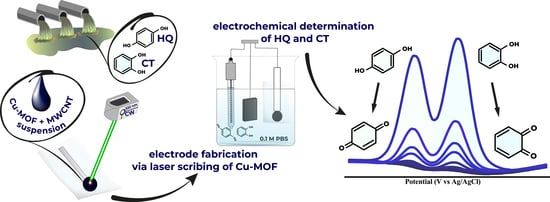
Simultaneous Catechol and Hydroquinone Detection with Laser Fabricated MOF-Derived Cu-CuO@C Composite Electrochemical Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [Cu4{1,4-C6H4(COO)2}3(4,4′-bipy)2]n
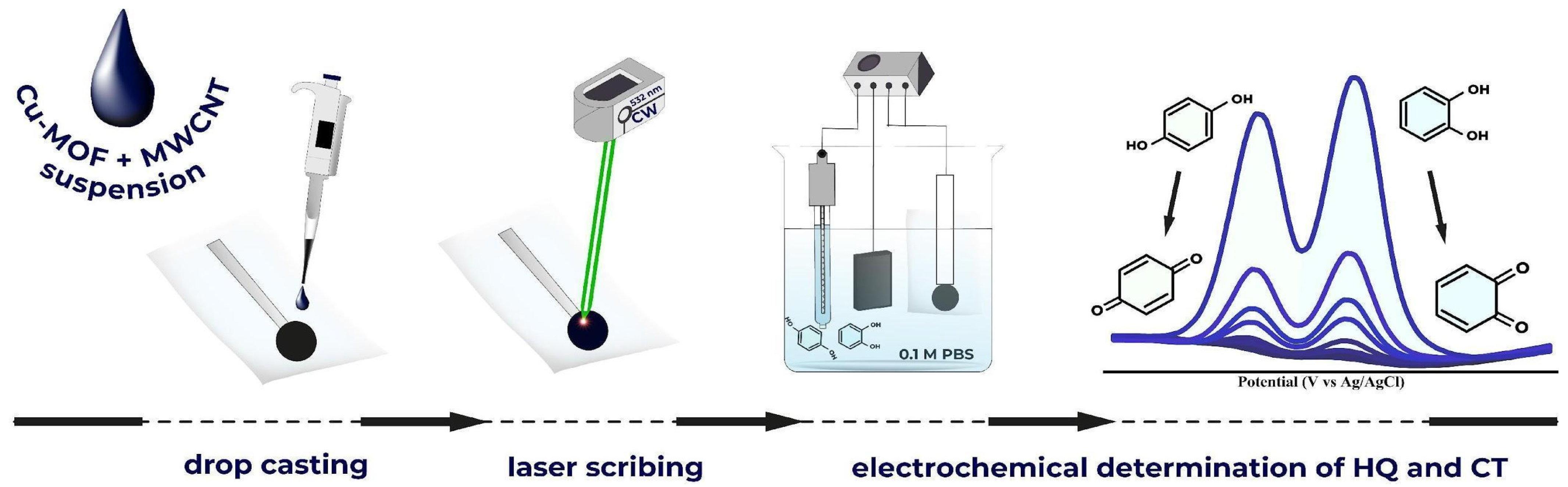
2.3. Electrode Fabrication
2.4. Characterization Techniques
2.5. Electrochemical Measurements
3. Results
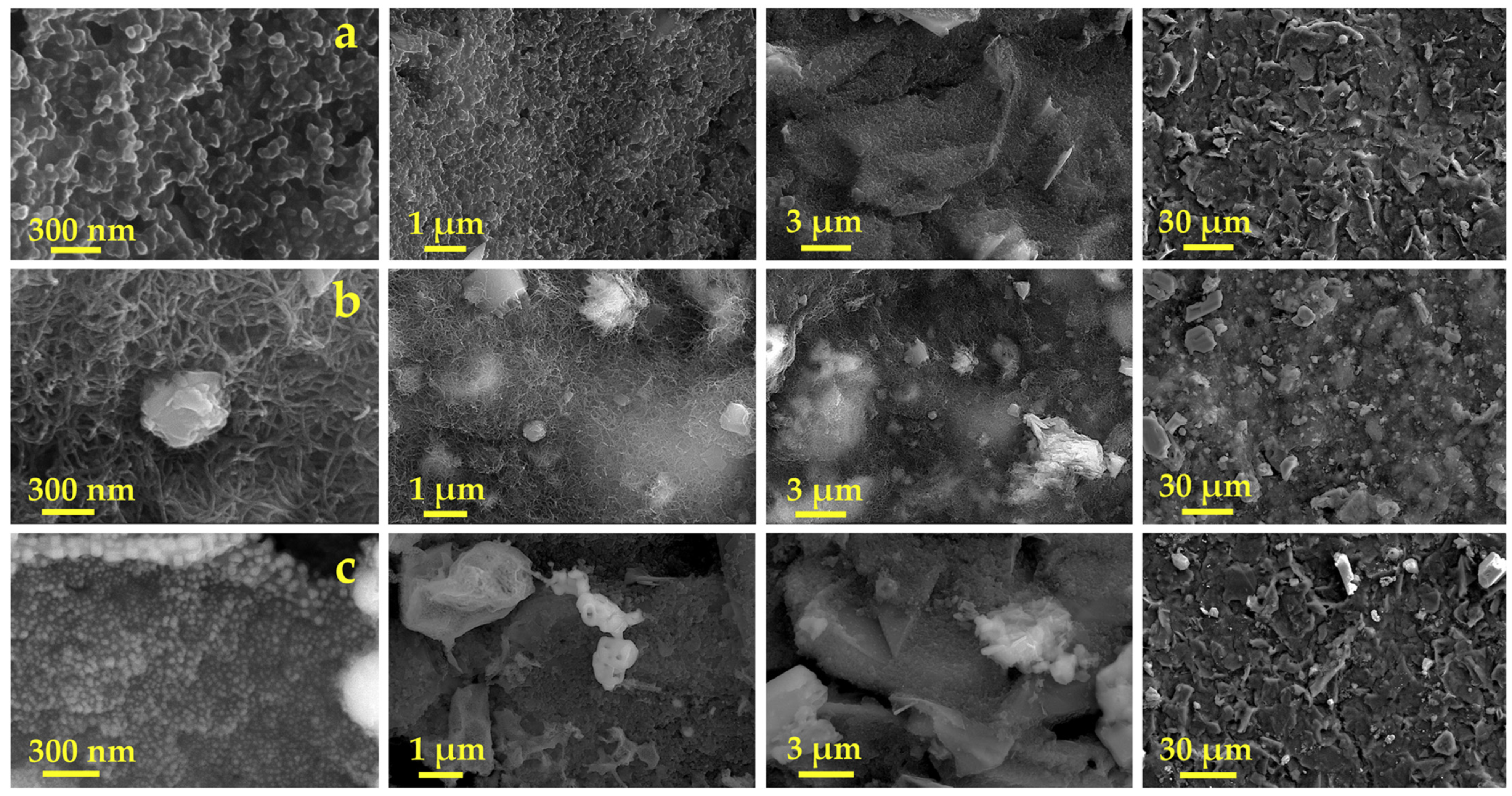
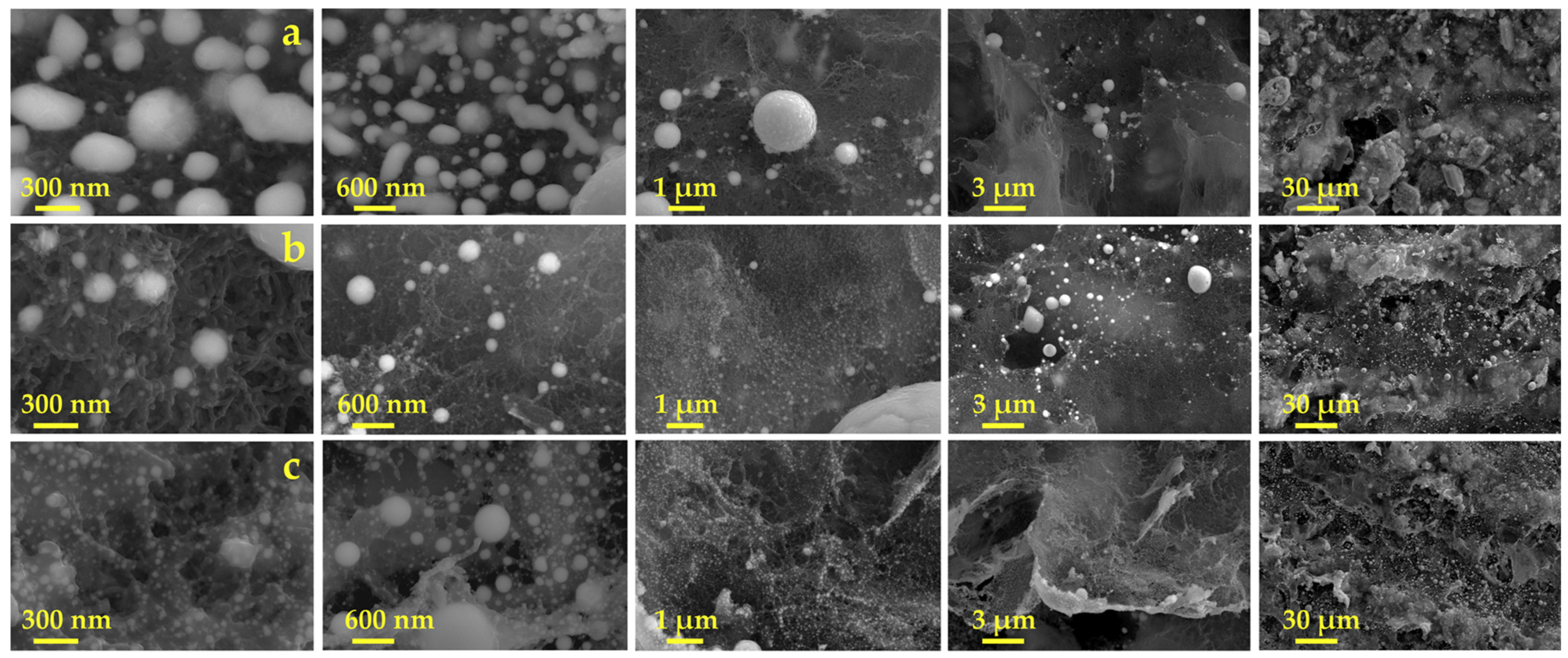
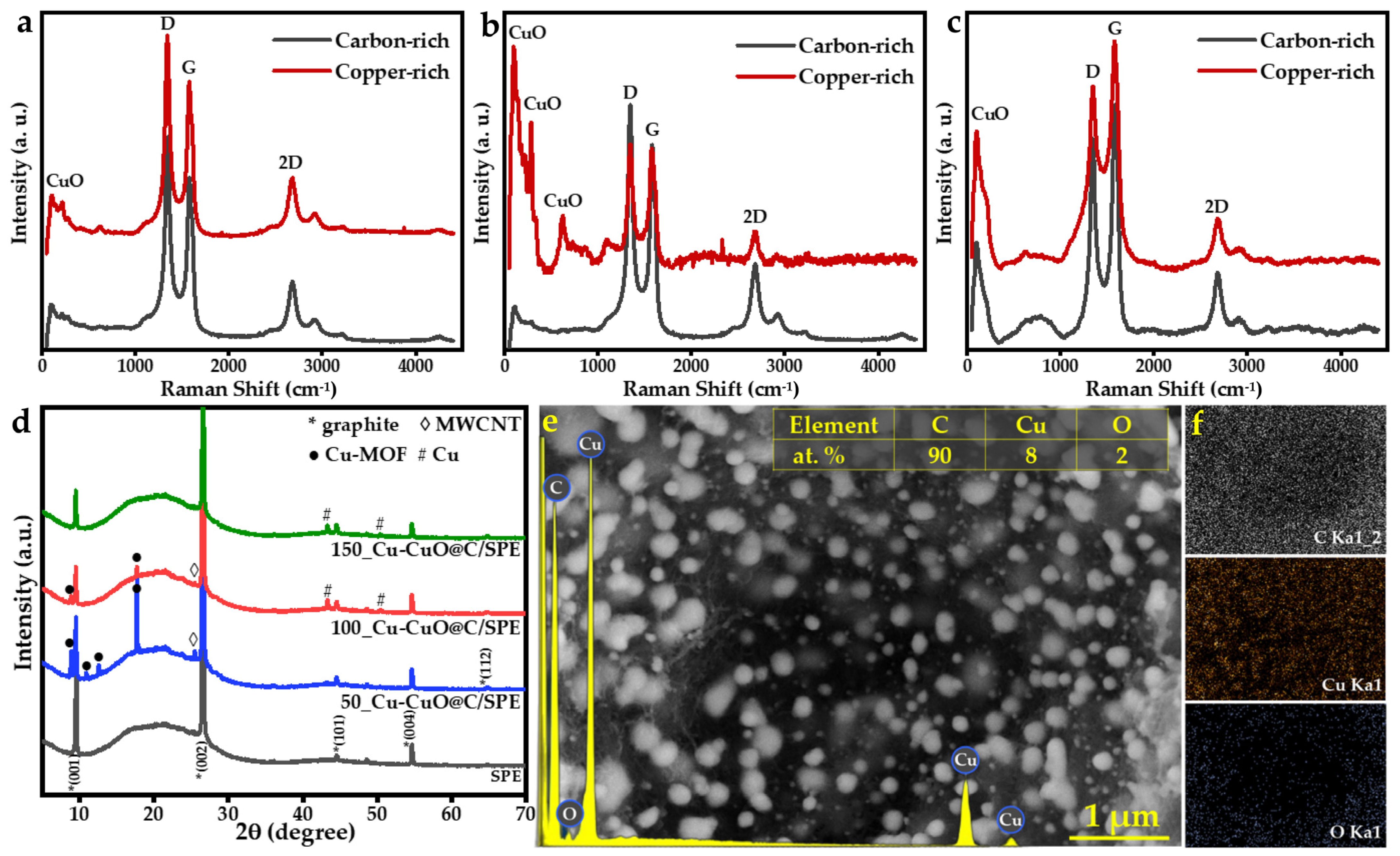
3.1. Preparation and Characterization of Cu-CuO@C/SPE via Laser Scribing
3.1.1. Composition Optimization
3.1.2. Laser Scribing Optimization
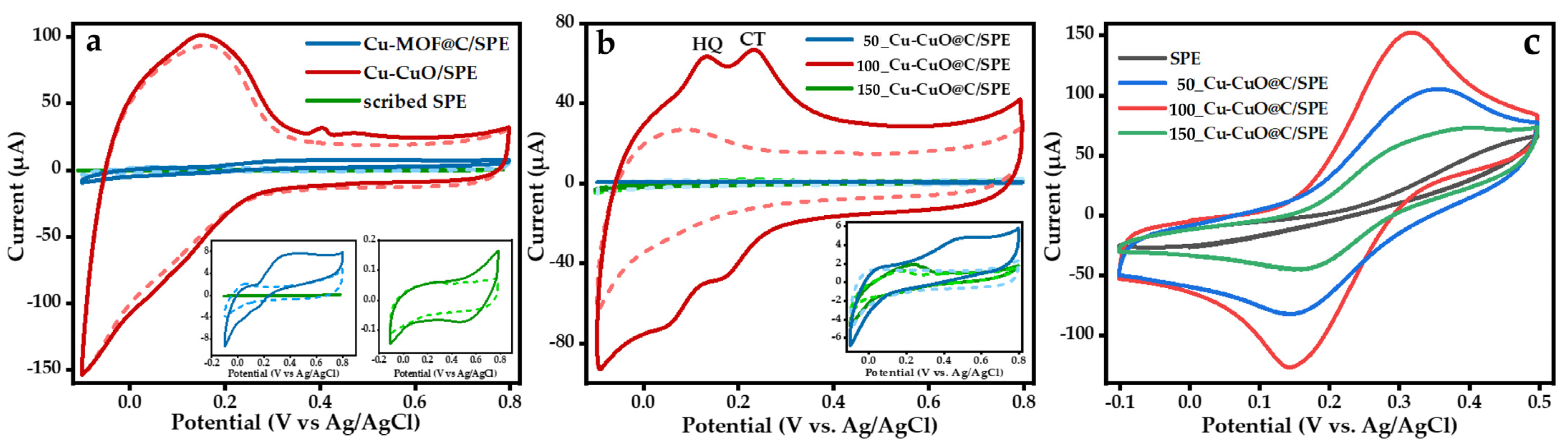
3.2. Electrochemical Properties of Cu-CuO@C/SPE
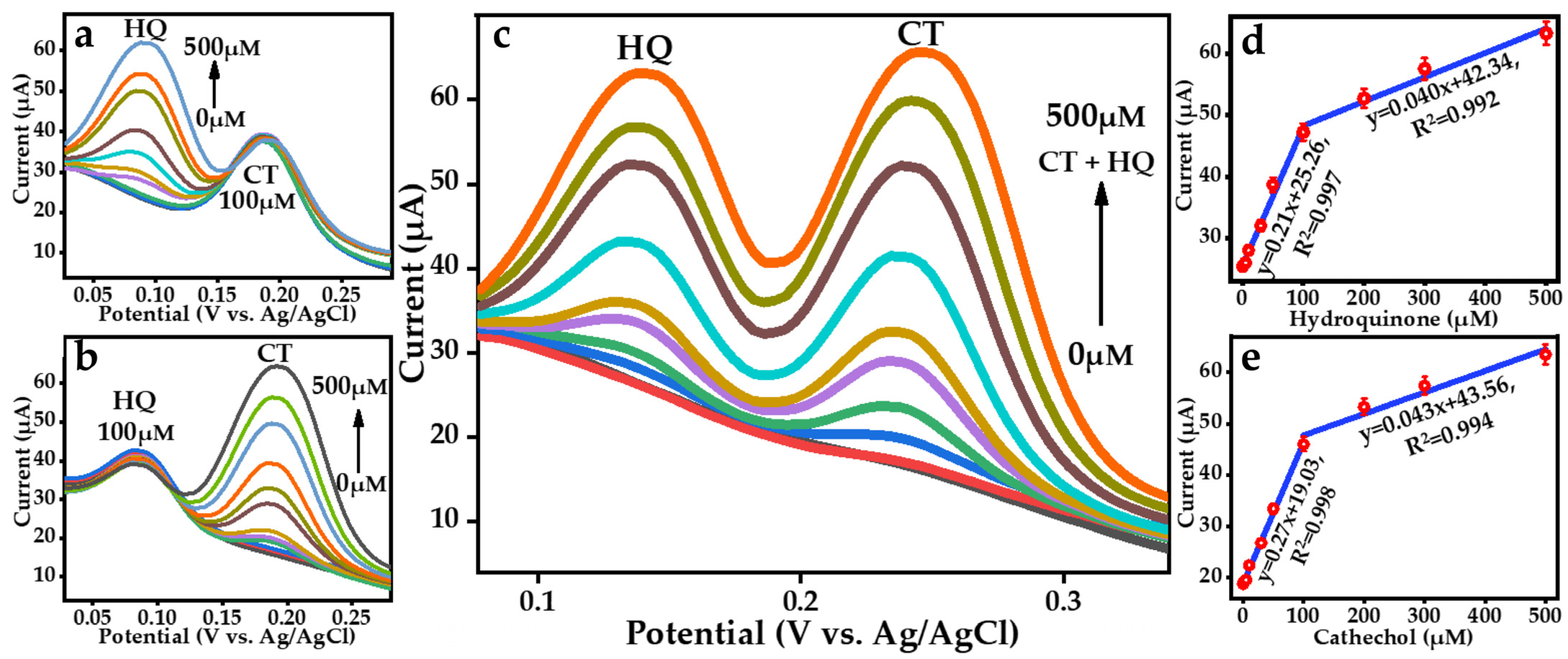
3.3. Electrochemical Determination of Hydroquinone and Catechol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent Advances in the Electrochemical Applications of Ni-Based Metal Organic Frameworks (Ni-MOFs) and Their Derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent Advances in Al(Iii)/In(Iii)-Based MOFs for the Detection of Pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Bloch, W.M.; Babarao, R.; Hill, M.R.; Doonan, C.J.; Sumby, C.J. Post-Synthetic Structural Processing in a Metal-Organic Framework Material as a Mechanism for Exceptional CO2/N2 Selectivity. J. Am. Chem. Soc. 2013, 135, 10441–10448. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Xu, S.; Dong, A.; Hu, Y.; Yang, Z.; Huang, S.; Qian, J. Multidimensional MOF-Derived Carbon Nanomaterials for Multifunctional Applications. J. Mater. Chem. A 2023, 11, 9721–9747. [Google Scholar] [CrossRef]
- Zhong, M.; Kong, L.; Li, N.; Liu, Y.Y.; Zhu, J.; Bu, X.H. Synthesis of MOF-Derived Nanostructures and Their Applications as Anodes in Lithium and Sodium Ion Batteries. Coord. Chem. Rev. 2019, 388, 172–201. [Google Scholar] [CrossRef]
- Jiao, S.; Kang, N.; Zhang, Y.; Li, Y.; Maryam, B.; Zhang, X.; Zhang, P.; Liu, X. Amalia Yunia Rahmawati Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts. Catalysts 2020, 13, 1339. [Google Scholar] [CrossRef]
