Integrated Laser Additive Manufacturing of α-Al2O3 Nanoparticle-Seeded β/γ’ Ni-Al Intermetallic Alloy with Enhanced High-Temperature Oxidation Performance
Abstract
:1. Introduction
2. Materials and Methods
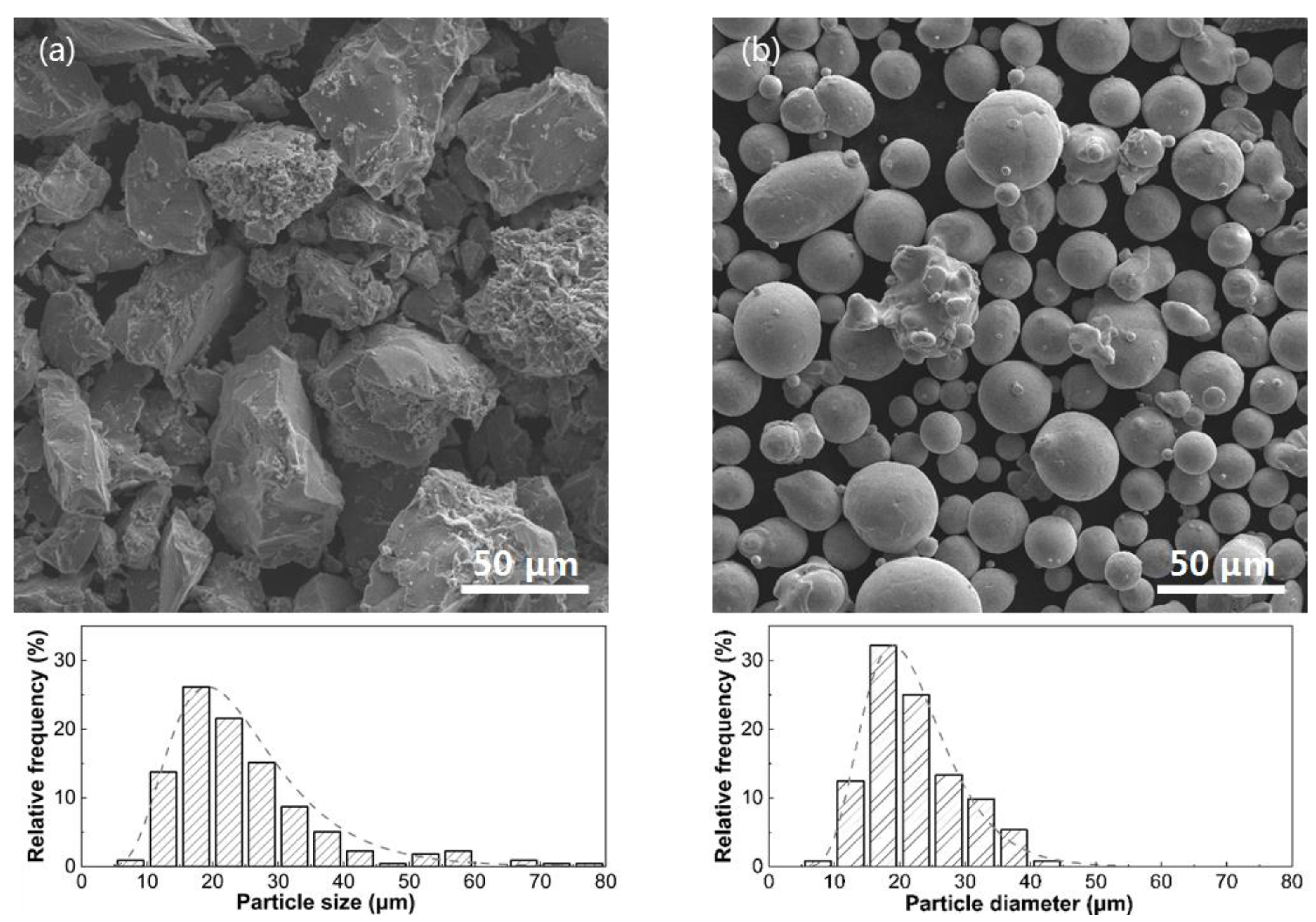
2.1. Materials
2.2. Methodology to Design β/γ’ Ni-Al Intermetallic Alloy
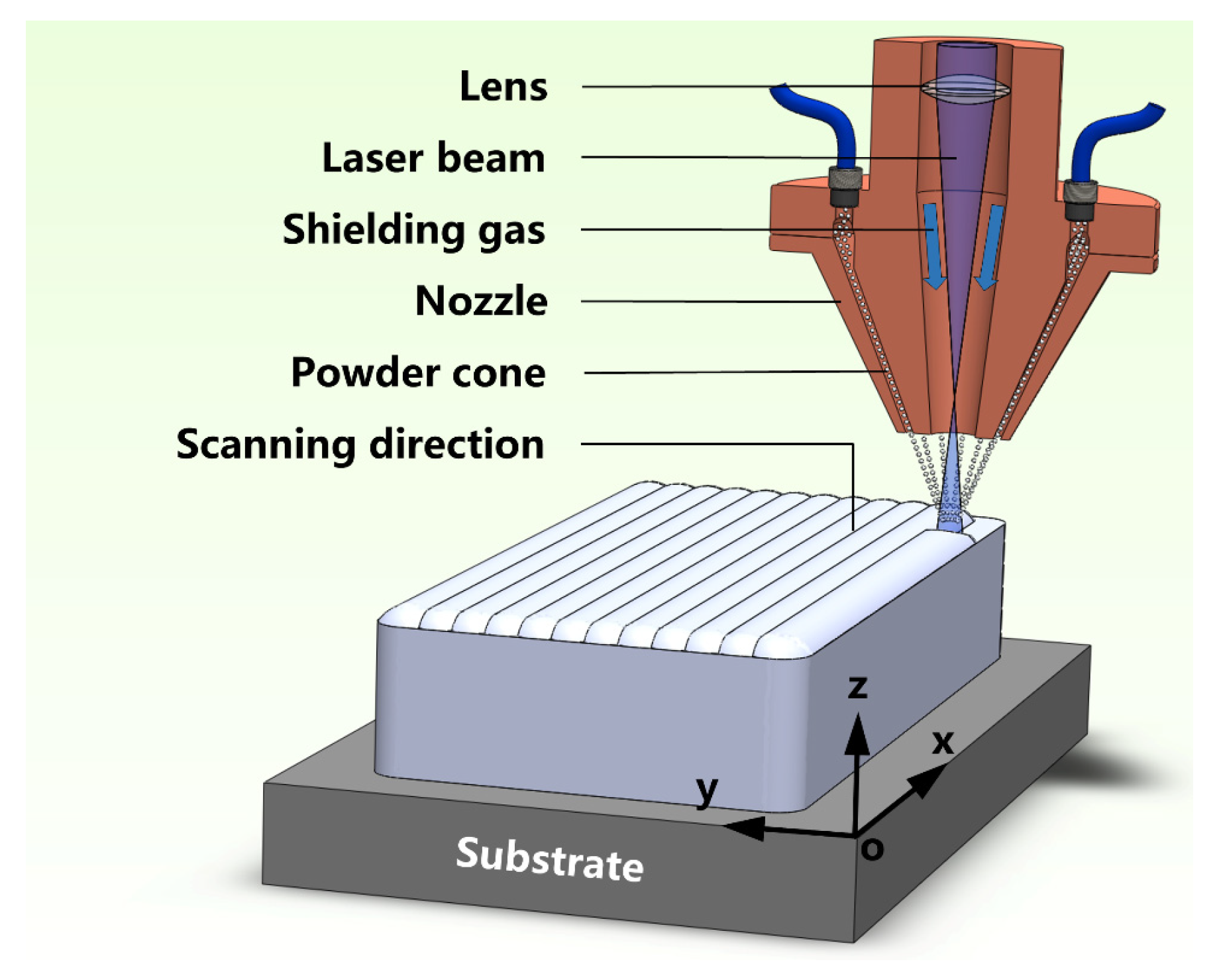
2.3. LAM Fabrication of β/γ’ Alloy without and with Seeding α-Al2O3 NPs
2.4. Oxidation Test
2.5. Microstructural Characterization
3. Results and Discussion
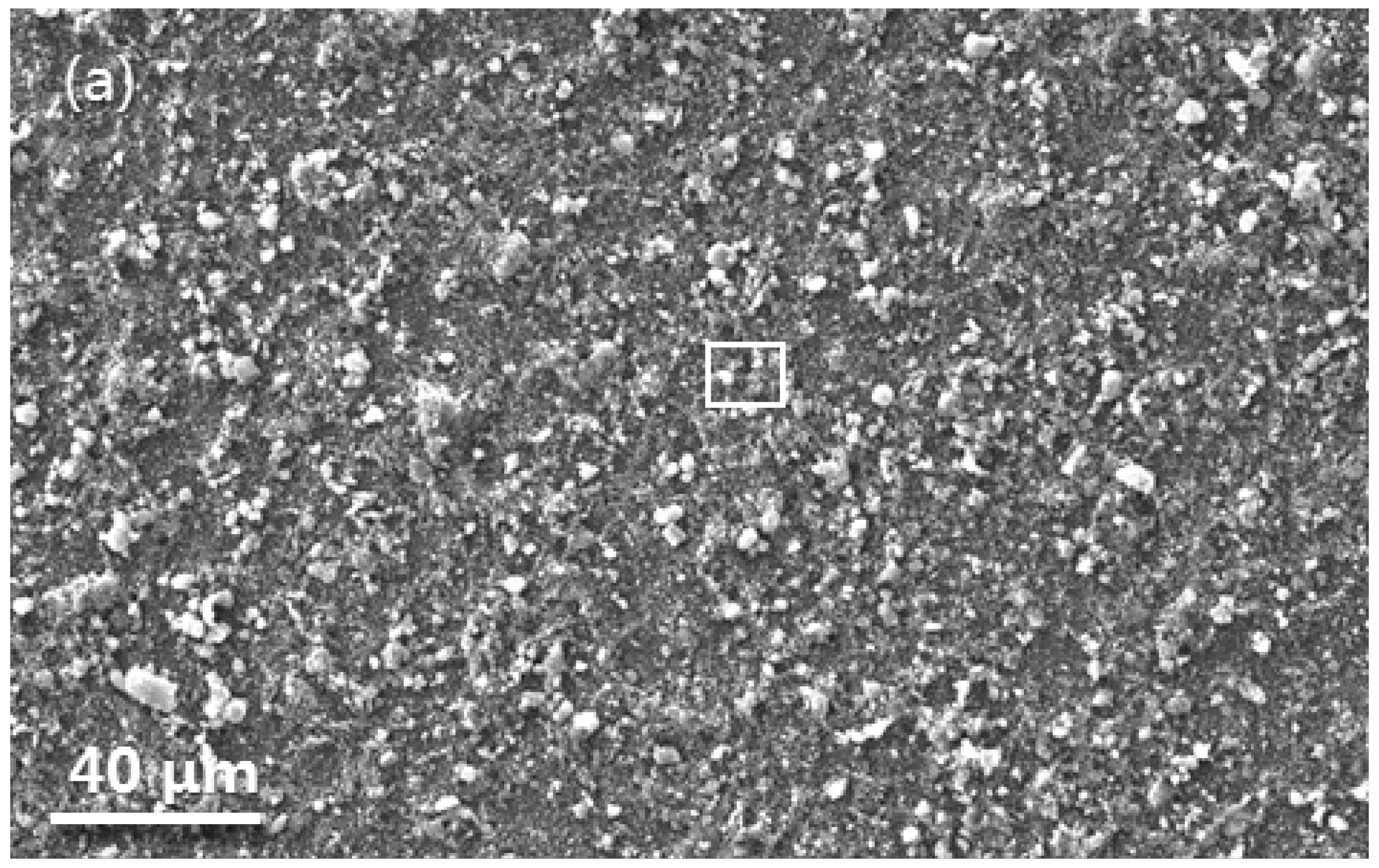
3.1. Microstructure
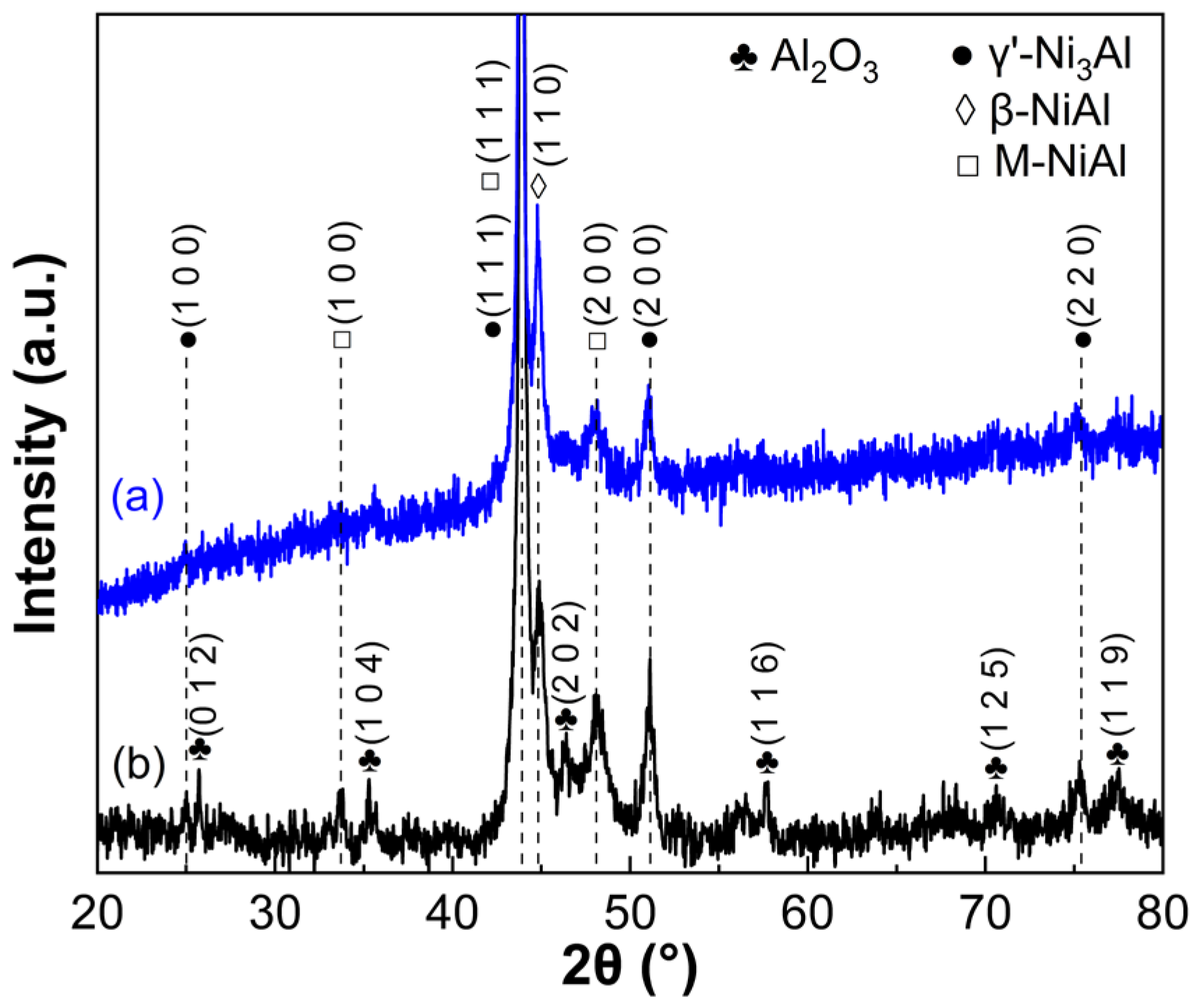
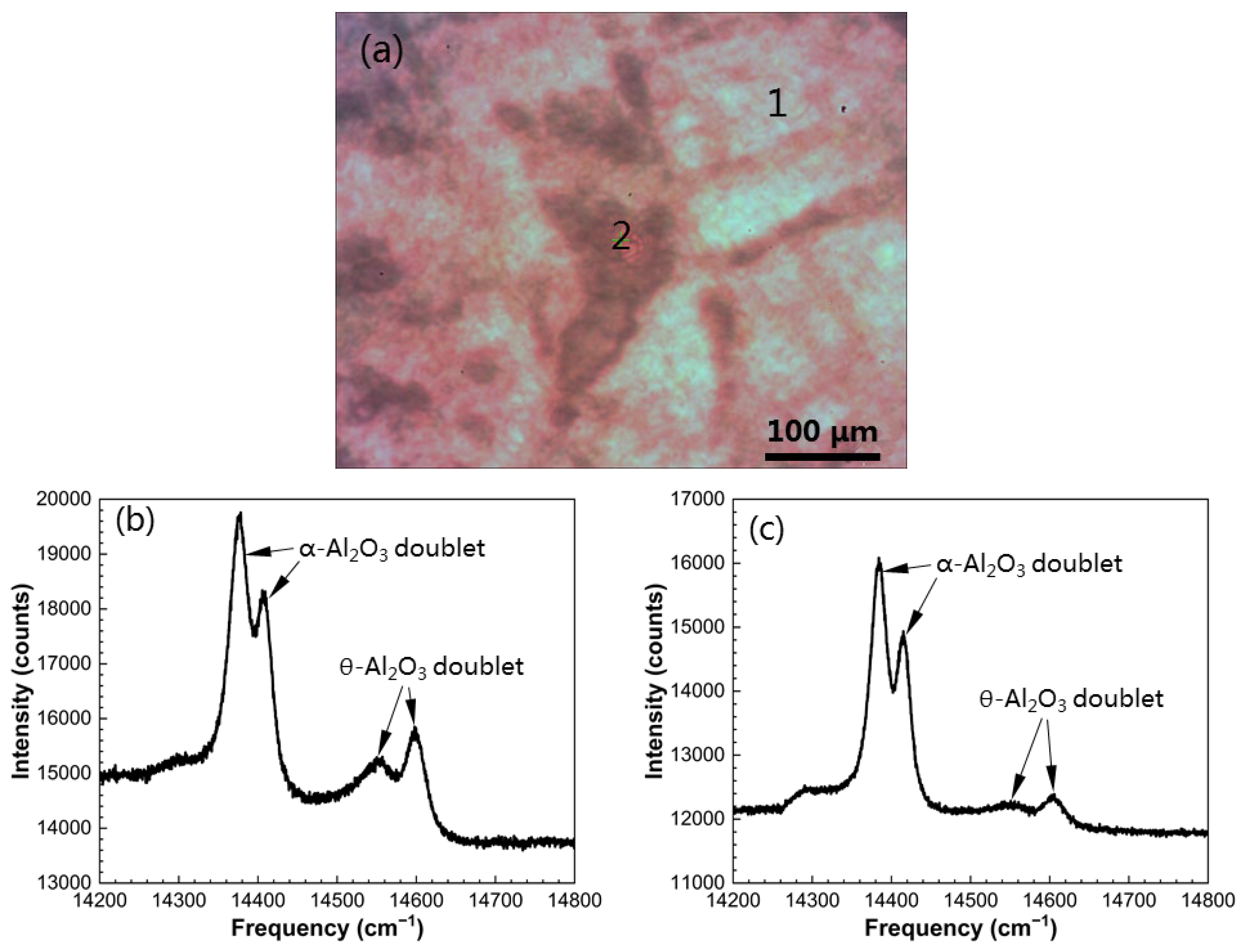
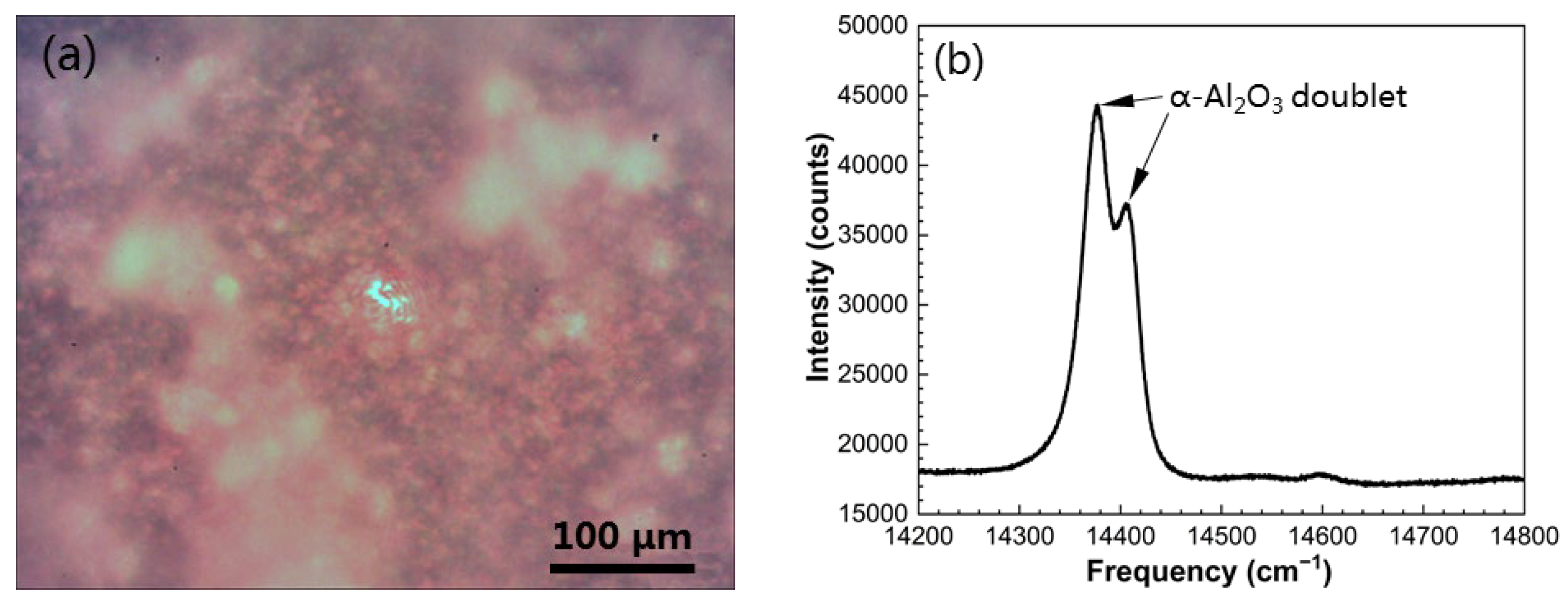
3.2. Growth of Initial Alumina Phases
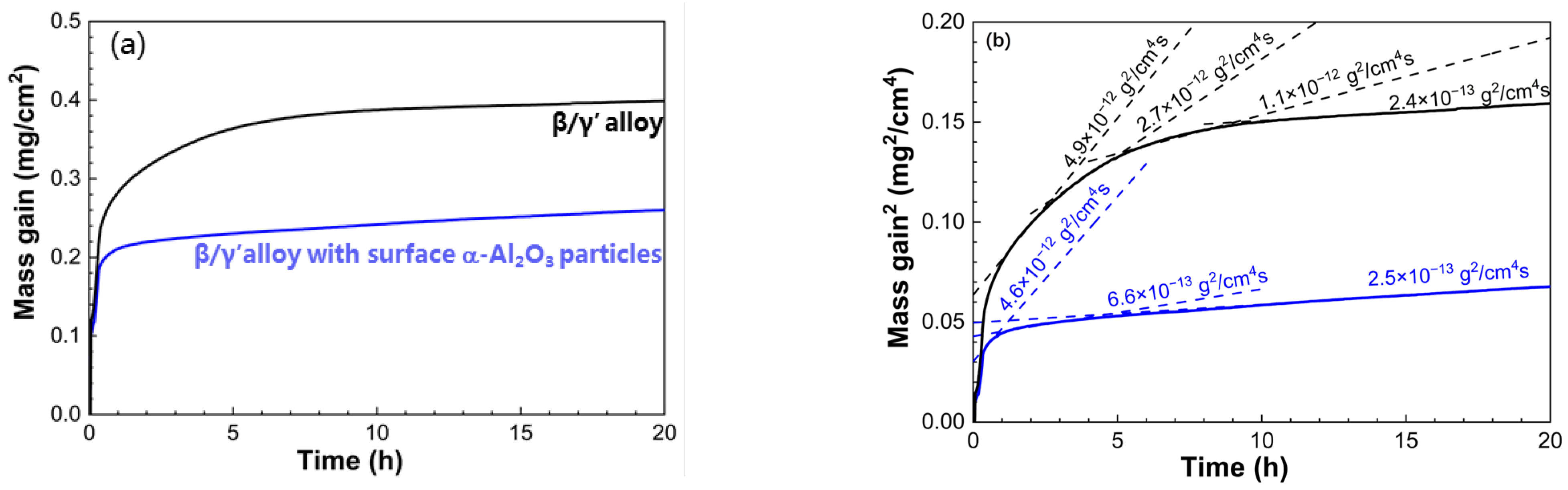
3.3. Oxidation Kinetics
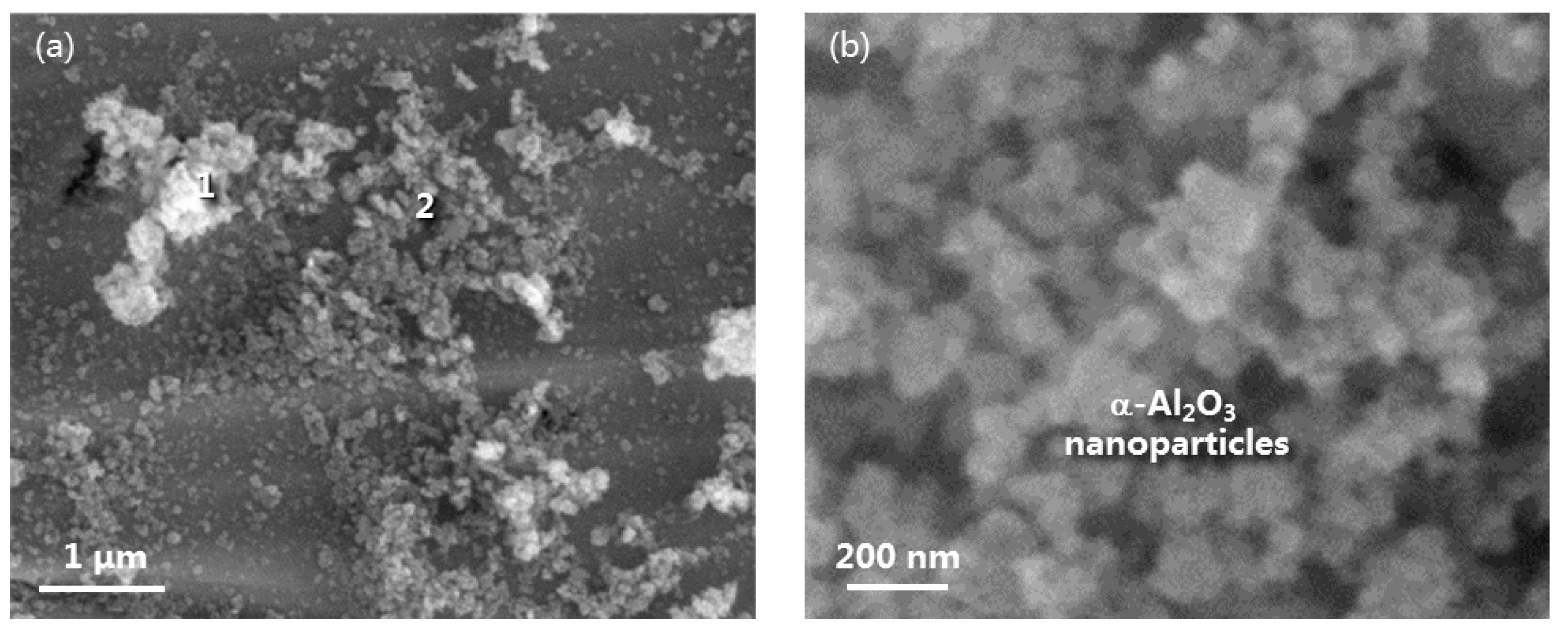
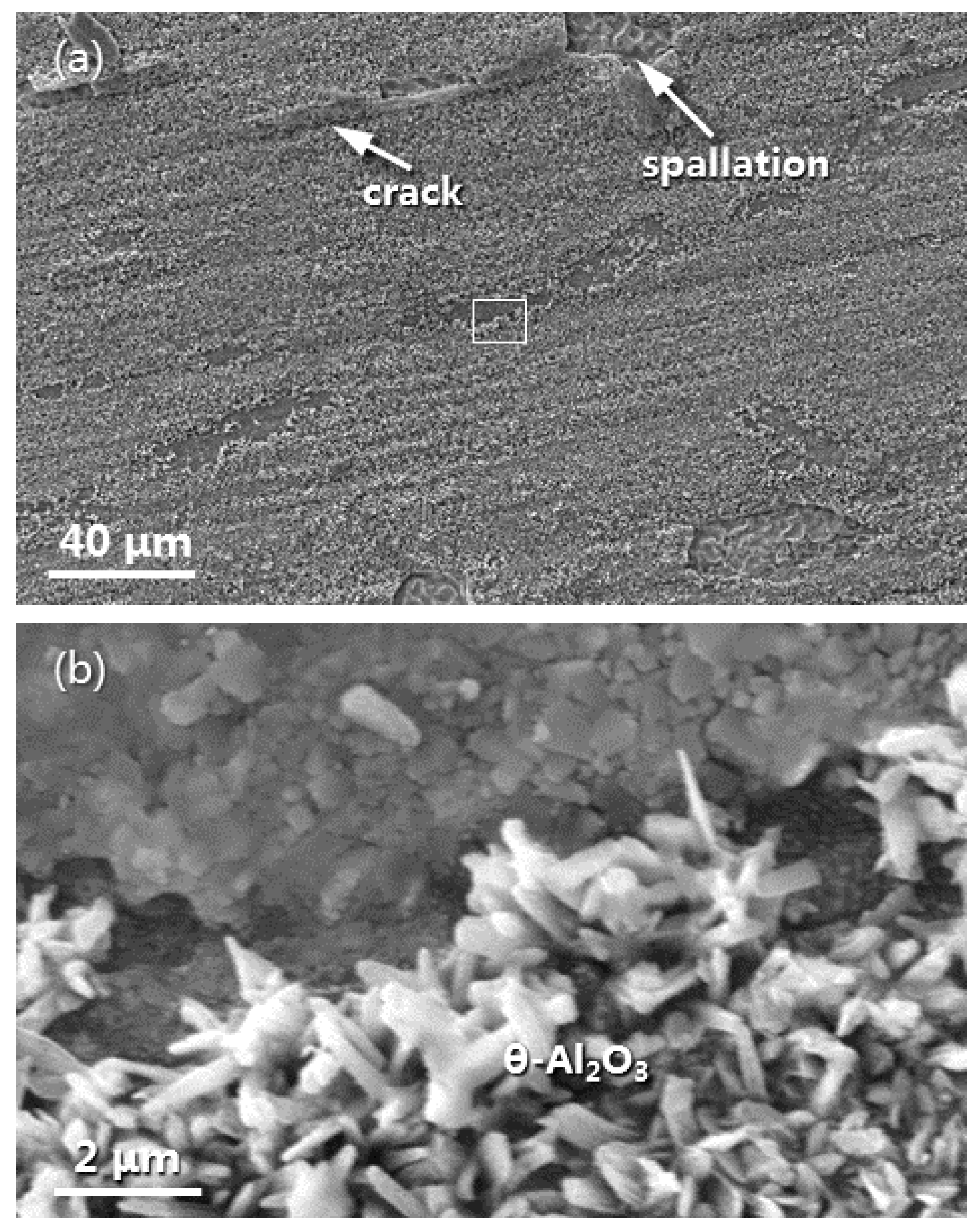
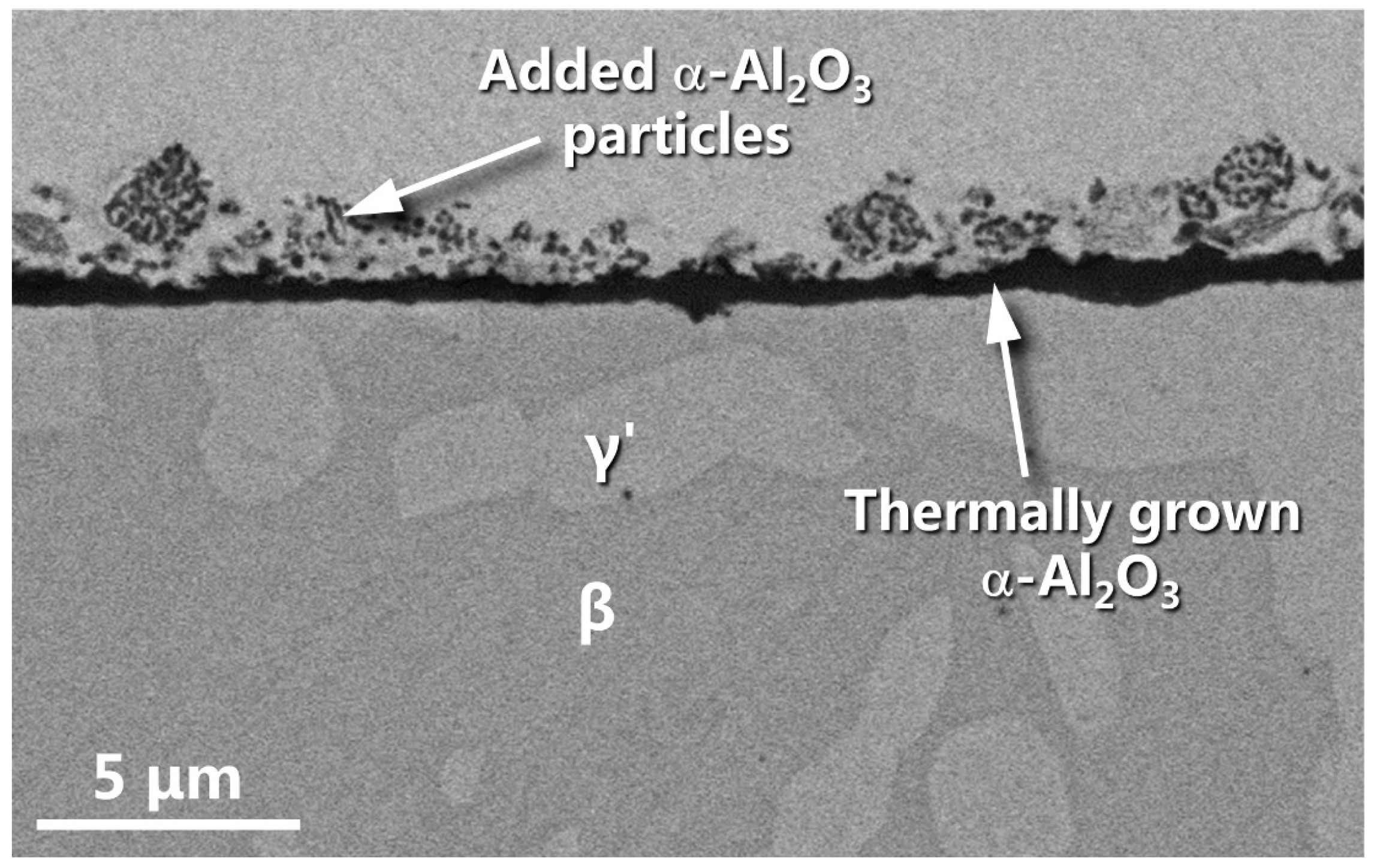
3.4. Oxide Morphology Characteristics and α-Al2O3 NPs Effect on Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, E.P.; Liu, C.T. Alloy design of ordered intermetallics. MRS Proc. 1990, 186, 309. [Google Scholar] [CrossRef]
- Liu, C.T. Recent advances in ordered intermetallics. Mater. Chem. Phys. 1995, 42, 77–86. [Google Scholar] [CrossRef]
- Azhagarsamy, P.; Sekar, K.; Murali, K.P. Nickel aluminide intermetallic composites fabricated by various processing routes—A review. Mater. Sci. Technol. 2022, 38, 556–571. [Google Scholar] [CrossRef]
