Rough and Porous Micropebbles of CeCu2Si2 for Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. CeCu2Si2 Preparation
2.2. Characterization
3. Results and Discussion
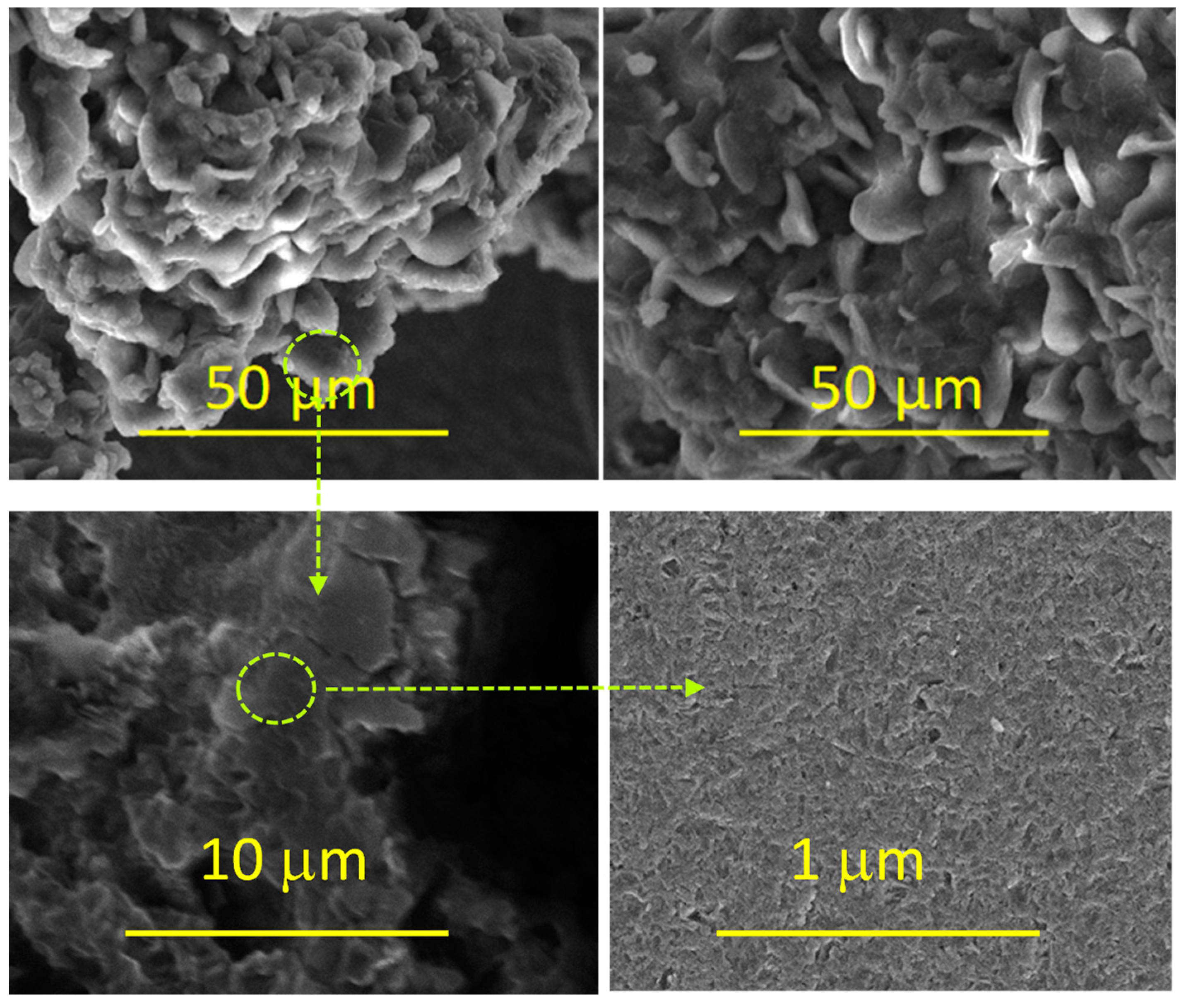
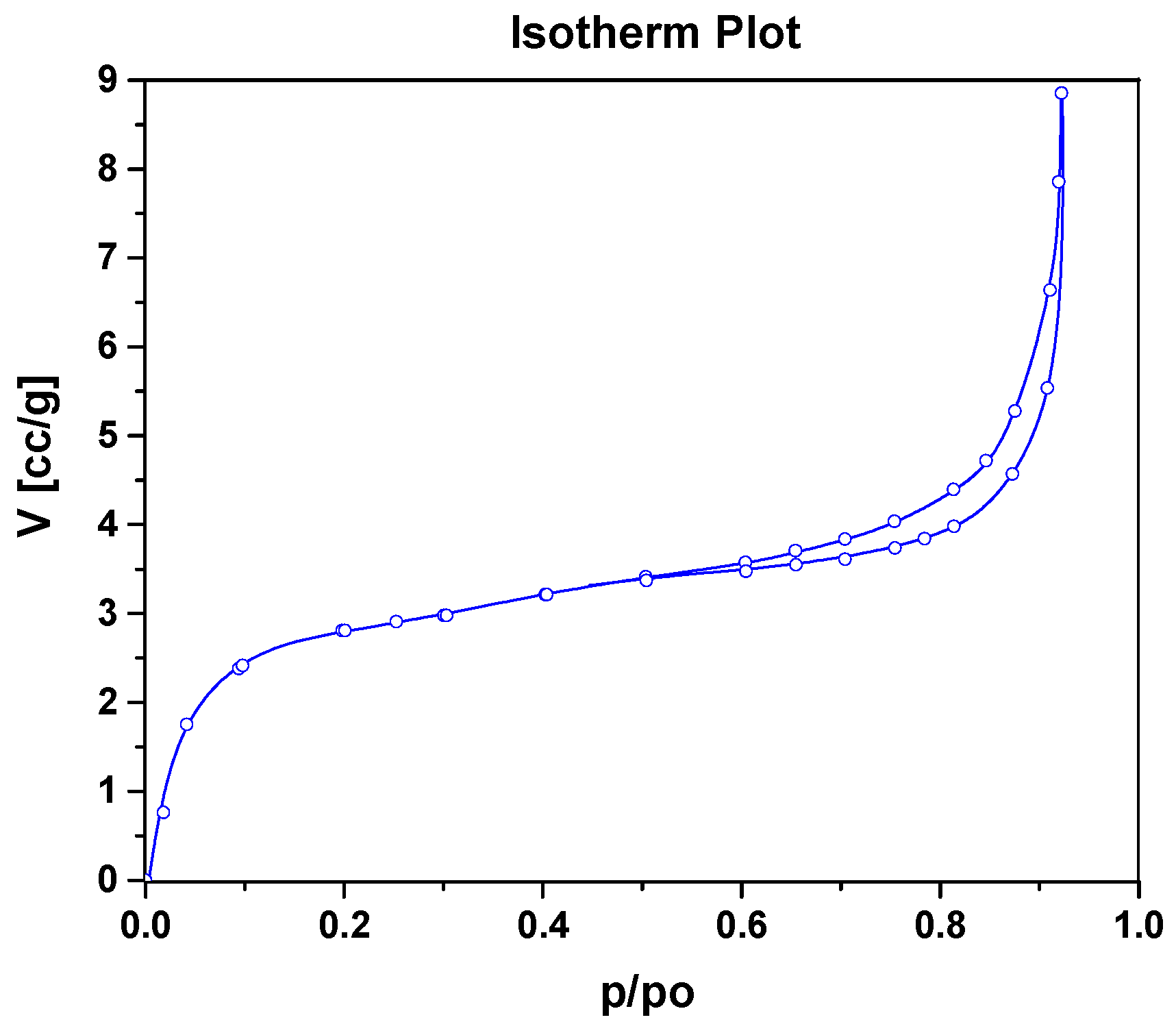
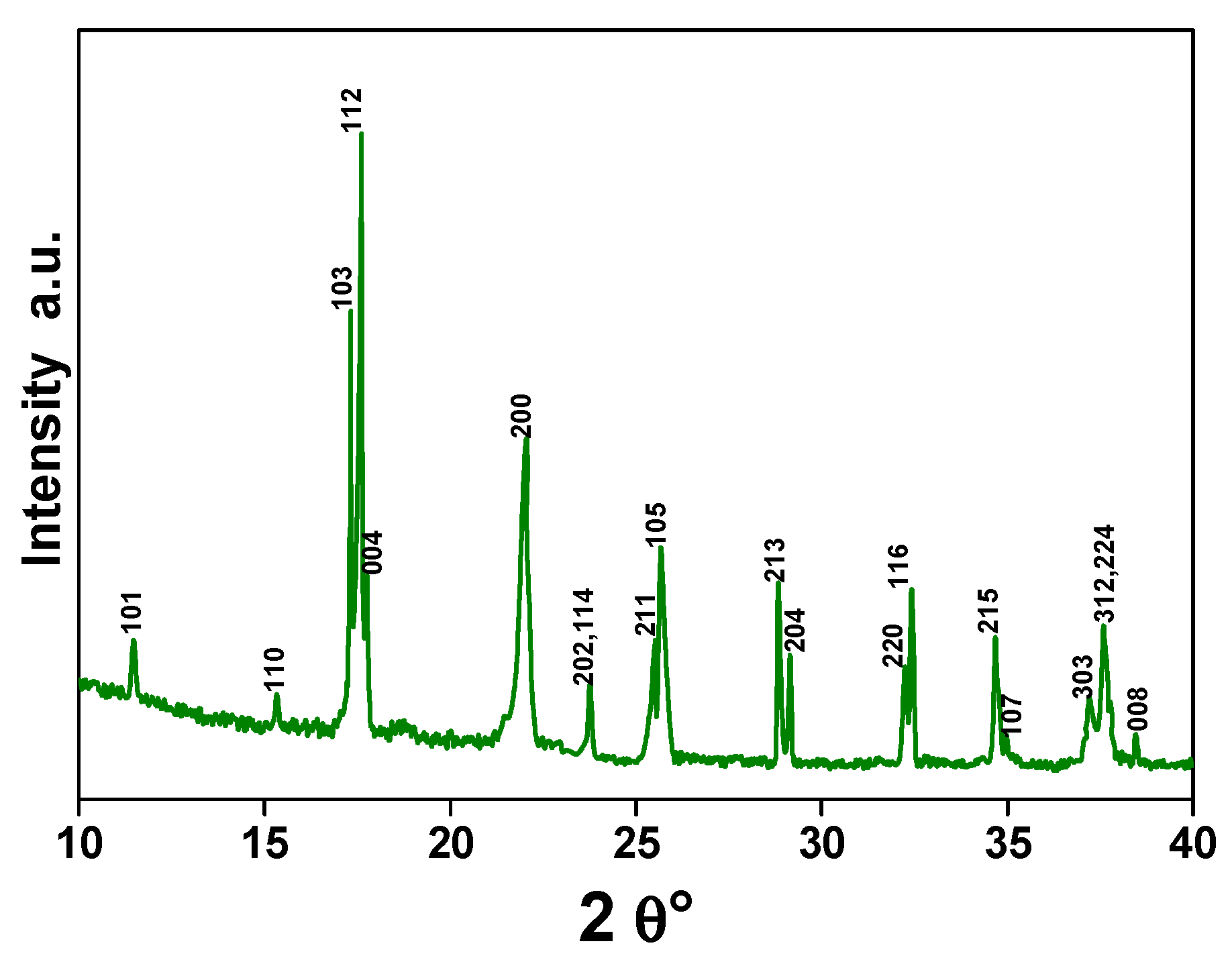
3.1. Morphological Characterization
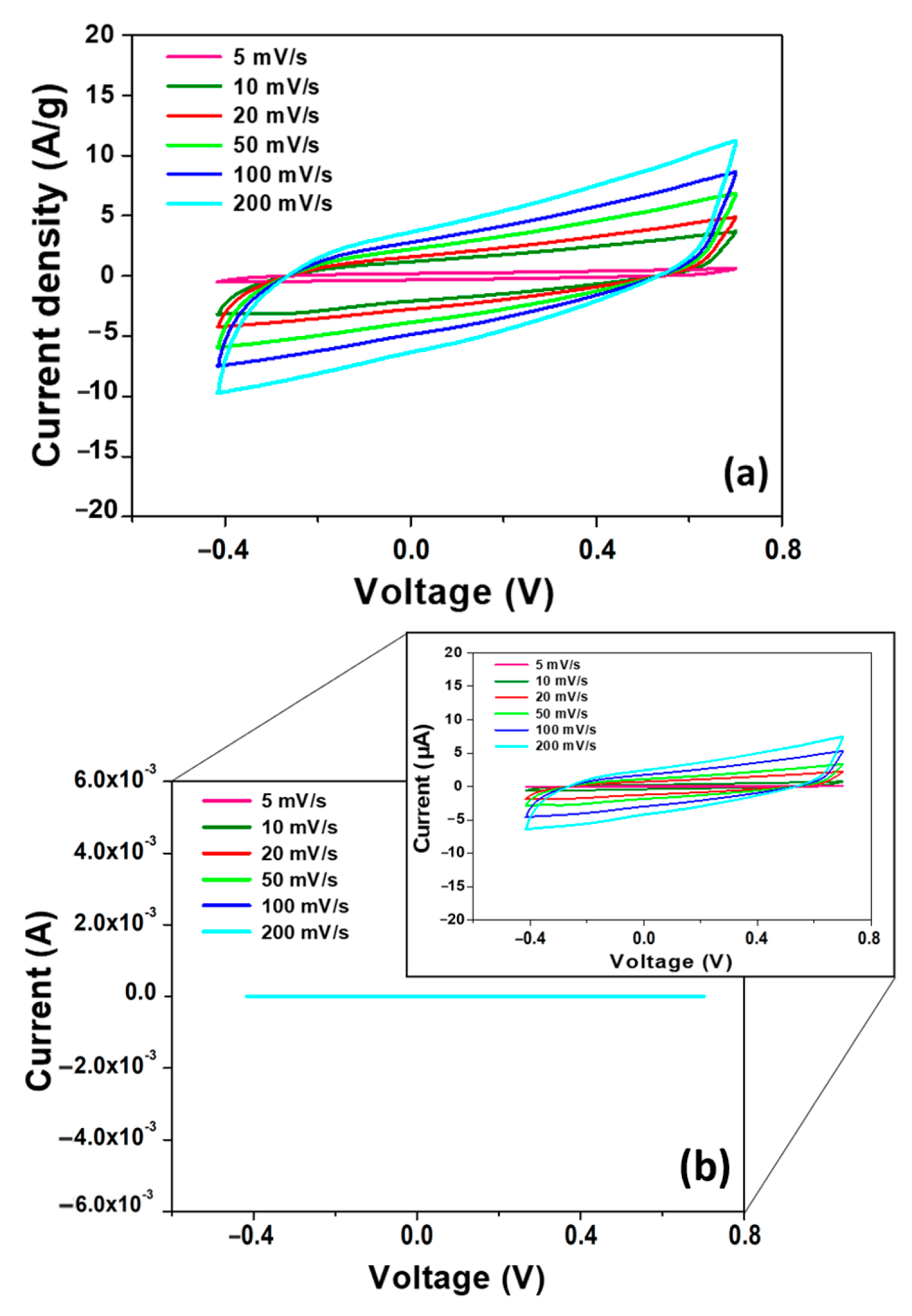
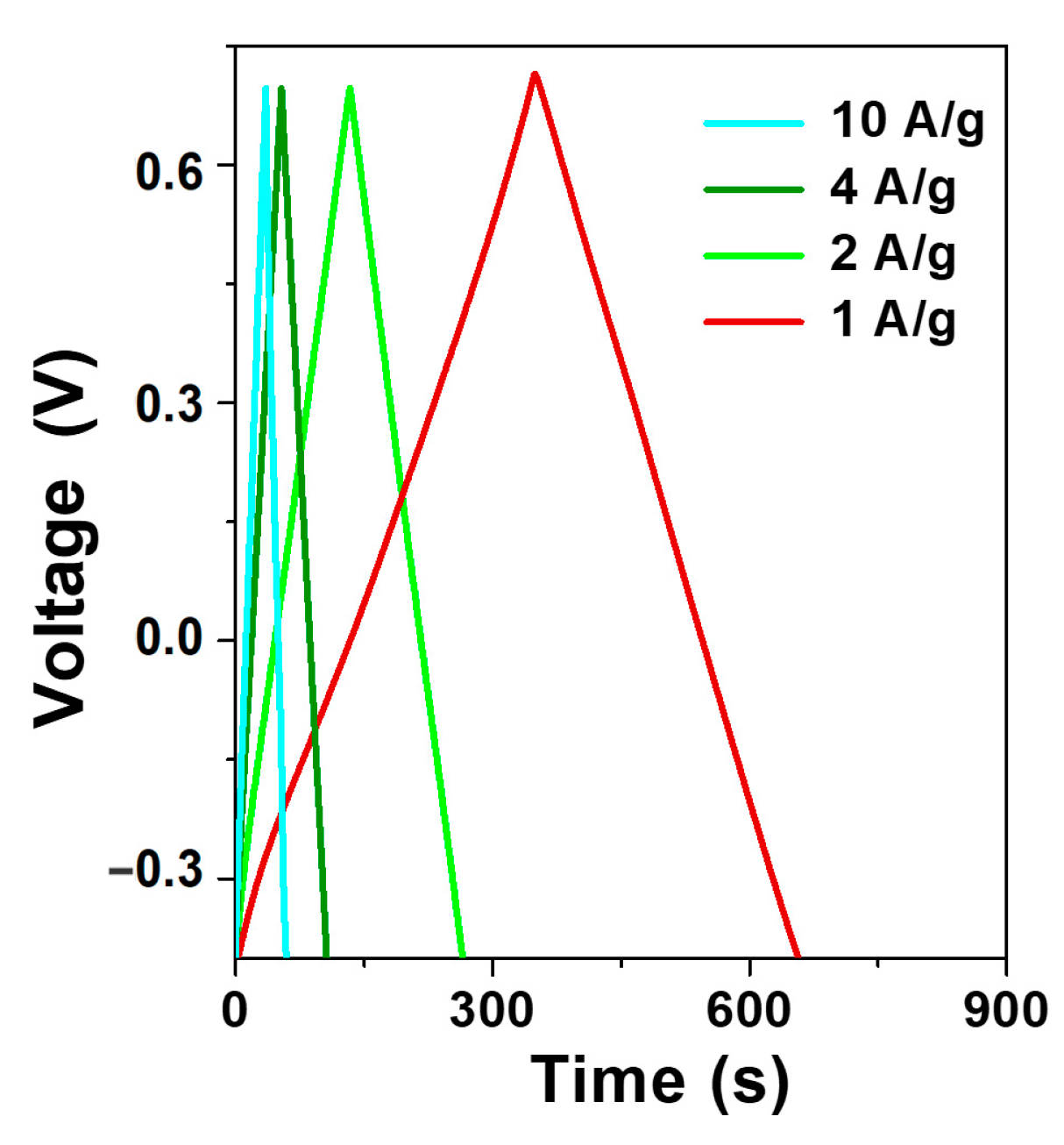
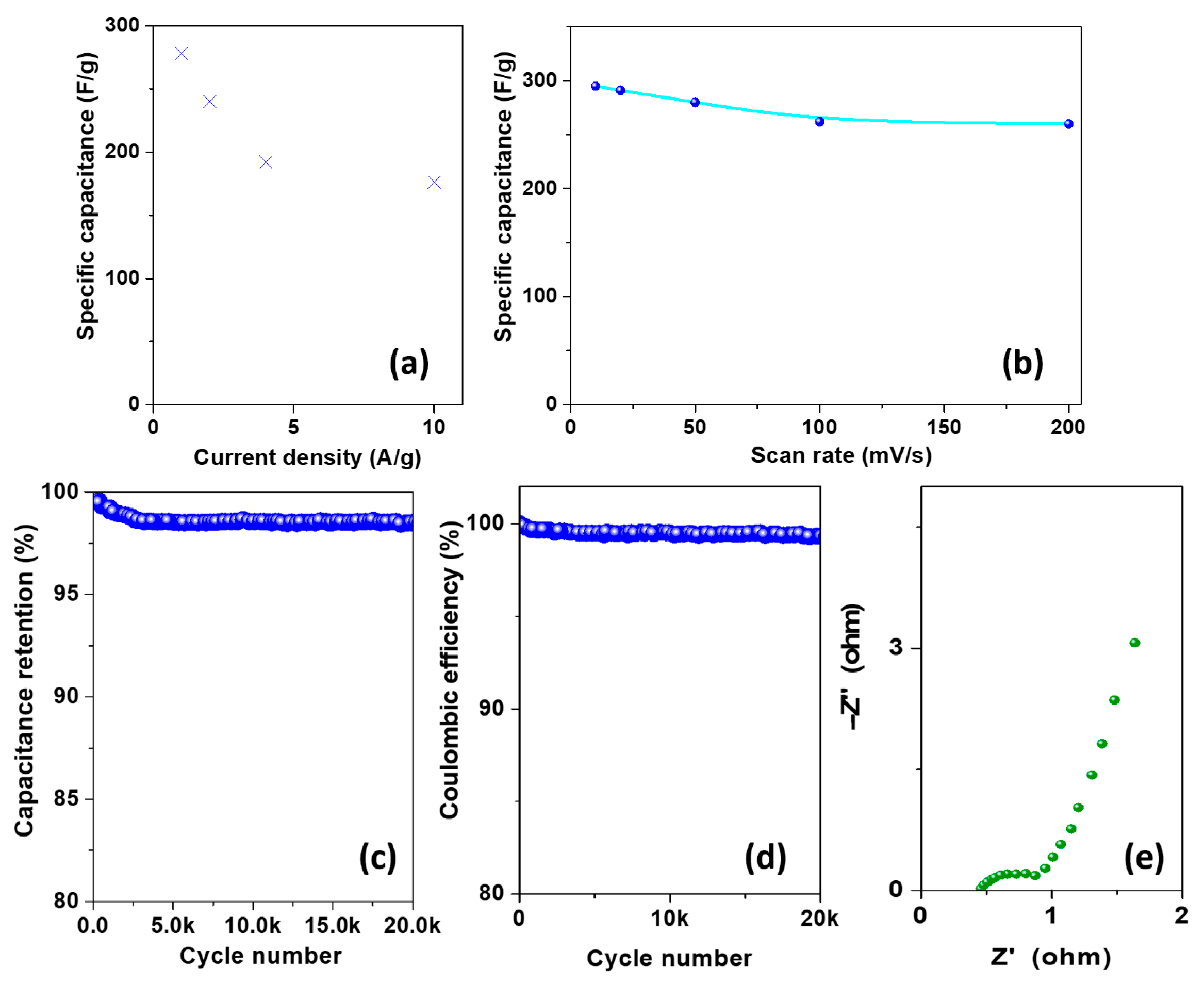
3.2. Electrochemical Characterization
3.3. Comparison with Literature and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernão Pires, V.; Romero-Cadaval, E.; Vinnikov, D.; Roasto, I.; Martins, J.F. Power converter interfaces for electrochemical energy storage systems—A review. Energy Convers. Manag. 2014, 86, 453–475. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Y.; Cheng, F.; Cheng, C.; Mia, Y.; Gao, F.; Yu, J. Inactive Al3+-doped La(CoCrFeMnNiAlx)1/(5+x)O3 high-entropy perovskite oxides as high performance supercapacitor electrodes. J. Adv. Ceram. 2022, 11, 742–753. [Google Scholar] [CrossRef]
- Han, C.; Xu, X.; Mu, H.; Tian, Q.; Li, Q.; Liu, Y.; Zhang, X.; Zhao, Z.; Su, X. Construction of hierarchical sea urchin-like manganese substituted nickel cobaltite@tricobalt tetraoxide core-shell microspheres on nickel foam as binder-free electrodes for high performance supercapacitors. J. Colloid. Interface Sci. 2021, 596, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Gao, S.; Zhang, H.; Wang, H.; Li, Q. Photo-assisted charging of carbon fiber paper-supported CeO2/MnO2 heterojunction and its long-lasting capacitance enhancement in dark. J. Adv. Ceram. 2022, 11, 1735–1750. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhangb, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185. [Google Scholar] [CrossRef]
