Flat-Band Potential Determination and Catalytical Properties of Sn3O4/SnO2 Heterostructures in the Photo-Electrooxidation of Small Organic Molecules under Ultraviolet (370 nm) and Blue (450 nm) Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Physico-Chemical Characterization
2.4. Photoelectrochemical Studies
3. Results
3.1. Physico-Chemical Properties
3.2. Flat-Band Potential Determination
3.3. Photoelectrocatalytic Oxidation of Organic Substrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Chikunova, I.O.; Semeykina, V.S.; Kuznetsov, A.N.; Kalinkin, P.N.; Gribov, E.N.; Parkhomchuk, E.V. Template-assisted synthesis and electrochemical properties of SnO2 as a cathode catalyst support for PEMFC. Ionics 2020, 26, 1861–1873. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review—Recent Advances in Tin Oxide Nanomaterials as Electrochemical/Chemiresistive Sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Reddy, M.V.; Linh, T.T.; Hien, D.T.; Chowdari, B.V.R. SnO2 Based Materials and Their Energy Storage Studies. ACS Sustain. Chem. Eng. 2016, 4, 6268–6276. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Sai Krishna, A.M.; Mukhopadhyay, S.; Dey, A.; et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Mishra, S.R.; Ahmaruzzaman, M. Tin oxide based nanostructured materials: Synthesis and potential applications. Nanoscale 2022, 14, 1566–1605. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Saravanakumar, K.; Lakshmi Prabavathi, S.; Abinaya, M.; Muthuraj, V. Fabrication of novel surface plasmon resonance induced visible light driven iridium decorated SnO2 nanorods for degradation of organic contaminants. J. Alloys Compd. 2018, 763, 512–524. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect engineering on SnO2 nanomaterials for enhanced gas sensing performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- Sethi, Y.A.; Kulkarni, A.K.; Ambalkar, A.A.; Panmand, R.P.; Kulkarni, M.V.; Gosavi, S.W.; Kale, B.B. Efficient solar light-driven hydrogen generation using an Sn3O4 nanoflake/graphene nanoheterostructure. RSC Adv. 2021, 11, 29877–29886. [Google Scholar] [CrossRef]