- Guo, S.; Gao, M.; Zhang, W.; Liu, F.; Guo, X.; Zhou, K. Recent Advances in Laser-Induced Synthesis of MOF Derivatives. Adv. Mater. 2023. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.K.; Wu, K.C.W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Van Lam, D.; Sohail, M.; Kim, J.H.; Lee, H.J.; Han, S.O.; Shin, J.; Kim, D.; Kim, H.; Lee, S.M. Laser Synthesis of MOF-Derived Ni@Carbon for High-Performance Pseudocapacitors. ACS Appl. Mater. Interfaces 2020, 12, 39154–39162. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, C.; Pan, M.; Lai, C.; Wang, Y.; Liao, G.; Ma, Z.; Zhang, F.; Suriyaprakash, J.; Guo, L.; et al. Sub-40 Nm Nanogratings Self-Organized in PVP-Based Polymer Composite Film by Photoexcitation and Two Sequent Splitting under Femtosecond Laser Irradiation. Appl. Surf. Sci. 2023, 609, 155395. [Google Scholar] [CrossRef]
- Jiang, H.; Jin, S.; Wang, C.; Ma, R.; Song, Y.; Gao, M.; Liu, X.; Shen, A.; Cheng, G.J.; Deng, H. Nanoscale Laser Metallurgy and Patterning in Air Using MOFs. J. Am. Chem. Soc. 2019, 141, 5481–5489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; Zheng, H.; Bao, J.; Tang, Y.; Zhou, K. Laser-Assisted Printing of Electrodes Using Metal–Organic Frameworks for Micro-Supercapacitors. Adv. Funct. Mater. 2021, 31, 2009057. [Google Scholar] [CrossRef]
- Kogolev, D.; Semyonov, O.; Metalnikova, N.; Fatkullin, M.; Rodriguez, R.D.; Slepicka, P.; Yamauchi, Y.; Guselnikova, O.; Boukherroub, R.; Postnikov, P.S. Waste PET Upcycling to Conductive Carbon-Based Composite through Laser-Assisted Carbonization of UiO-66. J. Mater. Chem. A 2022, 11, 1108–1115. [Google Scholar] [CrossRef]
- Kulachenkov, N.K.; Bruyere, S.; Sapchenko, S.A.; Mezenov, Y.A.; Sun, D.; Krasilin, A.A.; Nominé, A.; Ghanbaja, J.; Belmonte, T.; Fedin, V.P.; et al. Ultrafast Melting of Metal–Organic Frameworks for Advanced Nanophotonics. Adv. Funct. Mater. 2020, 30, 1908292. [Google Scholar] [CrossRef]
- Chen, K.; Wu, C. De Transformation of Metal-Organic Frameworks into Stable Organic Frameworks with Inherited Skeletons and Catalytic Properties. Angew. Chem. Int. Ed. 2019, 58, 8119–8123. [Google Scholar] [CrossRef]
