Reutilization of Reclaimed Asphalt Binder via Co-Pyrolysis with Rice Husk: Thermal Degradation Behaviors and Kinetic Analysis
Abstract
:1. Introduction
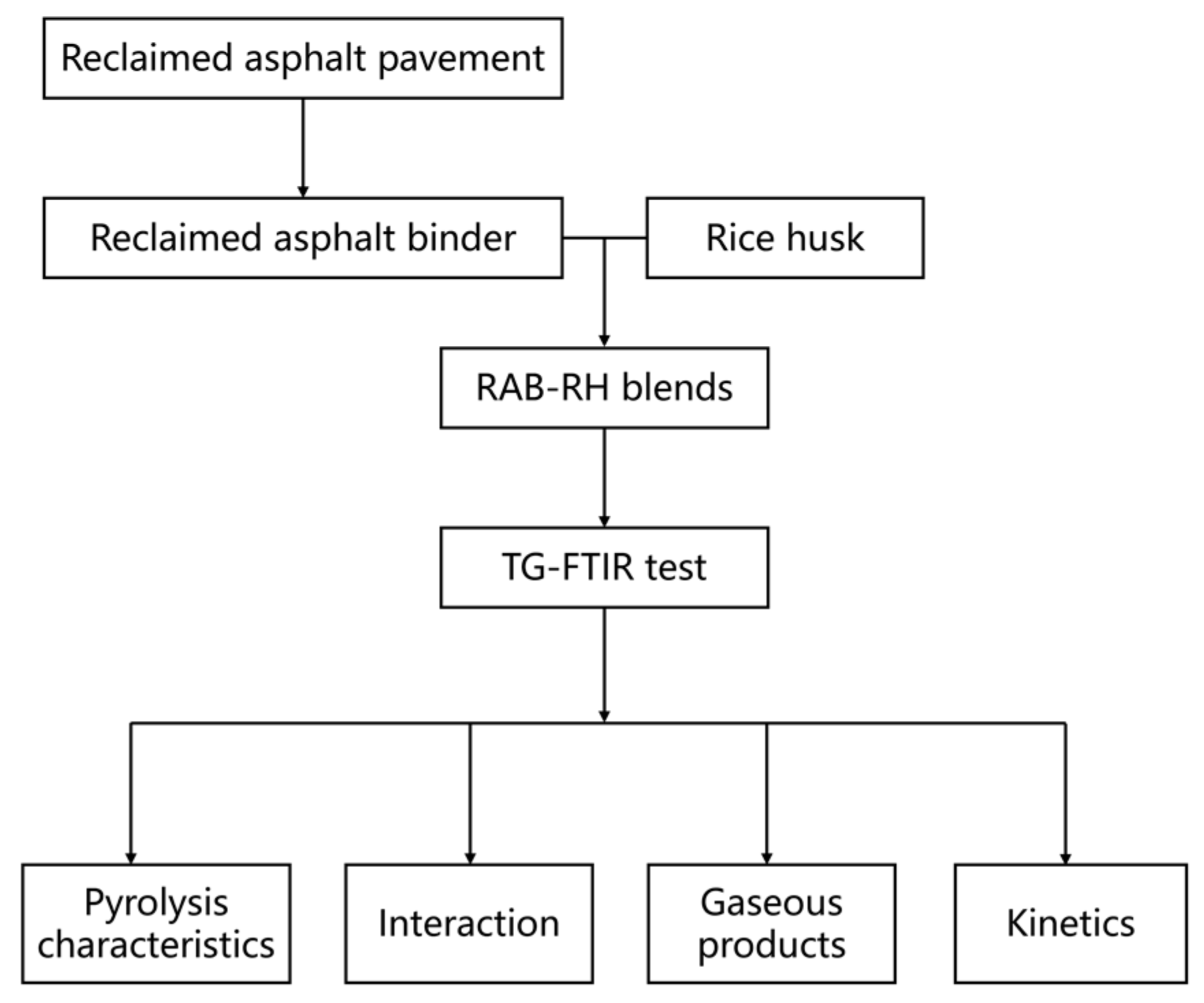
2. Materials and Methods
2.1. RAB and RH Materials
2.2. Preparation of RAB and RH Blends
2.3. TG-FTIR Method and Instrument
2.4. Pyrolysis Indices
2.5. Interaction Analysis
2.6. Kinetic Theory
2.6.1. Iso-Conversional Analysis
2.6.2. Combined Kinetic Analysis
2.7. Experimental Procedure
3. Results and Discussion
3.1. TG-DTG Analysis
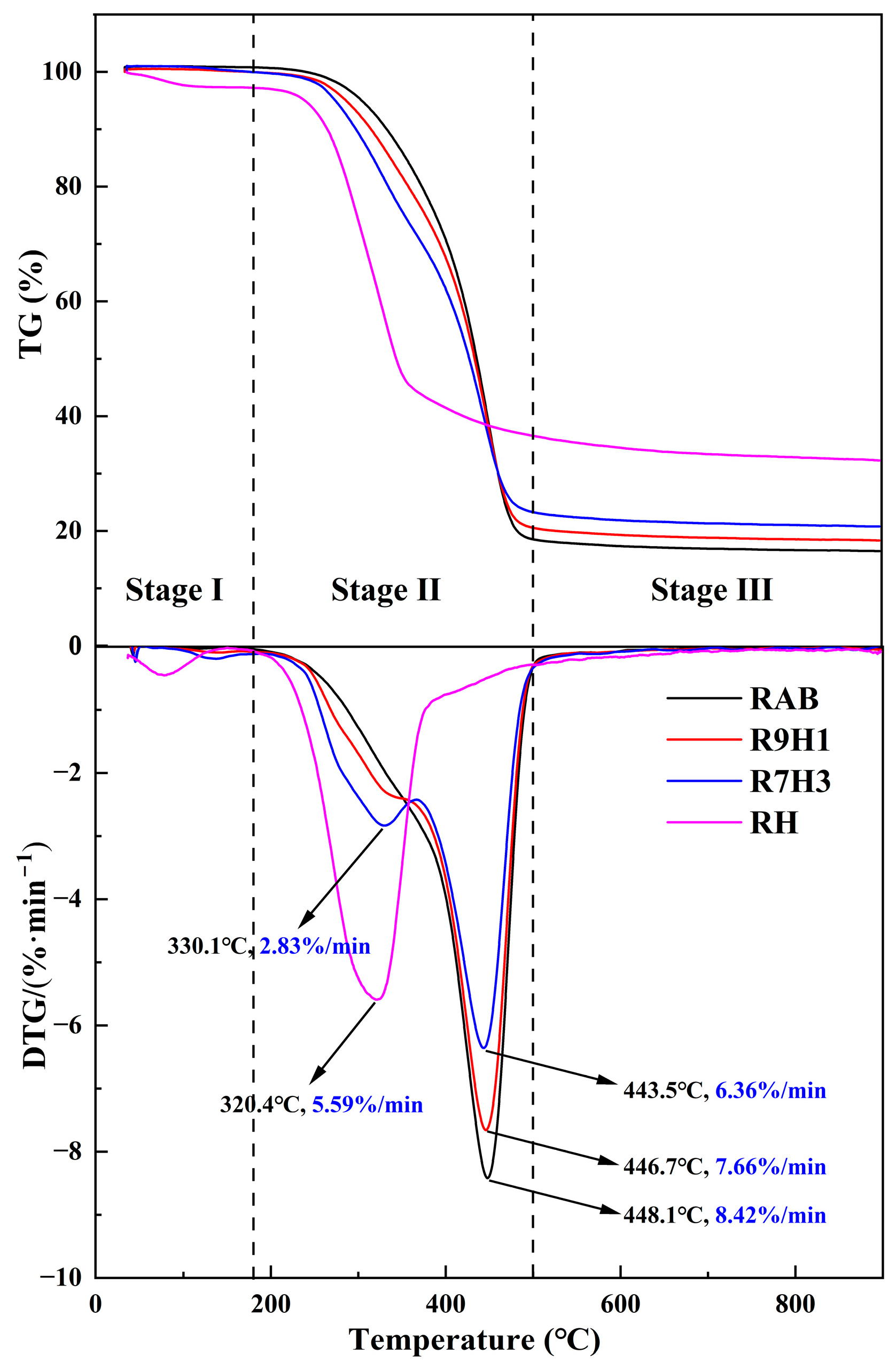
3.1.1. Pyrolysis Characteristics
3.1.2. Interactions between RAB and RH
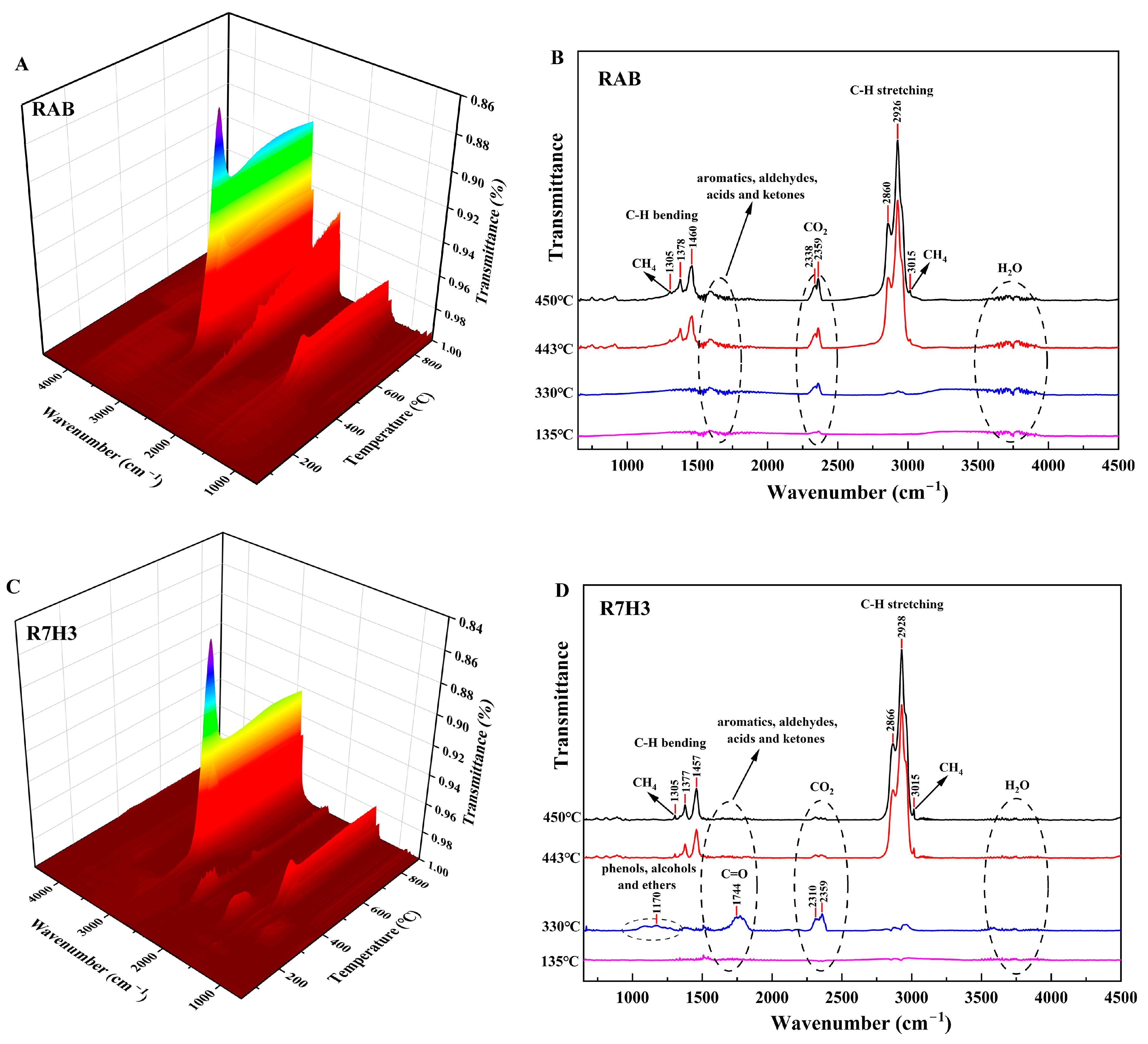
3.2. FTIR Results of Gaseous Products
3.3. Kinetic Analysis
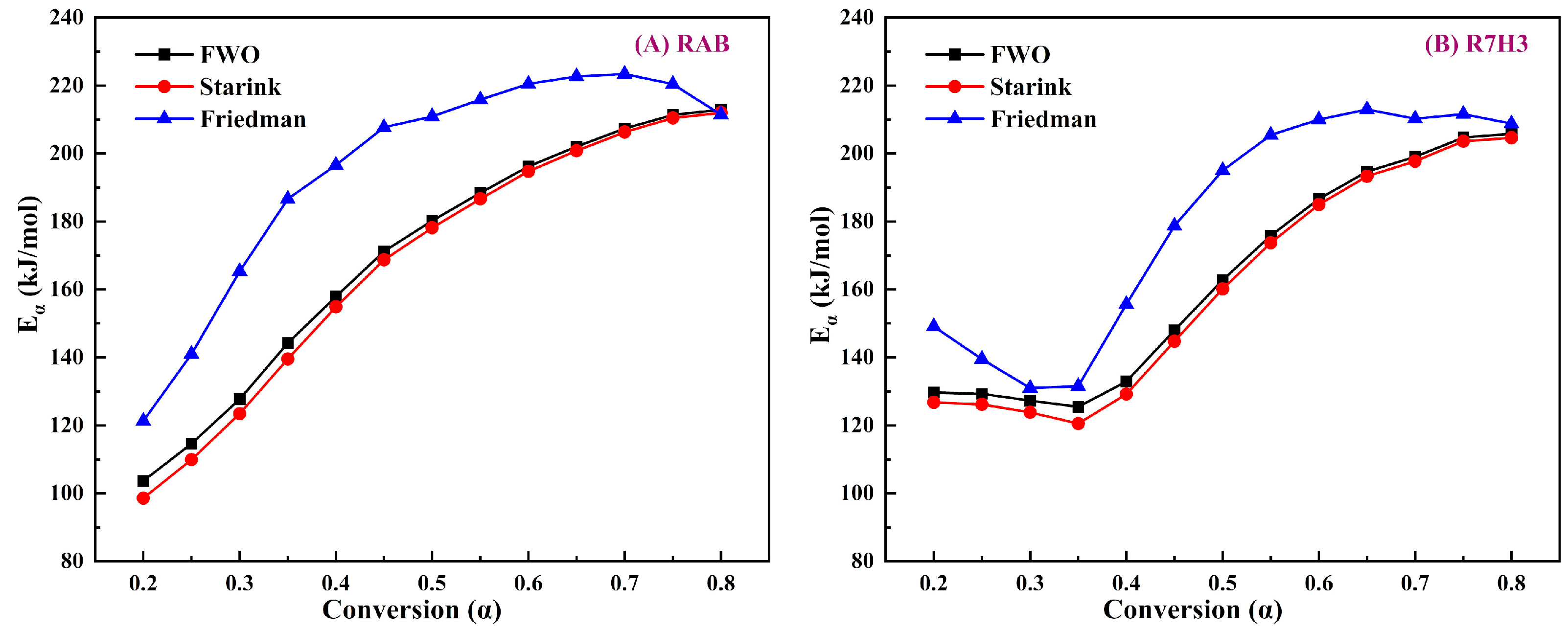
3.3.1. Estimation of Activation Energy
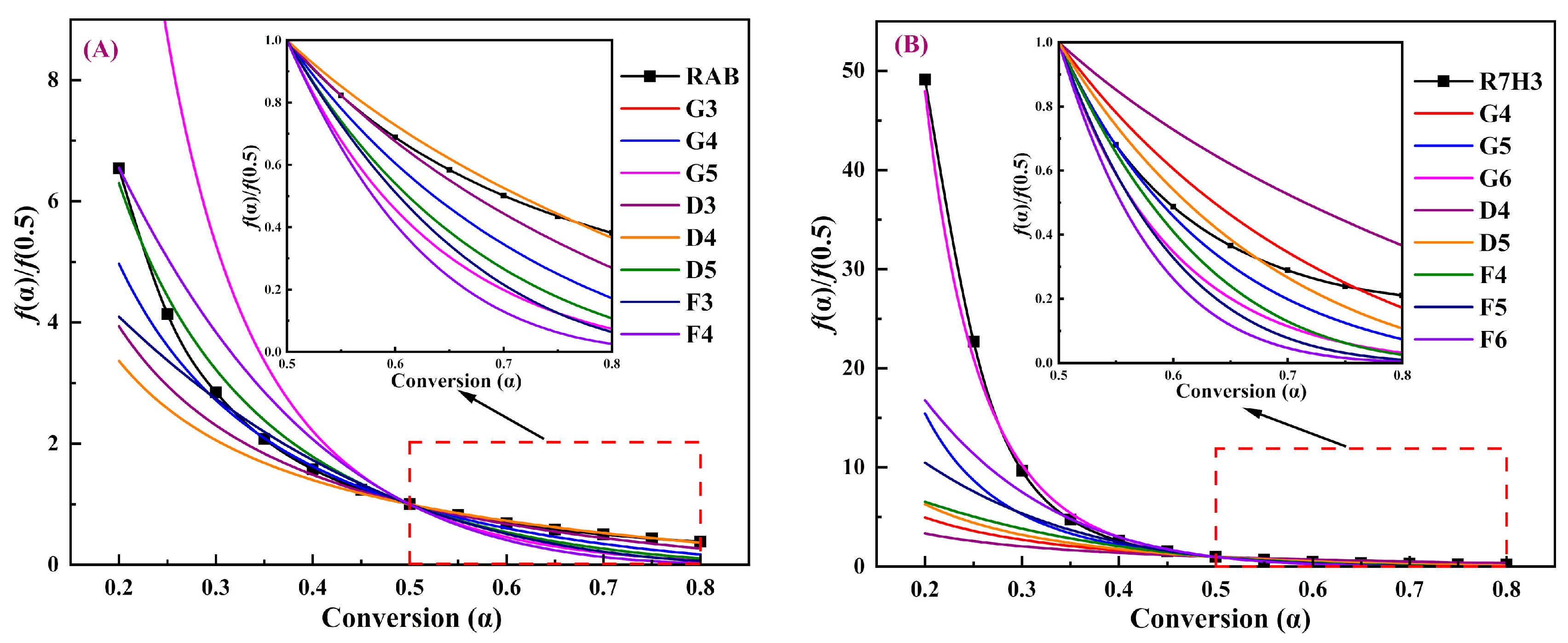
3.3.2. Determination of Reaction Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhao, B.; Xue, Y.; He, Y.; Ding, S.; Wen, Y.; Lv, S. Synchronous Method and Mechanism of Asphalt-Aggregate Separation and Regeneration of Reclaimed Asphalt Pavement. Constr. Build. Mater. 2023, 378, 131127. [Google Scholar] [CrossRef]
- Rout, M.D.; Biswas, S.; Shubham, K.; Sinha, A.K. A Systematic Review on Performance of Reclaimed Asphalt Pavement (RAP) as Sustainable Material in Rigid Pavement Construction: Current Status to Future Perspective. J. Build. Eng. 2023, 76, 107253. [Google Scholar] [CrossRef]
- Rout, M.K.D.; Sahdeo, S.K.; Biswas, S.; Roy, K.; Sinha, A.K. Feasibility Study of Reclaimed Asphalt Pavements (RAP) as Recycled Aggregates Used in Rigid Pavement Construction. Materials 2023, 16, 1504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lü, S.; Liu, C. Technical System, Key Scientific Problems and Technical Frontier of Long-Life Pavement. Chin. Sci. Bull. 2020, 65, 3219–3229. [Google Scholar] [CrossRef]
- Ameri, M.; Mansourkhaki, A.; Daryaee, D. Evaluation of Fatigue Behavior of High Reclaimed Asphalt Binder Mixes Modified with Rejuvenator and Softer Bitumen. Constr. Build. Mater. 2018, 191, 702–712. [Google Scholar] [CrossRef]
- Rahmani, E.; Darabi, M.K.; Little, D.N.; Masad, E.A. Constitutive Modeling of Coupled Aging-Viscoelastic Response of Asphalt Concrete. Constr. Build. Mater. 2017, 131, 1–15. [Google Scholar] [CrossRef]