- Tanabe, T.; Hashimoto, M.; Mibu, K.; Tanikawa, T.; Gunji, T.; Kaneko, S.; Abe, H.; Miyauchi, M.; Matsumoto, F. Synthesis of single phase Sn3O4: Native visible-light-sensitive photocatalyst with high photocatalytic performance for hydrogen evolution. J. Nanosci. Nanotechnol. 2017, 17, 3454–3459. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Jin, W.; Xia, W.; He, J.; Zeng, X. An excellent H2 production photoelectrode based on mixed valence Sn3O4 nanoflake arrays treated by H2O2 hydrothermal reaction. Int. J. Hydrogen Energy 2022, 47, 10594–10602. [Google Scholar] [CrossRef]
- Mone, P.; Mardikar, S.; Balgude, S. Morphology-controlled synthesis of Sn3O4 nanowires for enhanced solar-light driven photocatalytic H2 production. Nano-Struct. Nano-Objects 2020, 24, 100615. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Ren, N.; Liu, J.; Sun, D.; Ding, L.; Liu, H. Top or Bottom, Assembling Modules Determine the Photocatalytic Property of the Sheetlike Nanostructured Hybrid Photocatalyst Composed with Sn3O4 and rGO (GQD). ACS Sustain. Chem. Eng. 2018, 6, 11775–11782. [Google Scholar] [CrossRef]
- Manikandan, M.; Tanabe, T.; Li, P.; Ueda, S.; Ramesh, G.V.; Kodiyath, R.; Wang, J.; Hara, T.; Dakshanamoorthy, A.; Ishihara, S.; et al. Photocatalytic water splitting under visible light by mixed-valence Sn3O4. ACS Appl. Mater. Interfaces 2014, 6, 3790–3793. [Google Scholar] [CrossRef]
- He, Y.; Li, D.; Chen, J.; Shao, Y.; Xian, J.; Zheng, X.; Wang, P. Sn3O4: A novel heterovalent-tin photocatalyst with hierarchical 3D nanostructures under visible light. RSC Adv. 2014, 4, 1266–1269. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Huda, A.; Suman, P.H.; Torquato, L.D.M.; Silva, B.F.; Handoko, C.T.; Gulo, F.; Zanoni, M.V.B.; Orlandi, M.O. Visible light-driven photoelectrocatalytic degradation of acid yellow 17 using Sn3O4 flower-like thin films supported on Ti substrate (Sn3O4/TiO2/Ti). J. Photochem. Photobiol. A Chem. 2019, 376, 196–205. [Google Scholar] [CrossRef]
