Polyurethane Adhesives for Wood Based on a Simple Mixture of Castor Oil and Crude Glycerin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Polyols
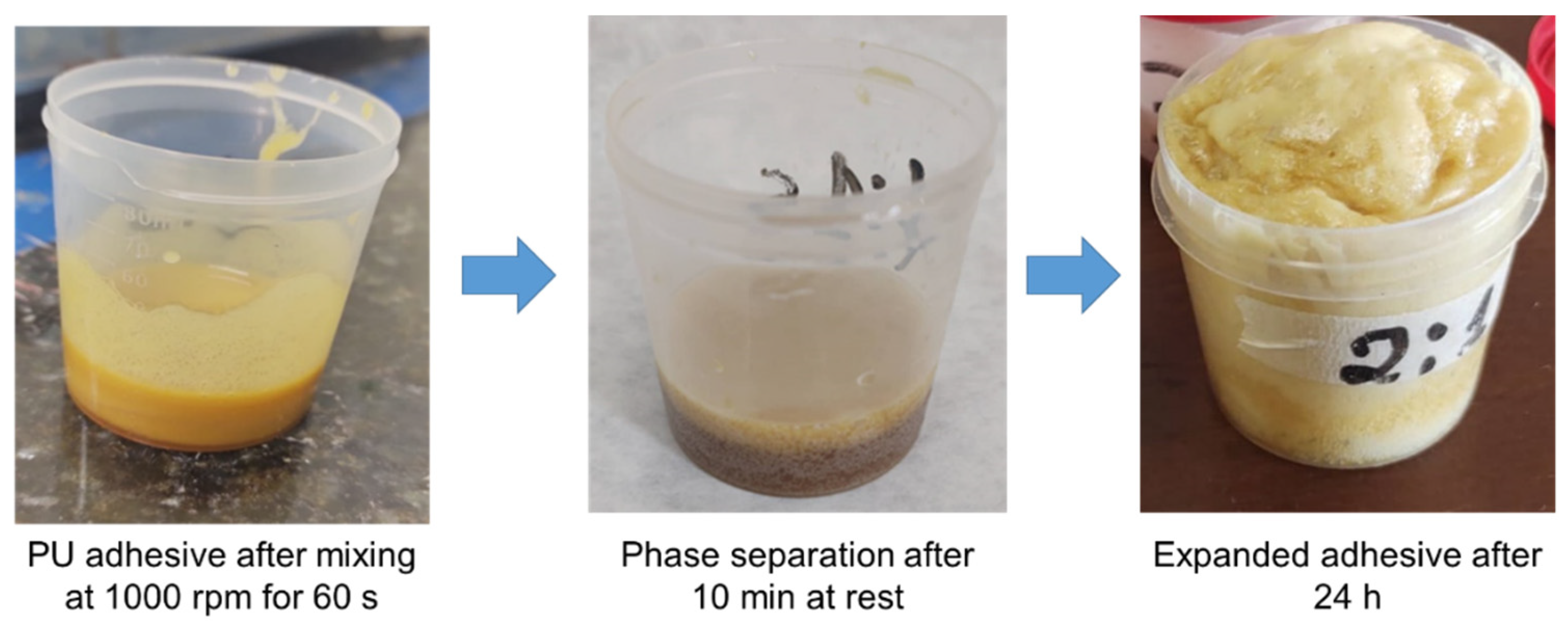
2.2. Preparation and Characterization of the Adhesives
2.3. Bond Properties at the Wood-to-Wood Interface for Adhesive Evaluation
3. Results and Discussion
3.1. Physical and Chemical Characteristics of Polyols
3.2. Adhesive Polymerization and Viscosity
3.3. Chemical and Physical Properties of Adhesives
3.4. Mechanical Strength of the Wood–Wood Adhesions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tout, R. Review of Adhesives for Furniture. Int. J. Adhes. Adhes. 2000, 20, 269–272. [Google Scholar] [CrossRef]
- de Avila Delucis, R.; Magalhães, W.L.E.; Petzhold, C.L.; Amico, S.C. Forest-Based Resources as Fillers in Biobased Polyurethane Foams. J. Appl. Polym. Sci. 2018, 135, 45684. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Cruz, J.A.; Amico, S.C.; Bianchi, O. Effect of the Aramid Pulp on the Physicochemical, Viscoelastic Properties and Rheokinetics of Polyurethanes. J. Polym. Res. 2023, 30, 12. [Google Scholar] [CrossRef]
- Kerche, E.F.; Bock, D.N.; de Avila Delucis, R.; Magalhães, W.L.E.; Amico, S.C. Micro Fibrillated Cellulose Reinforced Bio-Based Rigid High-Density Polyurethane Foams. Cellulose 2021, 28, 4313–4326. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of Bio-Based Polyurethanes: Perspective on Applications and Bio-Degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Tr, N.B. Structural Characterization of Castor Oil Nature of Intact Glycerides and Distribution of Hydroxyl Groups. Polymers 1997, 38, 2467–2473. [Google Scholar]
- Cui, S.; Liu, Z.; Li, Y. Bio-Polyols Synthesized from Crude Glycerol and Applications on Polyurethane Wood Adhesives. Ind. Crops Prod. 2017, 108, 798–805. [Google Scholar] [CrossRef]
- Delucis, R.d.A.; Magalhães, W.L.E.; Petzhold, C.L.; Amico, S.C. Thermal and Combustion Features of Rigid Polyurethane Biofoams Filled with Four Forest-Based Wastes. Polym. Compos. 2018, 39, E1770–E1777. [Google Scholar] [CrossRef]
- Acosta, A.; Aramburu, A.B.; Beltrame, R.; Gatto, D.A.; Amico, S.; Labidi, J.; Delucis, R.D.A. Wood Flour Modified by Poly (Furfuryl Alcohol) as a Filler in Rigid Polyurethane Foams: Effect on Water Uptake. Polymers 2022, 14, 5510. [Google Scholar] [CrossRef]
