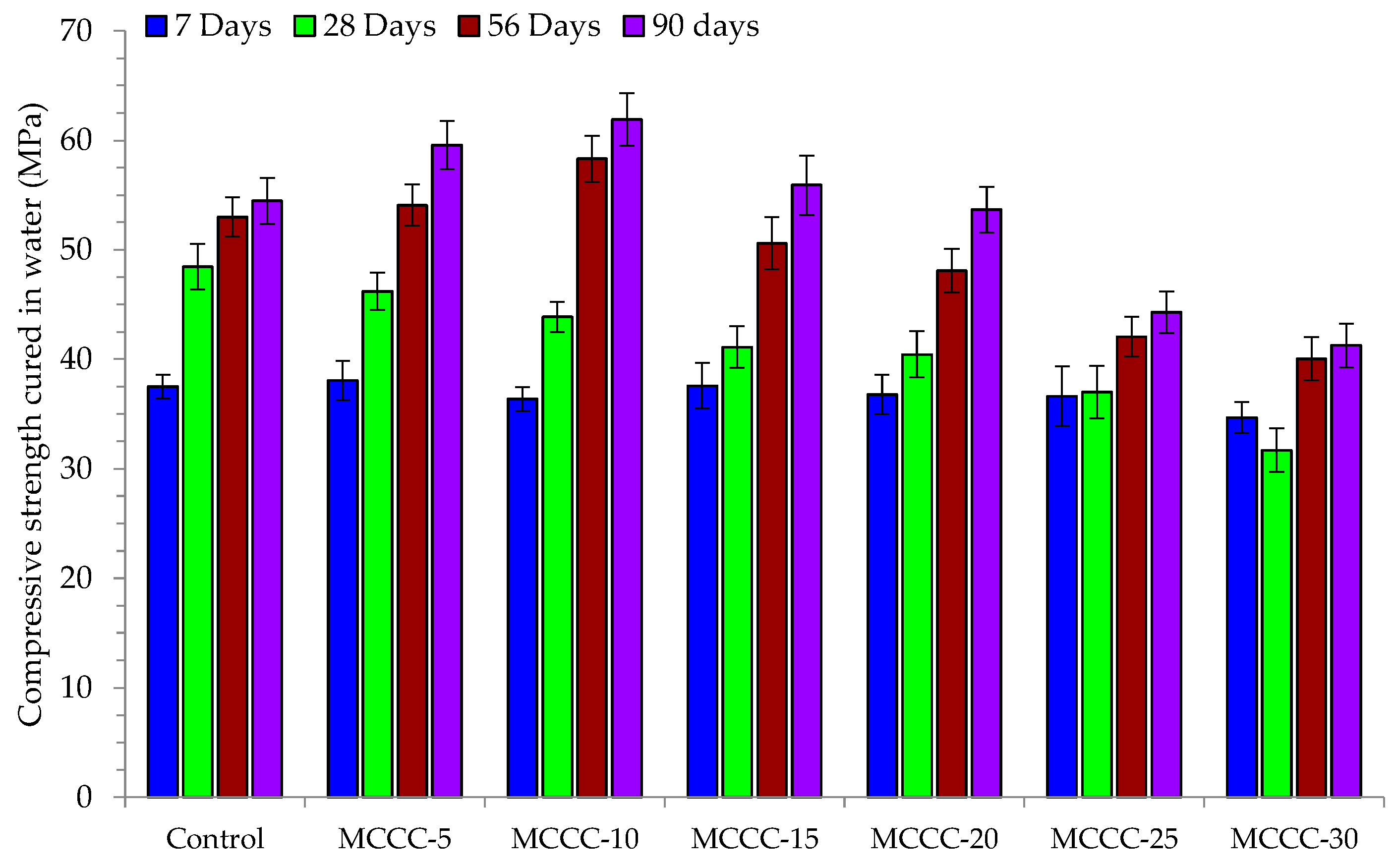
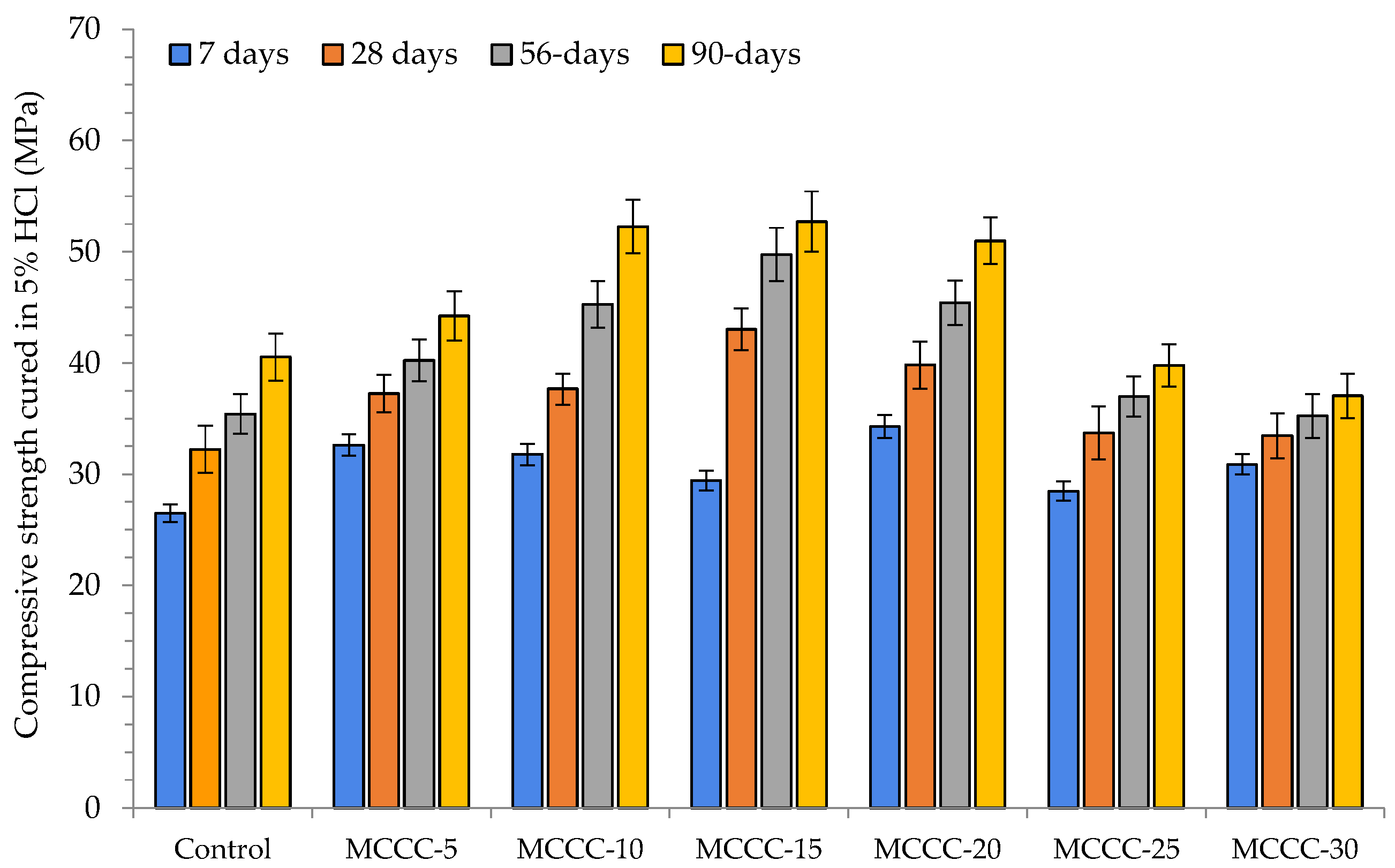
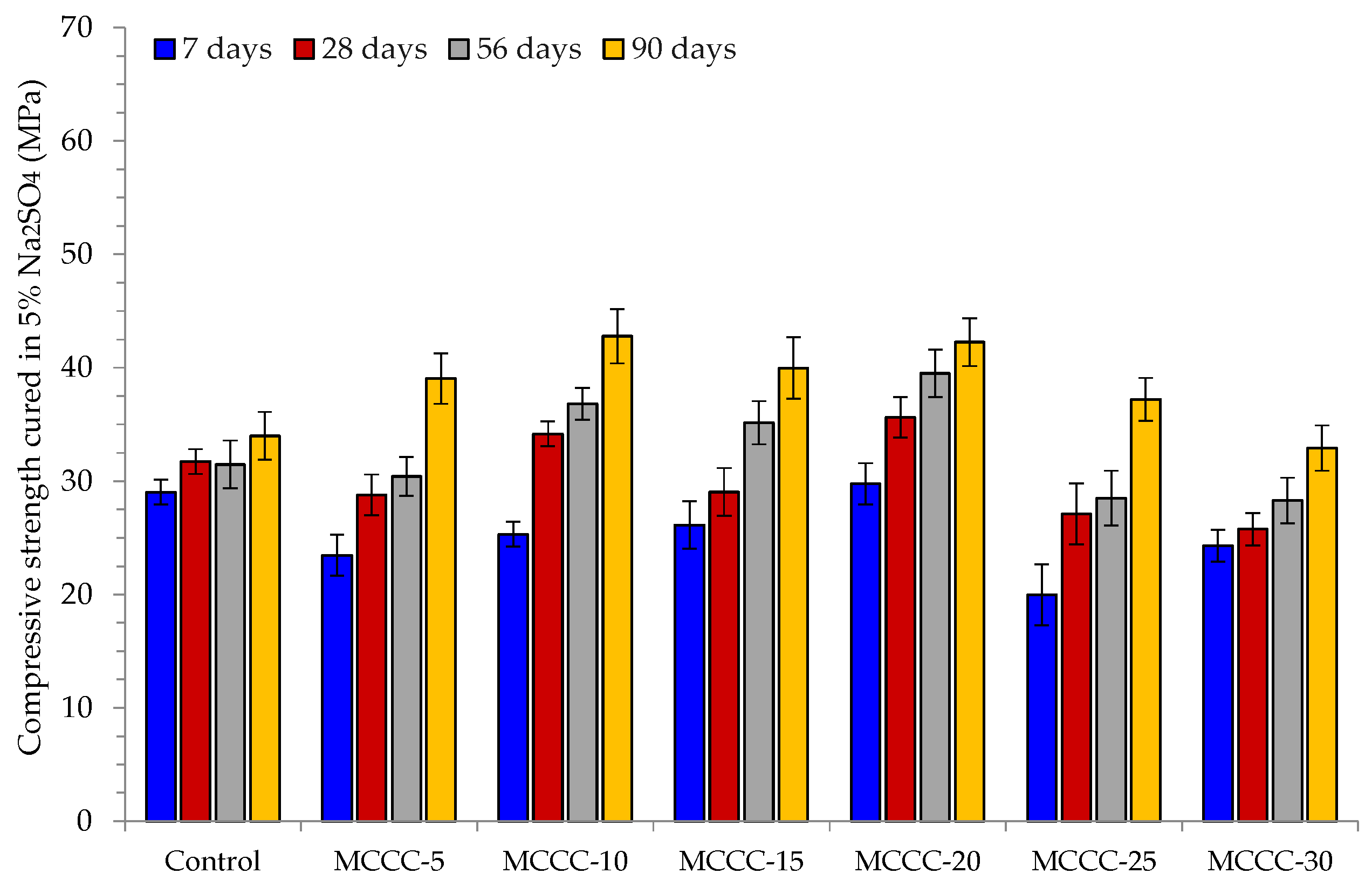
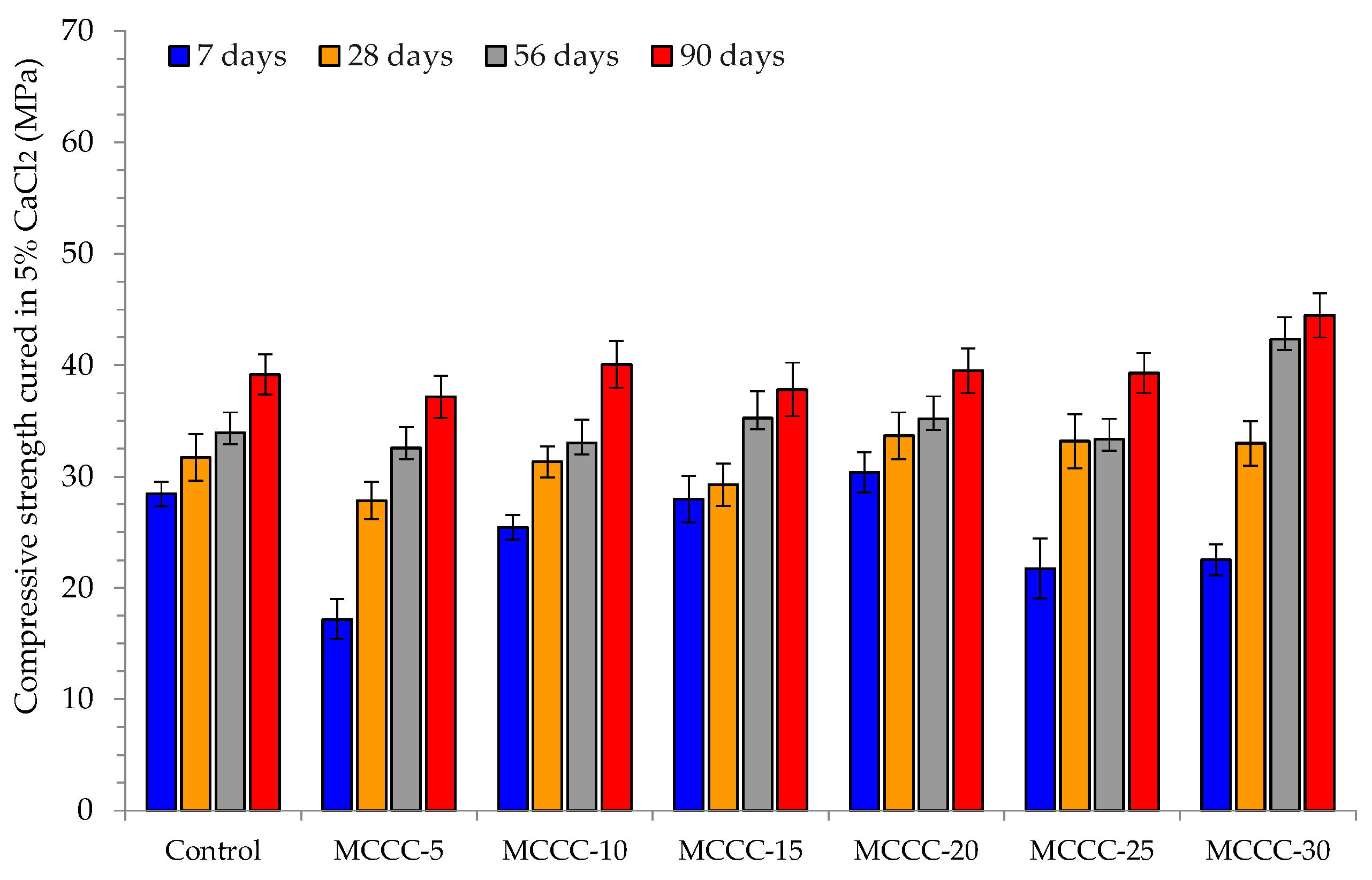
3.3.2. Compressive Strength Analysis
4. Conclusions
- According to ASTM C 642 [41], the water absorption values obtained in MCC-based HPCs are within the 2 to 5% acceptable range of water absorption for HPC.
- On all observed days, the 5–15% cement replacements had very close water sorptivity values attributable to the physical properties regarding the greater specific surface area and pore size distribution of MCC.