- Yang, R.; Ji, Y.; Zhang, J.; Zhang, R.; Liu, F.; Chen, Y.; Liang, L.; Han, S.; Yu, X.; Liu, H. Efficiently degradation of polyacrylamide pollution using a full spectrum Sn3O4 nanosheet/Ni foam heterostructure photoelectrocatalyst. Catal. Today 2019, 335, 520–526. [Google Scholar] [CrossRef]
- Yang, R.Q.; Liang, N.; Chen, X.Y.; Wang, L.W.; Song, G.X.; Ji, Y.C.; Ren, N.; Lü, Y.W.; Zhang, J.; Yu, X. Sn/Sn3O4−x heterostructure rich in oxygen vacancies with enhanced visible light photocatalytic oxidation performance. Int. J. Miner. Metall. Mater. 2021, 28, 150–159. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zhang, X.; Zhang, W.; Ma, J.; Wang, Q.; Su, S. Sulfur-doped Sn3O4 nanosheets for improved photocatalytic performance. J. Alloys Compd. 2023, 961, 170904. [Google Scholar] [CrossRef]
- Chen, G.; Lu, S.; Shang, Y.; Zeng, W.; Zhan, H.; Mei, J.; He, Z. Sn3O4-SnO2 heterostructure for highly sensitive and selective NO2 sensing at low operating temperature. Nano Express. 2021, 2, 020021. [Google Scholar] [CrossRef]
- Masteghin, M.G.; Silva, R.A.; Cox, D.C.; Godoi, D.R.M.; Silva, S.R.P.; Orlandi, M.O. The role of surface stoichiometry in NO2 gas sensing using single and multiple nanobelts of tin oxide. Phys. Chem. Chem. Phys. 2021, 23, 9733–9742. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Shi, Y.-T.; Xie, K.-F.; Wang, J.-Y.; Wang, Y.-F.; Zheng, Y.; Fang, S.-M.; Zhang, Y.-H. Engineering of in-plane SnO2-Sn3O4 hierarchical nanoflower heterojunctions for enhanced formaldehyde sensing. Appl. Surf. Sci. 2023, 614, 156110. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, K.; Feng, X.; Wei, C. Novel hierarchical flowers-like Sn3O4 firstly used as anode materials for lithium ion batteries. J. Alloys Compd. 2017, 690, 765–770. [Google Scholar] [CrossRef]
- Chothe, U.P.; Ambalkar, A.A.; Ugale, C.K.; Kulkarni, M.V.; Kale, B.B. Synergy of a heteroatom (P-F) in nanostructured Sn3O4 as an anode for sodium-ion batteries. Sustain. Energy Fuels 2021, 5, 2678–2687. [Google Scholar] [CrossRef]
