Microstructure and Efflorescence Resistance of Metakaolin Geopolymer Modified by 5A Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
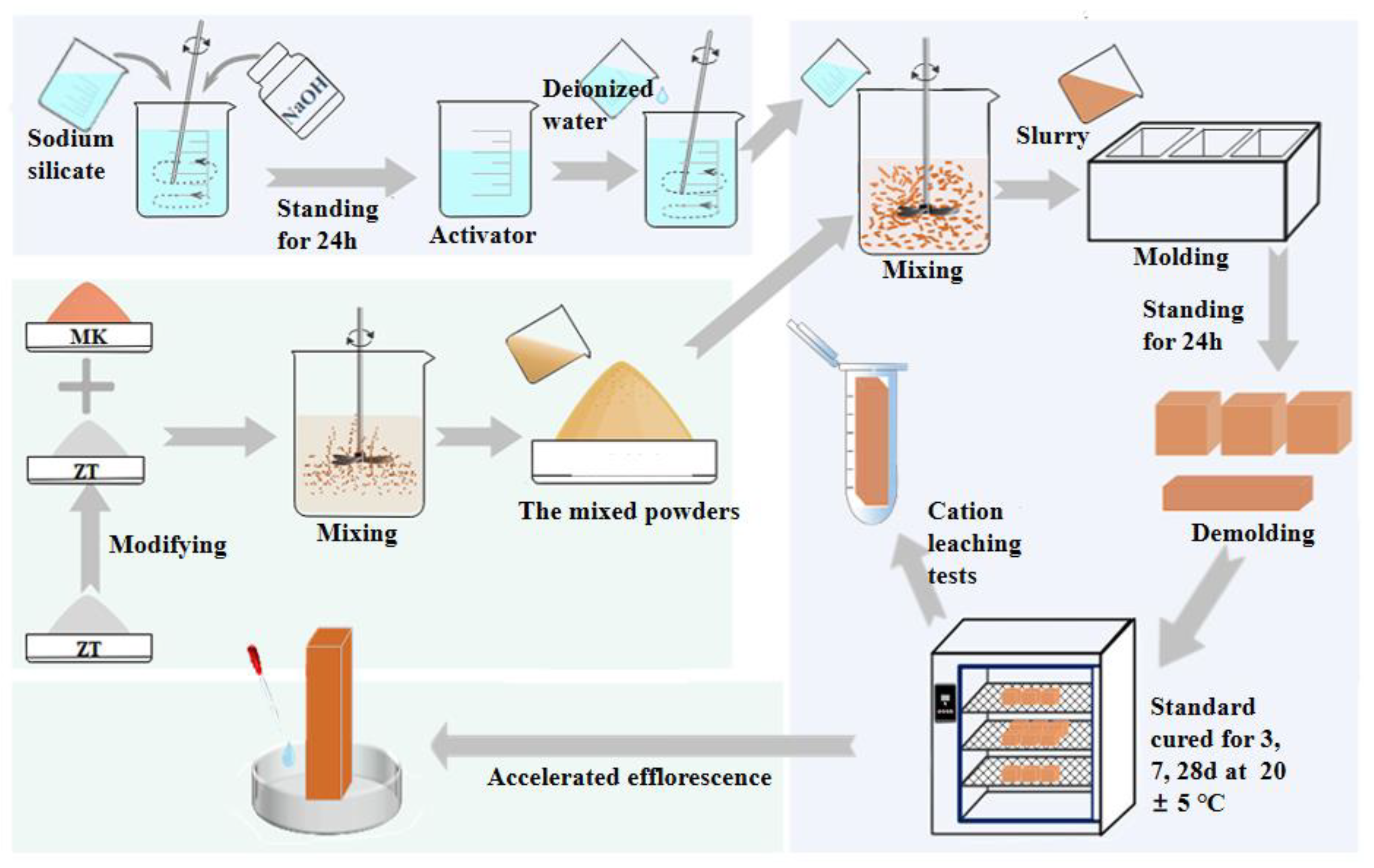
2.2. Experimental Procedure
2.2.1. Preparation of Activators
2.2.2. Modification of 5A Zeolite
2.2.3. Preparation of Modified 5A Zeolite–Metakaolin Geopolymer
2.2.4. Assessment of Anti-Efflorescence Performance of Geopolymers
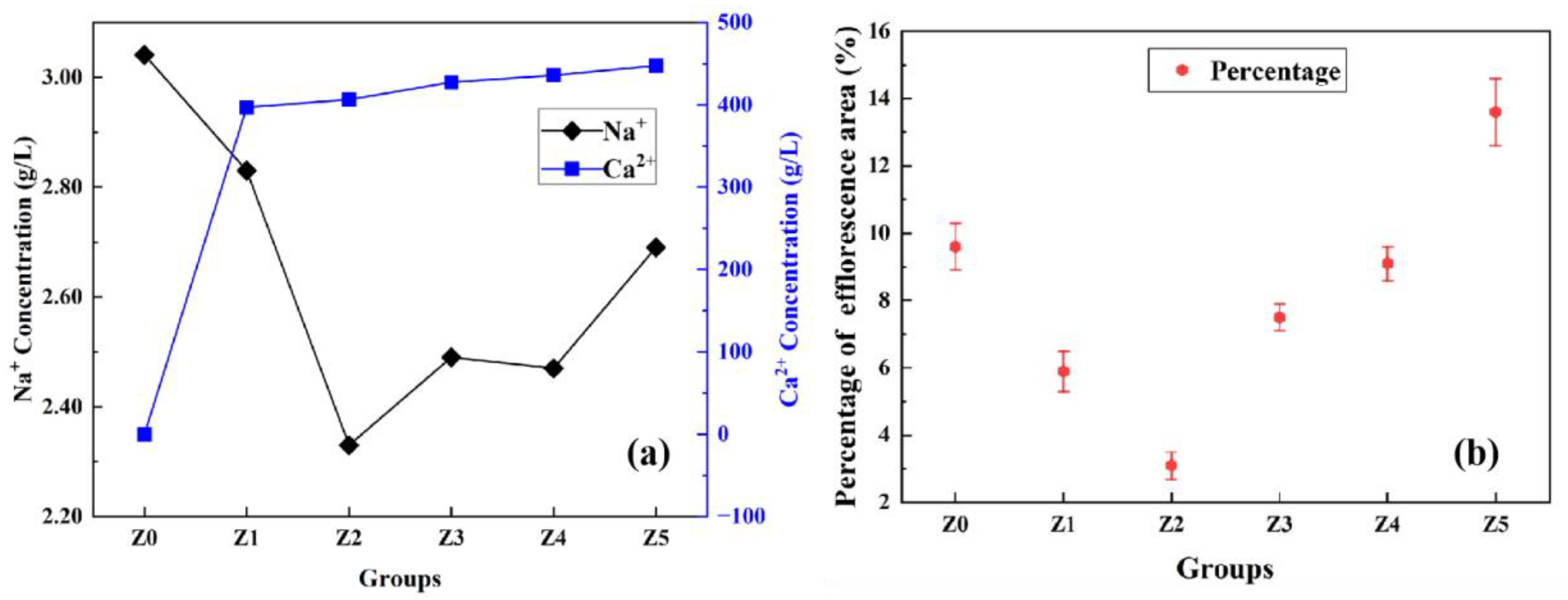
- After curing for 28 day, the samples were sealed in a large centrifuge tube and immersed in 40 mL deionized water for 7 day. Next, 10 mL of the top layer was removed and then filtered through a microporous membrane (0.45 µm). The pH was adjusted to the test range using 2% HNO3 and placed in a 25 mL volumetric flask. The corresponding dilution rates were calculated for the ICP test. And the leaching concentration of cations reflected the degree of efflorescence in the geopolymer.
