Adverse Effects of Non-Metallic Nanoparticles in the Central Nervous System
Abstract
:1. Introduction
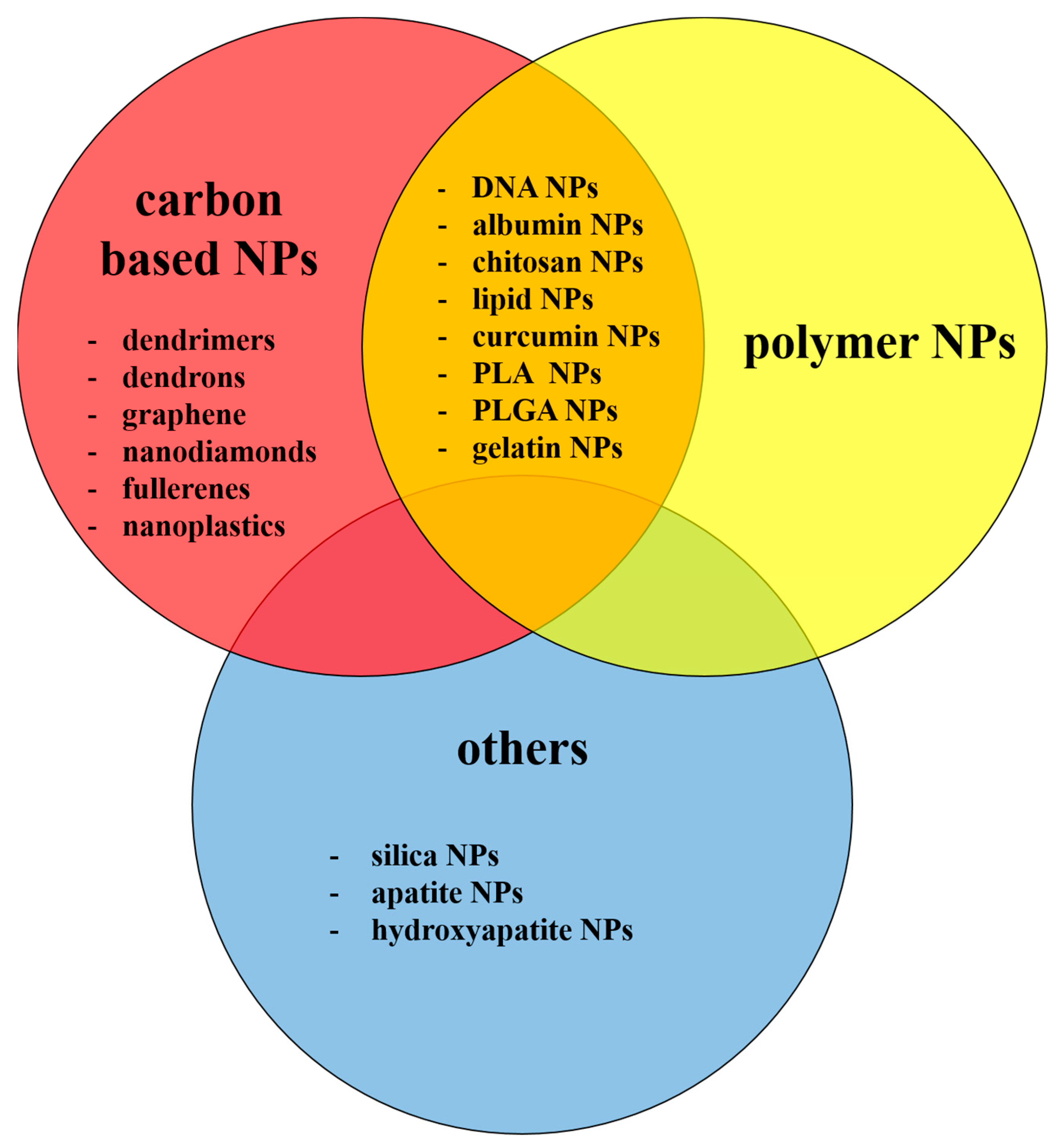
2. Non-Metallic NPs
3. Uptake of Non-Metallic NPs
3.1. Uptake of Non-Metallic NPs by Normal CNS-Derived Cells In Vitro
3.2. Uptake of Non-Metallic NPs by CNS-Derived Cancer Cells In Vitro
3.3. Uptake of Non-Metallic NPs by CNS In Vivo
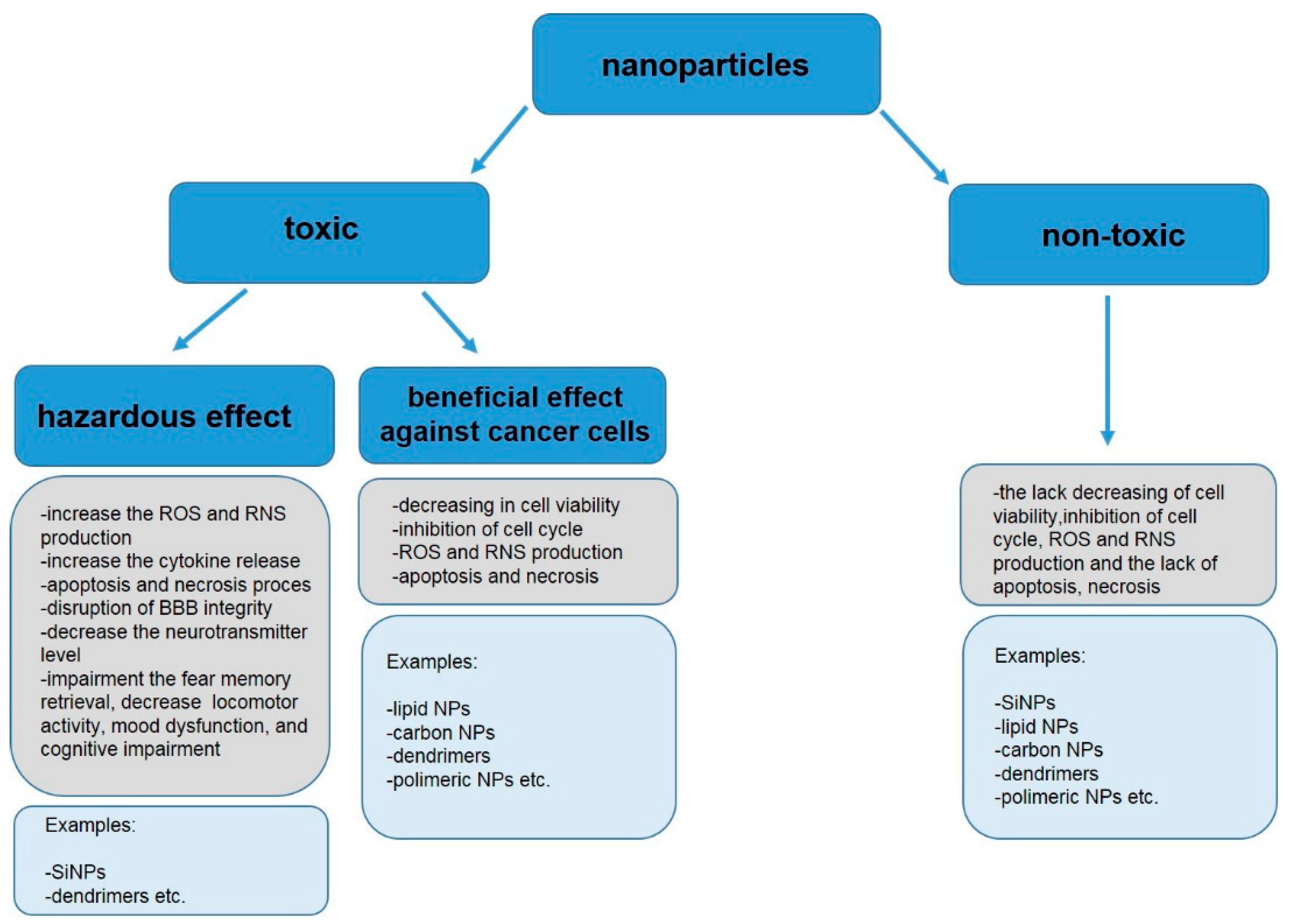
4. Toxicity of Non-Metallic NPs in Mammals
4.1. In Vitro and Ex Vivo Toxicity
4.2. Toxicity In Vivo
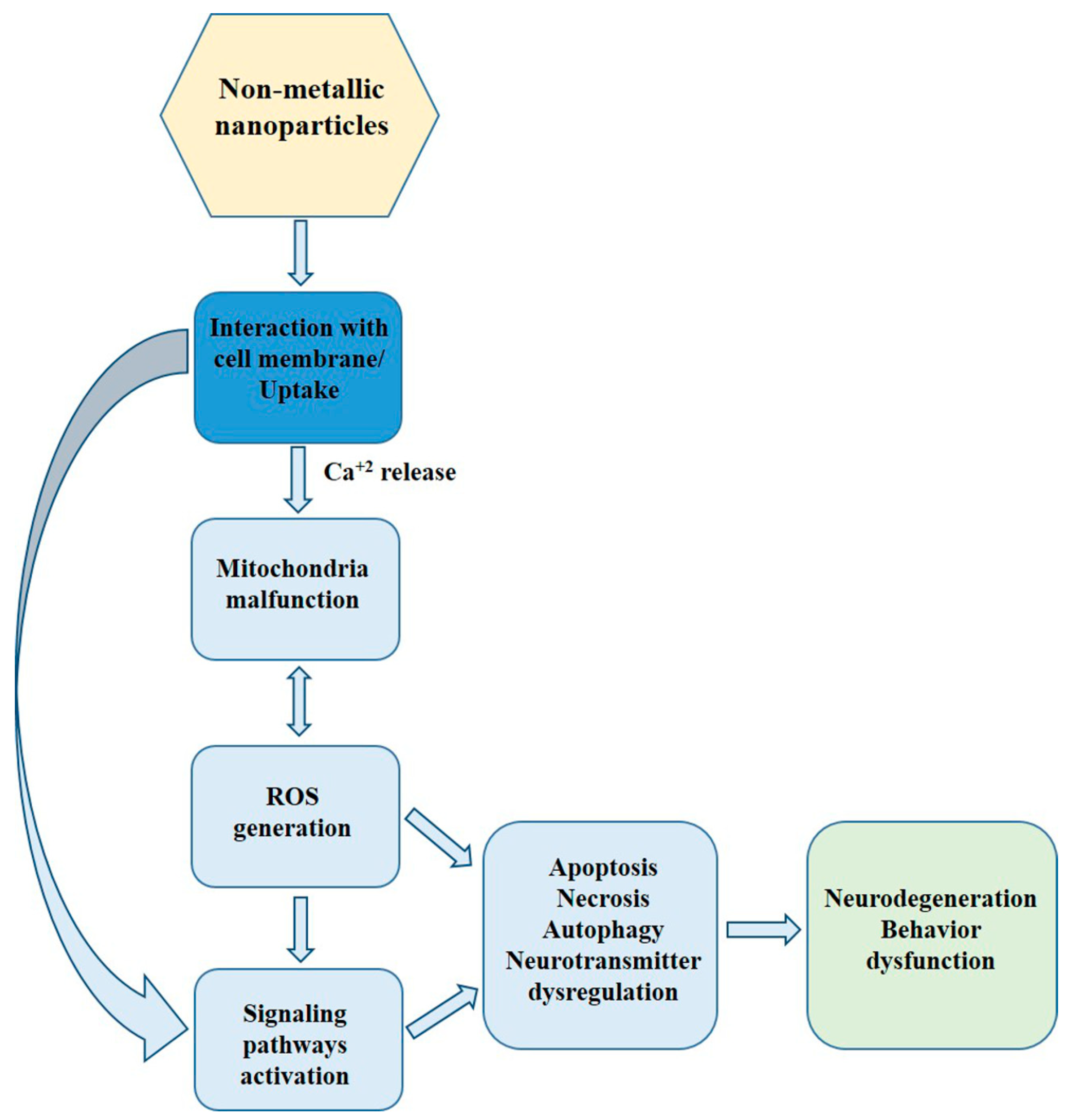
5. Mechanism of nmNPs Toxicity. Other Adverse Effects of Non-Metallic NPs in Mammals
6. Adverse Effects of Non-Metallic NPs in Non-Mammalian Organisms
7. Conclusions
8. Limitation of the Study and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.W.; Allaker, R.P.; Reip, P.; Oxford, J.; Ahmad, Z.; Ren, G. A review of nanoparticle functionality and toxicity on the central nervous system. J. R. Soc. Interface 2010, 7, 411–422. [Google Scholar] [CrossRef]
- Li, W.; Cheng, J.; He, F.; Zhang, P.; Zhang, N.; Wang, J.; Song, Q.; Hou, Y.; Gan, Z. Cell membrane-based nanomaterials for theranostics of central nervous system diseases. J. Nanobiotechnol. 2023, 21, 276. [Google Scholar] [CrossRef]
- Ekhator, C.; Qureshi, M.Q.; Zuberi, A.W.; Hussain, M.; Sangroula, N.; Yerra, S.; Devi, M.; Arsal Naseem, M.; Bellegarde, S.B.; Pendyala, P.R. Advances and Opportunities in Nanoparticle Drug Delivery for Central Nervous System Disorders: A Review of Current Advances. Cureus 2023, 15, e44302. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; He, J.; Gong, M.; Sun, J.; Yang, X. Central nervous system injury meets nanoceria: Opportunities and challenges. Regen. Biomater. 2022, 9, rbac037. [Google Scholar] [CrossRef]
- Annu Sartaj, A.; Qamar, Z.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front. Bioeng. Biotechnol. 2022, 10, 788128. [Google Scholar]
- Montegiove, N.; Calzoni, E.; Emiliani, C.; Cesaretti, A. Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases. J. Funct. Biomater. 2022, 13, 125. [Google Scholar] [CrossRef]
- Athira, S.S.; Prajitha, N.; Mohanan, P.V. Interaction of nanoparticles with central nervous system and its consequences. Am. J Res. Med. Sci. 2018, 4, 12–32. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; La Maestra, S.; Ceccarelli, M.; D’Aleo, F.; Nunnari, G.; Pellicanò, G.F.; Di Pietro, A. Carbon nanotubes and central nervous system: Environmental risks, toxicological aspects and future perspectives. Environ. Toxicol. Pharmacol. 2018, 65, 23–30. [Google Scholar] [CrossRef]
- Zia, S.; Islam Aqib, A.; Muneer, A.; Fatima, M.; Atta, K.; Kausar, T.; Zaheer, C.-N.F.; Ahmad, I.; Saeed, M.; Shafique, A. Insights into nanoparticles-induced neurotoxicity and cope up strategies. Front. Neurosci. 2023, 17, 1127460. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Zhang, W.; Sigdel, G.; Mintz, K.J.; Seven, E.S.; Zhou, Y.; Wang, C.; Leblanc, R.M. Carbon Dots: A Future Blood–Brain Barrier Penetrating Nanomedicine and Drug Nanocarrier. Int. J. Nanomed. 2021, 16, 5003–5016. [Google Scholar] [CrossRef]
- Zielinski, A.; Majkowska-Marzec, B. Whether Carbon Nanotubes Are Capable, Promising, and Safe for Their Application in Nervous System Regeneration. Some Critical Remarks and Research Strategies. Coatings 2022, 12, 1643. [Google Scholar] [CrossRef]
- Gauro, R.; Nandave, M.; Jain, V.K.; Jain, K. Advances in dendrimer-mediated targeted drug delivery to the brain. J. Nanoparticle Res. 2021, 23, 76. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Bussy, C.; Al-Jamal, K.T.; Boczkowski, J.; Lanone, S.; Prato, M.; Bianco, A.; Kostarelos, K. Microglia Determine Brain Region-Specific Neurotoxic Responses to Chemically Functionalized Carbon Nanotubes. ACS Nano. 2015, 9, 7815–7830. [Google Scholar] [CrossRef]
- Bussy, C.; Hadad, C.; Prato, M.; Bianco, A.; Kostarelos, K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale 2016, 8, 590–601. [Google Scholar] [CrossRef]
- Kateb, B.; Van Handel, M.; Zhang, L.; Bronikowski, M.J.; Manohara, H.; Badiea, B. Internalization of MWCNTs by microglia: Possible application in immunotherapy of brain tumors. NeuroImage 2007, 37, 9–17. [Google Scholar] [CrossRef]
- Goode, A.E.; Gonzalez Carter, D.A.; Motskin, M.; Pienaar, I.S.; Chen, S.; Hu, S.; Ruenraroengsak, P.; Ryan, M.P.; Shaffer, M.S.P.; Dexter, D.T.; et al. High resolution and dynamic imaging of biopersistence and bioreactivity of extra and intracellular MWNTs exposed to microglial cells. Biomaterials 2015, 70, 57–70. [Google Scholar] [CrossRef]
- Choi, J.; Zheng, Q.; Katz, H.E.; Guilarte, T.R. Silica-Based Nanoparticle Uptake and Cellular Response by Primary Microglia. Environ. Health Perspect. 2010, 118, 589–595. [Google Scholar] [CrossRef]
- Ducray, A.D.; Stojiljkovic, A.; Möller, A.; Stoffel, M.H.; Widmer, H.R.; Frenz, M.; Mevissen, M. Uptake of silica nanoparticles in the brain and effects on neuronal differentiation using different in vitro models. Nanomedicine 2017, 13, 1195–1204. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sato, S.S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In Vivo Distribution and Toxicity of PAMAM Dendrimers in the Central Nervous System Depend on Their Surface Chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine 2010, 5, 1317–1329. [Google Scholar] [CrossRef]
- Hollinger, K.; Sharma, A.; Tallon, C.; Lovell, L.; Thomas, A.G.; Zhu, X.; Kambhampati, S.P.; Liaw, K.; Sharma, R.; Rojas, C.; et al. Microglia-targeted dendrimer-2PMPA therapy robustly inhibits GCPII and improves cognition in a mouse model of multiple sclerosis. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Salgado, A.J.; Oliveira, J.M.; Pirraco, R.P.; Pereira, V.H.; Fraga, J.S.; Marques, A.P.; Neves, N.M.; Mano, J.F.; Reis, R.L.; Sousa, N. Carboxymethylchitosan/Poly(amidoamine) Dendrimer Nanoparticles in Central Nervous Systems-Regenerative Medicine: Effects on Neuron/Glial Cell Viability and Internalization Efficiency. Macromol. Biosci. 2010, 10, 1130–1140. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflamm. 2016, 13, 65. [Google Scholar] [CrossRef]
- Zhang, F.; Magruder, J.T.; Lin, Y.A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.A.; Blue, M.E.; Kannan, S.; Johnston, M.V.; et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 10, 173–182. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Kafa, H.; Wang, J.T.W.; Rubio, N.; Venner, K.; Anderson, G.; Pach, E.; Ballesteros, B.; Preston, J.E.; Abbott, N.J.; Al-Jamal, K.T. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 2015, 53, 437–452. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Sun, B.; Zhu, Z.; Zheng, D.; Li, X. Enhanced Neuroprotective Effects of Resveratrol Delivered by Nanoparticles on Hydrogen Peroxide-Induced Oxidative Stress in Rat Cortical Cell Culture. Mol. Pharm. 2013, 10, 2045–2053. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt A-beta aggregates. Int. J. Pharm. 2017, 30, 413–424. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1044–1055. [Google Scholar] [CrossRef]
- Kou, C.H.; Han, J.; Han, X.L.; Zhuang, H.J.; Zhao, Z.M. Preparation and characterization of the Adriamycin loaded amphiphilic chitosan nanoparticles and their application in the treatment of liver cancer. Oncol. Lett. 2017, 17, 7833–7841. [Google Scholar] [CrossRef]
- Huang, Y.A.; Kao, C.W.; Liu, K.K.; Huang, H.S.; Chiang, M.H.; Soo, C.R.; Chang, H.C.; Chiu, T.W.; Chao, J.I.; Hwang, E. The effect of fluorescent nanodiamonds on neuronal survival and morphogenesis. Sci. Rep. 2014, 4, 6919. [Google Scholar] [CrossRef]
- Zeng, Y.; Kurokawa, Y.; Zeng, Q.; Win-Shwe, T.T.; Nansai, H.; Zhang, Z.; Sone, H. Effects of Polyamidoamine Dendrimers on a 3-D Neurosphere System Using Human Neural Progenitor Cells. Toxicol. Sci. 2016, 152, 128–144. [Google Scholar] [CrossRef]
- Nyitrai, G.; Héja, L.; Jablonkai, I.; Pál, I.; Visy, J.; Kardos, J. Polyamidoamine dendrimer impairs mitochondrial oxidation in brain tissue. J. Nanobiotechnol. 2013, 11, 9. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In Vitro and in Vivo Studies on the Transport of PEGylated Silica Nanoparticles across the Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Ye, D.; Raghnaill, M.N.; Bramini, M.; Mahon, E.; Aberg, C.; Salvati, A.; Dawson, K.A. Nanoparticle accumulation and transcytosis in brain endothelial cell layers. Nanoscale 2013, 5, 11153–11165. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for Oligonucleotide Delivery to the Brain. Bioconjug. Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef]
- Lao, F.; Chen, L.; Li, W.; Ge, C.; Qu, Y.; Sun, Q.; Zhao, Y.; Han, D.; Chen, C. Fullerene Nanoparticles Selectively Enter Oxidation-Damaged Cerebral Microvessel Endothelial Cells and Inhibit JNK Related Apoptosis. ACS Nano 2009, 3, 3358–3368. [Google Scholar] [CrossRef]
- Van Arendonk, K.J.; Chung, D.H. Neuroblastoma: Tumor Biology and Its Implications for Staging and Treatment. Children 2019, 6, 12. [Google Scholar] [CrossRef]
- Wang, J.; Bettegowda, C. Genomic discoveries in adult astrocytoma. Curr. Opin. Genet. Dev. 2015, 30, 17–24. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 20, 280–293. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Paka, G.D.; Doggui, S.; Zaghmi, A.; Safar, R.; Dao, L.; Reisch, A.; Klymchenko, A.; Roullin, V.G.; Joubert, O.; Ramassamy, C. Neuronal Uptake and Neuroprotective Properties of Curcumin- Loaded Nanoparticles on SK-N-SH Cell Line: Role of Poly(lactide-coglycolide) Polymeric Matrix Composition. Mol. Pharm. 2016, 13, 391–403. [Google Scholar] [CrossRef]
- Dong, L.; Witkowski, C.M.; Craig, M.M.; Greenwade, M.M.; Joseph, K.L. Cytotoxicity Effects of Different Surfactant Molecules Conjugated to Carbon Nanotubes on Human Astrocytoma Cells. Nanoscale Res. Lett. 2009, 4, 1517–1523. [Google Scholar] [CrossRef]
- Listik, E. Development and optimization of G-1 polymeric nanoparticulated and liposomal systems for central nervous system applications. Neurol. Disord. Therap. 2018, 2, 1–11. [Google Scholar]