- Furukawa, M.; Iwamoto, D.; Inamori, K.; Tateishi, I.; Katsumata, H.; Kaneco, S. Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation. Catalysts 2023, 13, 1179. [Google Scholar] [CrossRef]
- Jia, H.; Cao, X.; Pan, A.; Huang, L.; Yin, B.; Chen, J.; Tan, X.; Tang, Y.; Han, M.; Liang, S. Vertically oriented Sn3O4 nanoflakes directly grown on carbon fiber cloth for high-performance lithium storage. Inorg. Chem. Front. 2019, 6, 1468–1474. [Google Scholar] [CrossRef]
- Reddy Pallavolu, M.; Tanaya Das, H.; Anil Kumar, Y.; Naushad, M.; Sambasivam, S.; Hak Jung, J.; Joo, S.W. Marigold flower-like Sn3O4 nanostructures as efficient battery-type electrode material for high-performing asymmetric supercapacitors. J. Electroanal Chem. 2022, 920, 116641. [Google Scholar] [CrossRef]
- Fadaam, S.A.; Ali, H.M.; Salih, A.A.; Obaid, M.A.; Sabeeh Ali, A.; Habubi, N.F. Synthesis and characterization of metastable phases of SnO and Sn3O4 thin films for solar cells applications. J. Phys. Conf. Ser. 2021, 1963, 012003. [Google Scholar] [CrossRef]
- Liu, L.X.; Zhou, Y.; Chang, Y.C.; Zhang, J.R.; Jiang, L.P.; Zhu, W.; Lin, Y. Tuning Sn3O4 for CO2 reduction to formate with ultra-high current density. Nano Energy 2020, 77, 105296. [Google Scholar] [CrossRef]
- Romeiro, F.C.; Silva, B.C.; Martins, A.S.; Zanoni, M.V.B.; Orlandi, M.O. Superior performance of rGO-tin oxide nanocomposite for selective reduction of CO2 to methanol. J. CO2 Util. 2021, 46, 101460. [Google Scholar] [CrossRef]
- Xia, W.; Qian, H.; Zeng, X.; Dong, J.; Wang, J.; Xu, Q. Visible-Light Self-Powered Photodetector and Recoverable Photocatalyst Fabricated from Vertically Aligned Sn3O4 Nanoflakes on Carbon Paper. J. Phys. Chem. C 2017, 121, 19036–19043. [Google Scholar] [CrossRef]