- Malik, M.; Kaur, R. Influence of Aliphatic and Aromatic Isocyanates on the Properties of Poly(Ether Ester) Polyol Based PU Adhesive System. Polym. Eng. Sci. 2018, 58, 112–117. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. PU Foam Derived from Renewable Sources: Perspective on Properties Enhancement: An Overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The Influence of Crude Glycerol and Castor Oil-Based Polyol on the Structure and Performance of Rigid Polyurethane-Polyisocyanurate Foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y. Synthesis and Characterization of Polyols and Polyurethane Foams from PET Waste and Crude Glycerol. J. Polym. Environ. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Polyols and Polyurethane Foams from Base-Catalyzed Liquefaction of Lignocellulosic Biomass by Crude Glycerol: Effects of Crude Glycerol Impurities. Ind. Crops Prod. 2014, 57, 188–194. [Google Scholar] [CrossRef]
- Uprety, B.K.; Reddy, J.V.; Dalli, S.S.; Rakshit, S.K. Utilization of Microbial Oil Obtained from Crude Glycerol for the Production of Polyol and Its Subsequent Conversion to Polyurethane Foams. Bioresour. Technol. 2017, 235, 309–315. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, Detachable, and Self-Healing Dynamic Crosslinked Hot Melt Polyurethane Adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Cure and Performance of Castor Oil Polyurethane Adhesive. Int. J. Adhes. Adhes. 2019, 95, 102413. [Google Scholar] [CrossRef]
- Desai, S.D.; Patel, J.V.; Sinha, V.K. Polyurethane Adhesive System from Biomaterial-Based Polyol for Bonding Wood. Int. J. Adhes. Adhes. 2003, 23, 393–399. [Google Scholar] [CrossRef]
- Khoon Poh, A.; Choy Sin, L.; Sit Foon, C.; Cheng Hock, C. Polyurethane Wood Adhesive from Palm Oil-Based Polyester Polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Cui, S.; Luo, X.; Li, Y. Synthesis and Properties of Polyurethane Wood Adhesives Derived from Crude Glycerol-Based Polyols. Int. J. Adhes. Adhes. 2017, 79, 67–72. [Google Scholar] [CrossRef]
- EPA. Proposed Designation of Di-Ethylhexyl Phthalate (DEHP) (1,2-Benzene- Dicarboxylic Acid, 1,2-Bis (2-Ethylhexyl) Ester) (CASRN 117-81-7) as a High-Priority Substance for Risk Evaluation; EPA: Washington, DC, USA, 2019; pp. 1–41.