- Feng, C.; Wu, Y.; Gao, L.; Guo, X.; Wang, Z.; Xing, B. Publication Landscape Analysis on Gliomas: How Much Has Been Done in the Past 25 Years? Front. Oncol. 2020, 9, 1463. [Google Scholar] [CrossRef]
- Hong, S.; Rattan, R.; Majoros, I.J.; Mullen, D.G.; Peters, J.L.; Shi, X.; Bielinska, A.U.; Blanco, L.; Orr, B.O.; Baker, J.R., Jr.; et al. The Role of Ganglioside GM1 in Cellular Internalization Mechanisms of Poly(amidoamine) Dendrimers. Bioconjug. Chem. 2009, 19, 1503–1513. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, biodistribution, in vitro cytotoxicity andbiocompatibility of Vitamin E TPGS coated trans resveratrol liposomes. Colloids Surf. B Biointerfaces 2016, 145, 479–491. [Google Scholar] [CrossRef]
- Shao, J.; Li, X.; Lu, X.; Jiang, C.; Hu, Y.; Li, Q.; You, Y.; Fu, Z. Enhanced growth inhibition effect of Resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf. B Biointerfaces 2009, 72, 40–47. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosanhydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef]
- Gan, C.W.; Feng, S.S. Transferrin-conjugated nanoparticles of Poly(lactide)-D-a-Tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials 2010, 31, 7748–7757. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Kosuru, R.; Singh, S.K.; Prasad, C.B.; Narayan, G.; Muthua, M.S.; Singh, S. Resveratrol loaded PLGA:D-a-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016, 6, 74254–74268. [Google Scholar] [CrossRef]
- Liang, J.; Gao, C.; Zhu, Y.; Ling, C.; Wang, Q.; Huang, Y.; Qin, J.; Wang, J.; Lu, W.; Wang, J. Natural Brain Penetration Enhancer-Modified Albumin Nanoparticles for Glioma Targeting Delivery. ACS Appl. Mater. Interfaces 2018, 10, 30201–30213. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Chen, S.; Auriat, A.; Koudrina, A.; DeRosa, M.; Cao, X.; Tsai, E.C. Nano-engineering Nanoparticles for Clinical Use in the Central Nervous System: Clinically Applicable Nanoparticles and Their Potential Uses in the Diagnosis and Treatment of CNS Aliments. In Nanoengineering Materials for Biomedical Uses; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–145. [Google Scholar]
- Yang, S.T.; Guo, W.; Lin, Y.; Deng, X.Y.; Wang, H.F.; Sun, H.F.; Liu, Y.J.; Wang, X.; Wang, W.; Chen, M.; et al. Biodistribution of Pristine Single-Walled Carbon Nanotubes In Vivo. J. Phys. Chem. 2007, 111, 17761–17764. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, Y.; Tan, Y.Z.; Hu, K.L.; Jiang, X.G.; Fu, S.K. Cationic albumin conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J. Control. Release 2005, 107, 428–448. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Sun, J.; Xue, J. Neurotoxicity of Silica Nanoparticles: Brain Localization and Dopaminergic Neurons Damage Pathways. ACS Nano 2011, 5, 4476–4489. [Google Scholar] [CrossRef]
- Koffie, R.M.; Farrar, C.T.; Saidi, L.J.; William, C.M.; Hyman, B.T.; Spires-Jones, T.L. Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18837–18842. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurons. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, T.; Yang, C. Development of PLGA-lipid nanoparticles with covalently conjugated indocyanine green as a versatile nanoplatform for tumor-targeted imaging and drug delivery. Int. J. Nanomed. 2016, 11, 5807–5821. [Google Scholar] [CrossRef]
- Sun, W.; Xie, C.; Wang, H.; Hu, Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004, 25, 3065–3071. [Google Scholar] [CrossRef]
- Bardi, G.; Nunes, A.; Gherardini, L.; Bates, K.; Al-Jamal, K.T.; Gaillard, C.; Kostarelos, K. Functionalized Carbon Nanotubes in the Brain: Cellular Internalization and Neuroinflammatory Responses. PLoS ONE 2013, 8, e80964. [Google Scholar] [CrossRef]
- Sarlo, K.; Blackburn, K.L.; Clark, E.D.; Grothaus, J.; Chaney, J.; Neu, S.; Flood, J.; Abbott, D.; Bohne, C.; Casey, K.; et al. Tissue distribution of 20nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology 2009, 263, 117–126. [Google Scholar] [CrossRef]
- Kafa, H.; Wang, J.T.W.; Rubio, N.; Klippstein, R.; Costa, P.M.; Hassan, H.A.F.M.; Sosabowski, J.K.; Bansal, S.S.; Preston, J.E.; Abbott, N.J.; et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood–brain barrier in vitro and in vivo. J. Control. Release 2016, 225, 217–229. [Google Scholar] [CrossRef]
- Gupta, Y.; Jain, A.; Jain, S.K. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J. Pharm. Pharmacol. 2007, 59, 935–940. [Google Scholar] [CrossRef]
- Helm, F.; Fricker, G. Liposomal Conjugates for Drug Delivery to the Central Nervous System. Pharmaceutics 2015, 7, 27–42. [Google Scholar] [CrossRef]
- Pang, Z.; Gao, H.; Chen, J.; Shen, S.; Zhang, B.; Ren, J.; Guo, L.; Qian, Y.; Jiang, X.; Mei, H. Intracellular delivery mechanism and brain delivery kinetics of biodegradable cationic bovine serum albumin-conjugated polymersomes. Int. J. Nanomed. 2012, 7, 3421–3432. [Google Scholar]
- Lozić, I.; Hartz, R.V.; Bartlett, C.A.; Shaw, J.A.; Archer, M.; Naidu, P.S.R.; Smith, N.M.; Dunlop, S.A.; Swaminathan Iyer, K.; Kilburn, M.R.; et al. Enabling dual cellular destinations of polymeric nanoparticles for treatment following partial injury to the central nervous system. Biomaterials 2016, 74, 200–216. [Google Scholar] [CrossRef]
- Tahara, K.; Miyazaki, Y.; Kawashima, Y.; Kreuter, J.; Yamamoto, H. Brain targeting with surface-modified poly(D,L-lactic-co-glycolic acid) nanoparticles delivered via carotid artery administration. Eur. J. Pharm. Biopharm. 2011, 77, 84–88. [Google Scholar] [CrossRef]
- Calvo, P.; Gouritin, B.; Chacun, H.; Desmaele, D.; D’Angelo, J.; Noel, J.P.; Georgin, D.; Fattal, E.; Andreux, J.P.; Couvreur, P. Long-Circulating PEGylated Polycyanoacrylate Nanoparticles as New Drug Carrier for Brain Delivery. Pharm. Res. 2001, 18, 1157–1166. [Google Scholar] [CrossRef]
- Nance, E.; Zhang, C.; Shih, T.Y.; Xu, Q.; Schuster, B.S.; Hanes, J. Brain-Penetrating Nanoparticles Improve Paclitaxel Efficacy in Malignant Glioma Following Local Administration. ACS Nano 2014, 8, 10655–10664. [Google Scholar] [CrossRef]
- Inoue, Y.; Ezure, H.; Ito, J.; Sawa, C.; Yamamoto, M.; Hata, H.; Moriyama, H.; Manome, Y.; Otsuka, N. Effect of Silica Nanoparticles on Cultured Central Nervous System Cells. J. Neurosci. 2018, 8, 146–156. [Google Scholar] [CrossRef]
- Du, Q.; Ge, D.; Mirshafiee, V.; Chen, C.; Li, M.; Xue, C.; Ma, X.; Sun, B. Assessment of neurotoxicity induced by differentsized Stöber silica nanoparticles: Induction of pyroptosis in microglia. Nanoscale 2019, 11, 12965–12972. [Google Scholar] [CrossRef]
- Bittner, A.; Ducray, A.D.; Widmer, H.R.; Stoffel, M.H.; Mevissen, M. Effects of gold and PCL- or PLLA-coated silica nanoparticles on brain endothelial cells and the blood–brain barrier. Beilstein J. Nanotechnol. 2019, 10, 941–954. [Google Scholar] [CrossRef]
- Gilardino, A.; Catalano, F.; Ruffinatti, F.A.; Alberto, G.; Nilius, B.; Antoniotti, S.; Martra, G.; Lovisolo, D. Interaction of SiO2 nanoparticles with neuronal cells: Ionic mechanisms involved in the perturbation of calcium homeostasis. Int. J. Biochem. Cell Biol. 2015, 66, 101–111. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Y.; Xia, D.; Meng, L.; He, M.; Liu, C.; Zhang, Z. Silica Nanoparticles Promote α-Synuclein Aggregation and Parkinson’s Disease Pathology. Front. Neurosci. 2022, 15, 807988. [Google Scholar] [CrossRef]
- Bang, J.; Yeyeodu, S.; Gilyazova, N.; Witherspoon, S.; Ibeanu, G. Effects of Carbon Nanotubes on a Neuronal Cell Model In Vitro. J. Biol. 2011, 1, 70–77. [Google Scholar] [CrossRef]
- Villegas, J.C.; Álvarez-Montes, L.; Rodríguez-Fernández, L.; González, J.; Valiente, R.; Fanarraga, M.L. Multiwalled Carbon Nanotubes Hinder Microglia Function Interfering with Cell Migration and Phagocytosis. Adv. Healthcare Mater. 2014, 3, 424–432. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Yang, Y.; Wang, Q.; Gao, L.; Yang, Y.; Chang, T.; Zhang, X.; Xiang, G.; Cao, Y.; et al. Single-wall carbon nanohorns inhibited activation of microglia induced by lipopolysaccharide through blocking of Sirt3. Nanoscale Res. Lett. 2013, 8, 100. [Google Scholar] [CrossRef]
- Zheng, W.; McKinney, W.; Kashon, M.; Salmen, R.; Castranova, V.; Kan, H. The influence of inhaled multi-walled carbon nanotubes on the autonomic nervous system. Part. Fibre Toxicol. 2016, 13, 8. [Google Scholar] [CrossRef]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomedicine 2013, 9, 461–464. [Google Scholar] [CrossRef]
- Serramía, M.J.; Álvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, J.; Muñoz-Fernández, M.A. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef]
- Gomes, M.J.; Fernandes, C.; Martins, S.; Borges, F.; Sarmento, B. Tailoring Lipid and Polymeric Nanoparticles as siRNA Carriers towards the Blood-Brain Barrier—From Targeting to Safe Administration. J. Neuroimmune Pharmacol. 2017, 12, 107–119. [Google Scholar] [CrossRef]
- Mastorakos, P.; Zhang, C.; Berry, S.; Oh, Y.; Lee, S.; Eberhart, C.H.; Woodworth, G.F.; Suk, J.S.; Hanes, J. Highly PEGylated DNA Nanoparticles Provide Uniform and Widespread Gene Transfer in the Brain. Adv. Healthc. Mater. 2015, 4, 1023–1033. [Google Scholar] [CrossRef]
- Murali, K.; Kenesei, K.; Li, Y.; Demeter, K.; Környei, Z.; Madarász, E. Uptake and bioreactivity of polystyrene nanoparticles is affected by surface modifications, ageing and LPS adsorption: In vitro studies on neural tissue cells. Nanoscale 2015, 7, 4199–4210. [Google Scholar] [CrossRef]
- Jung, B.K.; Han, S.W.; Park, S.H.; Bae, J.S.; Choi, J.; Ryu, K.Y. Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. NeuroToxicology 2020, 81, 189–196. [Google Scholar] [CrossRef]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Rosenholm, J.M. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood- Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef]
- Bardi, G.; Tognini, P.; Ciofani, G.; Raffa, V.; Costa, M.; Pizzorusso, T. Pluronic-coated carbon nanotubes do not induce degeneration of cortical neurons in vivo and in vitro. Nanomedicine 2009, 5, 96–104. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef]