- Song, W.; Huang, B.; Shu, X. Influence of Warm-Mix Asphalt Technology and Rejuvenator on Performance of Asphalt Mixtures Containing 50% Reclaimed Asphalt Pavement. J. Clean. Prod. 2018, 192, 191–198. [Google Scholar] [CrossRef]
- Tarsi, G.; Tataranni, P.; Sangiorgi, C. The Challenges of Using Reclaimed Asphalt Pavement for New Asphalt Mixtures: A Review. Materials 2020, 13, 4052. [Google Scholar] [CrossRef]
- Xu, S.; Wu, H.; Song, W.; Zhan, Y. Investigation of the Aging Behaviors of Reclaimed Asphalt. J. Clean. Prod. 2022, 356, 131837. [Google Scholar] [CrossRef]
- Rinaldini, E.; Schuetz, P.; Partl, M.N.; Tebaldi, G.; Poulikakos, L.D. Investigating the Blending of Reclaimed Asphalt with Virgin Materials Using Rheology, Electron Microscopy and Computer Tomography. Compos. Part B Eng. 2014, 67, 579–587. [Google Scholar] [CrossRef]
- Kusam, A.; Malladi, H.; Tayebali, A.A.; Khosla, N.P. Laboratory Evaluation of Workability and Moisture Susceptibility of Warm-Mix Asphalt Mixtures Containing Recycled Asphalt Pavements. J. Mater. Civ. Eng. 2017, 29, 04016276. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Koting, S.; Katman, H.Y.B.; Ibrahim, M.R.; Babalghaith, A.M.; Asqool, O. Performance of High Content Reclaimed Asphalt Pavement (RAP) in Asphaltic Mix with Crumb Rubber Modifier and Waste Engine Oil as Rejuvenator. Appl. Sci. 2021, 11, 5226. [Google Scholar] [CrossRef]
- Yi, X.; Chen, H.; Wang, H.; Shi, C.; Yang, J. The Feasibility of Using Epoxy Asphalt to Recycle a Mixture Containing 100% Reclaimed Asphalt Pavement (RAP). Constr. Build. Mater. 2022, 319, 126122. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, L.; Zhao, Z.; Hou, X. Recent Applications and Developments of Reclaimed Asphalt Pavement in China, 2010–2021. Sustain. Mater. Technol. 2023, 37, e00697. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Chen, J.; Li, J.; Yu, B.; Qiu, G.; Zhang, Y.; Xu, B. Investigation into Rheological Behavior of Warm-Mix Recycled Asphalt Binders with High Percentages of RAP Binder. Materials 2023, 16, 1599. [Google Scholar] [CrossRef]
- Mohammadafzali, M.; Ali, H.; Musselman, J.A.; Sholar, G.A.; Rilko, W.A. Aging of Rejuvenated Asphalt Binders. Adv. Mater. Sci. Eng. 2017, 2017, e8426475. [Google Scholar] [CrossRef]
- Wei, X.; Ma, X.; Peng, X.; Yao, Z.; Yang, F.; Dai, M. Comparative Investigation between Co-Pyrolysis Characteristics of Protein and Carbohydrate by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2018, 135, 209–218. [Google Scholar] [CrossRef]