- Ma, Y.; Sung, K.W.; Ahn, H.J. MOF-Derived Co Nanoparticles Catalyst Assisted by F- and N-Doped Carbon Quantum Dots for Oxygen Reduction. Nanomaterials 2023, 13, 2093. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Ren, Z.; Shen, H.; Sheng, J.; Zhang, K.; Wang, J.; Wang, X. Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis. Coatings 2023, 13, 1437. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent Advances on Preparation and Environmental Applications of MOF-Derived Carbons in Catalysis. Sci. Total Environ. 2021, 760, 143333. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, J.; Pan, P.; Wang, Z.; Yang, Z.; Wei, J.; Li, P.; Cao, S.; Shen, H.; Zhou, J.; et al. Electrochemical Sensor Based on Laser-Induced Preparation of MnOx/RGO Composites for Simultaneous Recognition of Hydroquinone and Catechol. Microchem. J. 2023, 185, 108234. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, N.; Li, L.; Liu, T.; Zhang, Y.; Tang, J.; Guo, J.; Su, S. Synthesis of Graphene Oxide-Coupled CoNi Bimetallic MOF Nanocomposites for the Simultaneous Analysis of Catechol and Hydroquinone. Sensors 2023, 23, 6957. [Google Scholar] [CrossRef]
- Lei, Y.Y.; Zhan, X.; Wu, Y.W.; Yu, X.X. N,Si Co-Doped GQDs: Facile Green Preparation and Application in Visual Identifying Dihydroxybenzene Isomers and Selective Quantification of Catechol, Hydroquinone and Antioxidants. Talanta 2024, 268, 125287. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical Properties of Catechols and Their Molecular Modes of Toxic Action in Cells, from Microorganisms to Mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, T.J.; Westerhof, W. Toxicology and Health Risks of Hydroquinone in Skin Lightening Formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Meskher, H.; Achi, F. Electrochemical Sensing Systems for the Analysis of Catechol and Hydroquinone in the Aquatic Environments: A Critical Review. Crit. Rev. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Lo, S.M.F.; Chui, S.S.Y.; Shek, L.Y.; Lin, Z.; Zhang, X.X.; Wen, G.H.; Williams, I.D. Solvothermal Synthesis of a Stable Coordination Polymer with Copper-I Copper-II Dimer Units:[Cu4{1,4-C6H4(COO)2}3(4,4′-bipy)2]n. J. Am. Chem. Soc. 2000, 122, 6293–6294. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Zhang, J.; Qu, J. MOF-Derived Cu/N–C Composite Based Electrochemical Sensor for Simultaneous Detection of Catechol and Hydroquinone. J. Electrochem. Soc. 2023, 170, 077509. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M.A. Poly(Safranine T)-Deep Eutectic Solvent/Copper Oxide Nanoparticle-Carbon Nanotube Nanocomposite Modified Electrode and Its Application to the Simultaneous Determination of Hydroquinone and Catechol. Microchem. J. 2022, 179, 107531. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Xue, S.; Ma, X.; Tao, H. Innovative Electrochemical Sensor Based on Graphene Oxide Aerogel Wrapped Copper Centered Metal-Organic Framework to Detect Catechol. J. Electroanal. Chem. 2021, 899, 115686. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Yang, L.; Yan, S.; Wang, M.; Cheng, D.; Chen, Q.; Dong, Y.; Liu, P.; Cai, W.; et al. The Cu-MOF-199/Single-Walled Carbon Nanotubes Modified Electrode for Simultaneous Determination of Hydroquinone and Catechol with Extended Linear Ranges and Lower Detection Limits. Anal. Chim. Acta 2015, 899, 57–65. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent Advances in Non-Enzymatic Electrochemical Glucose Sensors Based on Non-Precious Transition Metal Materials: Opportunities and Challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Jin, W. Earth-Abundant Transition Metal and Metal Oxide Nanomaterials: Synthesis and Electrochemical Applications. Prog. Mater. Sci. 2019, 106, 100574. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Synergetic Effects of Combined Nanomaterials for Biosensing Applications. Sensors 2017, 17, 1010. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and Metal Oxide Nanoparticle Synthesis from Metal Organic Frameworks (MOFs): Finding the Border of Metal and Metal Oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A Concise Review of the Raman Spectra of Carbon Allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Khairullina, E.M.; Ratautas, K.; Panov, M.S.; Andriianov, V.S.; Mickus, S.; Manshina, A.A.; Račiukaitis, G.; Tumkin, I.I. Laser-Assisted Surface Activation for Fabrication of Flexible Non-Enzymatic Cu-Based Sensors. Microchim. Acta 2022, 189, 259. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing Graphene, Graphite, and Carbon Nanotubes by Raman Spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon N. Y. 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Mahmoudi Alemi, F.; Mousavi Dehghani, S.A.; Rashidi, A.; Hosseinpour, N.; Mohammadi, S. Synthesize of MWCNT-Fe2O3 Nanocomposite for Controlling Formation and Growth of Asphaltene Particles in Unstable Crude Oil. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126295. [Google Scholar] [CrossRef]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and Size Control of Monodisperse Copper Nanoparticles by Polyol Method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of Temperature on the Size of Biosynthesized Silver Nanoparticle: Deep Insight into Microscopic Kinetics Analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Wei, X.; Wang, M.S.; Bando, Y.; Golberg, D. Thermal Stability of Carbon Nanotubes Probed by Anchored Tungsten Nanoparticles. Sci. Technol. Adv. Mater. 2011, 12, 044605. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Thermal Stability and Structural Changes of Double-Walled Carbon Nanotubes by Heat Treatment. Chem. Phys. Lett. 2004, 398, 87–92. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Yoshidomi, K.; Darkhanbaatar, N.; Khairullina, E.M.; Tumkin, I.I. Effect of Substrates on Femtosecond Laser Pulse-Induced Reductive Sintering of Cobalt Oxide Nanoparticles. Nanomaterials 2021, 11, 3356. [Google Scholar] [CrossRef]
- Pérez-de León, A.; Plasencia, J.; Vázquez-Durán, A.; Méndez-Albores, A. Comparison of the In Vitro Antifungal and Anti-Fumonigenic Activities of Copper and Silver Nanoparticles Against Fusarium Verticillioides. J. Clust. Sci. 2020, 31, 213–220. [Google Scholar] [CrossRef]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic Heating of Nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, K.; Zhang, L.; Yang, Y.; Yuan, H.; Li, H.; Wang, L.; Zeng, J. Copper-Based Plasmonic Catalysis: Recent Advances and Future Perspectives. Adv. Mater. 2021, 33, 2008145. [Google Scholar] [CrossRef]