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Dodangeh, F.; Seyed Dorraji, M.S.; Rasoulifard, M.H.; Ashjari, H.R. Synthesis and Characterization of Alkoxy Silane Modified Polyurethane Wood Adhesive Based on Epoxidized Soybean Oil Polyester Polyol. Compos. Part B Eng. 2020, 187, 107857. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Marinović-Cincović, M.; Špírková, M. The Effects of the Structure and Molecular Weight of the Macrodiol on the Properties Polyurethane Anionic Adhesives. Int. J. Adhes. Adhes. 2013, 41, 132–139. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Impact of the Processing Method on the Properties of Castor Oil/Cellulose Acetate Polyurethane Adhesives for Bonding Wood. Int. J. Adhes. Adhes. 2022, 116, 103153. [Google Scholar] [CrossRef]
- Gurgel, D.; Bresolin, D.; Sayer, C.; Cardozo Filho, L.; Hermes de Araújo, P.H. Flexible Polyurethane Foams Produced from Industrial Residues and Castor Oil. Ind. Crops Prod. 2021, 164, 113377. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and Characterization of Polyurethane Foams from a Simple Mixture of Castor Oil, Crude Glycerol and Untreated Lignin as Bio-Based Polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Carvalho, V.E.; Pasa, V.M.D. Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols. Molecules 2017, 22, 1091. [Google Scholar] [CrossRef]
- ASTM-D1544; Standard Test Method for Color of Transparent Liquids (Gardner Color Scale). ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D7487; Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test. 2021. Available online: https://compass.astm.org/document/?contentCode=ASTM%7CD7487-18%7Cen-US&proxycl=https%3A%2F%2Fsecure.astm.org&fromLogin=true (accessed on 26 September 2023).
- Acosta, A.P.; Labidi, J.; Schulz, H.R.; Gallio, E.; Barbosa, K.T.; Beltrame, R.; de Avila Delucis, R.; Gatto, D.A. Thermochemical and Mechanical Properties of Pine Wood Treated by in Situ Polymerization of Methyl Methacrylate (MMA). Forests 2020, 11, 768. [Google Scholar] [CrossRef]
- Schulz, H.R.; Gallio, E.; Acosta, A.P.; Barbosa, K.T.; Gatto, D.A. Efeito Da Furfurilação Em Propriedades Físicas e Mecânicas Da Madeira de Pinus Elliottii. Matéria 2019, 24. [Google Scholar] [CrossRef]
- ASTM D905-08; Standard Test Method for Strength Properties of Adhesive Bonds in Shear by Compression Loading. 2021. Available online: https://compass.astm.org/document/?contentCode=ASTM%7CD0905-08R21%7Cen-US (accessed on 26 September 2023).