- Schlepp, L.; Elie, M.; Landais, P.; Romero, M.A. Pyrolysis of Asphalt in the Presence and Absence of Water. Fuel Process. Technol. 2001, 74, 107–123. [Google Scholar] [CrossRef]
- Jing-Song, G.; Wei-Biao, F.; Bei-Jing, Z. A Study on the Pyrolysis of Asphalt. Fuel 2003, 82, 49–52. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Y.; Sit, S.P.; Lineberry, Q.; Pan, W. Thermal Characteristics of Bitumen Pyrolysis. J. Therm. Anal. Calorim. 2011, 107, 541–547. [Google Scholar] [CrossRef]
- Cardona, M.; Boffito, D.C.; Patience, G.S. Thermogravimetric Heat and Mass Transfer: Modeling of Bitumen Pyrolysis. Fuel 2015, 143, 253–261. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Hu, H.; Jin, L.; Wang, D.; Wang, G. Co-Pyrolysis of Baiyinhua Lignite and Pine in an Infrared-Heated Fixed Bed to Improve Tar Yield. Fuel 2020, 272, 117739. [Google Scholar] [CrossRef]
- Tauseef, M.; Ansari, A.A.; Khoja, A.H.; Naqvi, S.R.; Liaquat, R.; Nimmo, W.; Daood, S.S. Thermokinetics Synergistic Effects on Co-Pyrolysis of Coal and Rice Husk Blends for Bioenergy Production. Fuel 2022, 318, 123685. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Zhao, J.; Chen, L.; Meng, H. Product Distribution during Co-Pyrolysis of Bituminous Coal and Lignocellulosic Biomass Major Components in a Drop-Tube Furnace. Energy Fuels 2015, 29, 4168–4180. [Google Scholar] [CrossRef]
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M. Interaction and Kinetic Analysis for Coal and Biomass Co-Gasification by TG–FTIR. Bioresour. Technol. 2014, 154, 313–321. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Grace, J.R.; Bi, X.T.; Lim, C.J.; Ellis, N. From Fossil Fuels towards Renewables: Inhibitory and Catalytic Effects on Carbon Thermochemical Conversion during Co-Gasification of Biomass with Fossil Fuels. Appl. Energy 2015, 140, 196–209. [Google Scholar] [CrossRef]
- Martínez, J.D.; Veses, A.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Puy, N.; Artigues, A.; Bartrolí, J.; García, T. Co-Pyrolysis of Biomass with Waste Tyres: Upgrading of Liquid Bio-Fuel. Fuel Process. Technol. 2014, 119, 263–271. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the Thermal Behavior, Kinetics, and Product Characterization of Biomass and Low-Density Polyethylene Co-Pyrolysis by Thermogravimetric Analysis and Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Sandström, L.; Öhrman, O.G.W.; Jilvero, H. Co-Pyrolysis of Woody Biomass and Plastic Waste in Both Analytical and Pilot Scale. J. Anal. Appl. Pyrolysis 2018, 134, 102–113. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, P.; Dong, S.; Li, Y.; Yuan, G. Asphalt Binder Micro-Characterization and Testing Approaches: A Review. Measurement 2020, 151, 107255. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, C.; Xu, J.; Yang, X. Beneficial Synergetic Effect on Gas Production during Co-Pyrolysis of Sewage Sludge and Biomass in a Vacuum Reactor. Bioresour. Technol. 2015, 183, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Quispe, I.; Navia, R.; Kahhat, R. Energy Potential from Rice Husk through Direct Combustion and Fast Pyrolysis: A Review. Waste Manag. 2017, 59, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Y.; Cui, S.; Tao, Y.; Kai, X.; Yang, T. Study on the Co-Pyrolysis of Oil Shale and Corn Stalk: Pyrolysis Characteristics, Kinetic and Gaseous Product Analysis. J. Anal. Appl. Pyrolysis 2022, 163, 105456. [Google Scholar] [CrossRef]
- ASTM D2172–05; Standard Test Methods for Quantitative Extraction of Bitumen from Bituminous Paving Mixtures. ASTM International: West Conshohocken, PA, USA, 2005. [CrossRef]
- ASTM D5404-03; Standard Practice for Recovery of Asphalt from Solution Using the Rotary Evaporator. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Wang, T.; Ma, H.; Ren, L.; Chen, Z.; Chen, S.; Liu, J.; Mei, M.; Li, J.; Xue, Y. Insights into In-Situ Sulfur Retention by Co-Combustion of Dyeing Sludge and Wood Sawdust. J. Clean. Prod. 2021, 323, 129114. [Google Scholar] [CrossRef]
- Boom, Y.J.; Enfrin, M.; Xuan, D.L.; Grist, S.; Robert, D.; Giustozzi, F. Laboratory Evaluation of PAH and VOC Emission from Plastic-Modified Asphalt. J. Clean. Prod. 2022, 377, 134489. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-Pyrolysis Behavior of Sewage Sludge and Rice Husk by TG-MS and Residue Analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Characterizing and Optimizing (Co-)Pyrolysis as a Function of Different Feedstocks, Atmospheres, Blend Ratios, and Heating Rates. Bioresour. Technol. 2019, 277, 104–116. [Google Scholar] [CrossRef]