- Pojo, M.; Cerqueira, S.R.; Mota, T.; Xavier-Magalhães, A.; Ribeiro-Samy, S.; Mano, J.F.; Oliveira, J.M.; Reis, R.L.; Sousa, N.; Costa, B.M.; et al. In vitro evaluation of the cytotoxicity and cellular uptake of CMCht/PAMAM dendrimer nanoparticles by glioblastoma cell models. J. Nanopart. Res. 2013, 15, 1621. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Fan, L.; Zha, H.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X.; et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, X.; Zhang, X.; Li, S.; Song, Y.; Du, D.; Dutta, P.; Lin, Y. Synthetic Polymer Nanoparticles Functionalized with Different Ligands for Receptor-Mediated Transcytosis across the Blood–Brain Barrier. ACS Appl. Bio Mater. 2018, 1, 1687–1694. [Google Scholar] [CrossRef]
- Wong, L.R.; Ho, P.C. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: Implications for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2018, 70, 59–69. [Google Scholar] [CrossRef]
- Tian, X.; Wei, F.; Wang, T.; Wang, P.; Lin, X.; Wang, J.; Wang, D.; Ren, L. In vitro and in vivo studies on gelatin-siloxane nanoparticles conjugated with SynB peptide to increase drug delivery to the brain. Int. J. Nanomed. 2012, 7, 1031–1041. [Google Scholar]
- Cha, K.E.; Heejoon, M. Cytotoxic Effects of Nanoparticles Assessed In Vitro and In Vivo. J. Microbiol. Biotechnol. 2007, 17, 1573–1578. [Google Scholar]
- He, H.; Li, Y.; Jia, X.R.; Du, J.; Ying, X.; Lu, W.L.; Lou, J.N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Zhang, Z.; Liaw, K.; Kambhampati, S.P.; Porterfield, J.E.; Lin, K.C.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020, 6, eaay8514. [Google Scholar] [CrossRef]
- Ganesan, P.; Kim, B.; Ramalaingam, P.; Karthivashan, G.; Revuri, V.; Park, S.; Kim, J.S.; Ko, Y.T.; Choi, D.K. Antineuroinflammatory Activities and Neurotoxicological Assessment of Curcumin Loaded Solid Lipid Nanoparticles on LPS-Stimulated BV-2 Microglia Cell Models. Molecules 2019, 24, 1170. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fan, J.; Wang, Z.; Zeng, X.; Sun, Y.; Song, P.; Ju, D. The role of autophagy in the neurotoxicity of cationic PAMAM Dendrimers. Biomaterials 2014, 35, 7588–7597. [Google Scholar] [CrossRef]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon Nanotubes Enhance CpG Uptake and Potentiate Anti-Glioma Immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef]
- Ma, X.; Zhong, L.; Guo, H.; Wang, Y.; Gong, N.; Wang, Y.; Cai, J.; Liang, X.J. Multiwalled Carbon Nanotubes Induced Hypotension by Regulating the Central Nervous System. Adv. Funct. Mater. 2018, 28, 1705479. [Google Scholar] [CrossRef]
- Vittorio, O.; Raffa, V.; Cuschieri, A. Influence of purity and surface oxidation on cytotoxicity of multiwalled carbon nanotubes with human neuroblastoma cells. Nanomedicine 2009, 5, 424–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived PC12 Cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Li, Z.; Chen, T.; Lantz, S.M.; Howard, P.C.; Paule, M.G.; Slikker, W.; Watanabe, F.; Mustafa, T.; et al. Mechanistic Toxicity Evaluation of Uncoated and PEGylated Single-Walled Carbon Nanotubes in Neuronal PC12 Cells. ACS Nano 2011, 5, 7020–7033. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.; Kang, S.H.; Hwang, E.J.; Hwang, Y.S.; Lee, M.H.; Han, D.W.; Park, J.C. Enhanced Neural Cell Adhesion and Neurite Outgrowth on Graphene-Based Biomimetic Substrates. BioMed Res. Int. 2014, 2014, 212149. [Google Scholar] [CrossRef]
- Athira, S.S.; Biby, T.E.; Mohanan, P.V. Effect of polymer functionalized fullerene soot on C6 glial cells. Eur. Polym. J. 2020, 127, 109572. [Google Scholar] [CrossRef]
- Waite, C.L.; Sparks, S.M.; Uhrich, K.E.; Roth, C.M. Acetylation of PAMAM dendrimers for cellular delivery of siRNA. BMC Biotechnol. 2009, 9, 38. [Google Scholar] [CrossRef]
- Janiszewska, J.; Posadas, I.; Játiva, P.; Bugaj- Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second Generation Amphiphilic Poly-Lysine Dendrons Inhibit Glioblastoma Cell Proliferation without Toxicity for Neurons or Astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Kumari, L.; Patel, K.K.; Vuddanda, P.R.; Vajanthri, K.Y.; Mahtoc, S.K.; Singh, S. Intravenous administration of trans-resveratrol loaded TPGS-coated solid lipid nanoparticles for prolonged systemic circulation, passive brain targeting and improved in vitro cytotoxicity against C6 glioma cell lines. RSC Adv. 2016, 6, 50336–50348. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, J.; Zhang, L.Y.; Zhang, P.L.; Zhou, J.L.; Liu, B. Preparation of N, N, N-trimethyl chitosan-functionalized retinoic acid-loaded lipid nanoparticles for enhanced drug delivery to glioblastoma. Trop. J. Pharm. Res. 2017, 16, 1765–1772. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control Release. 2018, 10, 89–101. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Ghaemi, S.R.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Lim, K.J.; Bisht, S.; Bar, E.E.; Maitra, A.; Eberhart, C.G. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol. Ther. 2011, 11, 464–473. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. Nuclear and cytoplasmic delivery of lactoferrin in glioma using chitosan nanoparticles: Cellular location dependent-action of lactoferrin. Eur. J. Pharm. Biopharm. 2018, 129, 74–79. [Google Scholar] [CrossRef]
- Nejat, H.; Rabiee, M.; Varshochian, R.; Tahriri, M.; Jazayeri, H.E.; Rajadas, J.; Ye, H.; Cui, Z.; Tayebi, L. Preparation and characterization of cardamom extract-loaded gelatin nanoparticles as effective targeted drug delivery system to treat glioblastoma. React. Funct. Polym. 2017, 120, 46–56. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Liu, Y.; Sun, J.; Xie, W.; Zhao, P.; Liu, J. Ultrasound-Excited Protoporphyrin IX-Modified Multifunctional Nanoparticles as a Strong Inhibitor of Tau Phosphorylation and β-Amyloid Aggregation. ACS Appl. Mater. Interfaces 2018, 10, 32965–32980. [Google Scholar] [CrossRef]
- Patel, D.A.; Henry, J.E.; Good, T.A. Attenuation of β-amyloid induced toxicity by sialic acidconjugated dendrimers: Role of sialic acid attachment. Brain Res. 2007, 3, 95–105. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Tsai, H.C. Rosmarinic acid- and curcumin-loaded polyacrylamide-cardiolipin-poly (lactide-co-glycolide) nanoparticles with conjugated 83-14 monoclonal antibody to protect β-amyloid-insulted neurons. Mater. Sci. Eng. 2018, 91, 445–457. [Google Scholar] [CrossRef]
- Saraiva, A.M.; Cardoso, I.; Saraiva, M.J.; Tauer, K.; Pereira, M.K.; Coelho, M.A.N.; Möhwald, H.; Brezesinski, G. Randomization of Amyloid-b-Peptide(1-42)Conformation by Sulfonated and SulfatedNanoparticles Reduces Aggregation and Cytotoxicity. Macromol. Biosci. 2010, 10, 1152–1163. [Google Scholar] [CrossRef]
- Shyam, R.; Ren, Y.; Lee, J.; Braunstein, K.E.; Mao, H.Q.; Wong, P.C. Intraventricular Delivery of siRNA Nanoparticles to the Central Nervous System. Mol. Ther. Nucleic Acids. 2015, 4, e242. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Jha, A.; Ghormade, V.; Kolge, H.; Paknikar, K.M. Dual effect of chitosan-based nanoparticles on the inhibition of b-amyloid peptide aggregation and disintegration of the preformed fibrils. J. Mater. Chem. B 2019, 7, 3362–3373. [Google Scholar] [CrossRef]
- Sookhaklari, R.; Geramizadeh, B.; Abkar, M.; Moosavi, M. The neuroprotective effect of BSA-based nanocurcumin against 6-OHDA-induced cell death in SH-SY5Y cells. Avicenna J. Phytomed. 2019, 9, 92–100. [Google Scholar]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 Mediated Poly(butyl) Cyanoacrylate Nanoparticles Containing Curcumin: Study of Enhanced Activity of Curcumin against Beta Amyloid Induced Cytotoxicity Using In Vitro Cell Culture Model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef]
- You, R.; Ho, Y.S.; Hung, C.H.L.; Liu, Y.; Huang, C.X.; Chan, H.N.; Ho, S.L.; Lui, S.Y.; Li, H.W.; Chang, R.C.C. Silica nanoparticles induce neurodegeneration-like changes in behavior, neuropathology, and affect synapse through MAPK activation. Part. Fibre Toxicol. 2018, 15, 28. [Google Scholar] [CrossRef]
- Çömelekoğlu, Ü.; Ball, E.; Yalın, S.; Eroğlu, P.; Bayrak, G.; Yaman, S.; Söğüt, F. Effects of different sizes silica nanoparticle on the liver, kidney and brain in rats: Biochemical and histopathological evaluation. J. Res. Pharm. 2019, 23, 344–353. [Google Scholar] [CrossRef]
- Wei, W.; Yan, Z.; Liu, X.; Qin, Z.; Tao, X.; Zhu, X.; Song, E.; Chen, C.; Chun Ke, P.; Leong, D.T.; et al. Brain Accumulation and Toxicity Profiles of Silica Nanoparticles: The Influence of Size and Exposure Route. Environ. Sci. Technol. 2022, 56, 8319–8325. [Google Scholar] [CrossRef]
- Maurya, M.K.; Langeh, R.; Anuradha, J.; Sanjeevani, R.; Sanjeevi, R.; Tripathi, S.; Chauhan, D.S. Silica Nanoparticles Induced Oxidative Stress in Different Brain Regions of Male Albino Rats. Sch. Acad. J. Biosci. 2021, 9, 139–144. [Google Scholar] [CrossRef]
- Aragon, M.J.; Topper, L.; Tyler, C.R.; Sanchez, B.; Zychowski, K.; Young, T.; Herbert, G.; Hall, P.; Erdely, A.; Eye, T.; et al. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood–brain barrier impairment. Proc. Natl. Acad. Sci. USA 2017, 21, E1968–E1976. [Google Scholar] [CrossRef]
- Dal Bosco, L.; Weber, G.E.B.; Parfitt, G.M.; Paese, K.; Gonçalves, C.O.F.; Serodre, T.M.; Furtado, C.A.; Santos, A.P.; Monserrat, J.M.; Barros, D.M. PEGylated Carbon Nanotubes Impair Retrieval of Contextual Fear Memory and Alter Oxidative Stress Parameters in the Rat Hippocampus. BioMed Res. Int. 2015, 2015, 104135. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Hu, Y.L.; Gao, J.Q. Brain Localization and Neurotoxicity Evaluation of Polysorbate 80-Modified Chitosan Nanoparticles in Rats. PLoS ONE 2015, 10, e0134722. [Google Scholar] [CrossRef]