- To accelerate efflorescence, the samples cured for 28 day were immersed in a Petri dish with a water level of 2 mm and exposed to ambient air at 20 ± 5 °C, 50 ± 15% RH for 28 days. The initial water level was maintained by adding water daily, and physical photos of the specimens were taken after 28 days. Each sample had five surfaces where efflorescence products could be observed (the surface submerged in water was difficult to detect). Image Pro Plus software 6.0 was used to accurately quantify the visual degree of efflorescence by calculating the percentage of all efflorescence areas on the surface of each specimen. And the result is the average of three times of calculation.
2.3. Analysis and Testing
3. Results and Discussion
3.1. Effect of Modification Temperature on Cation-Exchange Property of 5A Zeolite
3.2. Basic Properties and Microstructure of Geopolymers
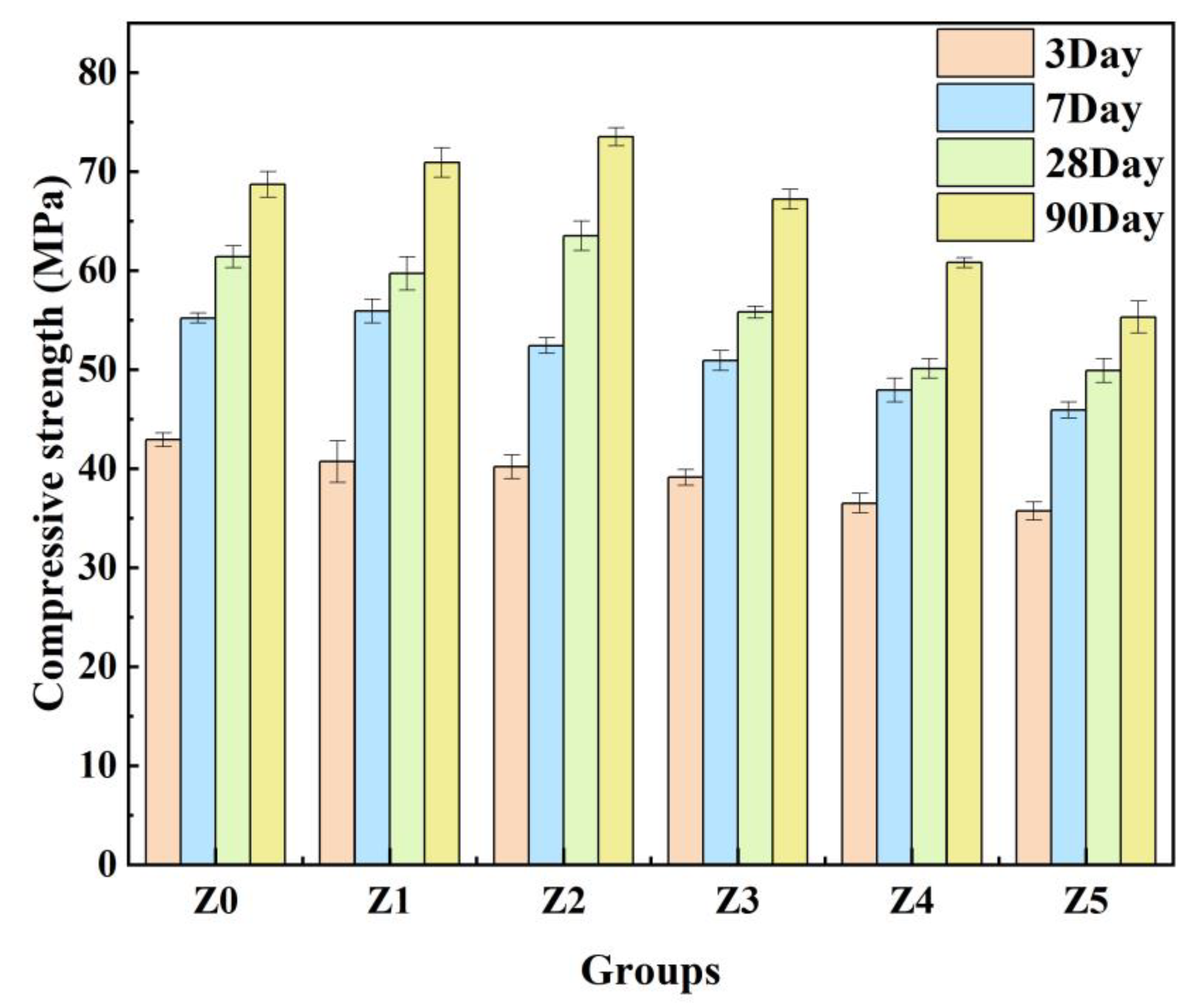
3.2.1. Analysis of the Compressive Strength of Geopolymers
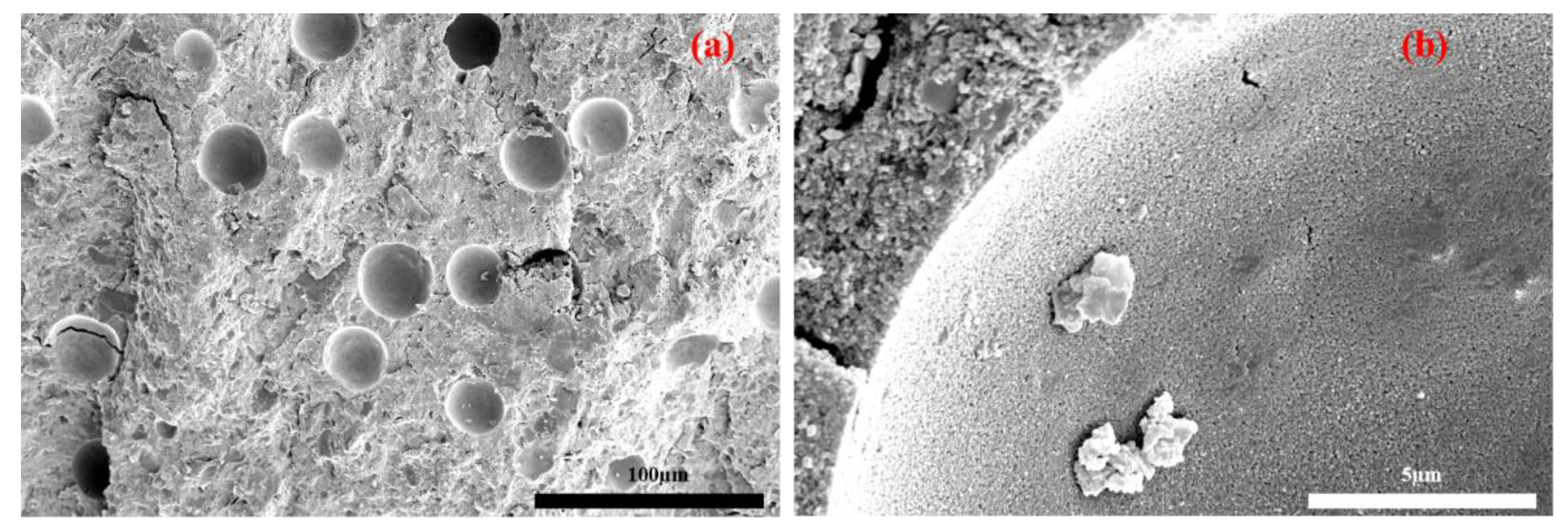
- Zeolites with a specific surface area of 1.1 m2/g can be distributed as micro-aggregates in the matrix to fill the pores of specimen, which can make the structure tightly packed and cause the specimen to be compacted [27].
- Due to the water absorption of 3ZT, a moderate addition of 3ZT can cause water deficit in partial microregions and increase the alkalinity of the solution. A higher alkali content promotes the dissolution of active amorphous silica-alumina materials in MK and subsequently promotes the geopolymerization reaction [28]. This may be one of the reasons for the formation of a more homogeneous microstructure. In addition, the cavities and pores in 5A zeolite can absorb and desorb water [29]. The 5A zeolite can exchange water effectively with its surroundings because of this property and its porous structure. Under the water deficit condition, the zeolite water present in the pores and cavities will be gradually released with the extension of curing time, providing basic conditions for a continuous geopolymerization reaction to achieve an internal curing effect, which is macroscopically manifested by an enhancement in the compressive strength of specimens with an appropriate 3ZT content.