- Ricci, P.C. Assessment of crystalline materials for solid state lighting applications: Beyond the rare earth elements. Crystals 2020, 10, 559. [Google Scholar] [CrossRef]
- Monteiro, J.H.S.K. Recent Advances in Luminescence Imaging of Biological Systems Using Lanthanide (III) Luminescent Complexes. Molecules 2020, 25, 2089. [Google Scholar] [CrossRef]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and CHF2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef]
- Tigaa, R.A.; Ortega, R.E.; Lin, X.; Strouse, G.F. A Versatile Tripodal Ligand for Sensitizing Lanthanide (LnIII) Ions and Color Tuning. Chemistry 2021, 3, 138–145. [Google Scholar] [CrossRef]
- Kaya, E.E.; Kaya, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals 2021, 11, 716. [Google Scholar] [CrossRef]
- Choi, E.Y.; Jeong, S.M. Electrochemical processing of spent nuclear fuels: An overview of oxide reduction in pyroprocessing technology. Prog. Nat. Sci. 2015, 25, 572–582. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Han, D.H.; Lee, J.; Cho, M.H. Defect-induced band gap narrowed CeO2 nanostructures for visible light activities. Ind. Eng. Chem. Res. 2014, 53, 9754–9763. [Google Scholar] [CrossRef]
- Vita, A. Catalytic Applications of CeO2-Based Materials. Catalysts 2020, 10, 576. [Google Scholar] [CrossRef]
- Zou, F.R.; Wang, S.N.; Wang, F.F.; Liu, D.; Li, Y. Synthesis of Lanthanide Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application. Catalysts 2020, 10, 833. [Google Scholar] [CrossRef]
- Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials 2021, 11, 723. [Google Scholar] [CrossRef]