- Singh, R.K.; Patil, T.; Pandey, D.; Tekade, S.P.; Sawarkar, A.N. Co-Pyrolysis of Petroleum Coke and Banana Leaves Biomass: Kinetics, Reaction Mechanism, and Thermodynamic Analysis. J. Environ. Manag. 2022, 301, 113854. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Analysis of Multi-Step Kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A Quick, Direct Method for the Determination of Activation Energy from Thermogravimetric Data. J. Polym. Sci. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating Isothermal Life from Thermogravimetric Data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Starink, M.J. A New Method for the Derivation of Activation Energies from Experiments Performed at Constant Heating Rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined Kinetic Analysis of Solid-State Reactions: A Powerful Tool for the Simultaneous Determination of Kinetic Parameters and the Kinetic Model without Previous Assumptions on the Reaction Mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the Kinetics of the Mechanism of Solid-State Reactions at Increasing Temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; García-Garrido, C.; Pérez-Maqueda, L.A. Kinetic Study of Complex Processes Composed of Non-Independent Stages: Pyrolysis of Natural Rubber. Polym. Degrad. Stab. 2021, 188, 109590. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Combined Kinetic Analysis of Thermal Degradation of Polymeric Materials under Any Thermal Pathway. Polym. Degrad. Stab. 2009, 94, 2079–2085. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Zeman, S.; Sánchez Jiménez, P.E.; Zhao, F.-Q.; Pérez-Maqueda, L.A.; Málek, J. The Effect of Polymer Matrices on the Thermal Hazard Properties of RDX-Based PBXs by Using Model-Free and Combined Kinetic Analysis. J. Hazard. Mater. 2014, 271, 185–195. [Google Scholar] [CrossRef]
- He, W.; Guo, J.-H.; Cao, C.-K.; Liu, X.-K.; Lv, J.-Y.; Chen, S.-W.; Liu, P.-J.; Yan, Q.-L. Catalytic Reactivity of Graphene Oxide Stabilized Transition Metal Complexes of Triaminoguanidine on Thermolysis of RDX. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Li, J.; Yao, X.; Chen, S.; Xu, K.; Fan, B.; Yang, D.; Geng, L.; Qiao, H. Investigation on the Co-Pyrolysis of Agricultural Waste and High-Density Polyethylene Using TG-FTIR and Artificial Neural Network Modelling. Process. Saf. Environ. Prot. 2022, 160, 341–353. [Google Scholar] [CrossRef]