- Sampath, S.; Ravi, V.P.; Sundararajan, S. An overview on synthesis, processing and applications of nickel aluminides: From fundamentals to current prospects. Crystals 2023, 13, 435. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, M.H.; Wee, D.M. Microstructure control of DS NiAl/Ni3Al alloy by fabrication of columnar-grained NiAl martensite. Mater. Sci. Forum 2003, 426–432, 1813–1818. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R.; Noebe, R.D. Deformation and fracture behavior of a directionally solidified β/γ’ Ni-30 at. pct Al alloy. Metall. Mater. Trans. A 1999, 30, 1003–1015. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.Y.; Lee, Z.H.; Kim, H.M. Ni3Al precipitation behavior in the two-phase (NiAl + Ni3Al) alloy. High Temp. Mater. Process. 1999, 18, 125–130. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, H.M.; Lee, Z.H. Boron effect on solidification in the two phase (NiAl + Ni3Al) alloy. High Temp. Mater. Process. 1999, 18, 145–150. [Google Scholar] [CrossRef]
- Ochiai, S.; Yamada, I.; Kojima, Y. Development of high strength and superplasticity in the heat-refined (NiAl + Ni3Al) two-phase alloy. Nippon Kinzoku Gakkai-si 1990, 54, 301–306. [Google Scholar]