- Arunachalam, S.; Kirubasankar, B.; Pan, D.; Liu, H.; Yan, C.; Guo, Z.; Angaiah, S. Research progress in rare earths and their composites based electrode materials for supercapacitors. Green. Energy Environ. 2020, 5, 259–273. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Magdum, V.V.; Khot, S.D.; Kim, J.H.; Lokhande, C.D. Doping of rare earth elements: Towards enhancing the electrochemical performance of pseudocapacitive materials. J. Alloys Compd. 2023, 960, 170601. [Google Scholar] [CrossRef]
- Steglich, F.; Aarts, J.; Bredl, C.D.; Lieke, W.; Meschede, D.; Franz, W.; Schäfer, H. Superconductivity in the Presence of Strong Pauli Paramagnetism: CeCu2Si2. Phys. Rev. Lett. 1979, 43, 1892. [Google Scholar] [CrossRef]
- He, D.; Xing, S.; Sun, B.; Cai, H.; Suo, H.; Zhao, C. Design and Construction of Three-Dimensional Flower-like CuO Hierarchical Nanostructures on Copper Foam for High Performance Supercapacitor. Electrochim. Acta 2016, 210, 639–645. [Google Scholar] [CrossRef]
- Yuan, M.; Gui, X.; Liu, Y.; Pang, H. Si-based materials derived from biomass: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2019, 7, 22123–22147. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Jin, X.; Li, T.; Ge, J.; Li, Z. A Review on Cutting Edge Technologies of Silicon-Based Supercapacitors. J. Nanomater. 2021, 2021, 6650131. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Peng, X. Highly Enhanced Capacitance of CuO Nanosheets by Formation of CuO/SWCNT Networks through Electrostatic Interaction. Electrochim. Acta 2013, 104, 289–294. [Google Scholar] [CrossRef]