- Liang, F.; Wang, R.; Hongzhong, X.; Yang, X.; Zhang, T.; Hu, W.; Mi, B.; Liu, Z. Investigating Pyrolysis Characteristics of Moso Bamboo through TG-FTIR and Py-GC/MS. Bioresour. Technol. 2018, 256, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liao, Y.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y.; Peng, X.; Ma, X. Co-Pyrolysis Kinetics of Sewage Sludge and Oil Shale Thermal Decomposition Using TGA–FTIR Analysis. Energy Convers. Manag. 2016, 118, 345–352. [Google Scholar] [CrossRef]
- Peng, X.; Ma, X.; Lin, Y.; Guo, Z.; Hu, S.; Ning, X.; Cao, Y.; Zhang, Y. Co-Pyrolysis between Microalgae and Textile Dyeing Sludge by TG–FTIR: Kinetics and Products. Energy Convers. Manag. 2015, 100, 391–402. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-Pyrolysis of Sewage Sludge and Rice Husk by TG–FTIR–MS: Pyrolysis Behavior, Kinetics, and Condensable/Non-Condensable Gases Characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Fang, S.; Ma, X. Behaviors, Product Characteristics and Kinetics of Catalytic Co-Pyrolysis Spirulina and Oil Shale. Energy Convers. Manag. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Pyrolytic Kinetics, Reaction Mechanisms and Products of Waste Tea via TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 184, 436–447. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.; Gao, W.; Lu, X. FTIR and Simultaneous TG/MS/FTIR Study of Late Permian Coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of Pyrolysis Characteristics and Kinetics of Palm Kernel Shell Using TGA–FTIR and Model-Free Integral Methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Douda, J.; Llanos, M.E.; Alvarez, R.; Franco, C.L.; de la Fuente, J.A.M. Pyrolysis Applied to the Study of a Maya Asphaltene. J. Anal. Appl. Pyrolysis 2004, 71, 601–612. [Google Scholar] [CrossRef]
- Scaccia, S. TG–FTIR and Kinetics of Devolatilization of Sulcis Coal. J. Anal. Appl. Pyrolysis 2013, 104, 95–102. [Google Scholar] [CrossRef]
- Li, T.; Song, F.; Zhang, J.; Liu, S.; Xing, B.; Bai, Y. Pyrolysis Characteristics of Soil Humic Substances Using TG-FTIR-MS Combined with Kinetic Models. Sci. Total Environ. 2020, 698, 134237. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Bi, H.; Jiang, C.; Tian, J.; Sun, H.; Zhou, W.; Lin, Q. Research on the Co-Pyrolysis of Coal Gangue and Coffee Industry Residue Based on Machine Language: Interaction, Kinetics, and Thermodynamics. Sci. Total Environ. 2022, 804, 150217. [Google Scholar] [CrossRef]