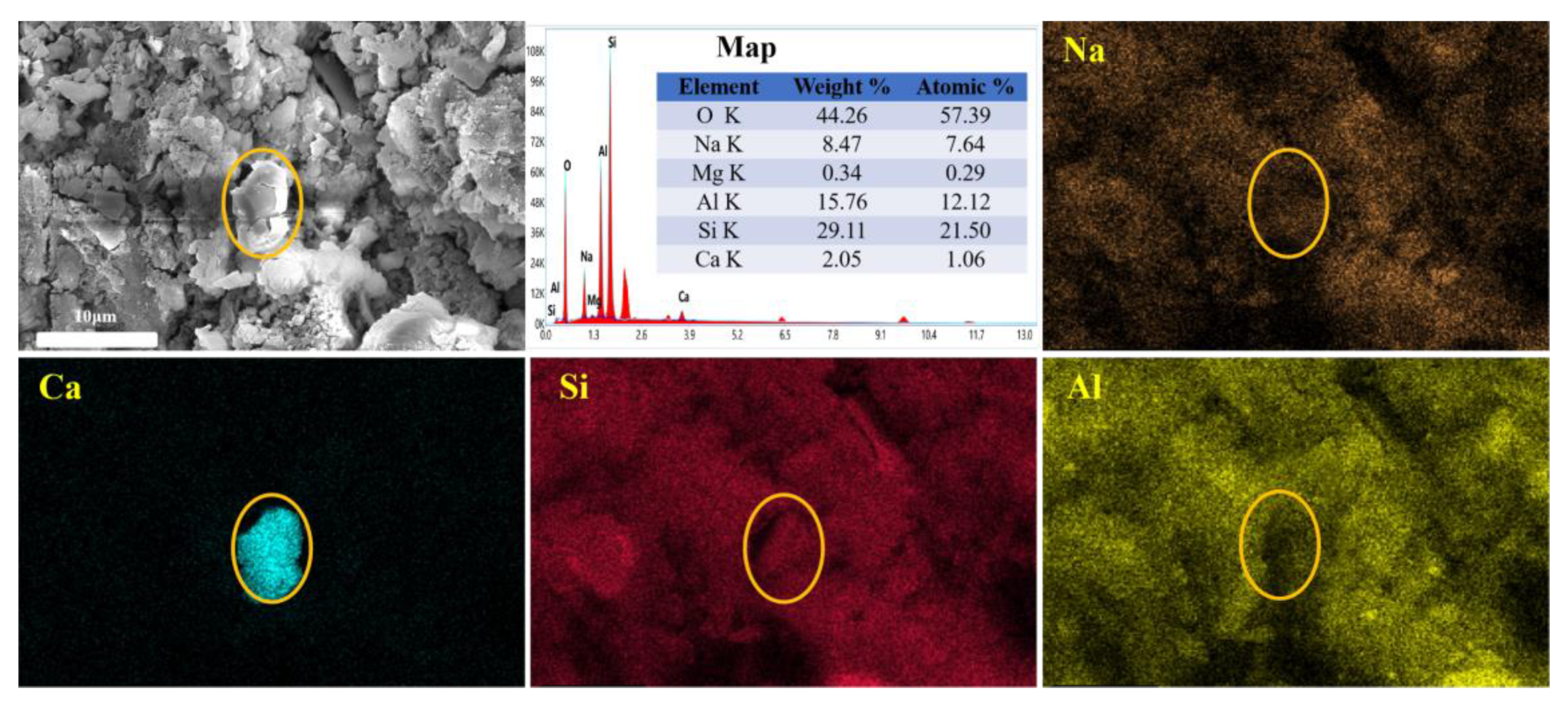
- Temuujin et al. [30] showed that the addition of Ca2+ led to a more homogeneous and denser microstructure of the material. Yip et al. [31] suggested that the Ca(OH)2 formed by Ca2+ in an alkaline environment could provide additional nucleation sites for the binder system to form (N,C)-A-S-H gels and then promote the rapid formation of geopolymer gels. Combined with the microstructure of each curing stage, it is also evident that 3ZT has a cation-exchange effect in the system, which can absorb the alkali metal Na+ from the matrix and gradually diffuse Ca2+ into the surroundings. In the early stage of the reaction, the geopolymerization reaction is imperfect, with fewer oligomers depolymerized by the zeolite. The exchanged Ca2+ forms Ca(OH)2 in an alkaline environment [31]. As the geopolymerization proceeds, the number of oligomers gradually increases. The solubility product of the gel is much smaller than that of Ca(OH)2, resulting in a decrease in Ca2+ concentration in the matrix and the continuous dissolution of Ca(OH)2 crystals [32]. The low Ca2+ concentration is not sufficient to form C-S-H but may form (N,C)-A-S-H gels and may be immobilized as charge-balancing cations captured by surrounding N-A-S-H gels. During this process, excess metal cations are immobilized, and gels are formed to optimize the pore space. It is one of the reasons why an appropriate admixture of 3ZT enhances the strength [30,33].