- Romanitan, C.; Varasteanu, P.; Mihalache, I.; Culita, D.; Somacescu, S.; Pascu, R.; Tanasa, E.; Eremia, S.A.V.; Boldeiu, A.; Simion, M.; et al. High-performance solid state supercapacitors assembling graphene interconnected networks in porous silicon electrode by electrochemical methods using 2,6-dihydroxynaphthalen. Sci. Rep. 2018, 8, 9654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, Y.; Li, H.; Xu, Z.; Sun, S.; Yin, S. Construction of Cu-Doped Ni−Co-Based Electrodes for High-Performance Supercapacitor Applications. ACS Appl. Energy Mater. 2022, 5, 6642–6653. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4 Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011–3019. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Supercapacitor Behavior of Cerium Oxide Nanoparticles in Neutral Aqueous Electrolytes. Energy Fuels 2015, 29, 8246–8253. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Kim, Y.H.; Park, C.J. One Step Hydrothermal Synthesis of a Carbon Nanotube/Cerium Oxide Nanocomposite and its Electrochemical Properties. Nanotechnology 2013, 24, 365401. [Google Scholar] [CrossRef]
- Cui, S.; Wang, F.; Sun, K.; Wang, X.; Hu, Q.; Peng, H.; Ma, G.; Lei, Z. High-Performance Hybrid Supercapacitors Based on Ce-Doped NiMoO4 Nanosheets and Fe3O4@Bi2O3 Nanoarrays. J. Phys. Chem. C 2021, 125, 18129c18140. [Google Scholar] [CrossRef]
- Saranya, P.E.; Selladurai, S. Mesoporous 3D network Ce-doped NiO nanoflakes as high performance electrodes for supercapacitor applications. New J. Chem. 2019, 43, 7441. [Google Scholar] [CrossRef]
- Shi, J.W.; Wang, Y.; Duan, R.; Gao, C.; Wang, B.; He, C.; Niu, C. The synergistic effects between Ce and Cu in CuyCe1−yW5Ox catalysts for enhanced NH3-SCR of NOx and SO2 tolerance. Catal. Sci. Technol. 2019, 9, 718–730. [Google Scholar] [CrossRef]