- Olivier, J.C.; Fenart, L.; Chauvet, R.; Pariat, C.; Cecchelli, R.; Couet, W. Indirect evidence that drug-brain targeting using Polysorbate 80-coated Polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm. Res. 1999, 16, 1836–1842. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Dou, J.; Hou, Q.; Cheng, J.; Jiang, X. Bioeffects of Inhaled Nanoplastics on Neurons and Alteration of Animal Behaviors through Deposition in the Brain. Nano Lett. 2022, 22, 1091–1099. [Google Scholar] [CrossRef]
- Van Handel, M.; Alizadeh, D.; Zhang, L.; Kateb, B.; Bronikowski, M.; Manohara, H.; Badie, B. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. J. Neuroimmunol. 2009, 208, 3–9. [Google Scholar] [CrossRef]
- Qu, G.; Bai, Y.; Zhang, Y.; Jia, Q.; Zhang, W.; Yan, B. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon 2009, 47, 2060–2069. [Google Scholar] [CrossRef]
- Yamago, S.; Tokuyama, H.; Nakamuralr, E.; Kikuchi, K.; Kananishl, S.; Sueki, K.; Nakahara, H.; Enomoto, S.; Ambe, F. In vivo biological behavior of a water-miscible fullerene:14C labeling, absorption, distribution, excretion and acute toxicity. Chem. Biol. 1995, 2, 385–389. [Google Scholar] [CrossRef]
- Shytikov, D.; Shytikova, I.; Rohila, D.; Kulaga, A.; Dubiley, T.; Pishel, I. Effect of Long-Term Treatment with C60 Fullerenes on the Lifespan and Health Status of CBA/Ca Mice. Rejuv. Res. 2021, 24, 345–353. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Liu, J.H.; Wang, H.; Liu, Y. Biodistribution and fate of nanodiamonds in vivo. Diam. Relat. Mater. 2009, 18, 95–100. [Google Scholar] [CrossRef]
- Pakhira, B.; Ghosh, M.; Allam, A.; Sarkar, S. Carbon nano onions cross the blood brain barrier. RSC Adv. 2016, 6, 29779–29782. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef]
- Mishra, M.K.; Beaty, C.A.; Lesniak, W.G.; Kambhampati, S.P.; Zhang, F.; Wilson, M.A.; Blue, M.E.; Troncoso, J.C.; Kannan, S.; Johnston, M.V.; et al. Dendrimer Brain Uptake and Targeted Therapy for Brain Injury in a Large Animal Model of Hypothermic Circulatory Arrest. ACS Nano 2014, 8, 2134–2147. [Google Scholar] [CrossRef]
- MacKay, J.A.; Deen, D.F.; Szoka, F.C., Jr. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005, 1035, 139–153. [Google Scholar] [CrossRef]
- Lee, S.; Ashizawa, A.T.; Kim, K.S.; Falk, D.J.; Notterpek, L. Liposomes to Target Peripheral Neurons and Schwann Cells. PLoS ONE 2013, 8, e78724. [Google Scholar] [CrossRef]
- Salvalaio, M.; Rigon, L.; Belletti, D.; D’Avanzo, F.; Pederzoli, F.; Ruozi, B.; Tosi, G. Targeted Polymeric Nanoparticles for Brain Delivery of High Molecular Weight Molecules in Lysosomal Storage Disorders. PLoS ONE 2016, 11, e0156452. [Google Scholar] [CrossRef]
- Tosi, G.; Bondioli, L.; Ruozi, B.; Badiali, L.; Severini, G.M.; Biffi, S.; De Vita, A.; Bortot, B.; Dolcetta, D.; Forni, F.; et al. NIR-labeled nanoparticles engineered for brain targeting: In vivo optical imaging application and fluorescent microscopy evidences. J. Neural Transm. 2011, 118, 145–153. [Google Scholar] [CrossRef]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Oğuz, K.K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. 2017, 78, 32–38. [Google Scholar] [CrossRef]
- Lu, W.; Wan, J.; She, Z.; Jiang, X. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J. Control. Release 2007, 118, 38–53. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Guccione, C.; Grossi, C.; Piazzini, V.; Torracchi, A.; Luccarini, I.; Casamenti, F.; Bilia, A.R. Albumin Nanoparticles for Brain Delivery: A Comparison of Chemical versus Thermal Methods and in vivo Behavior. ChemMedChem 2016, 11, 1840–1849. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, M.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Guo, C.; Cao, F.; Song, Z.; Xi, Y.; Yu, A.; Li, A.; Zhai, G. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanopart. Res. 2010, 12, 3111–3122. [Google Scholar] [CrossRef]
- Kanakia, S.; Toussaint, J.; Chowdhury, S.M.; Tembulkar, T.; Lee, S.; Jiang, Y.P.; Lin, R.Z.; Shroyer, K.R.; Moore, W.; Sitharaman, B. Dose Ranging, Expanded Acute Toxicity and Safety Pharmacology Studies for Intravenously Administered Functionalized Graphene Nanoparticle Formulations. Biomaterials 2014, 35, 7022–7031. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Kruszewski, M. Nanoplastic Impact on the Gut-Brain Axis: Current Knowledge and Future Directions. Int. J. Mol. Sci. 2021, 22, 12795. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Hamed, M.; Martyniuk, C.J.; Naguib, M.; Lee, J.S.; Sayed, A.E.H. Neurotoxic effects of different sizes of plastics (nano, micro, and macro) on juvenile common carp (Cyprinus carpio). Front. Mol. Neurosci. 2022, 15, 1028364. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M.L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar] [CrossRef]
- Oberdörster, E. Manufactured Nanomaterials (Fullerenes, C60) Induce Oxidative Stress in the Brain of Juvenile Largemouth Bass. Environ. Health Perspect. 2004, 112, 1058–1062. [Google Scholar] [CrossRef]
- Li, J.; Ying, G.G.; Jones, K.C.; Martin, F.L. Real-world carbon nanoparticle exposures induce brain and gonadal alterations in zebrafish (Danio rerio) as determined by biospectroscopy techniques. Analyst 2015, 140, 2687–2695. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Sun, Z. Toxic Effects of Silica Nanoparticles on Zebrafish Embryos and Larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.M.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.L.; Zhang, Y.; Baker, T.R. Nanoplastics impact the zebrafish (Danio rerio) transcriptome: Associated developmental and neurobehavioral consequences. Environ. Pollut. 2020, 266, 115090. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, S.; Jin, Y.; Xue, W.; Zhong, Y.; Wang, H.; An, J.; Li, H. Polystyrene nanoparticles induced neurodevelopmental toxicity in Caenorhabditis elegans through regulation of dpy-5 and rol-6. Ecotoxicol. Environ. Saf. 2021, 222, 112523. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.C.; Hsiao, C.D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef]
- Sokmen, T.O.; Sulukan, E.; Turkoğlu, M.; Baran, A.; Ozkaraca, M.; Ceyhun, S.B. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). NeuroToxicology 2020, 77, 51. [Google Scholar] [CrossRef]
- Qu, M.; Kong, Y.; Yuan, Y.; Wang, D. Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Environ. Sci. Nano 2019, 6, 2591. [Google Scholar] [CrossRef]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; Defu, H. Polystyrene (nano)microplastics cause sizedependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Environment. Nanoplastics: State of Knowledge and Environmental and Human Health Impacts; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
| Toxic Effect | |||||
|---|---|---|---|---|---|
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | |
| SILICA NPs | |||||
| None | 30 nm, 0.01, 0.1 and 1 mg/mL, 10 min, 30 min and 1 h | primary cultures of cortical neurons isolated from rats | Decreased neuron dendrite and induction of ROS production. Early stage apoptosis process and necrosis. | [80] | |
| None | 50, 100, 300 nm, 25–200 μg/mL, 24 h | microglia (N9 and BV-2), endothelial cells (bEnd.3), neuronal cells (HT22 cells) | Size- and cell type dependent cytotoxicity. Increased ROS production in microglia, decreased GSH level, lysosomal damage. Induction of release of IL-1β and N-terminal GSDMD, a marker of pyroptosis. | [81] | |
| ICG/PCL- (PCL-SiNPs) or ICG/PLLA-coated SiNPs (PLLA-SiNPs) | 90 nm (PCL-SiNPs) 95 nm (PLLA-SiNPs), 2.49 × 10−7 μg/mL to 24.9 × 10−7μg/mL, 2 and 24 h | brain endothelial cells and rat brain capillary endothelial cells (rBCEC4) | Time- and concentration-dependent decrease in cell viability, but not proliferation, differentiation. No inflammation process. | [82] | |
| None | 150–200 nm, 4 × 1010 NPs/mL to 7 × 1010 NPs/mL, 24 h | primary rat microglia | Increased production of ROS and RNS. Decrease in TNFα gene expression and cyclooxygenase-2 gene. Increased IL-1β cytokine release. | [23] | |
| None | 47; 49; 55; 500; 2000 nm, 20 µg/mL, 30 min and 4 h | neuronal cell lines (GT1–7 and GN11 cells) | Long lasting but reversible calcium signaling, independ on NP internalization. | [83] | |
| None | 509.3, 356.0, 469.0 nm, 100 and 200 µg/mL, 24 and 48 h 7 days | HEK293 cells and primary mouse cortical neurons | α-Synucleinopathy in HEK293 cells and neurons. | [84] | |
| CARBON NPs | |||||
| Carboxylated and aminated MWNTs | 200–300 nm, 5–100 μg/mL, 24 h | primary neuronal and glial cells isolated from fetal rat frontal cortex and striatum | Decreased microglia viability at concentration of 20 μg/mL or higher. Release of NO. | [19] | |
| MWNTs | 85–115 nm, 190 and 295 ppm, 24 and 48 h | NeuroScreen-1 (NS-1) cells | Reduced viability, apoptosis. Mitochondria depolarization and disruption of membranes. | [85] | |
| MWNTs | 5 to 15 nm, 0.6 μg/mL, 70 h | microglial cells (BV-2) | Dose-dependent cell division arrest and apoptosis. Perturbation of cell migration and phagocytosis. | [86] | |
| Single-wall carbon nanohorns | 80 to 100 nm, 16 h | microglial cells (N9 and BV-2) | Proliferation inhibition, promotion of apoptosis. Perturbation of cell cycle. | [87] | |
| DENDRIMERS | |||||
| G4 and G4-C12 PAMAM | 1 to 100 nM, 24 h | primary neuronal cultures | Apoptosis and cell death. | [25] | |
| PAMAM-NH2 G4 (PAMAM-NH2) or (PAMAM-NH2) modified with sodium carboxylate (PAMAM-S.C.) | 26.1, 38.2, 104.8 nm (PAMAM-NH2) 45.3, 173.4, 267.7 nm (PAMAM-SC), 0.3, 1, 3, and 10 µg/mL, 72 h | human neural progenitor cells cultured as a 3D neurosphere model | Inhibition of neurosphere growth, cell proliferation and neuronal migration. Down-regulation of early growth response gene 1, insulin-like growth factor-binding protein 3 and tissue factor pathway inhibitor (TFPI2). | [88] | |
| PAMAM G4, bare or modified with 4-carbomethoxypyrrolidone surface groups | 10, 40, 80, 120, 200 µM, 24 h | embryonic mouse hippocampal cells (mHippoE-18) | Minor toxicity. No effect on ROS production nor mitochondrial membrane potential. | [89] | |
| Cationic carbosilane dendrimer G2 loaded with FITC | 100 nM, 24 and 48 h | human primary astrocytes | Cell death. | [90] | |