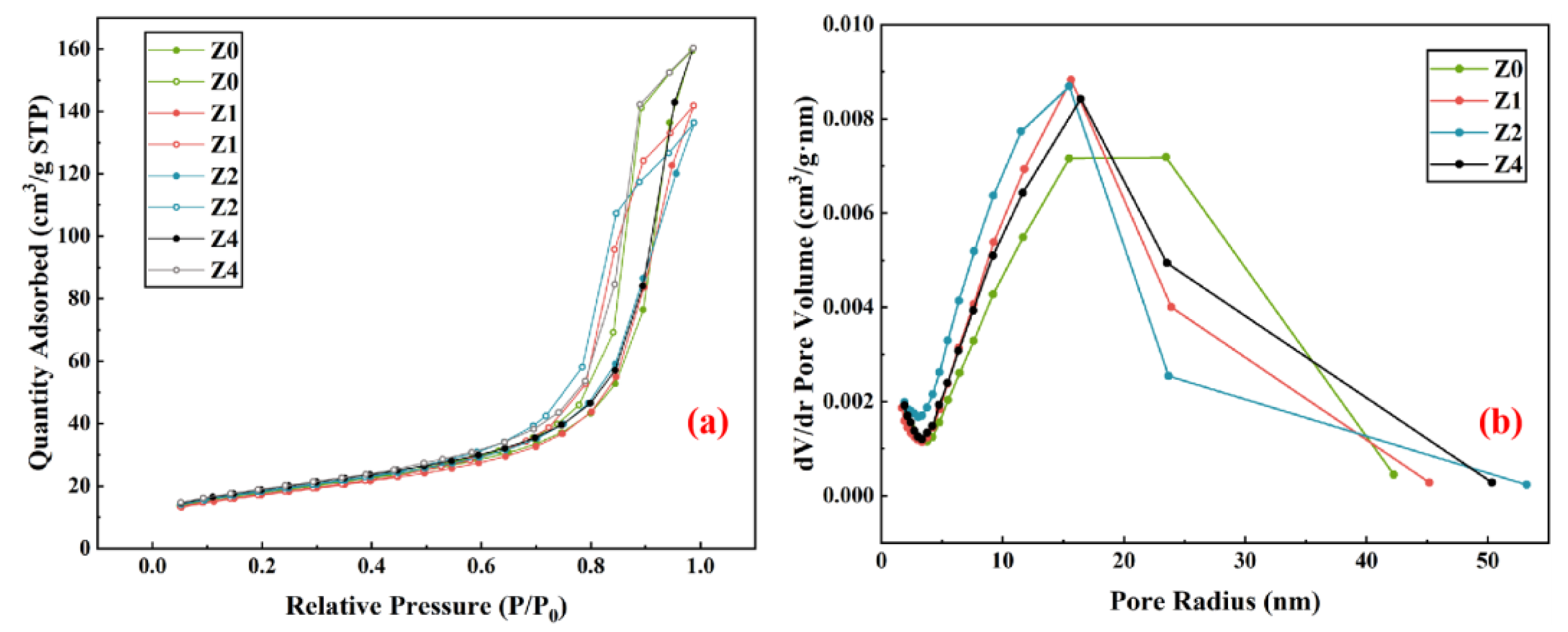
3.2.2. Analysis of the Pore Structure of Geopolymers
3.2.3. Microstructure Analysis of Geopolymers
3.3. The Property of Anti-Efflorescence of Geopolymers
4. Conclusions
- The water–air exchange of zeolites can provide nucleation conditions for bubbles, which can introduce defects that are detrimental to the early compressive strength of the geopolymer. An appropriate amount of 3ZT facilitates the enhancement of compressive strength at a later stage, and the optimal content was 4 wt.%. Compared to the MK geopolymer (group Z0), the compressive strength of samples in group Z2 increased by 3.4% and 7% at 28 and 90 days, respectively.
- The appropriate amount of 3ZT facilitates the geopolymerization reaction and the formation of more gel pores to reduce the average pore size. This is probably the result of the synergistic effect of 3ZT as a micro-aggregate, internal curing, and cation exchange in the matrix.
- The cation exchange of 3ZT in the matrix is effective in reducing the Na+ concentration and the degree of efflorescence in the pore solution. The Z2 group (3ZT dosed at 4 wt.%) performed best with a 19.4% reduction in Na+ leaching and a 57.3% reduction in efflorescence.