- Rauda, I.E.; Augustyn, V.; Dunn, B.; Tolbert, S.H. Enhancing pseudocapacitive charge storage in polymer templated mesoporous materials. Acc. Chem. Res. 2013, 46, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y.; Dai, D.; Zhang, F.; Zou, H.; Yang, X.; Ji, Y.; Li, B.; Wei, X. Surface Roughness: A Crucial Factor To Robust Electric Double Layer Capacitors. ACS Appl. Mater. Interfaces 2020, 12, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Controlled PtIr nanoalloy as an electro-oxidation platform for methanol reaction and ammonia detection. Nanotechnology 2019, 30, 394004. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Ru and Os based new electrode for electrochemical flow supercapacitors. Chem. Eng. J. 2019, 3771, 120050. [Google Scholar] [CrossRef]
- Shi, X.; Yu, J.; Huang, J.; Chen, B.; Fang, L.; Shao, L.; Sun, Z. Metal-organic framework derived high-content N, P and O-codoped Co/C composites as electrode materials for high performance supercapacitors. J. Power Sources 2020, 467, 228304. [Google Scholar] [CrossRef]
- Tornheim, A.; O’Hanlon, D.C. What do Coulombic efficiency and capacity retention truly measure? A deep dive into cyclable lithium inventory, limitation type, and redox side-reactions. J. Electrochem. Soc. 2020, 167, 110520. [Google Scholar] [CrossRef]
- Holter, G.; Adrian, H. Thin film preparation, superconductivity and transport properties of the heavy-fermion system CeCu2Si2. Solid. State Commun. 1986, 58, 45–49. [Google Scholar] [CrossRef]
- Stockert, O.; Arndt, J.; Faulhaber, E.; Geibel, C.; Jeevan, H.S.; Kirchner, S.; Loewenhaupt, M.; Schmalzl, K.; Schmidt, W.; Si, Q. Magnetically driven superconductivity in CeCu2Si2. Nat. Phys. 2010, 7, 119–124. [Google Scholar] [CrossRef]