- He, J.; Peng, H.; Gong, S.; Guo, H. Synergistic effect of reactive element co-doping in two-phase (γ’ + β) Ni-Al alloys. Corros. Sci. 2017, 120, 130–138. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Peng, H.; Gong, S.; Guo, H. The role of Dy and Hf doping on oxidation behavior of two-phase (γ’ + β) Ni-Al alloys. Corros. Sci. 2015, 98, 699–707. [Google Scholar] [CrossRef]
- Zhou, B.Y.; He, J.; Zhou, Q.J.; Guo, H.B. Effects of laser shock processing on θ-Al2O3 to α-Al2O3 transformation and oxide scale morphology evolution in (γ’ + β) two-phase Ni-34Al-0.1Dy alloys. J. Mater. Sci. Technol. 2022, 109, 157–166. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wei, L.L.; Li, Q.S.; Gong, S.K.; Guo, H.B. Microstructure stability of γ’ + β Ni-Al coated single-crystal superalloy N5 annealed at 1100 °C. Rare Metals 2021, 40, 693–700. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, Q.; He, J.; Wang, W.; Guo, H. A comprehensive study on the oxidation behavior and failure mechanism of (γ’ + β) two-phase Ni-34Al-0.1Dy coating treated by laser shock processing. J. Mater. Sci. Technol. 2023, 162, 131–144. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Askari, M.; Hutchins, D.A.; Thomas, P.J.; Astolfi, L.; Watson, R.L.; Abdi, M.; Ricci, M.; Laureti, S.; Nie, L.; Freear, S.; et al. Additive manufacturing of metamaterials: A review. Addit. Manuf. 2020, 36, 101562. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Blakey-Milner, B.; Gradl, P.; Snedden, G.; Brooks, M.; Pitot, J.; Lopez, E.; Leary, M.; Berto, F.; Du Plessis, A. Metal additive manufacturing in aerospace: A review. Mater. Des. 2021, 209, 110008. [Google Scholar] [CrossRef]