- Chuang, C.C.; Chu, H.C.; Huang, S.B.; Chang, W.S.; Tuan, H.Y. Laser-Induced Plasmonic Heating in Copper Nanowire Fabric as a Photothermal Catalytic Reactor. Chem. Eng. J. 2020, 379, 122285. [Google Scholar] [CrossRef]
- Jorio, A.; Souza Filho, A.G. Raman Studies of Carbon Nanostructures. Annu. Rev. Mater. Res. 2016, 46, 357–382. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Raman Spectra of CuO Nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T. Phase Transition of Cu2O to CuO Nanocrystals by Selective Laser Heating. Mater. Sci. Semicond. Process. 2016, 46, 6–9. [Google Scholar] [CrossRef]
- Tumkin, I.I.; Khairullina, E.M.; Panov, M.S.; Yoshidomi, K.; Mizoshiri, M. Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials 2021, 14, 2493. [Google Scholar] [CrossRef] [PubMed]
- Khairullina, E.; Shishov, A.; Gordeychuk, D.; Logunov, L.; Levshakova, A.; Sosnovsky, V.B.; Koroleva, A.; Mikhailovsky, V.; Gurevich, E.L.; Chernyshov, I.; et al. Rapid and Effective Method of Laser Metallization of Dielectric Materials Using Deep Eutectic Solvents with Copper Acetate. J. Mater. Sci. 2023, 58, 9322–9336. [Google Scholar] [CrossRef]
- Fan, J.; Pang, J.; Zhang, Y.; Zhang, L.; Xu, W.; Wang, J. Simultaneous Detection of Hydroquinone and Catechol with Decreasing PH at a Bare Glassy Carbon Electrode Surface. Anal. Methods 2019, 11, 604–609. [Google Scholar] [CrossRef]
- Bukhari, S.A.B.; Nasir, H.; Pan, L.; Tasawar, M.; Sohail, M.; Shahbaz, M.; Gul, F.; Sitara, E. Supramolecular Assemblies of Carbon Nanocoils and Tetraphenylporphyrin Derivatives for Sensing of Catechol and Hydroquinone in Aqueous Solution. Sci. Rep. 2021, 11, 5044. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Wu, Q.; Zhu, J.; Chen, S.; Lei, Y.; Li, Y.; Yi, H.; Chen, L.; Shi, Z.Q.; et al. Simultaneous Detection of Acetaminophen, Catechol and Hydroquinone Using a Graphene-Assisted Electrochemical Sensor. RSC Adv. 2022, 12, 23762–23768. [Google Scholar] [CrossRef] [PubMed]