- Chen, J.; Mu, L.; Jiang, B.; Yin, H.; Song, X.; Li, A. TG/DSC-FTIR and Py-GC Investigation on Pyrolysis Characteristics of Petrochemical Wastewater Sludge. Bioresour. Technol. 2015, 192, 1–10. [Google Scholar] [CrossRef]
- Janković, B.; Mentus, S.; Jelić, D. A Kinetic Study of Non-Isothermal Decomposition Process of Anhydrous Nickel Nitrate under Air Atmosphere. Phys. B Condens. Matter 2009, 404, 2263–2269. [Google Scholar] [CrossRef]
- Huidobro, J.A.; Iglesias, I.; Alfonso, B.F.; Espina, A.; Trobajo, C.; Garcia, J.R. Reducing the Effects of Noise in the Calculation of Activation Energy by the Friedman Method. Chemom. Intell. Lab. Syst. 2016, 151, 146–152. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, W.; Bi, H.; Ni, Z.; Sun, H.; Lin, Q. Co-Pyrolysis of Coal Slime and Cattle Manure by TG–FTIR–MS and Artificial Neural Network Modeling: Pyrolysis Behavior, Kinetics, Gas Emission Characteristics. Energy 2022, 247, 123203. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Hu, Y.M.; Xu, S.N.; He, Z.X.; Ji, H.S. Study on the Synergistic Co-Pyrolysis Behaviors of Mixed Rice Husk and Two Types of Seaweed by a Combined TG-FTIR Technique. J. Anal. Appl. Pyrolysis 2015, 114, 109–118. [Google Scholar] [CrossRef]
- Dimitra, K.; Konstantinos, C. Nonisothermal Crystallization Kinetics: Studying the Validity of Different Johnson–Mehl–Avrami–Erofeev–Kolmogorov (JMAEK) Based Equations. Thermochim. Acta 2021, 704, 179030. [Google Scholar] [CrossRef]
- Criado, J.M.; Ortega, A. Non-Isothermal Crystallization Kinetics of Metal Glasses: Simultaneous Determination of Both the Activation Energy and the Exponent n of the JMA Kinetic Law. Acta Met. 1987, 35, 1715–1721. [Google Scholar] [CrossRef]
- Gao, R.; Liu, B.; Zhan, L.; Guo, J.; Zhang, J.; Xu, Z. Catalytic effect and mechanism of coexisting copper on conversion of organics during pyrolysis of waste printed circuit boards. J. Hazard. Mater. 2021, 403, 123465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Wu, S.; Wang, B.; Wang, Z. Pyrolysis kinetics of phenol–formaldehyde resin by non-isothermal thermogravimetry. Carbon 2010, 48, 352–358. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Yeganeh, N.; Rezaei, M. Kinetic analysis of the complex process of poly(vinyl alcohol) pyrolysis using a new coupled peak deconvolution method. J. Therm. Anal. Calorim. 2014, 118, 1733–1746. [Google Scholar] [CrossRef]