- The relationship between HPCs sorptivity and MCC content highlighted a high correlation coefficient for 90 days of hydration (R2 > 0.96), while 56 and 28 days observation attested to moderate correlation coefficients of 0.89, respectively. In the same vein, MCC replacement and the water absorption relationship depicted a positive relationship with the water absorption (R2 = 0.81).
- At 56 and 90 days curing ages, the HPC specimen MCCC-10 had the highest compressive strength. The 10% replacement mix (MCCC-10) had the highest compressive strength across all curing ages, indicating that MCC is best for realising HPC at this level of CEM II replacement.
- The various MCC-blended mixtures (MCCC-5 to MCCC-30) demonstrated minimal weight reductions of less than or equal to 10% in the different chemical environment exposures across the curing ages.
- The HPC mixes (MCCC-10, MCCC-15 and MCCC-20) recorded reduced compression strength variation in the 1% to 14.59% range, indicating that ~15% strength was lost in HCl, Na2SO4 and CaCl2 over the 90 days of curing.
- For all mix types, the compressive strength of the tested samples was most affected by the acidic environment with approximately 15% strength lost in HCl, Na2SO4 and CaCl2 over the 90 days of curing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Design Floor. Different Concrete Grades Based on Different International Codes. 2023. Available online: https://designfloor.org/concrete-grades/ (accessed on 31 October 2023).