- Khairullina, E.M.; Tumkin, I.I.; Stupin, D.D.; Smikhovskaia, A.V.; Mereshchenko, A.S.; Lihachev, A.I.; Vasin, A.V.; Ryazantsev, M.N.; Panov, M.S. Laser-Assisted Surface Modification of Ni Microstructures with Au and Pt toward Cell Biocompatibility and High Enzyme-Free Glucose Sensing. ACS Omega 2021, 6, 18099–18109. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, K.M.; Abdel Hameed, R.M. Development of Cu2O/Carbon Vulcan XC-72 as Non-Enzymatic Sensor for Glucose Determination. Biosens. Bioelectron. 2011, 26, 3542–3548. [Google Scholar] [CrossRef]
- Karim, M.N.; Lee, J.E.; Lee, H.J. Amperometric Detection of Catechol Using Tyrosinase Modified Electrodes Enhanced by the Layer-by-Layer Assembly of Gold Nanocubes and Polyelectrolytes. Biosens. Bioelectron. 2014, 61, 147–151. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Liu, L.; Sathishkumar, P.; Nan, J.; Gu, F.L. Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem. 2018, 815, 68–75. [Google Scholar] [CrossRef]
- Zuo, J.; Shen, Y.; Wang, L.; Yang, Q.; Cao, Z.; Song, H.; Ye, Z.; Zhang, S. Flexible electrochemical sensor constructed using an active copper center instead of unstable molybdenum carbide for simultaneous detection of toxic catechol and hydroquinone. Microchem. J. 2023, 187, 108443. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Huang, Q.; Hu, S.; Zhang, H.; Zhang, W.; Wang, Z.; Zhu, M.; Dai, P.; Huang, L. Simultaneous electrochemical determination of hydroquinone, catechol and resorcinol at Nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes modified glassy carbon electrode. Electrochim. Acta 2014, 149, 237–244. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Min, X.; Cheng, D.; Zhou, J.; Li, Y.; Xie, Z.; Liu, P.; Cai, W.; Zhang, C. Determination of catechol and hydroquinone with high sensitivity using MOF-graphene composites modified electrode. J. Electroanal. Chem. 2017, 789, 114–122. [Google Scholar] [CrossRef]
- Chen, W.; Li, R.; Li, Z.; Yang, Y.; Zhu, H.; Liu, J. Promising copper oxide-histidine functionalized graphene quantum dots hybrid for electrochemical detection of hydroquinone. J. Alloys Compd. 2019, 777, 1001–1009. [Google Scholar] [CrossRef]