- Li, L.; Guan, C.; Zhang, A.; Chen, D.; Qing, Z. Thermal stabilities and the thermal degradation kinetics of polyimides. Polym. Degrad. Stab. 2004, 84, 369–373. [Google Scholar] [CrossRef]



| Item | RAB | RH |
|---|---|---|
| Ultimate analysis | ||
| Carbon | 82.08 | 38.12 |
| Hydrogen | 9.15 | 5.05 |
| Oxygen a | 1.13 | 43.14 |
| Nitrogen | 0.48 | 0.56 |
| Sulfur | 5.13 | 0.18 |
| Proximate analysis | ||
| Volatile | 87.62 | 65.44 |
| Fixed carbon a | 11.83 | 18.22 |
| Ash | 0.55 | 16.34 |
| Ash analysis | ||
| Na2O | - | 0.25 |
| K2O | - | 3.86 |
| CaO | 0.28 | 2.38 |
| MgO | 0.02 | 0.75 |
| Samples | (°C) | (°C) | (°C) | (%) | (10−11/min2 °C3) |
|---|---|---|---|---|---|
| RAB | 376.3 | 520.2 | 448.1 | 16.6 | 5.61 |
| R9H1 | 273.9 | 531.1 | 446.7 | 18.5 | 6.74 |
| R7H3 | 256.1 | 550.9 | 443.5 | 21.1 | 8.03 |
| RH | 271.9 | 669.3 | 320.4 | 32.8 | 4.48 |
| Samples | Combined Kinetic Analysis | Friedman Method | |||
|---|---|---|---|---|---|
| (kJ/moL) | (s−1) | m | n | Average (kJ/moL) | |
| RAB | 188.64 ± 3.45 | 28.51 ± 0.59 | −2.050 | 0 | 195.69 |
| R7H3 | 174.03 ± 2.51 | 24.02 ± 0.45 | −5.015 | −0.874 | 179.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Mi, B.; Li, N.; Wang, T.; Xue, Y. Reutilization of Reclaimed Asphalt Binder via Co-Pyrolysis with Rice Husk: Thermal Degradation Behaviors and Kinetic Analysis. Materials 2023, 16, 7160. https://doi.org/10.3390/ma16227160
Zhao H, Mi B, Li N, Wang T, Xue Y. Reutilization of Reclaimed Asphalt Binder via Co-Pyrolysis with Rice Husk: Thermal Degradation Behaviors and Kinetic Analysis. Materials. 2023; 16(22):7160. https://doi.org/10.3390/ma16227160
Chicago/Turabian StyleZhao, Hui, Bao Mi, Na Li, Teng Wang, and Yongjie Xue. 2023. "Reutilization of Reclaimed Asphalt Binder via Co-Pyrolysis with Rice Husk: Thermal Degradation Behaviors and Kinetic Analysis" Materials 16, no. 22: 7160. https://doi.org/10.3390/ma16227160
APA StyleZhao, H., Mi, B., Li, N., Wang, T., & Xue, Y. (2023). Reutilization of Reclaimed Asphalt Binder via Co-Pyrolysis with Rice Husk: Thermal Degradation Behaviors and Kinetic Analysis. Materials, 16(22), 7160. https://doi.org/10.3390/ma16227160