- Zhang, Y.; Zhu, P.; Liao, Z.; Wang, L. Interfacial bond properties between normal strength concrete substrate and ultra-high performance concrete as a repair material. Constr. Build. Mater. 2020, 235, 117431. [Google Scholar] [CrossRef]
- Oyebisi, S.; Ede, A.; Olutoge, F.; Omole, D. Geopolymer concrete incorporating agro-industrial wastes: Effects on mechanical properties, microstructural behaviour and mineralogical phases. Constr. Build. Mater. 2020, 256, 119390. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Shi, C.; Yuan, Q.; Zhang, Z. Durability of ultra-high performance concrete—A review. Constr. Build. Mater. 2020, 255, 119296. [Google Scholar] [CrossRef]
- Nduka, D.O.; Olawuyi, B.J.; Joshua, O.O.; Omuh, I.O. A Study on Gel/Space Ratio Development in Binary Mixture Containing Portland Cement and Meta-Illite Calcined Clay/Rice Husk Ash. Gels 2022, 8, 85. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, M.; Cui, J. A review on the deterioration of mechanical and durability performance of marine-concrete under the scouring action. J. Build. Eng. 2023, 66, 105924. [Google Scholar] [CrossRef]
- Hao, H.; Bi, K.; Chen, W.; Pham, T.M.; Li, J. Towards next generation design of sustainable, durable, multi-hazard resistant, resilient, and smart civil engineering structures. Eng. Struct. 2023, 277, 115477. [Google Scholar] [CrossRef]
- Sohail, M.G.; Kahraman, R.; Al Nuaimi, N.; Gencturk, B.; Alnahhal, W. Durability characteristics of high and ultra-high performance concretes. J. Build. Eng. 2020, 33, 101669. [Google Scholar] [CrossRef]
- Dushimimana, A.; Niyonsenga, A.A.; Nzamurambaho, F. A review on strength development of high performance concrete. Constr. Build. Mater. 2021, 307, 124865. [Google Scholar] [CrossRef]
- Nduka, D.O.; Olawuyi, B.J.; Fagbenle, O.I.; Fonteboa, B.G. Assessment of the Durability Dynamics of High-Performance Concrete Blended with a Fibrous Rice Husk Ash. Crystals 2022, 12, 75. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiao, P. Durability of ultra-high-performance concrete in tension under cold weather conditions. Cem. Concr. Compos. 2018, 94, 94–106. [Google Scholar] [CrossRef]
- ASTM C1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C666/C666M; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015.
- ASTM C672/C672M; Standard Test Method for Scaling Resistance of Concrete Surfaces Exposed to Deicing Chemicals. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- CEN/TR 15177; Technical Report of Testing the Freeze Thaw Resistance of Concrete Internal Structural Damage. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- SS 137244; Standard Concrete Testing Hardened Concrete Scaling at Freezing. Swedish Standards Institute: Stockholm, Sweden, 2005.
- CEN/TS 12390-9; Technical Specifications of Testing Hardened Concrete-Part 9: Freeze-Thaw Resistance–Scaling. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- Khan, M.I. Mix proportions for HPC incorporating multi-cementitious composites using artificial neural networks. Constr. Build. Mater. 2012, 28, 14–20. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Pozzolanic reactivity of a calcined interstratified illite/smectite (70/30) clay. Cem. Concr. Res. 2016, 79, 101–111. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Marchetti, G.; Rahhal, V.; Pavlík, Z.; Pavlíková, M.; Irassar, E.F. Assessment of packing, flowability, hydration kinetics, and strength of blended cements with illitic calcined shale. Constr. Build. Mater. 2020, 254, 119042. [Google Scholar] [CrossRef]
- Rossetti, A.; Ikumi, T.; Segura, I.; Irassar, E.F. Sulfate performance of blended cements (limestone and illite calcined clay) exposed to aggressive environment after casting. Cem. Concr. Res. 2021, 147, 106495. [Google Scholar] [CrossRef]
- Vejmelková, E.; Keppert, M.; Rovnaníková, P.; Ondráček, M.; Keršner, Z.; Černý, R. Properties of high performance concrete containing fine-ground ceramics as supplementary cementitious material. Cem. Concr. Compos. 2012, 34, 55–61. [Google Scholar] [CrossRef]
- Laidani, Z.E.A.; Benabed, B.; Abousnina, R.; Gueddouda, M.K.; Kadri, E.H. Experimental investigation on effects of calcined bentonite on fresh, strength and durability properties of sustainable self-compacting concrete. Constr. Build. Mater. 2020, 230, 117062. [Google Scholar] [CrossRef]
- Msinjili, N.S.; Vogler, N.; Sturm, P.; Neubert, M.; Schröder, H.-J.; Kühne, H.-C.; Hünger, K.-J.; Gluth, G.J. Calcined brick clays and mixed clays as supplementary cementitious materials: Effects on the performance of blended cement mortars. Constr. Build. Mater. 2021, 266, 120990. [Google Scholar] [CrossRef]
- Nduka, D.O.; Olawuyi, B.J.; Fagbenle, O.I.; Fonteboa, B.G. Effect of KyAl4(Si8-y)O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete. Crystals 2021, 11, 1152. [Google Scholar] [CrossRef]
- EN, BS 197-1; Cement–Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee For Standardisation: London, UK, 2011.
- DMS 29-1:2018; Composition, Specification and Conformity Criteria for Common Cements. Standards Organization of Nigeria: Abuja, Nigeria, 2018.