- Shatruk, M. ThCr2Si2 Structure Type: The “Perovskite” of Intermetallics. J. Solid. State Chem. 2019, 272, 198–209. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Ahuja, P.; Sahu, V.; Ujjain, S.K.; Sharma, R.K.; Singh, G. Performance evaluation of Asymmetric Supercapacitor based on Cobalt manganite modified graphene nanoribbons. Electrochim. Acta 2014, 146, 429–436. [Google Scholar] [CrossRef]
- Ujjain, S.K.; Singh, G.; Sharma, R.K. Co3O4@Reduced Graphene Oxide Nanoribbon for high performance Asymmetric Supercapacitor. Electrochim. Acta 2015, 169, 276–282. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R. Electrochemical Behaviour of Manganese & Ruthenium mixed Oxide@ Reduced Graphene Oxide Nanoribbon Composite in Symmetric and Asymmetric Supercapacitor. Appl. Surf. Sci. 2017, 427, 102–111. [Google Scholar]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate Dependent Self-Organization of Mesoporous Cobalt Oxide Nanowires with Remarkable Pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, W.; Niu, H.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ultrahigh energy density battery-type asymmetric supercapacitors: NiMoO4 nanorod-decorated graphene and graphene/Fe2O3 quantum dots. Nano Res. 2018, 11, 4744–4758. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary Transition Metal Sulfides Embedded in Graphene Nanosheets as Both the Anode and Cathode for High-Performance Asymmetric Supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Masarapu, C.; Zeng, H.F.; Hung, K.H.; Wei, B. Effect of Temperature on the Capacitance of Carbon Nanotube Supercapacitors. ACS Nano 2009, 3, 2199–2206. [Google Scholar] [CrossRef]
- Chen, K.; Pan, W.; Xue, D. Phase Transformation of Ce3+-Doped MnO2 for Pseudocapacitive Electrode Materials. J. Phys. Chem. C 2016, 120, 20077–20081. [Google Scholar] [CrossRef]