- Müller, M.; Heinen, B.; Riede, M.; López, E.; Brückner, F.; Leyens, C. Additive manufacturing of β-NiAl by means of laser metal deposition of pre-alloyed and elemental powders. Materials 2021, 14, 2246. [Google Scholar] [CrossRef]
- Kaplanskii, Y.Y.; Levashov, E.A.; Korotitskiy, A.V.; Loginov, P.A.; Sentyurina, Z.A.; Mazalov, A.B. Influence of aging and HIP treatment on the structure and properties of NiAl-based turbine blades manufactured by laser powder bed fusion. Addit. Manuf. 2020, 31, 100999. [Google Scholar] [CrossRef]
- Park, J.U.; Jun, S.Y.; Lee, B.H.; Jang, J.H.; Lee, B.S.; Lee, H.J.; Lee, J.H.; Hong, H.U. Alloy design of Ni-based superalloy with high γ′ volume fraction suitable for additive manufacturing and its deformation behavior. Addit. Manuf. 2022, 52, 102680. [Google Scholar] [CrossRef]
- Meng, Y.; Li, J.; Gao, M.; Zeng, X. Microstructure characteristics of wire arc additive manufactured NiAl intermetallic compounds. J. Manuf. Process. 2021, 68, 932–939. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Chen, J.; Zhou, H.; Guo, C.; Guo, B. Preparation, Microstructure and tribological properties of Ni3Al intermetallic compound coating by laser cladding. Intermetallics 2010, 18, 871–876. [Google Scholar] [CrossRef]