- The cation-exchange pattern of 3ZT in the matrix is as follows: In the cement slurry, the previous thermal activation modification reduces the resistance of water films on the zeolite surface. Then, 3ZT adsorbs part of the hydrated Na+ in exchange for a part of the Ca2+ to balance the charge. With the dissolution and reorganization of the surrounding matrix, Ca2+ exists as Ca(OH)2 in the alkaline environment. As the matrix gradually gelates, the highly soluble Ca(OH)2 gradually dissolves to release Ca2+, and the matrix is captured to form (N, C)-A-S-H gels, making 5A zeolite embed well within the hydration products. This process both fixes excess free Na+ in the matrix and optimizes the pore size distribution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A state-of-the-art review of recent advances in porous geopolymer: Applications in adsorption of inorganic and organic contaminants in water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Philip, S.; Nidhi, M. A review on the material performance of geopolymer concrete as green building materials. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Maged, A.; Eloffy, M.G.; Zahran, M.; Kharbish, S.; Elwakeel, K.Z.; Bhatnagar, A. Geopolymers as sustainable eco-friendly materials: Classification, synthesis routes, and applications in wastewater treatment. Sep. Purif. Technol. 2023, 324, 124631. [Google Scholar] [CrossRef]
- Kang, S.-P.; Kwon, S.-J. Effects of red mud and Alkali-Activated Slag Cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Dow, C.; Glasser, F.P. Calcium carbonate efflorescence on Portland cement and building materials. Cem. Concr. Res. 2003, 33, 147–154. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.S.; Zhang, Z.; Wang, H. Phase changes under efflorescence in alkali activated materials with mixed activators. Constr. Build. Mater. 2021, 283, 122678. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Pino, F.; Pargoletti, E.; Pecchioni, E.; Fratini, F.; Ruffolo, S.A.; La Russa, M.F. On the role of hydrophobic Si-based protective coatings in limiting mortar deterioration. Environ. Sci. Pollut. Res. 2015, 22, 17733–17743. [Google Scholar] [CrossRef]
- Maghsoodloorad, H.; Allahverdi, A. Efflorescence Formation and Control in Alkali-Activated Phosphorus Slag Cement. Int. J. Civ. Eng. 2016, 14, 425–438. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Longhi, M.A.; Zhang, Z.; Walkley, B.; Kirchheim, A.P. Strategies for control and mitigation of efflorescence in metakaolin-based geopolymers. Cem. Concr. Res. 2021, 144, 106431. [Google Scholar] [CrossRef]
- Gao, K.; Lin, K.-L.; Wang, D.; Hwang, C.-L.; Anh Tuan, B.L.; Shiu, H.-S.; Cheng, T.-W. Effect of nano-SiO2 on the alkali-activated characteristics of metakaolin-based geopolymers. Constr. Build. Mater. 2013, 48, 441–447. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Abdulkareem, F.A.; Shariff, M.A.; Ullah, S.; See, T.L.; Keong, L.K.; Mellon, N. Adsorption performance of 5A molecular sieve zeolite in water vapor–binary gas environment: Experimental and modeling evaluation. J. Ind. Eng. Chem. 2018, 64, 173–187. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Baek, W.; Ha, S.; Hong, S.; Kim, S.; Kim, Y. Cation exchange of cesium and cation selectivity of natural zeolites: Chabazite, stilbite, and heulandite. Microporous Mesoporous Mater. 2018, 264, 159–166. [Google Scholar] [CrossRef]