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of Polyurethane Wastes Using Different Carboxylic Acids via Acidolysis to Produce Wood Adhesives. J. Polym. Sci. 2021, 59, 697–705. [Google Scholar] [CrossRef]
- Ang, K.P.; Lee, C.S.; Cheng, S.F.; Chuah, C.H. Synthesis of Palm Oil-Based Polyester Polyol for Polyurethane Adhesive Production. J. Appl. Polym. Sci. 2014, 131, 39967. [Google Scholar] [CrossRef]
- Mishra, D.; Kumar Sinha, V. Eco-Economical Polyurethane Wood Adhesives from Cellulosic Waste: Synthesis, Characterization and Adhesion Study. Int. J. Adhes. Adhes. 2010, 30, 47–54. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Producing Lignin-Based Polyols through Microwave-Assisted Liquefaction for Rigid Polyurethane Foam Production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Emanuel, A.L.; Sinha, V.K. Biomaterial Based Polyurethane Adhesive for Bonding Rubber and Wood Joints. J. Polym. Res. 2003, 10, 275–281. [Google Scholar] [CrossRef]
- Vale, M.; Mateus, M.M.; Galhano dos Santos, R.; Nieto de Castro, C.; de Schrijver, A.; Bordado, J.C.; Marques, A.C. Replacement of Petroleum-Derived Diols by Sustainable Biopolyols in One Component Polyurethane Foams. J. Clean. Prod. 2019, 212, 1036–1043. [Google Scholar] [CrossRef]
- Yan, Y.; Pang, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and Characterization of Water-Blown Polyurethane Foams from Liquefied Cornstalk Polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Huang, G.; Wang, P. Effects of Preparation Conditions on Properties of Rigid Polyurethane Foam Composites Based on Liquefied Bagasse and Jute Fibre. Polym. Test. 2017, 60, 266–273. [Google Scholar] [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; Brien, P.O. Castor Oil: A Suitable Green Source of Capping Agent for Nanoparticle Syntheses and Facile Surface Functionalization. R. Soc. Open Sci. 2018, 5, 180824. [Google Scholar] [CrossRef]
- Win, S.S.; Trabold, T.A. Sustainable Waste-to-Energy Technologies: Transesterification. In Sustainable Food Waste-to-Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–109. [Google Scholar]
- Malik, M.; Kaur, R. Mechanical and Thermal Properties of Castor Oil–Based Polyurethane Adhesive: Effect of TiO2 Filler. Adv. Polym. Technol. 2016, 37, 24–30. [Google Scholar] [CrossRef]
- Daneshvar, S.; Behrooz, R.; Najafi, S.K.; Sadeghi, G.M.M. Preparation of Polyurethane Adhesive from Wood Sawdust Polyol: Application of Response Surface Methodology for Optimization of Catalyst and Glycerol. Biointerface Res. Appl. Chem. 2022, 12, 1870–1883. [Google Scholar] [CrossRef]
- Silva, B.B.R.; Santana, R.M.C.; Forte, M.M.C. A Solventless Castor Oil-Based PU Adhesive for Wood and Foam Substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and Characterization of Jatropha (Jatropha curcas L.) Oil-Based Polyurethane Wood Adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Lopes, P.J.G.; Calegari, L.; de Medeiros Silva, W.A.; Gatto, D.A.; de Medeiros Neto, P.N.; de Melo, R.R.; Bakke, I.A.; de Avila Delucis, R.; Missio, A.L. Tannin-Based Extracts of Mimosa Tenuiflora Bark: Features and Prospecting as Wood Adhesives. Appl. Adhes. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Valero, M.F.; Gonzalez, A. Polyurethane Adhesive System from Castor Oil Modified by a Transesterification Reaction. J. Elastomers Plast. 2012, 44, 433–442. [Google Scholar] [CrossRef]
- Moghadam, P.N.; Yarmohamadi, M.; Hasanzadeh, R.; Nuri, S. Preparation of Polyurethane Wood Adhesives by Polyols Formulated with Polyester Polyols Based on Castor Oil. Int. J. Adhes. Adhes. 2016, 68, 273–282. [Google Scholar] [CrossRef]
- Juhaida, M.; Paridah, M.; Hilmi, M.M.; Sarani, Z.; Jalaluddin, H.; Zaki, A.M. Liquefaction of Kenaf (Hibiscus cannabinus L.) Core for Wood Laminating Adhesive. Bioresour. Technol. 2010, 101, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hosseinpourpia, R.; Biziks, V.; Ahmed, S.A.; Militz, H.; Adamopoulos, S. Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers 2021, 13, 3267. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, F.; Konnerth, J.; Gindl-Altmutter, W. Mechanical Properties of Adhesives for Bonding Wood—A Review. Int. J. Adhes. Adhes. 2013, 45, 32–41. [Google Scholar] [CrossRef]
- Jutrzenka Trzebiatowska, P.; Deuter, I.; Datta, J. Cast Polyurethanes Obtained from Reactive Recovered Polyol Intermediates via Crude Glycerine Decomposition Process. React. Funct. Polym. 2017, 119, 20–25. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and Characterization of Lignin-Polyurethane Based Wood Adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- ASTM D5751-99; Standard Specification for Adhesives Used for Laminate Joints in Nonstructural Lumber Products. 2019. Available online: https://compass.astm.org/document/?contentCode=ASTM%7CD5751-99R19%7Cen-US&proxycl=https%3A%2F%2Fsecure.astm.org&fromLogin=true (accessed on 26 September 2023).