- Liu, W.; Dupont, J.N. In-situ reactive processing of nickel aluminides by laser-engineered net shaping. Metall. Mater. Trans. A 2003, 34, 2633–2641. [Google Scholar] [CrossRef]
- Lee, D.; Santella, M.L.; Anderson, I.M.; Pharr, G.M. Long-term oxidation of an as-cast Ni3Al alloy at 900 °C and 1100 °C. Metall. Mater. Trans. A 2006, 37, 505–514. [Google Scholar] [CrossRef]
- Hayashi, S.; Narita, T.; Gleeson, B. Early-stage oxidation behavior of γ’-Ni3Al-based alloys with and without Pt addition. Mater. Sci. Forum 2006, 522–523, 229–238. [Google Scholar] [CrossRef]
- Rybicki, G.C.; Smialek, J.L. Effect of the θ-α-Al2O3 transformation on the oxidation behavior of β-NiAl + Zr. Oxid. Metals 1989, 31, 275–304. [Google Scholar] [CrossRef]
- Yang, J.C.; Schumann, E.; Levin, I.; Rühle, M. Transient oxidation of NiAl. Acta Mater. 1998, 46, 2195–2201. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I phase transformations in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Yan, K.; Guo, H.; Gong, S. High-temperature oxidation behavior of minor Hf doped NiAl alloy in dry and humid atmospheres. Corros. Sci. 2013, 75, 337–344. [Google Scholar] [CrossRef]
- Lipkin, D.M.; Clarke, D.R.; Hollatz, M.; Bobeth, M.; Pompe, W. Stress development in alumina scales formed upon oxidation of (111) NiAl single crystals. Corros. Sci. 1997, 39, 231–242. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Microstructural study of the theta-alpha transformation in alumina scales formed on nickel-aluminides. Mater. High Temp. 2000, 17, 59–70. [Google Scholar] [CrossRef]