- Nagase, T.; Ikeda, T.; Hiyoshi, N.; Abe, C.; Hanaoka, T. Structural changes in –LIT zeolites related to cation-exchange treatments under aqueous and non-aqueous conditions. Microporous Mesoporous Mater. 2014, 190, 92–98. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; El-kamash, A.M.; Hanafy, M.; Abdel-Monem, N.M. Examination of the use of synthetic Zeolite NaA–X blend as backfill material in a radioactive waste disposal facility: Thermodynamic approach. Chem. Eng. J. 2008, 144, 67–74. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, K.-L.; Cui, R.; Hwang, C.-L.; Chang, Y.-M.; Cheng, T.-W. The effects of SiO2/Na2O molar ratio on the characteristics of alkali-activated waste catalyst–metakaolin based geopolymers. Constr. Build. Mater. 2015, 95, 710–720. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Xu, F.; Song, X.; Ye, J.; Duan, P.; Zhang, Z. Sulfate resistance of hybrid fiber reinforced metakaolin geopolymer composites. Compos. Part B Eng. 2020, 183, 107689. [Google Scholar]
- Xue, B.; Qian, C. Mitigation of efflorescence of wallboard by means of bio mineralization. Front. Microbiol. 2015, 6, 1155. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Muñoz, G.; Calero-Berrocal, R.; Larriba, M.; Águeda, V.I.; Delgado, J.A. Influence of sodium and potassium proportion on the adsorption of methanol and water on LTA zeolites at high temperature. Microporous Mesoporous Mater. 2023, 360, 112669. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, K.; Gao, S.; Wang, F.; Wu, Z. Effect of pozzolanic reaction of zeolite on its internal curing performance in cement-based materials. J. Build. Eng. 2023, 63, 105503. [Google Scholar]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Panek, R.; Szerement, J.; Marcińska-Mazur, L.; Renata, J.; Tomasz, B.; Wojciech, F.; Mierzwa-Hersztek, M. The influence of zeolite X ion-exchangeable forms and impregnation with copper nitrate on the adsorption of phosphate ions from aqueous solutions. J. Water Process Eng. 2022, 50, 103299. [Google Scholar]
- Wang, J.; Li, F.; Zhou, Z.; Du, P.; Xu, D.; Xie, N.; Cheng, X.; Liu, Y. Effect of zeolite on waste based alkali-activated inorganic binder efflorescence. Constr. Build. Mater. 2018, 158, 683–690. [Google Scholar] [CrossRef]
- Islam, M.S.; Mohr, B.J.; VandenBerge, D. Performance of natural clinoptilolite zeolite in the cementitious materials: A comparative study with metakaolin, fly ash, and blast furnace slag. J. Build. Eng. 2022, 53, 104535. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Gao, M.; Bai, H.; Nagasaka, T. Synthesis of zeolite-geopolymer composites with high zeolite content for Pb(II) removal by a simple two-step method using fly ash and metakaolin. J. Clean. Prod. 2022, 378, 134528. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar]
- Huang, Y.; Han, M.; Yi, R. Microstructure and properties of fly ash-based geopolymeric material with 5A zeolite as a filler. Constr. Build. Mater. 2012, 33, 84–89. [Google Scholar]
- Rożek, P.; KrÓl, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar]
- Odler, I.; Rößler, M. Investigations on the relationship between porosity, structure and strength of hydrated Portland cement pastes. II. Effect of pore structure and of degree of hydration. Cem. Concr. Res. 1985, 15, 401–410. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Zhang, X.; Chen, H. Diverse zeolites derived from a circulating fluidized bed fly ash based geopolymer for the adsorption of lead ions from wastewater. J. Clean. Prod. 2021, 312, 127769. [Google Scholar]
- Li, Y.; Luo, Y.; Sun, P.; Peng, B.; Wang, J.; Shao, Z.; Zhou, L.; Qian, B.; Ma, B.; Hu, Y.; et al. Investigation of disused artificial zeolite on the properties of metakaolin-based geopolymer. J. Indian Chem. Soc. 2022, 99, 100627. [Google Scholar]
- Zhu, H.; Liang, G.; Zhang, Z.; Wu, Q.; Du, J. Partial replacement of metakaolin with thermally treated rice husk ash in metakaolin-based geopolymer. Constr. Build. Mater. 2019, 221, 527–538. [Google Scholar]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr contaminated clinoptilolite in geopolymers produced from metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar]