- Berengue, O.M.; Simon, R.A.; Chiquito, A.J.; Dalmaschio, C.J.; Leite, E.R.; Guerreiro, H.A.; Guimarães, F.E.G. Semiconducting Sn3O4 nanobelts: Growth and electronic structure. J. Appl. Phys. 2010, 107, 033717. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, M.; Li, F.; You, Y.; Jiang, D.; Yuan, H.; Ma, L.; Shen, W. SnOx/graphene anode material with multiple oxidation states for high-performance Li-ion batteries. Nanotechnology 2021, 32, 195407. [Google Scholar] [CrossRef]
- Xia, W.; Wang, H.; Zeng, X.; Han, J.; Zhu, J.; Zhou, M.; Wu, S. High-efficiency photocatalytic activity of type II SnO/ Sn3O4 heterostructures via interfacial charge transfer. Crystengcomm 2014, 16, 6841–6847. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Chen, G.; Zhan, H.; Mei, J.; Luo, N.; He, Z.; Tang, C. SnO- Sn3O4 heterostructural gas sensor with high response and selectivity to parts-per-billion-level NO2 at low operating temperature. RSC Adv. 2020, 10, 29843–29854. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Mei, J.; Tang, C.; Luo, K.; Li, S.; Zhan, H.; He, Z. Hierarchical SnO2– Sn3O4 heterostructural gas sensor with high sensitivity and selectivity to NO2. Sens. Actuators B Chem. 2019, 301, 127010. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Du, S.; Ren, Z.; Yu, P.; Wang, X.; Wang, G.; Fu, H. Heterophase engineering of SnO2/ Sn3O4 drives enhanced carbon dioxide electrocatalytic reduction to formic acid. Sci. China Mater. 2020, 63, 2314–2324. [Google Scholar] [CrossRef]
- Huda, A.; Mahendra, I.P.; Ichwani, R.; Handoko, C.T.; Ngoc, H.M.; Yudono, B.; Bustan, M.D.; Gulo, F. High efficient visible-light activated photo catalytic semiconductor SnO2/ Sn3O4 heterostructure in direct blue 71 (DB71) degradation. Rasayan J. Chem. 2019, 12, 308–318. [Google Scholar] [CrossRef]
- Yang, R.; Ji, Y.; Li, Q.; Zhao, Z.; Zhang, R.; Liang, L.; Liu, F.; Chen, Y.; Han, S.; Yu, X.; et al. Ultrafine Si nanowires/ Sn3O4 nanosheets 3D hierarchical heterostructured array as a photoanode with high-efficient photoelectrocatalytic performance. Appl. Catal. B Environ. 2019, 256, 117798. [Google Scholar] [CrossRef]