| None | 0.1 mg/mL, 30 min | neurons and glial cells from rats | Increase in intratracellular Ca2+ flux and mitochonria depolarization and impaired oxidative metabolism in pyramidal neurons and astrocytes. | [39] | |
| SOLID LIPID NPs | |||||
| PLGA NPs with a peptide-binding transferrin receptor, loaded with siRNA | 115 and 150 nm, 0.1; 1; 10; 100; 1000 µg/mL, 24 h | immortalized human cerebral microvascular endothelial cell line (hCMEC/D3 cell line) | Decreased metabolic activity of cells at the highest concentration. | [91] | |
| POLY(ETHYLENEGLYCOL)–POLY(LACTIDE) NPs | |||||
| PLGA NPs coated with BSA | 80 nm, 0.025 to 8.0 mg/mL, 4 h | capillary endothelial cell (BCEC) | Concentration dependent cytotoxicity. | [64] | |
| DNA NPs | |||||
| DNA NPs modified with PEG | <60 nm, 1, 5, 10 μg/mL, 24, 48 and 72 h | rabbit and rat primary astrocytes | Toxicity. | [92] | |
| NANOGEL | |||||
| PEG or PEG-PEI nanogel | <100 nm, 0.001; 0.01; 0.1; 1; 10 mg/mL, 2 h | bovine brain microvessel endothelial cells (BBMEC) | Neglectable cytotoxicity. | [43] | |
| NANOPLASTIC | |||||
| Carboxylated polystyrene NPs | 55 nm, 7.8–250 mg/L, 24 h | NE-4C neuroectodermal stem cells; primary brain cell cultures from mouse; microglia; brain vascular endothelial cell cultures | LDH leakage of neuronal cells. Microglial cells were able to internalize carboxylated polystyrene nanoparticles by phagocytosis. | [93] | |
| Polystyrene NPs | 100 nm, 100–200 μg/mL | GES-1, different primary brain cells | Reduced cell viability and defective neuronal development, reactive astrocytosis in astrocytes | [94] | |
| Non-toxic Effect | |||||
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | |
| SILICA NPs | |||||
| ICG/PCL-rhodaminedoped SiNPs (PCL-NPs) and ICG/PLLA-rhodamine-doped SiNPs (PLLA-NPs) | 90 nm (PCL-NPs) 95 nm (PLLA-NPs), 2.6 × 109, 5.2 × 109 and 2.6 × 1010 (PCL-NPs/mL) [2.9 × 109, 5.8 × 109 and 2.9 × 1010 (PLLA-NPs/mL), 6 and 24 h | rat primary hippocampal culture | No change in the release of IL-1β and TNFα. | [24] | |
| PEG and lactoferrin | 26; 53 and 105 nm, 1 to 10 nM, 24 h | in vitro blood−brain barrier (BBB) model consisting of three distinct types of cells: endocytes, pericytes, and astrocytes | Lactoferrin enhanced efficiency of NPs transport across the BBB. The cell viability at a level over 93%. | [95] | |
| Mesoporous silica NPs bare or coated with a PEG-PEI block copolymer | 50 to 240 nm, 10, 20, 50 μg/mL, 36 h | rat brain endothelial cells (RBE4) | No toxicity up to 50 μg/mL. No damage to BBB. | [96] | |
| CARBON NPs | |||||
| Carboxylated and aminated MWNTs | 20–30 nm, 5–100 μg/mL, 24 h | primary neuronal and glial cell populations isolated from fetal rat frontal cortex and striatum | Neurons from both brain regions were generally not affected by exposure to NPs. The viability of mixed glia was not reduced in frontal cortex. | [19] | |
| Prestine and pluronic F127 coated MWNTs | 20–30 nm, 3.5, 17.5 μg/mL, 24 h | neurons and glia | No toxicity and no apoptosis after co-treatment with Pluronic F127 and MWNTs. No toxicity after treatment with prestine MWNTs. | [97] | |
| Amino-functionalized MWNTs | 18.9, 20 and 50 µg/mL, 24 and 72 h | primary porcine brain endothelial cells (PBEC) and primary rat astrocytes | No statistically significant toxicity. | [32] | |
| MWNTs modified with plasmid DNA | 20 nm, 2.5 μg of MWNTs, 15, 24 and 48 h | microglial cells (BV-2) | No detrimental effect on proliferation or cytokine production. | [21] | |
| Acid-oxidized MWNTs | 5, 7 and 8 µm, 1–10 µg/mL, 24 h | microglial cells (N9) | Confirmed NP internalization without any effect on viability, pro-inflammatory cytokine release or NO production. | [22] | |
| Graphene modified with poly-L-lysine | 7 days | mouse hippocampal culture model | No effect on cell viability and morphology. No effect on neuron growth. | [98] | |
| Nanodiamonds | 114.7 nm, 1, 5, 10, 25, 50, 100, 250 µg/mL, 2–3 days | mouse hippocampal neurons and mouse dorsal root ganglion neurons | Low neuronal toxicity but disturbances in neuronal morphogenesis. | [37] | |
| DENDRIMERS | |||||
| CMCht/PAMAM | 22.0 to 30.7 nm, 200 µg/mL, 1, 2, 6, 12, 15, 18, 24 and 48 h | hippocampal neuron cultures and cortical glial cells | No cytotoxicity. | [28] | |
| CMCht/PAMAM dendrimer NPs | 45 and 250 nm, 200–400 µg/mL, 1, 6, 12, 24, and 48 h, 7 days | human immortalized astrocytes (hTERT/E6/E7) | Low level of cytotoxicity (20% of decrease in metabolic activity) after long-term exposures (7 days). | [99] | |
| POLY(LACTIDE-CO-GLYCOLIC ACID) NPs | |||||
| PLGA loaded with curcumin, and modified with g7 peptide | 200–250 nm, 10, 20, 40 μM, 24 h | primary hippocampal neurons from rats | Lack of cytotoxicity. No significant increase in apoptotic nor necrosis. | [34] | |
| PLGA loaded with curcumin | 200 nm, 0.001, 0.01, 0.1, 0.2, 0.5, 5, and 50 μM, 24 h | neurospheres in culture and neural stem cells | Eenhanced proliferation of the neural stem cells at doses as low as 0.001 μM, with the highest proliferation at 0.5 μM. 0.5 μM of NPs were non-cytotoxic. | [100] | |
| PLGA curcumin loaded, modified with PEG and conjugated with B6 peptide | less than 150 nm, 50, 100, 200, and 500 µg/mL, 24 h | HT22 cells | No effect on cell viability. | [35] | |
| PLGA modified with PEG and phage-displayed peptides | 121.46 nm, 0.1; 0.5, 1; 2.5 mg/mL, 4 h | endothelial cells (bEnd.3) | No cytotoxicity. | [101] | |
| POLYMER NPs | |||||
| TEB NPs, bare | 25 nm, 50, 100, 250, 500, 800 ng/mL, 24 h | endothelial cells (bEnd.3) | No obvious effect on cell viability. | [102] | |
| SOLID LIPID NPs | |||||
| PLGA functionalized with a transferrin receptor or peptide mimicking transferrin receptor and loaded with siRNA | 115, 150 nm, 0.1; 1; 10; 100; 1000 µg/mL, 24 h | immortalized human cerebral microvascular endothelial cell line (hCMEC/D3 cell line) brain capillary endothelial cell line (BCEC) | No toxicity. | [91] | |
| SERUM ALBUMIN NPs | |||||
| R-flurbiprofen | 284.4 nm, 25, 50, 100 and 200 μM, 48 h | chinese hamster ovary (CHO) cells stably transfected with mouse Ab precursor protein 695 | No effect on cell viability. | [103] | |
| Polysorbate 80 or with attached Apolipoprotein E | 249 nm, 0.1, 1 and 2 mg/mL, 24 h | endothelial cells (b.End3) | Stimulation of cell viability. | [67] | |
| OTHERS | |||||
| Gelatin-siloxane NPs modified with SynB peptides-cell penetrating peptides | 194.55 nm, 100–600 µg/mL, 4 and 12 h | primary cultures of rat brain capillary endothelial cells | Good biocompatibility with brain capillary endothelial cells. | [104] | |
| Biodegradable polymersomes conjugated with cationic albumin | 95 nm, 0.25, 0.5, 1, 2, 4, 8 mg/mL, 60 min | endothelial cells (bEnd.3) | Only little toxicity (viability above 85%). | [75] | |
| Toxic Effect | ||||||||
|---|---|---|---|---|---|---|---|---|
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | ||||
| SILICA NPs | ||||||||
| (ICG)/(PCL)-rhodamine-doped NPs and Si-ICG/PCL/ PLLA rhodamine-doped NPs | 90 and 95 nm, 2.6 × 109, 5.2 × 109, 2.6 × 1010 PCL-NPs/mL and 2.9 × 109, 5.8 × 109, 2.9 × 1010 PLLA-NPs/mL, 6 and 24 h | neuroblastoma (SH-SY5Y) | Neurite outgrowth was not significantly altered. Reduction in neuronal differentiation. | [24] | ||||
| Aminopropyltriethoxysilane | 92.6 nm, 25–200 μg/mL, 24 h | neuronal cells (PC12) | The accumulation of cells in the G2/M phase at a concentration of 100 and 200 μg/mL. Activation of the p53-mediated signaling pathway and apoptosis. | [65] | ||||
| None | 509.3, 356.0, 469.0 nm, 100 and 200 µg/mL, 24 and 48 h, 7 days | Neuroblastoma (SH-SY5Y) | Mitochondrial dysfunction, pathological autophagy and cell apoptosis. Oxidative stress. | [84] | ||||
| CARBON NPs | ||||||||
| MWNTs modified with carboxyl groups | 50 and 500 nm, 10 and 40 μg/mL, 24 h | neuroblastoma (N2a) | Up-regulation of nNOS level in cells via promoting nuclear translocation and acetylation of NF-κB. Increase in NO level. | [111] | ||||
| MWNTs with pluronic F127 solution | 5 μg/mL or 10 μg/mL, 3 days, 1 week, 2 weeks | Neuroblastoma (SH-SY5Y) | Higher concentrations of NPs (50 and 500 μg/mL) and longer incubation times (1 and 2 weeks) caused a decrease in the viability of cells. | [112] | ||||
| SWNTs with DNA conjugated with different surfactants sodium dodecyl sulfate, sodium dodecylbenzene sulfonate and sodium cholate | 0.5 mg/mL, 72 h | astrocytoma (1321N1) | NPs with SC did not affect cell morphology, proliferation, or growth. NPs with SDS and SDBS surfactants demonstrated irregular cell morphology, and they were toxic to cells. | [50] | ||||
| Graphene layers and SWNTs | 0.8–1.2; 0.01; 0.1; 1; 10; 100 μg/mL, 24 h | neuronal cells (PC12) | The generation of ROS after exposure of graphene layers. The upregulation of caspase 3 indicates an apoptosis of PC12 cells. | [113] | ||||
| PEG coated CNTs | 2.5–4.5 nm, 0.1; 1; 10; 100 μg/mL, 24 h | neuronal cells (PC12) | The decrease in metabolic activity and generation of ROS. NPs with PEG exhibited less cytotoxic potency than uncoated NPs. | [114] | ||||
| Graphene, SWNTs and MWNTs coated with an ultrathin layer of gold/platinum | 50 nm (graphene), ∼100 nm (SWCNTs), and 2–5; 10–15 nm (MWNTs), 0.5–500 ppm, 24 h | neuronal cells (PC12) | The significant dose-dependent decreases in the viability of cells. Graphene exerted adverse effects on the neural cells at a concentration over 62.5 ppm. | [115] | ||||
| Fullerenes C60 and C70 functionalized with dextran polymer | 30.29 nm | glioma (C6) | Dose-dependent reduction in cell viability. NPs affect the growth, proliferation, functional and phenotypic aspects of cells. The slight degeneration of actin filaments and cytoskeletal destruction. Lysosomal integrity as well as mitochondrial membrane potential was significantly affected. ROS level inside cells was slight. | [116] | ||||
| DENDRIMERS | ||||||||
| (G7) PAMAM with amine, acetamide, and carboxylate end groups, with GM1-pyrene | 4–8 nm, 100 nM, 200 nM, and 400 nM and 1 μM, 1 h | glioma (C6) | The cells exhibited a greater sensitivity to G5-NH2 and G7-NH2 exposure but no differential effect was observed as a function of the presence of GM1 in the membrane. | [53] | ||||