- Su, X.; Yu, L.; Cheng, G.; Zhang, H.; Sun, M.; Zhang, L.; Zhang, J. Controllable hydrothermal synthesis of Cu-doped δ-MnO2 films with different morphologies for energy storage and conversion using supercapacitors. Appl. Energy 2014, 134, 439–445. [Google Scholar] [CrossRef]
- Lavanya, T.; Ramaprabhu, S. Copper nanoparticles incorporated porous carbon nanofibers as a freestanding binder-free electrode for symmetric supercapacitor with enhanced electrochemical performance. Mater. Res. Express 2019, 6, 105005. [Google Scholar] [CrossRef]
- Oh, I.; Kim, M.; Kim, J. Carbon-coated Si/MnO2 nanoneedle composites with optimum carbon layer activation for supercapacitor applications. Chem. Eng. J. 2015, 273, 82–91. [Google Scholar] [CrossRef]
- Liu, Q.; Nayfeh, M.H.; Yau, S.T. Supercapacitor electrodes based on polyaniline–silicon nanoparticle composite. J. Power Sources 2010, 195, 3956–3959. [Google Scholar] [CrossRef]

| Cu, Ce, Si-Containing Electrode Material (with Average Size) | CV Potential Window (V) | GCD Current Density (A/g) | Mass Capacitance (F/gsample) | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| Ce-doped NiO nanoflakes (27–30 nm) | 0 ÷ 0.45 vs. Ag/AgCl | 1 | 1775 | retains about 93% after 2000 cycles at 5 A/g | [30] |
| CeO2 nanoparticles (14 nm) | 0 ÷ 0.8 vs. Ag/AgCl | 2 | 457 | retains about 82% after 2000 cycles at 10 A/g | [27] |
| Ce-doped MnO2 nanorods (10–20 nm) | 0 ÷ 0.8 vs. SCE | 1 | 101.1 | retains about 99.5% after 1000 cycles at 5 A/g | [49] |
| Cu-doped-MnO2 nanosheets | 0 ÷ 1 vs. SCE | 1 | 296 | retains about 79% after 1000 cycles at 2 A/g | [50] |
| Ni–Co–Cu oxide nanorods (10–40 nm) | −0.1 ÷ 0.65 vs. Hg/HgO | 3 | 6.54 F/cm2 | the device retains about 40% after 2000 cycles at 2 A/g | [25] |