- Nagarajan, S.; Vairamuthu, R.; Angamuthu, R.; Venkatachalam, G. Electrochemical fabrication of reusable pencil graphite electrodes for highly sensitive, selective and simultaneous determination of hydroquinone and catechol. J. Electroanal. Chem. 2019, 846, 113156. [Google Scholar] [CrossRef]
- Cheng, D.; Kan, X. Simultaneous determination of dihydroxybenzene isomers based on gold dendritic/pEDOT electrochemical sensor. J. Electroanal. Chem. 2020, 857, 113741. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Kumara Swamy, B.E.; Jayadevappa, H. Poly (Evans blue) sensor for catechol and hydroquinone: A voltammetric study. J. Electroanal. Chem. 2019, 833, 512–519. [Google Scholar] [CrossRef]
- Sumanth, G.S.; Kumara Swamy, B.E.; Chetankumar, K. Poly DY 11/Zn/CuO modified electrochemical sensor for the detection of catechol and hydroquinone: A voltammetric study. Mater. Chem. Phys. 2023, 296, 127349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levshakova, A.; Kaneva, M.; Borisov, E.; Panov, M.; Shmalko, A.; Nedelko, N.; Mereshchenko, A.S.; Skripkin, M.; Manshina, A.; Khairullina, E. Simultaneous Catechol and Hydroquinone Detection with Laser Fabricated MOF-Derived Cu-CuO@C Composite Electrochemical Sensor. Materials 2023, 16, 7225. https://doi.org/10.3390/ma16227225
Levshakova A, Kaneva M, Borisov E, Panov M, Shmalko A, Nedelko N, Mereshchenko AS, Skripkin M, Manshina A, Khairullina E. Simultaneous Catechol and Hydroquinone Detection with Laser Fabricated MOF-Derived Cu-CuO@C Composite Electrochemical Sensor. Materials. 2023; 16(22):7225. https://doi.org/10.3390/ma16227225
Chicago/Turabian StyleLevshakova, Aleksandra, Maria Kaneva, Evgenii Borisov, Maxim Panov, Alexandr Shmalko, Nikolai Nedelko, Andrey S. Mereshchenko, Mikhail Skripkin, Alina Manshina, and Evgeniia Khairullina. 2023. "Simultaneous Catechol and Hydroquinone Detection with Laser Fabricated MOF-Derived Cu-CuO@C Composite Electrochemical Sensor" Materials 16, no. 22: 7225. https://doi.org/10.3390/ma16227225