- Jana, S.; Konar, S.; Mitra, B.C.; Mondal, A.; Mukhopadhyay, S. Fabrication of a new heterostructure Au/Pt/SnO2: An excellent catalyst for fast reduction of para-nitrophenol and visible light assisted photodegradation of dyes. Mater. Res. Bull. 2021, 141, 111351. [Google Scholar] [CrossRef]
- Chen, L.; Yue, S.; Wang, J.; Chen, W.; Zhang, Y.; Xie, M.; Han, W. Overall water splitting on surface-polarized Sn3O4 through weakening the trap of Sn(II) to holes. Appl. Catal. B Environ. 2021, 299, 120689. [Google Scholar] [CrossRef]
- Tsybulya, S.V.; Cherepanova, S.V.; Soloviyova, L.P. Polycrystal software package for IBM/PC. J. Struct. Chem. 1996, 37, 332–334. [Google Scholar] [CrossRef]
- Eckold, P.; Rolff, M.; Niewa, R.; Hügel, W. Synthesis, characterization and in situ Raman detection of Sn3O2(OH)2-xClx phases as intermediates in tin corrosion. Corros. Sci. 2015, 98, 399–405. [Google Scholar] [CrossRef]
- Gaur, L.K.; Chandra Mathpal, M.; Kumar, P.; Gairola, S.P.; Agrahari, V.; Martinez, M.A.R.; Aragon, F.F.H.; Soler, M.A.G.; Swart, H.C.; Agarwal, A. Observations of phonon anharmonicity and microstructure changes by the laser power dependent Raman spectra in Co doped SnO2 nanoparticles. J. Alloys Compd. 2020, 831, 154836. [Google Scholar] [CrossRef]
- Yu, K.N.; Xiong, Y.; Liu, Y.; Xiong, C. Microstructural change of nano-SnO2 grain assemblages with the annealing temperature. Phys. Rev. B 1997, 55, 2666. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Wiercigroch, E.; Malek, K.; Kozieł, M.; Andrzejczuk, M.; Marzec, M.M.; Jarosz, M.; Brzózka, A.; Sulka, G.D. The effect of anodizing potential and annealing conditions on the morphology, composition and photoelectrochemical activity of porous anodic tin oxide films. Electrochim. Acta 2019, 319, 18–30. [Google Scholar] [CrossRef]
- Ilka, M.; Bera, S.; Kwon, S.H. Influence of surface defects and size on photochemical properties of SnO2 nanoparticles. Materials 2018, 11, 904. [Google Scholar] [CrossRef]