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson Educational Limited: Harlow, UK, 2012. [Google Scholar]
- Zeyad, A.M.; Johari, M.A.M.; Abutaleb, A.; Tayeh, B.A. The effect of steam curing regimes on the chloride resistance and pore size of high–strength green concrete. Constr. Build. Mater. 2021, 280, 122409. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Johari, M.A.M.; Alharbi, Y.R.; Abadel, A.A.; Amran, Y.M.; Tayeh, B.A.; Abutaleb, A. Influence of steam curing regimes on the properties of ultrafine POFA-based high-strength green concrete. J. Build. Eng. 2021, 83, 102204. [Google Scholar] [CrossRef]
- Arulmoly, B.; Konthesingha, C.; Nanayakkara, A. Performance evaluation of cement mortar produced with manufactured sand and offshore sand as alternatives for river sand. Constr. Build. Mater. 2021, 297, 123784. [Google Scholar] [CrossRef]
- Olawuyi, B.J.; Boshoff, W. Influence of SAP content and curing age on air void distribution of high performance concrete using 3D volume analysis. Constr. Build. Mater 2017, 135, 580–589. [Google Scholar] [CrossRef]
- Olawuyi, B.J. The Mechanical Behaviour of High-Performance Concrete with Superabsorbent Polymers (SAP). Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2016. Available online: scholar.sun.ac.za/handle/10019.1/98352 (accessed on 30 June 2023).
- EN, B. 12350-5; Testing Fresh Concrete Flow Table Test. British Standards Institution-BSI: London, UK, 2009.
- ASTM C403; Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. American Society for Testing and Materials: Easton, MD, USA, 2008.
- EN, B. 1097-6; Tests for Mechanical and Physical Properties of Aggregates Part 6: Determination of Particle Density and Water Absorption. BSI Standards Ltd.: Brussels, Belgium, 2013.
- ASTM C1585; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2004.
- EN, B. 12390-3; Testing Hardened Concrete. Compressive Strength of Test Specimens. British Standard Institute: London, UK, 2019.
- International Union of Testing and Research Laboratories for Materials and Structures (RILEM). CPC4—Compressive Strength of Concrete (1975). TC14-CPC, RILEM Technical Recommendations for the Testing and Use of Construction Materials; E FN, Spon: London, UK, 1994; pp. 17–18. [Google Scholar]
- ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2006.
- Kwan, W.H.; Wong, Y.S. Acid Leached Rice Husk Ash (ARHA) in Concrete: A Review. Mater. Sci. Energy Technol. 2020, 3, 501–507. [Google Scholar] [CrossRef]