| PAMAM | 0–100 μg/mL 24 h | human glioma cell lines (87MG, U251MG, U118 and A172) | Toxic. Akt/mTOR pathway was involved in the initiation of dendrimers-induced autophagy. The autophagy process induced by dendrimers is mediated by intracellular ROS generation. | [109] | ||||
| Complexed with siRNA | 200 nm, 0.005 and 0.01 mM, 4 h | glioblastoma (U87) | Primary amine acetylation of dendrimers reduced their cytotoxicity. | [117] | ||||
| Second generation amphiphilic polylysine dendrons with siRNA | 3 μM 72 h | glioblastoma (U87 and C6) | Inhibition of the proliferation of two glioblastoma cell lines. Non-toxic for non-tumoural CNS cells. Mitochondrial depolarization and the increase in ROS production. | [118] | ||||
| CMCht/PAMAM dendrimer | 45 and 250 nm, 200–400 µg/mL, 1, 6, 12, 24, and 48 h, 7 days | GBM cel line (U87MG) | Only long-term exposures (7 days) induced low levels of cytotoxicity (20% decrease in metabolic activity). | [99] | ||||
| LIPID NPs | ||||||||
| D-α-tocopheryl PEG 1000 succinate -coated solid lipid NPs with resveratrol | 128.6 to 429.1 nm, 0–200 µg/mL, 72 h | glioma (C6) | High toxicity in cancer cells. The metabolic activity significantly decreased depending on the concentration of NPs. | [119] | ||||
| Solid lipid NPs with retinoic acid and functionalized electrostatically with trimethyl chitosan | 214 nm, to 500 µg/mL, 24 h | glioblastoma (U87MG) | Anti-tumor inhibitory effect by decrease in cell viability and the presence of cells in early and late apoptotic and necrotic phases. | [120] | ||||
| D-α-tocopheryl PEG 1000 succinate (TPGS) coated liposomes with resveratrol | 61–262 nm, 0–200 µg/mL, 72 h | glioma (C6) | High toxicity in cancer cells. The metabolic activity significantly decreased depending on the concentration of NPs. | [54] | ||||
| Liposomes loaded with resveratrol PEGylated, modified with transferrin | 230 nm, 12.5 μM to 200 μM, 24 and 48 h | glioblastoma (U87MG) | Toxic. Higher levels of apoptosis accompanied by activation of caspases 3/7. NPs with transferrin were more effective in inducing toxicity. | [121] | ||||
| Liposomes and micelles with diatom microalgae-derived nanoporous biosilica | 90.5 and 115 nm, 1.70 mg /mL, 1 h | neuroblastoma (SH-SY5Y) | Toxic. The viability was 9–10%. | [122] | ||||
| PLGA lipid NPs conjugated with folic acid and ICG with resveratrol | 104.5–121.1 nm, 5, 10, 50 and 100 μg/mL, 24 h | glioblastoma (U87) | No changes in viability rate. Apoptosis process. | [68] | ||||
| POLY(LACTIDE-CO-GLYCOLIDE) NPs, POLIMERIC NPs | ||||||||
| PLGA, PLG -tocopheryl PEG NPs with resveratrol | 135–222 nm, 0–200 μg/mL, 72 h | glioma (C6) | High toxicity in cancer cells. The metabolic activity decreased depending on the concentration of NPs. | [59] | ||||
| TMC surface- PLGA, PLG NPs | 150 nm, 0.025–8.0 mg/mL, 24 h | neuroblastoma (SH-SY5Y) | Cell viability was slightly reduced at higher concentrations. | [123] | ||||
| Ephrin type-A receptor 3 (EPHA3) tyrosine kinase antibody-modified PLGA NPs | 145.9 nm, 4 ng/mL, 48 h | glioma (C6) | NPs inhibited of cell growth. | [57] | ||||
| Aptamer was conjugated to the surface of PEG-PLGA NPs | 156 nm, 0.019, 0.038, 0.38, 0.76, 3.8, 12 and 24 mg/mL, 24, 48 and 96 h | glioma (C6) | The IC50 value was detected at a concentration of 1.5 µg/mL (24 h incubation time). The cytotoxicity is dependent on incubation time. | [124] | ||||
| Polymeric NPs with curcumin | 5 or 10 μM, 24 h | medulloblastoma, glioblastoma (DAOY, D283Med) | A dose-dependent decrease in cell growth via programmed cell death and cell cycle arrest. Dose-dependent reduction in expression of both the IGF ligands and IGF-1R. Reduction in total STAT3α protein levels and increase phosphorylation of STAT3 at Tyr 705. | [125] | ||||
| Tf conjugated NPs, poly(lactide)-D-a-Tocopheryl PEG PLA-TPGS diblock copolymer | 137.6 nm, 0.05, 0.50 and 2.50 µg/mL, 24, 48 and 72 h | glioma (C6) | Toxic. 50% of death cells was observed at a concentration of 5 µg/mL. | [58] | ||||
| mPEG–PCL NPs with resveratrol | 87.5 nm, 0–31 μM 48 h | glioma (C6) | A dose-dependent cytotoxicity against cells. | [55] | ||||
| DNA NPs | ||||||||
| Highly PEGylated | 43, 47, 59 nm, 1, 5, 10 µg/mL, 24 h | rat gliosarcoma cells (9L) | Toxic. The viability was significant decreased. | [92] | ||||
| CHITOSAN NPs | ||||||||
| Lactoferrin | 300, 800, 1200 µg/mL, 24 h | glioma 261 | Results suggest that the cytotoxicity of lactoferrin and NPs on glioma is attributable to its cytoplasmic allocation. | [126] | ||||
| OTHERS | ||||||||
| Cardamom extract-loaded gelatin NPs | <200 nm | glioblastoma (U87MG) | Apoptosis process and inhibition of the viability. | [127] | ||||
| Lactoferrin-curcuminoid coated polysaccharide NPs based on chitosan hydrochloride/hyaluronic acid/PEG | 210–240 nm, 0–10 µg/mL | Glioma (C6) | Loaded NPs showed 50% cytotoxicity at concentration of 8–10 µg/mL. | [56] | ||||
| Non-toxic Effect | ||||||||
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | ||||
| SILICA NPs | ||||||||
| None | 10–20, 40–50 nm, and 90–110 nm, 0.24; 2.4; 24; 240; 2400 ppb, 24 h | glioblastoma (A-172) | NPs were not toxic but NPs alter the membrane permeability. | [105] | ||||
| CARBON NPs | ||||||||
| PKH26-labeled MWNTs | 200–400 μm length, 80 μg/mL 0–48 h | glioma (GL261) expressing eGFP | NPs did not affect to cells proliferation. | [21] | ||||
| SWNTs with CpG oligodeoxynucleotides | 5 µg/mL, 24 h | glioma (GL261) expressing eGFP and luciferase- | No toxicity in gliomas. | [110] | ||||
| Protoporphyrin IX (PX)-modified oxidized mesoporous carbon nanospheres | 90 nm, 0–50 µg/mL, 24 h | Neuroblastoma (SH-SY5Y) | No toxicity during a 24 h treatment. NPs with ultrasounds were protective through the decrease in Aβ-mediated cellular toxicity. | [128] | ||||
| DENDRIMERS | ||||||||
| Sialic acid | 0–15 µM 24 h | Neuroblastoma (SH-SY5Y) | Attenuation of Aβ induced neurotoxicity. | [129] | ||||
| POLY(LACTIDE-CO-GLYCOLIC ACID) NPs, POLIMERIC NPs | ||||||||
| G-protein estrogen receptor (GPER-1) selective agonist was encapsulated in polymeric NPs and liposomes, coated with polyssorbate-80 | 84.36 nm, 1, 10, 100 mg/mL 15 h and 24 h for N2a; 12 h and 24 h for SHSY5Y | neuroblastoma (N2a and SHSY5Y) | NPs were not toxic to cells at 324 µg/mL, but were taken up by cells. | [51] | ||||
| PLGA, PLG NPs with curcumin | 101 nm, 0.5 µM, 1 h | human neuroblastoma (SK-N-SH) | Prevention of the phosphorylation of Akt and Tau proteins in cells after induction by H2O2. anti-inflammatory and antioxidant activities of NPs. | [49] | ||||
| Rosmarinic acid- and curcumin-loaded polyacrylamide-cardiolipin- PLGA, PLG NPs with conjugated 83-14 monoclonal antibody | 40 μg/mL, 24, 36 h | neuroblastoma (SK-N-MC) | The protective role of NPs in cells induced with Aβ peptide. NPs caused the recovery of pp38 and p-S202 expressions to normal levels. | [130] | ||||
| Polymeric nanostructures sulfonated and sulfated NPs | 40 nm, 1.6 µg/mL 24 h | neuroblastoma (SH-SY5Y) | The protective role of NPs in cells induced by toxic Aβ peptide through decreasing caspase-3 activity and increasing cell viability. | [131] | ||||
| LIPID NPs | ||||||||
| Linear polyethyleneimine (LPEI)-g-PEG copolymer-based micellar nanoparticle with siRNA | <100 nm, 24 h | neuroblastoma (N2a) | No toxicity. The viability was not decreased. | [132] | ||||
| Spongosome and cubosome lipid NPs co-encapsulate curcumin and fish oil, rich in ω-3 polyunsaturated fatty acids | 100 and 400 nm, 300 and 500 nM, 24 h | neuroblastoma (SH-SY5Y) | The cytotoxicity of the blank and antioxidant-loaded nanocarriers was negligible. The protective effect in cells induced by H2O2. | [133] | ||||
| OTHERS | ||||||||
| Chitosan NPs copolymerized with PLGA, PLG | 110 nm, 40 µg/mL 24 h, 96 h | neuroblastoma (SH-SY5Y) | The protective effect in cells against toxicity induced by Aβ peptide. | [134] | ||||
| Lactoferrin NPs with curcumin | 43–60 nm, 2 µM 24 h | neuroblastoma (SK-N-SH) | The cells were rescued from rotenone-induced neurotoxicity after NPs treatment. Antioxidant activity of NPs. | [48] | ||||
| Nanocurcumin with BSA | 153 nm, 0–500 nM, 24 h | neuroblastoma (SH-SY5Y) | NPs prevented cell death induced by 6-hydroxydopamine. The reversion of decrement p-Akt/t-Akt ratio in cells. | [135] | ||||
| Apolipoprotein E3 mediated poly(butyl) cyanoacrylate NPs containing curcumin | 178 nm, 10, 100, 1000 nM, 24 h | neuroblastoma (SH-SY5Y) | The protective role in cells induced by Aβ peptide (antioxidant effect of NPs). The decrease in apoptotic cell population. | [136] | ||||
| Natural brain penetration enhancer-modified | 100–200 nm, 50 ng/mL, 4 h | glioma (C6) | NPs showed good biocompatibility and negligible cytotoxicity. | [60] | ||||
| Toxic Effect | |||||||
|---|---|---|---|---|---|---|---|
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | |||
| SILICA NPs | |||||||
| SiO2NPs modified with aminopropyl-triethoxysilane | 15 nm, 20 μg/rat, 1 day, 7 days | adult rats | Induction of the oxidative stress and an increased inflammatory response in the striatum. The decrease in neurotransmitter dopamine and the downregulation of tyrosine hydroxylase protein in the brain. | [65] | |||
| None | 115 nm, 8 mg/kg, 1 and 2 months | male C57BL/6 N mice | Mood dysfunction and cognitive impairment and neurodegeneration-like pathology and synaptic changes via ERK activation. | [137] | |||
| None | 6, 20 and 50 nm, 150 μg/mL, 28 days | Wistar male rats | SOD and CAT activity was increased in the brain. The MDA level was increased in the brain and degenerative changes in the nerve fibers. | [138] | |||
| None | 509.3, 356.0, 469.0 nm, 5 μg/mL, 3 months | male transgenic mice expressing A53T human a-Syn (a-Syn A53T Tg mice) | NPs promoted PD-like pathology including α-Synuclein aggregation and dopaminergic neuronal degeneration. Mitochondria impairment, oxidative stress, autophagy dysfunction, and neuronal apoptosis. | [84] | |||