- Matos, G.d.S.; Molina, J.C. Resistência Da Madeira Ao Cisalhamento Paralelo Às Fibras Segundo as Normas ABNT NBR 7190:1997 e ISO 13910:2005. Matéria 2016, 21, 1069–1079. [Google Scholar] [CrossRef]
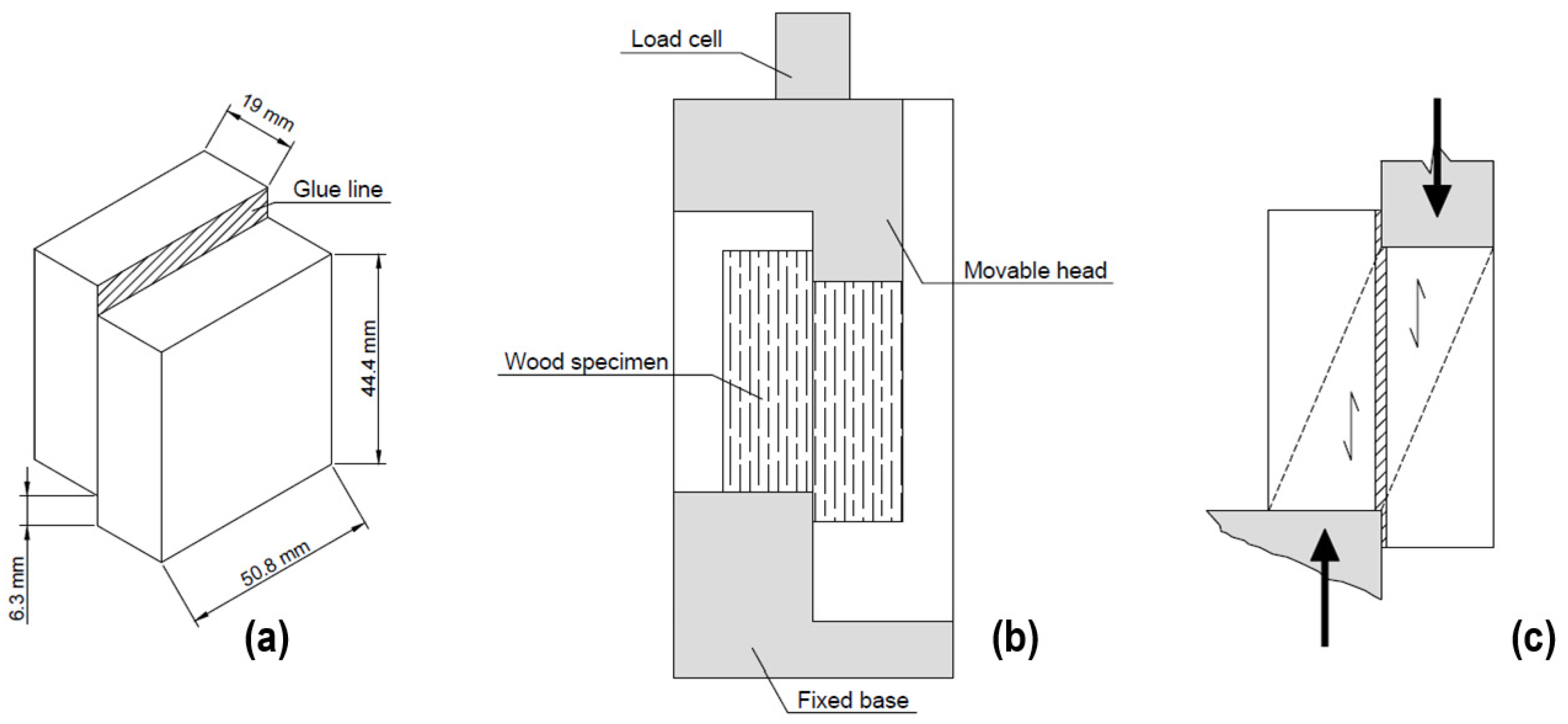







| Adhesive | Exposure Environment | Exposure Time | N° of Samples |
|---|---|---|---|
| 2:1 | Ambient water (30 °C) | 24 h | 10 |
| 2:1 | Hot water (100 °C) | 24 h | 10 |
| 2:1 | Acidic solution H2SO4 (pH 3 at 70 °C) | 1 h | 10 |
| 2:1 | Alkaline solution NaOH (pH 13 at 70 °C) | 1 h | 10 |
| 3:1 | Ambient water (30 °C) | 24 h | 10 |
| 3:1 | Hot water (100 °C) | 24 h | 10 |
| 3:1 | Acidic solution H2SO4 (pH 3 at 70 °C) | 1 h | 10 |
| 3:1 | Alkaline solution NaOH (pH 13 at 70 °C) | 1 h | 10 |
| 4:1 | Ambient water (30 °C) | 24 h | 10 |
| 4:1 | Hot water (100 °C) | 24 h | 10 |
| 4:1 | Acidic solution H2SO4 (pH 3 at 70 °C) | 1 h | 10 |
| 4:1 | Alkaline solution NaOH (pH 13 at 70 °C) | 1 h | 10 |
| CO:CG Ratio | 2:1 | 3:1 | 4:1 |
|---|---|---|---|
| Hydroxyl content (mg KOH/g) | 582 | 480 | 416 |
| Cinematic viscosity (cP) | 20,958 | 20,210 | 19,817 |
| Apparent density (g/cm3) | 1.042 | 1.016 | 1.019 |
| Moisture content (%) | 0.040 | 0.030 | 0.024 |
| Color | 16 | 14 | 5 |
| CO:CG Ratio | 2:1 | 3:1 | 4:1 |
|---|---|---|---|
| Gel time (min) | 8 | 10 | 10 |