- Kar, A.; Olszówka, J.; Sain, S.; Sloman, S.R.I.; Montes, O.; Fernández, A.; Pradhan, S.K.; Wheatley, A.E.H. Morphological effects on the photocatalytic properties of SnO2 nanostructures. J. Alloys Compd. 2019, 810, 151718. [Google Scholar] [CrossRef]
- Liu, L.Z.; Wu, X.L.; Gao, F.; Shen, J.C.; Li, T.H.; Chu, P.K. Determination of surface oxygen vacancy position in SnO2 nanocrystals by Raman spectroscopy. Solid State Commun. 2011, 151, 811–814. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Miao, C.; Jiang, W.; Li, H.; Liu, C.; Che, G. An S-scheme photocatalyst constructed by modifying Ni-doped Sn3O4 micro-flowers on g-C3N4 nanosheets for enhanced visible-light-driven hydrogen evolution. J. Ind. Eng. Chem. 2022, 113, 380–388. [Google Scholar] [CrossRef]
- Babu, B.; Neelakanta Reddy, I.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Du, C.; Zhao, Q.; Liu, Z. Self-doping for visible light photocatalytic purposes: Construction of SiO2/SnO2/SnO2:Sn2+ nanostructures with tunable optical and photocatalytic performance. RSC Adv. 2014, 4, 30820–30827. [Google Scholar] [CrossRef]
- Davar, F.; Salavati-Niasari, M.; Fereshteh, Z. Synthesis and characterization of SnO2 nanoparticles by thermal decomposition of new inorganic precursor. J. Alloys Compd. 2010, 496, 638–643. [Google Scholar] [CrossRef]
- Chuvenkova, O.A.; Domashevskaya, E.P.; Ryabtsev, S.V.; Yurakov, Y.A.; Popov, A.E.; Koyuda, D.A.; Nesterov, D.N.; Spirin, D.E.; Ovsyannikov, R.Y.; Turishchev, S.Y. XANES and XPS investigations of surface defects in wire-like SnO2 crystals. Phys. Solid State 2015, 57, 153–161. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Choso, T.; Karasuda, T.; Shimomura, S.; Ouyang, F.; Tabata, K.; Yamaguchi, Y. Photoemission study of the interaction of a reduced thin film SnO2 with oxygen. Surf. Sci. 1999, 433, 226–229. [Google Scholar] [CrossRef]
- Gribov, E.N.; Koshevoy, E.I.; Chikunova, I.O.; Parmon, V.N. Template-Assisted SnO2: Synthesis, Composition, and Photoelectrocatalytical Properties. Catalysts 2023, 13, 168. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Koshevoy, E.; Gribov, E.; Polskikh, D.; Lyulyukin, M.; Solovyeva, M.; Cherepanova, S.; Kozlov, D.; Selishchev, D. Photoelectrochemical Methods for Determination of Flat-Band Potential in Semiconducting Photocatalysts: A Comparison Study. Langmuir 2023, 39, 13466–13480. [Google Scholar] [CrossRef]