| None | 50 and 500 nm, 20 mg/kg, 1 h, 2 h, 24 h and 28 days | mice | Degeneration of neurons. The regulation of apoptosis by regulating Bax and Bcl-XL expression. Elevation of the autophagic responses. NPs administered via the intranasal instillation route resulted in more severe brain lesions compared to the intravenous injection route. | [139] | |||
| None | 40 and 80 mg/kg, 14 days | rats | NPs passed from the BBB into the brain. The reduction in the activity of SOD and CAT. Cellular morphological modifications, mitochondrial dysfunction, and oxidative stress. | [140] | |||
| CARBON NPs | |||||||
| MWNTs modified with carboxyl groups | 5–40 µg/mL, 2, 4, 7 days | C57BL/6J mice | The neurotransmitter level was decreased. The increase in the NOS release. High levels of nNOS expression in regions associated with regulation of sympathetic nerve activity. | [111] | |||
| MWNTs | 5 mg/m3, 5 h | male Sprague–Dawley rats | Inhalation of NPs significantly changes the balance between sympathetic and parasympathetic nervous system. | [88] | |||
| MWNTs shortened by oxidation and functionalized with amino groups (oxMWNT-NH3+) or functionalized with amino groups (MWNT-NH3+) | 20–30 nm, 500 ng/mouse, 30 days post-injection | female C57/Bl6 mice | The increase in pro-inflammatory cytokine gene expression. oxMWNT-NH3+ induced a higher expression of pro-inflammatory cytokines compared to MWNT-NH3+. In addition, oxMWNT-NH3+ induced higher expression of GFAP and CD11b. | [70] | |||
| MWNTs | 49 nm, 10 or 40 μg/mouse, 4 h | male C57BL/6J mice | Neuroinflammatory responses depend on the disruption of BBB integrity. | [141] | |||
| SWNTs modified with PEG | 10 to 1000; or 1000 to 10,000 nm, 0.5, 1.0, and 2.1 mg/mL, 24 h and 30 min | Wistar male rats | Impairment of fear memory retrieval. Lipid peroxidation in the hippocampus. | [142] | |||
| DENDRIMERS | |||||||
| G4 and G4-C12 modified PAMAM | 1 μM, 24 h | C57/BL6-j mice | Higher concentrations of G4-C12 PAMAM dendrimer were toxic and caused the apoptosis process, while G4 PAMAM accumulation did not show any sign of apoptosis. Low level of glial activation. | [25] | |||
| OTHERS | |||||||
| Chitosan NP modified with Polysorbate 80 | 251 nm, 3, 10, 30 mg/kg, 0.5, 2, 4, 8, and 24 h, 7 days | Sprague-Dawley male rats | NPs can enter the brain and induce the apoptosis and necrosis of neurons, slight inflammatory response in the frontal cortex. The decrease in GFAP expression in the cerebellum. | [143] | |||
| Polibuthylcyanoacrylate NPs modified with Polysorbate 80 and polystyrene NPs modified with dalargin | 200 nm, 13.5 mg/kg, 5 min | mice | The locomotor activity decreased. | [144] | |||
| Polystyrene NPs, COOH-modified | 80, 100, 200 nm, 7 days | mice after the aerosol inhalation | NPs with a size of 80 nm can deposit in the brain of mice via aerosol inhalation triggering neuron toxicity and altering the animal behavior. Inhibition of aChE activities. | [145] | |||
| Non-toxic Effect | |||||||
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | |||
| CARBON NPs | |||||||
| MWNTs, NH2-functionalized | 18.9 nm, [111In]- MWNTs (50 µg, 0.5 MBq) in 100 mL PBS,5 min, 30 min, 1 h, 4 h and 24 h | C57/Bl6 mice | NPs were present in both brain capillaries and parenchyma fractions. NPs are potential nanocarriers to use for the delivery of drugs. | [32] | |||
| MWNTs, coated with Pluronic F127 | 10–30 nm, 0.5 mg/mL, 3 days | mice | NPs provoked no damage to the overall organization of the brain. | [97] | |||
| MWNTs | ~20 nm, 5 µg, 72 h | GL261-bearing mice | NP uptake occurred by tumor-associated macrophages. No toxicity to mice with glioma. | [146] | |||
| MWNTs, COOH-modified | 40 nm, 1 mg/mL, 7, 28 days | BALB/C mice | The lack of pathological changes in the brain. | [147] | |||
| Fullerene C60 | 200–500 mg/kg, 1, 3, 6, 16, 30 and 160 h | rats | NPs were able to penetrate the BBB, but the toxicity was found to be quite low. | [148] | |||
| Fullerene C60 | 3.4 mg/kg, 10–12 month | mice | No health deterioration in mice. No significant effect on body weight, spontaneous locomotor activity, and grip strength. Fasting blood glucose and glucose tolerance are not affected. No changes in the blood parameters in mice. No significant influence of NPs on organ weight except for a higher kidney weight in males compared with females. | [149] | |||
| Nanodiamond | 50 nm, 20 mg/kg, 28 days | mice | Very low NPs concentration was detected in the brain. | [150] | |||
| Nanodiamond | 114.7 nm, 10 μL of NPs at a concentration of 100 mg/mL, 10 min | rats | NPs did not induce cytotoxicity in primary neurons from either central (CNS) or peripheral nervous system (PNS) and did not affect animal behavior. | [37] | |||
| Carbon nano-onion | 15 nm, 10 mL per g body weight of 1.0 mg/mL of NPs, 4, 12, 24 h | FVB/N mice | Carbon nano-onion crosses not only through the BBB into the brain of leukoencephalopathy mice but also through the glioblastoma multiforme-induced mice. | [151] | |||
| DENDRIMERS | |||||||
| PEG | 1.9 nm, 55 mg/kg, 1, 4 and 24 h | cerebral palsy rabbits model, murine orthotopic model of glioblastoma | NPs were taken up by the brain and accumulated in the corpus callosum (white matter), hippocampus, and cortex. They fully penetrate and distribute throughout the solid tumor. | [107] | |||
| Modified with PEG, lactoferrin and DNA | 50 µg/mouse, 2 h | Balb/c mice | Lactoferrin improved the NP uptake. NPs can be a potential non-viral gene vector to the brain via noninvasive administration. | [152] | |||
| Dendrimers and dendriplexes loaded with siRNA | 15 mg/kg in 200μL PBS, 1 and 24 h | BALB/c mice | NPs were present inside the brain, but there was no specific brain histology alterations. | [90] | |||
| N-acetyl cysteine and valproic acid | ∼5 mg/kg dogs, 48 h | dogs | NPs were not toxic but were present in the brain. Dendrimers with drugs improved neurological outcome in injured brain. | [153] | |||
| None | ~6.7 and ~4.3 nm, 6 mg/kg, 48 h | dogs (brain injury model) | Generation 6 dendrimers showed extended blood circulation times and increased accumulation in the injured brain compared to generation 4 dendrimers, which were undetectable in the brain by 48 h after final administration. | [30] | |||
| Ethylenediamine-core | 4 nm, 2.5 µg of dendrimer in 5 µL of PBS, 24 h | white rabbits | The high uptake of NPs into astrocytes and microglia cells. | [26] | |||
| LIPID NPs | |||||||
| Liposomes modified with BSA | 118.2–185.8 nm, 11 and 25 mg lipid /kg, 1, 3, 6 and 24 h | Wistar rats | NPs were able to move into brain tissue. NP uptake is dependent on the presence of BSA. NPs are a promising tool in drug delivery to the CNS. | [74] | |||
| Liposomes modified with PEG | 40, 80, 200 nm, 1 mM, 1, 6, 24 and 48 h | rats | Liposomes accumulated in a subpopulation of perivascular cells within the brain dependent on charge and PEG coated. NPs are a promising tool in drug delivery to the CNS. | [154] | |||
| D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) coated liposomes with resveratrol | 107.8, 212.5 and 262.3 nm, 2 mg/kg of resveratrol, 0, 0.083, 0.25, 1, 2, 4, 8, 12, 24, 36 and 48 h | Charles Foster rats | NPs showed high accumulation in the brain but are biocompatible and safe. | [54] | |||
| Liposomes with phospholipid, a polymer surfactant and cholesterol | <200 nm, 10 and 150 µL form 20 nM solution, 4 and 24 h | rats and C57/BL6 mice | NPs were delivered to myelinated peripheral nerves. The liposomes were presented in choroid epithelium, but not in myelinated white matter regions or in brain parenchyma. NPs are a promising tool in drug delivery to the CNS. | [155] | |||
| Micellar linear polyethyleneimine (LPEI)-g-polyethylene glycol (PEG) copolymer NPs | below 100 nm, 0.6%, 0.8% and 1.2% PEG grafting density in LPEI-g-PEG/Bace NPs, from 2 to 7 days | C57BL/6J mice | NPs did not impact on the activation of astrocytes and microglial cells in ipsilateral hippocampus of mice. No inflammation and no cytotoxicity in the brain. Normal morphology of astrocytes and microglia. | [132] | |||
| POLY(LACTIC-CO-GLYCOLIC ACID) AND POLYMERIC NPs | |||||||
| PLGA, PLG NPs modified with a 7-aminoacid glycopeptide and with albumin | 234 nm, 2 mg NPs, 2 h | C57BL/6 mouse | NPs were able to deliver low molecular weight molecules-albumin across the BBB in two murine models of lysosomal storage disorders. NPs are a promising tool in drug delivery to the CNS. | [156] | |||
| PEG/PLA NPs, modified with cationic BSA | 80 nm, 60 mg/kg, 30 min | mice | NPs were observed in the lateral ventricle, third ventricle and periventricular region of the brain. NPs are a promising tool in drug delivery to the CNS with low toxicity. | [64] | |||
| PLGA, PLG NPs modified with glycopeptide (g7) | 200 nm, 1 mL of NPs (8 mg) suspension, 3 h | Balb/c mice | NPs can enter to the brain. NPs are a promising tool in drug delivery to the CNS. | [157] | |||
| PLGA, PLG -tocopheryl PEG 1000 succinate blend NPs with resveratrol | 175.5, 199.7 and 222.5 nm, 2 mg/kg of resveratrol, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36 and 48 h | Charles Foster rats | NPs showed high accumulation in the brain. NPs are a promising tool in cancer therapy, and they are safe. | [59] | |||
| PLGA, PLG tocopheryl PEG succinate diblock copolymer modified with transferrin | 161.5, 121.6, 137.6 nm, 5 mL/kg, 4 h | Sprague-Dawley rats | NPs were present in the brain. NPs could be able to deliver imaging/therapeutic agents across the BBB. | [58] | |||
| 12-amino-acid-peptide conjugated onto the surface of PLGA, PLG PEG NPs | 121 nm, 30 µg/kg, 0.083 h, 0.25 h, 0.5 h,1 h, 2 h, 4 h, 8 h,12 h and 24 h | nude mice | Enhanced brain accumulation efficiency together with lower accumulation in the liver and spleen was observed in the mice intravenously. Injection with peptide- conjugated NPs compared with plain NPs, shows powerful brain selectivity. NPs could be able to deliver imaging/therapeutic agents across the BBB. | [101] | |||