| Surface drying time (min) | 90 | 110 | 110 |
| Tack-free time (min) | 520 | 540 | 540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, T.L.C.; Ribeiro, F.V.; Aramburu, A.B.; Barbosa, K.T.; Acosta, A.P.; Missio, A.L.; Subhani, M.; Delucis, R.d.A. Polyurethane Adhesives for Wood Based on a Simple Mixture of Castor Oil and Crude Glycerin. Materials 2023, 16, 7251. https://doi.org/10.3390/ma16237251
Peres TLC, Ribeiro FV, Aramburu AB, Barbosa KT, Acosta AP, Missio AL, Subhani M, Delucis RdA. Polyurethane Adhesives for Wood Based on a Simple Mixture of Castor Oil and Crude Glycerin. Materials. 2023; 16(23):7251. https://doi.org/10.3390/ma16237251
Chicago/Turabian StylePeres, Tábata Larissa Corrêa, Felipe Vahl Ribeiro, Arthur Behenck Aramburu, Kelvin Techera Barbosa, Andrey Pereira Acosta, André Luiz Missio, Mahbube Subhani, and Rafael de Avila Delucis. 2023. "Polyurethane Adhesives for Wood Based on a Simple Mixture of Castor Oil and Crude Glycerin" Materials 16, no. 23: 7251. https://doi.org/10.3390/ma16237251
APA StylePeres, T. L. C., Ribeiro, F. V., Aramburu, A. B., Barbosa, K. T., Acosta, A. P., Missio, A. L., Subhani, M., & Delucis, R. d. A. (2023). Polyurethane Adhesives for Wood Based on a Simple Mixture of Castor Oil and Crude Glycerin. Materials, 16(23), 7251. https://doi.org/10.3390/ma16237251