| Cu nanoparticles/PCNFs | 0 ÷ 1 | 1 | 333.5 | retains about 95.8% after 10,000 cycles at 3 A/g | [51] |
| Si/MnO2 nanoneedle (20–40 nm) | −0.2 ÷ 0.8 vs. Ag/AgCl | 1 | 240.1 | retains about 85.2% after 2000 cycles at 1 A/g | [52] |
| PANI-Si nanoparticles (1–2.8 nm) | −0.2 ÷ 1 vs. Ag/AgCl | 5 mA/cm2 | 470 | retains 78% after 1500 cycles | [53] |
| CeCu2Si2 rough micro pebbles | −0.4 ÷ 0.7 vs. SCE | 1 | 278 | retains about 98% after 20,000 cycles at 10 A/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, D.; Cirillo, C.; Luciano, C.; Nigro, A.; Adami, R.; Cirillo, C.; Attanasio, C.; Iuliano, M.; Ponticorvo, E.; Sarno, M. Rough and Porous Micropebbles of CeCu2Si2 for Energy Storage Applications. Materials 2023, 16, 7182. https://doi.org/10.3390/ma16227182
Scarpa D, Cirillo C, Luciano C, Nigro A, Adami R, Cirillo C, Attanasio C, Iuliano M, Ponticorvo E, Sarno M. Rough and Porous Micropebbles of CeCu2Si2 for Energy Storage Applications. Materials. 2023; 16(22):7182. https://doi.org/10.3390/ma16227182
Chicago/Turabian StyleScarpa, Davide, Claudia Cirillo, Christopher Luciano, Angela Nigro, Renata Adami, Carla Cirillo, Carmine Attanasio, Mariagrazia Iuliano, Eleonora Ponticorvo, and Maria Sarno. 2023. "Rough and Porous Micropebbles of CeCu2Si2 for Energy Storage Applications" Materials 16, no. 22: 7182. https://doi.org/10.3390/ma16227182
APA StyleScarpa, D., Cirillo, C., Luciano, C., Nigro, A., Adami, R., Cirillo, C., Attanasio, C., Iuliano, M., Ponticorvo, E., & Sarno, M. (2023). Rough and Porous Micropebbles of CeCu2Si2 for Energy Storage Applications. Materials, 16(22), 7182. https://doi.org/10.3390/ma16227182