- Nikookar, M.; Brake, N.A.; Adesina, M.; Rahman, A.; Selvaratnam, T.; Snyder, H.A.; Günaydın-Sen, O. Reutilization of oil and gas produced water in cement composite manufacturing. J. Clean. Prod. 2022, 381, 135113. [Google Scholar] [CrossRef]
- Vivek, S.S.; Dhinakaran, G. Durability Characteristics of Binary Blend High Strength SCC. Constr. Build. Mater. 2017, 146, 1–8. [Google Scholar] [CrossRef]
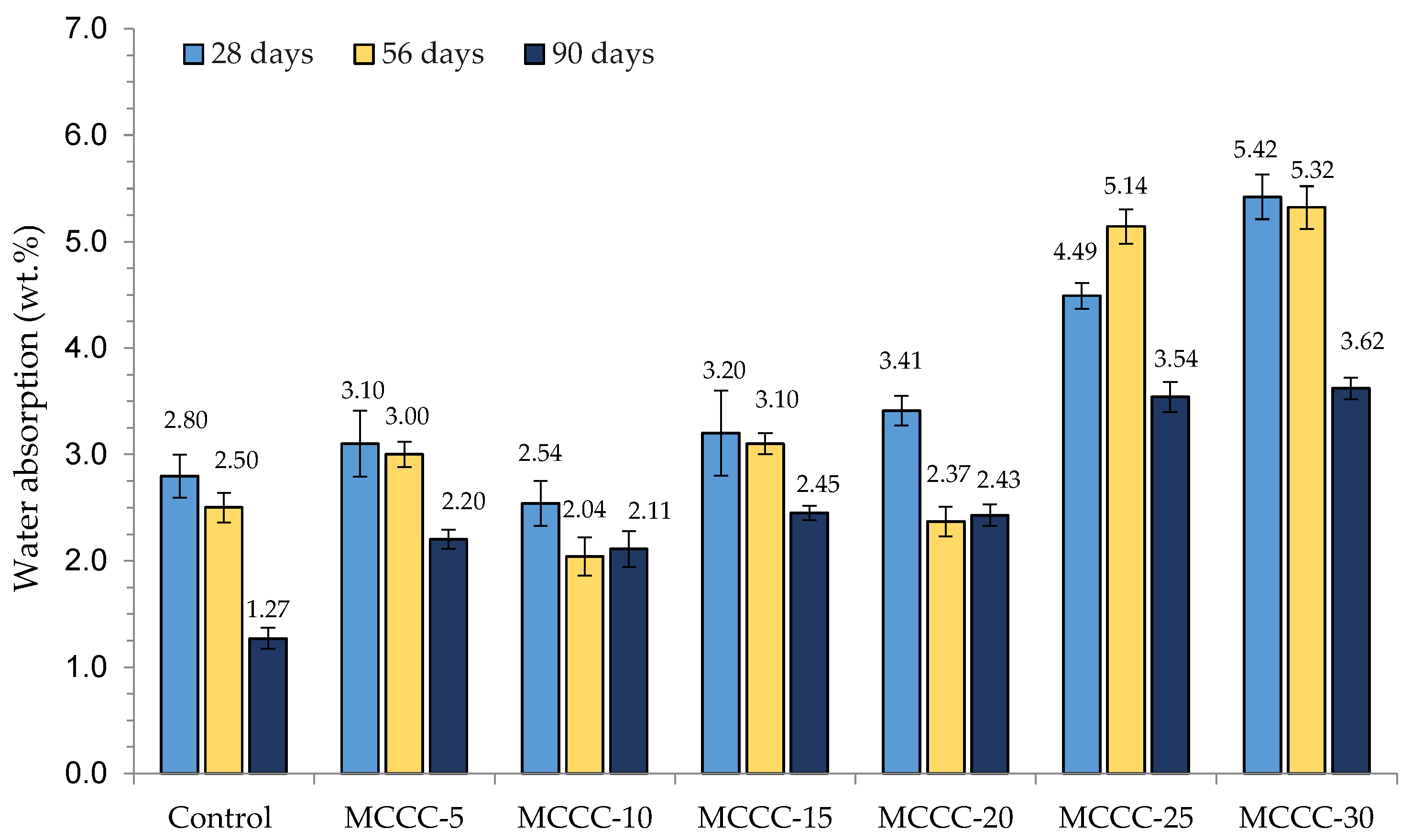
| Material Properties | MCC | CEM II | Sand | Coarse Aggregate |
|---|---|---|---|---|
| Fineness modulus | - | - | 2.87 | - |
| Specific gravity | 2.81 | 3.12 | 2.65 | 2.7 |
| Water absorption, % | - | - | 1.44 | 1.26 |
| Aggregate crushing value, % | - | - | - | 28 |
| Aggregate impact value, % | - | 11 | ||
| BET SSA (m2/g) MultiPoint | 4.649 × 102 | 8.182 × 102 | - | - |
| Pore diameter mode—DA (nm) | 2.88 | 2.92 | - | - |
| Particle size distribution of binders | ||||
| D90 (µm) | 42.35 | 48.00 | ||
| D50 (µm) | 15.40 | 20.05 | ||
| D10 (µm) | 2.04 | 1.48 | ||
| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | P2O5 | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| MCC (%) | 60.92 | 3.20 | 3.43 | 1.83 | 0.24 | 0.35 | 0.18 | 0.12 | 7.68 |
| CEM II (%) | 15.38 | 4.14 | 3.19 | 56.92 | 2.44 | 0.21 | 0.28 | 0.04 | 15.59 |
| Constituents | Mixes (kg/m3) | ||||||
|---|---|---|---|---|---|---|---|
| Control | MCCC-5 | MCCC-10 | MCCC-15 | MCCC-20 | MCCC-25 | MCCC-30 | |
| Water | 156 | 156 | 156 | 156 | 156 | 156 | 156 |
| Cement | 540 | 513 | 486 | 459 | 432 | 405 | 378 |
| MCC | 0 | 27 | 54 | 81 | 108 | 135 | 162 |
| Coarse aggregate | 1050 | 1050 | 1050 | 1050 | 1050 | 1050 | 1050 |
| Sand | 700 | 700 | 700 | 700 | 700 | 700 | 700 |
| SAP (0.3% bwob) | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 |
| SP (1.5% bwob) | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 |
| Water/binder (W/B) * | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Additional water | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 |
| HPC Mix | 28 Days | 56 Days | 90 Days | |||
|---|---|---|---|---|---|---|
| S (mm/min0.5) | R2 | S (mm/min0.5) | R2 | S (mm/min0.5) | R2 | |
| Control | 0.128 ± 0.010 | 0.941 | 0.113 ± 0.014 | 0.916 | 0.097 ± 0.011 | 0.982 |
| MCCC-5 | 0.132 ± 0.011 | 0.965 | 0.121 ± 0.012 | 0.947 | 0.101 ± 0.011 | 0.926 |
| MCCC-10 | 0.130 ± 0.014 | 0.984 | 0.128 ± 0.018 | 0.952 | 0.111 ± 0.012 | 0.988 |
| MCCC-15 | 0.148 ± 0.016 | 0.921 | 0.143 ± 0.013 | 0.941 | 0.107 ± 0.015 | 0.927 |
| MCCC-20 | 0.138 ± 0.015 | 0.945 | 0.133 ± 0.010 | 0.921 | 0.131 ± 0.014 | 0.955 |