- Wei, X.-L.; Pan, W.-Y.; Pan, M.; Peng, L.-L.; Niu, C.-Q.; Chao, Z.-S. Effects of bubbles on the structure and performance of zeolite membranes. J. Eur. Ceram. Soc. 2020, 40, 1709–1716. [Google Scholar]
- Caputo, D.; Liguori, B.; Colella, C. Some advances in understanding the pozzolanic activity of zeolites: The effect of zeolite structure. Cem. Concr. Compos. 2008, 30, 455–462. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar]
- Provis, J.; Van Deventer, J. Geopolymers: Structures, Processing, Properties and Industrial Applications; CRC Press: Boca Raton, FL, USA; Woodhead Publishing: Sawston, UK, 2009; pp. 1–454. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Chang, J. Variation in mineral composition by hydration and carbonation in calcium hydroxide matrix containing zeolite. J. Build. Eng. 2022, 56, 104491. [Google Scholar] [CrossRef]
- Erfanimanesh, A.; Sharbatdar, M.K. Mechanical and microstructural characteristics of geopolymer paste, mortar, and concrete containing local zeolite and slag activated by sodium carbonate. J. Build. Eng. 2020, 32, 101781. [Google Scholar] [CrossRef]
- Weise, K.; Ukrainczyk, N.; Koenders, E. Pozzolanic Reactions of Metakaolin with Calcium Hydroxide: Review on Hydrate Phase Formations and Effect of Alkali Hydroxides, Carbonates and Sulfates. Mater. Des. 2023, 231, 112062. [Google Scholar]
- Huang, X.; Hu, S.; Wang, F.; Liu, Y.; Mu, Y. Properties of alkali-activated slag with addition of cation exchange material. Constr. Build. Mater. 2017, 146, 321–328. [Google Scholar] [CrossRef]
- Xu, W.; Lo, Y.T.; Ouyang, D.; Memon, S.A.; Xing, F.; Wang, W.; Yuan, X. Effect of rice husk ash fineness on porosity and hydration reaction of blended cement paste. Constr. Build. Mater. 2015, 89, 90–101. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Yan, S.; Zhang, M.; Xie, Y. Properties of microscopic particle morphology and particle contact of renewable construction waste mixtures. Constr. Build. Mater. 2019, 207, 190–205. [Google Scholar] [CrossRef]
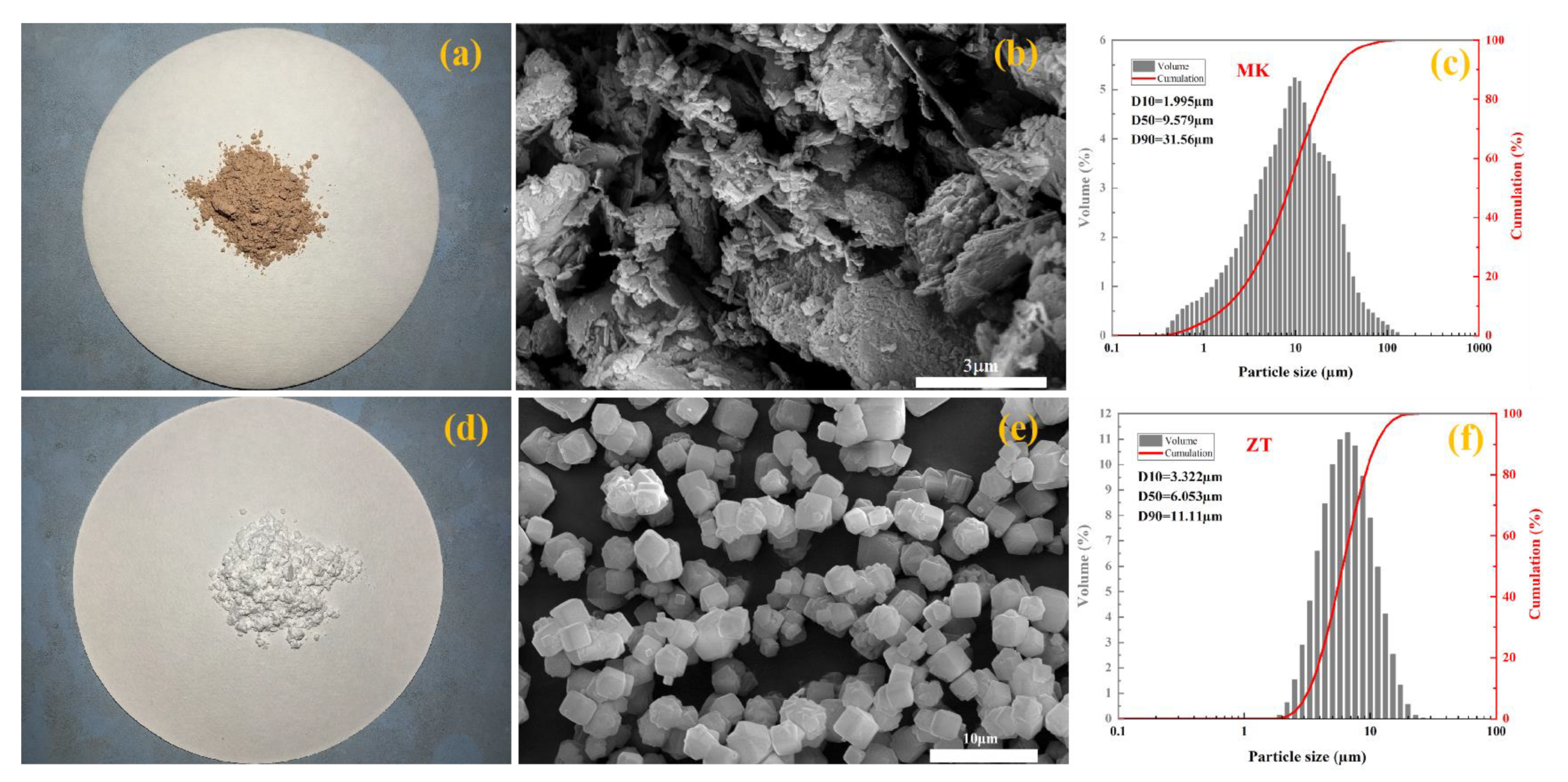


| Oxide | ZT | MK |
|---|---|---|
| Na2O | 5.29 | 0.57 |
| MgO | - | 0.9 |
| Al2O3 | 37.05 | 41.94 |
| SiO2 | 42.24 | 53.73 |
| P2O5 | - | 0.06 |
| SO3 | 0.13 | 0.37 |
| K2O | 0.63 | 0.05 |
| CaO | 14.25 | 0.54 |
| Fe2O3 | 0.03 | 1.55 |
| LOI | 0.38 | 0.29 |
| Sample No. | Heating Rate/°C/min | Thermal Activation Temperature/°C | Holding Time/h |
|---|---|---|---|
| ZT | 0 | 50 | 72 |
| 1ZT | 5 | 100 | 2 |
| 2ZT | 5 | 200 | 2 |
| 3ZT | 5 | 300 | 2 |
| 4ZT | 5 | 400 | 2 |
| 5ZT | 5 | 500 | 2 |
| 6ZT | 5 | 600 | 2 |
| 7ZT | 5 | 700 | 2 |
| 8ZT | 5 | 800 | 2 |
| Sample No. | Modified Zeolite/wt.% | MK/wt.% | Alkali Activator/wt.% | Water/wt.% |
|---|---|---|---|---|
| Z0 | 0 | 100 | 90 | 10 |
| Z1 | 2 | 98 | 90 | 10 |
| Z2 | 4 | 96 | 90 | 10 |
| Z3 | 6 | 94 | 90 | 10 |
| Z4 | 8 | 92 | 90 | 10 |
| Z5 | 10 | 90 | 90 | 10 |
| Group | Z0 | Z1 | Z2 | Z4 |
|---|---|---|---|---|
| Average pore size/nm | 13.8611 | 12.1878 | 11.0179 | 13.1219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Song, L.; Xu, Y.; Duan, P.; Wang, X. Microstructure and Efflorescence Resistance of Metakaolin Geopolymer Modified by 5A Zeolite. Materials 2023, 16, 7243. https://doi.org/10.3390/ma16227243
Lu Y, Song L, Xu Y, Duan P, Wang X. Microstructure and Efflorescence Resistance of Metakaolin Geopolymer Modified by 5A Zeolite. Materials. 2023; 16(22):7243. https://doi.org/10.3390/ma16227243
Chicago/Turabian StyleLu, Yuwei, Luxia Song, Yuan Xu, Ping Duan, and Xiaoming Wang. 2023. "Microstructure and Efflorescence Resistance of Metakaolin Geopolymer Modified by 5A Zeolite" Materials 16, no. 22: 7243. https://doi.org/10.3390/ma16227243
APA StyleLu, Y., Song, L., Xu, Y., Duan, P., & Wang, X. (2023). Microstructure and Efflorescence Resistance of Metakaolin Geopolymer Modified by 5A Zeolite. Materials, 16(22), 7243. https://doi.org/10.3390/ma16227243