- Specht, E.D.; Tortorelli, P.F.; Zschack, P. In Situ measurement of growth stress in alumina scale. Powder Diffr. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Hou, P.Y.; Paulikas, A.P.; Veal, B.W. Growth strains and stress relaxation in alumina scales during high temperature oxidation. Mater. Sci. Forum 2004, 461–464, 671–680. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, X. The promoted formation of an α-Al2O3 scale on a nickel aluminide with surface Cr2O3 particles. Corros. Sci. 2016, 112, 226–232. [Google Scholar] [CrossRef]
- Khan, A.; Huang, Y.; Dong, Z.; Peng, X. Effect of Cr2O3 nanoparticle dispersions on oxidation kinetics and phase transformation of thermally grown alumina on a nickel aluminide coating. Corros. Sci. 2019, 150, 91–99. [Google Scholar] [CrossRef]
- Tan, X.; Peng, X.; Wang, F. The mechanism for self-formation of a CeO2 diffusion barrier layer in an aluminide coating at high temperature. Surf. Coat. Technol. 2013, 224, 62–70. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Li, Y.; Peng, X. A novel method to promote selective oxidation of Ni-Cr alloys: Surface spreading α-Al2O3 nanoparticles. Corros. Sci. 2021, 190, 109717. [Google Scholar] [CrossRef]