| MCCC-25 | 0.182 ± 0.014 | 0.904 | 0.174 ± 0.014 | 0.988 | 0.197 ± 0.021 | 0.971 |
| MCCC-30 | 0.190 ± 0.019 | 0.912 | 0.178 ± 0.020 | 0.941 | 0.180 ± 0.020 | 0.991 |
| HPC Mix | Control | MCCC-5 | MCCC-10 | MCCC-15 | MCCC-20 | MCCC-25 | MCCC-30 |
|---|---|---|---|---|---|---|---|
| H2O curing (WC%) | |||||||
| 7 days | 7.3 | 7.5 | 7.4 | 7.8 | 7.8 | 8.1 | 8.7 |
| 28 days | 9.2 | 9.4 | 9.7 | 9.6 | 10.4 | 10.8 | 11.1 |
| 56 days | 12.7 | 13.1 | 13 | 13.4 | 13.7 | 13.9 | 14.1 |
| 90 days | 13.7 | 13.9 | 13.7 | 14.5 | 14.9 | 15.2 | 15.4 |
| 5% HCL curing (WC%) | |||||||
| 7 days | −0.5 | −0.9 | −1.1 | −1.4 | −1.7 | −2.2 | −2.4 |
| 28 days | −4.7 | −5.1 | −5.4 | −5.9 | −5.9 | −6.4 | −6.7 |
| 56 days | −5.5 | −6.2 | −6.7 | −6.6 | −6.9 | −7.4 | −8.1 |
| 90 days | −7.4 | −7.8 | −8.4 | −8.7 | −8.9 | −9.9 | −10.4 |
| 5% Na2SO4 curing (WC%) | |||||||
| 7 days | 1.4 | 1.7 | 1.7 | 2.1 | 2.3 | 2.4 | 2.6 |
| 28 days | −2.4 | −2.8 | −2.8 | −2.9 | −3.1 | −3.7 | −3.9 |
| 56 days | −3.8 | −3.9 | −4.1 | −4.0 | −4.4 | −4.8 | −4.9 |
| 90 days | −4.9 | −5.4 | −5 | −5.1 | −5.7 | −5.9 | −6.1 |
| 5% CaCl2 curing (WC%) | |||||||
| 7 days | 0.9 | 1.1 | 1.4 | 1.9 | 2.1 | 2.1 | 2.5 |
| 28 days | −2.7 | −2.9 | −3.1 | −3.4 | −3.9 | −4.4 | −5.1 |
| 56 days | −3.9 | −4.1 | −4.4 | −3.9 | −4.2 | −5.1 | −5.9 |
| 90 days | −5.1 | −5.2 | −5.3 | −5.4 | −5.4 | −5.9 | −6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nduka, D.O.; Olawuyi, B.J.; Cantero, B.; González-Fonteboa, B. Assessment of Water Transport and Chemical Attack of Meta-Illite Calcined Clay Blended Cement in High-Performance Concrete. Materials 2023, 16, 7149. https://doi.org/10.3390/ma16227149
Nduka DO, Olawuyi BJ, Cantero B, González-Fonteboa B. Assessment of Water Transport and Chemical Attack of Meta-Illite Calcined Clay Blended Cement in High-Performance Concrete. Materials. 2023; 16(22):7149. https://doi.org/10.3390/ma16227149
Chicago/Turabian StyleNduka, David O., Babatunde J. Olawuyi, Blas Cantero, and Belén González-Fonteboa. 2023. "Assessment of Water Transport and Chemical Attack of Meta-Illite Calcined Clay Blended Cement in High-Performance Concrete" Materials 16, no. 22: 7149. https://doi.org/10.3390/ma16227149
APA StyleNduka, D. O., Olawuyi, B. J., Cantero, B., & González-Fonteboa, B. (2023). Assessment of Water Transport and Chemical Attack of Meta-Illite Calcined Clay Blended Cement in High-Performance Concrete. Materials, 16(22), 7149. https://doi.org/10.3390/ma16227149