| PLGA, PLG NPs modified with ephrin type-A receptor 3 tyrosine kinase antibody | 145 nm, 0.5 mg/kg, 1, 2, 4, and 8 h | Sprague-Dawley rats | Anti-EPHA3-modified NPs showed high fluorescence intensity in the brain and effectively accumulated in glioma tissues. A significantly higher tumor cell apoptosis. | [57] | |||
| PLGA, PLG 1,2-distearoyl-glycerol-3-phospho-ethanolamine-N-[methoxy(PEG)-2000] ammonium salt hybrid NPs modified with curcumin | 169 nm, 25 µM, 5 days | Wistar rats | Tumor size decreased significantly after 5 days. | [158] | |||
| PLGA, PLG NPs modified with polysorbate 80, poloxamer 188 and chitosan | 250 to 400 nm, 100 mg/mL, 0, 15, 60, 120, 240 min | rats | NPs concentrations in the brain were increased by NP surface modification. NPs could be able to deliver therapeutic agents across the BBB. | [77] | |||
| PLGA, PLG NPs modified with cationic BSA | 97, 98, 104 nm, 30 mg/ kg, 0.033 h and 1 h | BALB/c mice | The increase in surface density of the NPs enhanced the BBB permeability. NPs are a promising tool in drug delivery to the CNS. | [159] | |||
| PEG-poly(ε-caprolactone) polymersomes modified with cationic BSA | 95 nm, 10 mg/kg, 0.5, 1, 2, and 4 h | Sprague-Dawley rats | NPs can enter to the brain and might be a promising carrier for drug delivery to the brain. | [75] | |||
| ALBUMIN NPs | |||||||
| None | 143 and 151 nm, 1 µL from 10 µg/mL rats and 100 mg/ kg mice, 1,7, 14 days | adult rats and mice | NPs located in different brain tissues but did not induce an inflammatory response. No locomotor, explorative, or cognitive function impairment. | [160] | |||
| Polysorbate 80 or with attached apolipoprotein E | 249 nm, 200 μg of NPs/g body weight, 15 and 30 min | female SV 129 mice | The uptake was dependent on the presence of apolipoprotein E. NPs with apolipoprotein E were taken up less by animal brain cells. NPs are a promising tool in drug delivery to the CNS. | [67] | |||
| borneol, muscone and menthol | 100–200 nm, 40 mg/kg, 1, 2, 4, 8, 12, and 24 h | BALB/c mice | Accumulate in glioma cells with a much higher targeting efficiency than that of transferrin-modified NPs. | [60] | |||
| CHITOSAN NPs | |||||||
| Thymoquinone-encapsulated | between 150 and 200 nm, 500 μg of TQ/25 μL, 0.25, 0.5, 2, 4, 6, 24 h | Wistar rats | Taken up by brain tissue. NPs are a promising tool in drug delivery to the CNS. | [161] | |||
| OTHERS | |||||||
| Poly(n-butyl cyanoacrylate) dextran polymers coated with polysorbate 80 | 48 nm, 200 µL from 60 mg/mL of NPs, 2 days | APP/PS1 mice (AD model) | No induction of non-specific BBB disruption. NPs collaborate with plasma apolipoprotein E to facilitate BBB crossing. | [66] | |||
| Cyanoacrylate NPs, PEGylated and coated with polysorbate 80 or poloxamine 908 | 137 nm, 30 mg/kg, 60 mg/kg, 30 min; 1 and 4 h | OF1 mice and rats dark agouti DA/R | NPs can penetrate into the brain. Localization in the ependymal cells of the choroid plexuses, in the epithelial cells of pia mater and ventricles and to a lower extent in the capillary endothelial cells of BBB. NPs are a promising tool in drug delivery to the CNS. | [78] | |||
| Polybutylcyanoacrylate NPs coated with polysorbate 80, loaded with curcumin | 152 nm, 5 mg/kg, 0.033; 0.083; 0.25; 0.5; 1.25; 0.75; 1; 2; 3; 4; 8; h | mice | NPs inside the brain tissue. NPs are a promising tool in drug delivery to the CNS. | [162] | |||
| Polysaccharide NPs based on hyaluronic acid and chitosan hydrochloride, functionalized with lactoferrin and loaded with curcumin | >200 nm, 1.25 mg/kg, 24 h | ICR mice | Accumulated in the brain. The presence of lactoferrin improved the NP uptake. NPs can be potentially used for cancer therapy. | [56] | |||
| PLA NPs coated with T-80 | 162 nm, 8 mg/kg, 45 min | mice | Present in the brain. The specific role of T-80 coating on NPs in brain targeting. NPs are a promising tool in drug delivery to the CNS. | [69] | |||
| Polystyrene latex nanospheres | 20, 100 and 1000 nm, 90–120 days | Fischer F344 rats | Low numbers of particles in the brain. | [71] | |||
| Polystyrene NPs COOH-modified and methoxy (MeO)-PEG amine (NH2) | 60 nm, 2.5 mg/kg, 1 and 24 h | Fischer F344 rats | Diffusion in normal brain tissue. Delayed tumor growth following local administration, this effect was dependent on the presence of PEG. | [79] | |||
| PEG and polyethylenimine (complexes of oligonucleotides with 3H-labeled nanogel) | <100 nm, 200 µL, 1 h | mice | Accumulation in the brain. Nanogel is a promising system for the delivery of oligonucleotides to the brain. | [43] | |||
| Gelatin-siloxane NPs modified with SynB peptide | 194 nm, 1 mL, 60 mg/kg, 0.5, 1, 2, and 4 h | nude mice and Sprague-Dawley rats | The presence of peptide improved the NP uptake. No toxicity. | [104] | |||
| Toxic Effect | ||||||
|---|---|---|---|---|---|---|
| Surface Coating and/or NPs | Size, Concentration and Exposure Time | Model | Results | Ref. | ||
| SILICA NPs | ||||||
| None | 62 nm, 25, 50, 100, 200 µg/mL, 4–96 h | zebrafish embryos (D. rerio) | Embryonic developmental toxicity, resulted in persistent effects on larval behavior. | [173] | ||
| CARBON NPs | ||||||
| Fullerenes aqueous suspended colloids (nC60) | 0.5 ppm and 1 ppm, 48 h | juvenile largemouth bass | Lipid peroxidation in brain after 48 h of exposure. | [171] | ||
| Fullerenes (C60), long or short | MWNTs, SWNTs and 0.001 mg/L, 21 days | D. rerio | High level of alterations in the DNA/ RNA region, especially in the brain. | [172] | ||
| POLYSTYRENE NPs | ||||||
| None | 50 nm, 0, 1, 10, 100 and 1000 μg/L, 72 h | Caenorhabditis elegans used as a model organism to evaluate the neurodevelopmental toxicity | Significant inhibition in body length, survival rate, head thrashes, and body bending. The increase in ROS production. The lipofuscin accumulation, apoptosis and decrease in dopamine contents. pink-1 gene was involved in the polystyrene NPs-induced neurotoxicity. NPs at concentration of 100 μg/L caused up-regulation of 89 genes and down-regulation of 56 genes regulated differently expressed genes correlated with biological function of cuticle development and molting cycle. The neurodevelopmental toxicity and oxidative stress responses induced by NPs were regulated by dpy-5 and rol-6. | [175] | ||
| NH2-modified, positively charged | 25–330 nm, 0.005 g/L to 0.150 g/L, 24 h | Daphnia magna | The alterations in behavioral patterns, decreased brain mass and morphological changes in the cerebral gyri. | [167] | ||
| None | <100 nm, 100 mg/L NPs, 15 days | Cyprinus carpio | Degrees of necrosis, fibrosis, changes in blood capillaries, tissue detachment, edema, degenerated connective tissues, and necrosis in large cerebellar neurons and ganglion cells. The activity of acetylcholinesterase (aChE) significantly decreased after exposure to 100 mg/L. | [168] | ||
| None | 50 and 200 nm, 10–10,000 parts per billion, 5 days | larval zebrafish (D. rerio) | Accumulation in the tissues of larval zebrafish, alteration of their transcriptome, and changes in behavior and physiology. Potentially decreasing organismal fitness in contaminated ecosystems. | [174] | ||
| None | 50 nm, 1 mg/L, till 120 h post fertilization | D. rerio | Neurotoxicity. Suppression of locomotion activity. | [176] | ||
| Fluorescent NPs | 51 nm, 0.1, 1, 10 ppm, until 120 h post fertilization | D. rerio | Accumulation in the brain and alteration of larval behavior. | [177] | ||
| Fluorescent NPs | 70 nm, 0.5, 1.5, 5 ppm, 7 days, 30 days and 7 week | D. rerio | Alteration of neuro-behavior and neurotransmitter regulation. | [178] | ||
| Fluorescent NPs | 20 nm, 270 ppm, 120 h | D. rerio | NPs can reach brain, oxidative damage and apoptosis. | [179] | ||
| None | 100 nm, 1, 10, 100, 1000 µg/L, Prolonged exposure:from L1-larvae to adult day-1 | C. elegans | Induction of neurodegeneration of D-type GABAergic motor neurons. Alteration of forward and backward movement. | [180] | ||
| None | 0.1, 0.5, 1, 2 and 5 μm, 1 mg/L, 3 days | C. elegans | Excitatory toxicity on locomotive behavior. Damage to cholinergic and GABAergic neurons. | [181] | ||
| None | 0,11 µm, 0.005, 0.05, 0.5, 5 and 50 mg/L, 96 h | Mediterranean mussel (Mytilus galloprovincialis) | Changes in gene expression. Cholinesterase inhibition in hemolymph. | [169] | ||
| NH2-modified, positively charged | 50 nm, 0.1, 1.0, 3.0 and 10.0 mg/L, 48 h up to 14 days | Brine shrimp (Artemia fransiscana) | Cholinesterase inhibition. Glutathione S-Transferase and catalase decreased. | [170] | ||
| None | 40 nm, 10 mg/L, 7 days | Japanese rice fish (Oryzias latipes) | Particle presence in the brain, suggesting penetration of BBB. | [166] | ||
| None | 0,1 µm, 1, 10 and 100 μg/L, 1–14 days | Red tilapia (Oreochromis niloticus) | Particle presence in brain tissue. Inhibition of aChE activity in the brain. | [182] | ||
| NH2-modified, positively charged | 100 mg/L, 64 days | Crucian carp (Carassius carassius) | Particle were present in the brain and caused brain weight loss. Behavioural changes and enlarged cerebral gyri. | [167] | ||
| None | 50 nm, 1 mg/L, 3 days | Zebrafish, larvae (D. rerio) | Particle were present in head, gills and muscle. Decrease aChE activity. | [183] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, K.; Sawicki, K.; Czajka, M.; Kapka-Skrzypczak, L.; Kruszewski, M.; Brzóska, K. Adverse Effects of Non-Metallic Nanoparticles in the Central Nervous System. Materials 2023, 16, 7264. https://doi.org/10.3390/ma16237264
Sikorska K, Sawicki K, Czajka M, Kapka-Skrzypczak L, Kruszewski M, Brzóska K. Adverse Effects of Non-Metallic Nanoparticles in the Central Nervous System. Materials. 2023; 16(23):7264. https://doi.org/10.3390/ma16237264
Chicago/Turabian StyleSikorska, Katarzyna, Krzysztof Sawicki, Magdalena Czajka, Lucyna Kapka-Skrzypczak, Marcin Kruszewski, and Kamil Brzóska. 2023. "Adverse Effects of Non-Metallic Nanoparticles in the Central Nervous System" Materials 16, no. 23: 7264. https://doi.org/10.3390/ma16237264