- He, X.; Peng, X.; Fang, J. Study on the microstructure and high-temperature oxidation performance of β-NiAl/γ’-Ni3Al intermetallic compounds fabricated by laser metal deposition. Metals 2023, 13, 1461. [Google Scholar] [CrossRef]
- Peng, X.; Clarke, D.R.; Wang, F. Transient-alumina transformations during the oxidation of magnetron-sputtered CoCrAl nanocrystalline coatings. Oxid. Metals 2003, 60, 225–240. [Google Scholar] [CrossRef]
- Smialek, J.; Hehemann, R. Transformation temperatures of martensite in beta phase nickel aluminide. Metall. Mater. Trans. B 1973, 4, 1571–1575. [Google Scholar] [CrossRef]
- Enami, K.; Nenno, S.; Shimizu, K. Crystal structure and internal twins of the Ni-36.8 at% Al martensite. Trans. Jpn. Inst. Metals 1973, 14, 161–165. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, X.; Chen, X.Q. TiO2 nanoparticles-assisted α-Al2O3 direct thermal growth on nickel aluminide intermetallics: Template effect of the oxide with the hexagonal oxygen sublattice. Corros. Sci. 2019, 153, 109–117. [Google Scholar] [CrossRef]
- Pérez, P.; González-Carrasco, J.L.; Adeva, P. Oxidation behavior of a Ni3Al PM alloy. Oxid. Metals 1997, 48, 143–170. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, W.; Zhu, S.; Wang, F. The scaling behavior of sputtered Ni3Al coatings with and without Pt modification. Corros. Sci. 2012, 58, 115–120. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der oxydation von legierungen. Z. Elektrochem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Pint, B.A.; Treska, M.; Hobbs, L.W. The effect of various oxide dispersions on the phase composition and morphology of Al2O3 scales grown on β-NiAl. Oxid. Metals 1997, 47, 1–20. [Google Scholar] [CrossRef]
- Peng, X.; Huang, Y.; Wang, X.; Xie, Y. Nanoparticles application in promoting the growth of a more protective oxide scale at high temperatures. High Temp. Corros. Mater. 2023. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Shu, X.; Zhou, Z.; Yang, S.; You, L.; Peng, X. Integrated Laser Additive Manufacturing of α-Al2O3 Nanoparticle-Seeded β/γ’ Ni-Al Intermetallic Alloy with Enhanced High-Temperature Oxidation Performance. Materials 2023, 16, 7205. https://doi.org/10.3390/ma16227205
He X, Shu X, Zhou Z, Yang S, You L, Peng X. Integrated Laser Additive Manufacturing of α-Al2O3 Nanoparticle-Seeded β/γ’ Ni-Al Intermetallic Alloy with Enhanced High-Temperature Oxidation Performance. Materials. 2023; 16(22):7205. https://doi.org/10.3390/ma16227205
Chicago/Turabian StyleHe, Xun, Xiaoyong Shu, Ziyi Zhou, Shouhua Yang, Limei You, and Xiao Peng. 2023. "Integrated Laser Additive Manufacturing of α-Al2O3 Nanoparticle-Seeded β/γ’ Ni-Al Intermetallic Alloy with Enhanced High-Temperature Oxidation Performance" Materials 16, no. 22: 7205. https://doi.org/10.3390/ma16227205