- Patel, M.Y.; Mortelliti, M.J.; Dempsey, J.L. A compendium and meta-analysis of flatband potentials for TiO2, ZnO, and SnO2 semiconductors in aqueous media. Chem. Phys. Rev. 2022, 3, 011303. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yamaguchi, A.; Yang, Y.; Aisnada, A.N.E.; Uchida, S.; Abe, H.; Ueda, S.; Yamaguchi, K.; Tanabe, T.; Miyauchi, M. Synthesis and Characterization of the Orthorhombic Sn3O4 Polymorph. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300640. [Google Scholar] [CrossRef] [PubMed]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Al-Azri, Z.H.N.; Dosado, A.G.; Nadeem, M.A.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohol–water mixtures. J. Catal. 2015, 329, 499–513. [Google Scholar] [CrossRef]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Xu, W.; Raftery, D.; Francisco, J.S. Effect of Irradiation Sources and Oxygen Concentration on the Photocatalytic Oxidation of 2-Propanol and Acetone Studied by in Situ FTIR. J. Phys. Chem. B 2003, 107, 4537–4544. [Google Scholar] [CrossRef]
- Gu, Q.; Fu, X.; Wang, X.; Chen, S.; Leung, D.Y.C.; Xie, X. Photocatalytic reforming of C3-polyols for H2 production. Appl. Catal. B Environ. 2011, 106, 689–696. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.; Zhu, Y.; Wu, Y. Hydrogen production by photoelectrochemically splitting solutions of formic acid. ChemSusChem 2011, 4, 1475–1480. [Google Scholar] [CrossRef]
- Zielinska, B.; Borowiakpalen, E.; Kalenczuk, R. Photocatalytic hydrogen generation over alkaline-earth titanates in the presence of electron donors. Int. J. Hydrogen Energy 2008, 33, 1797–1802. [Google Scholar] [CrossRef]
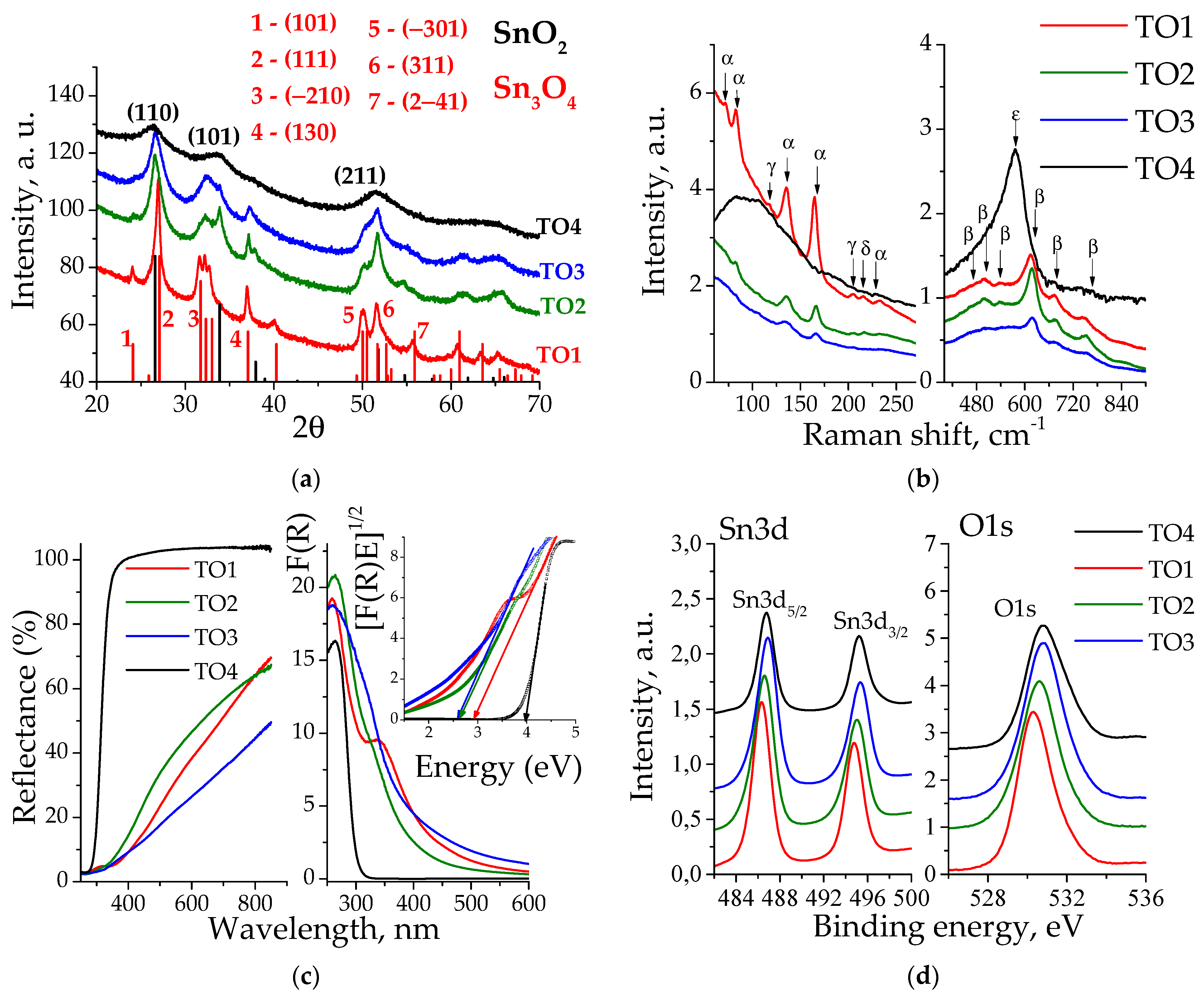

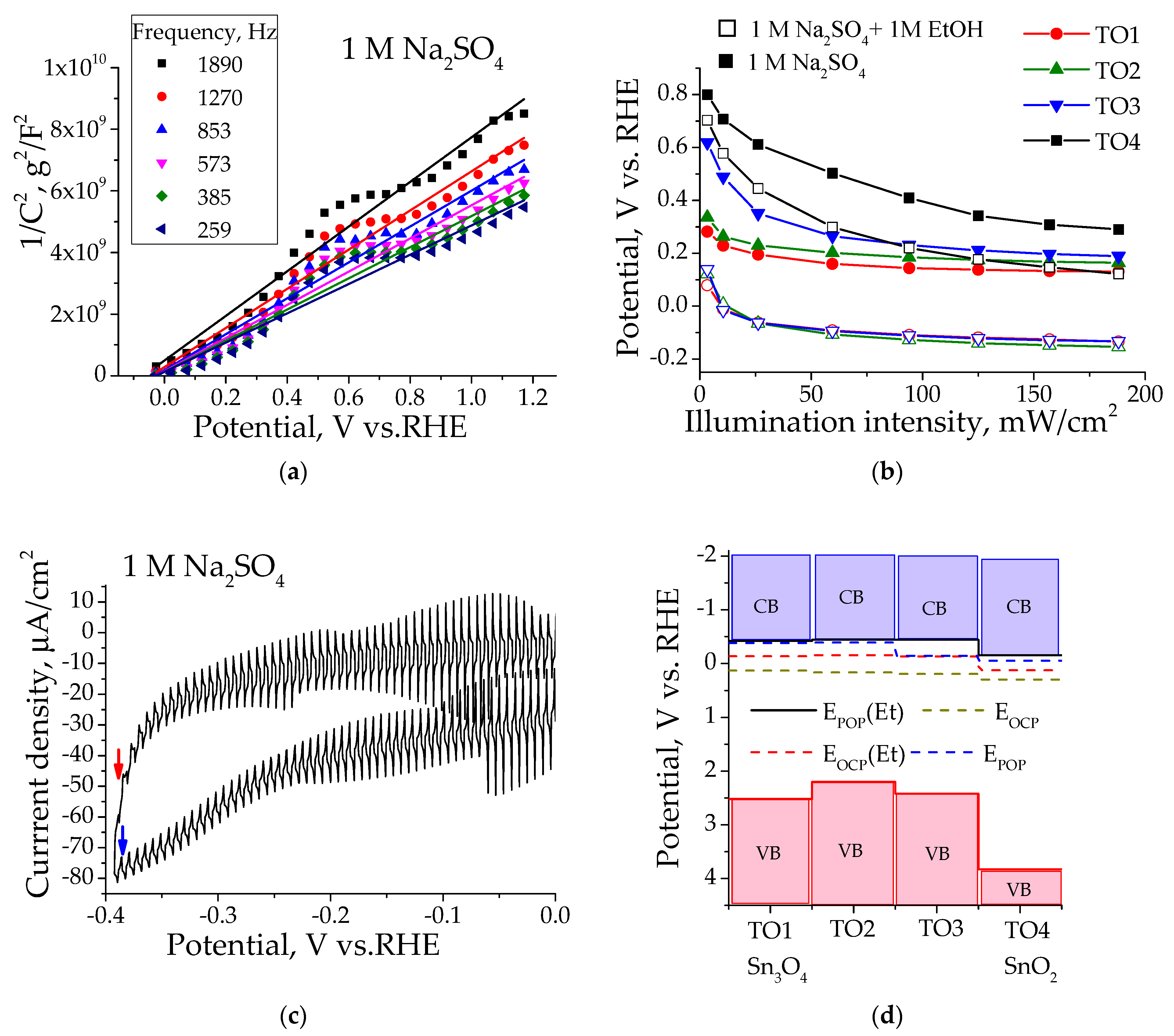
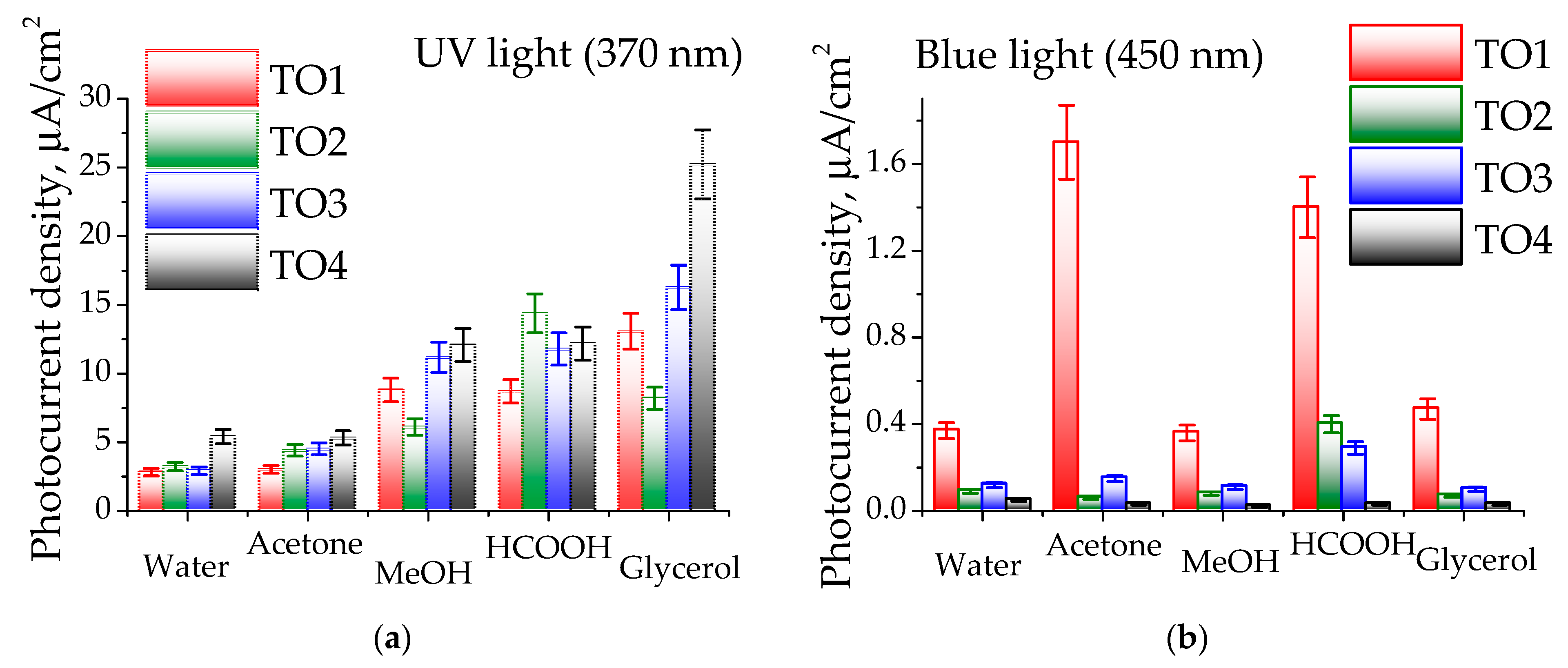
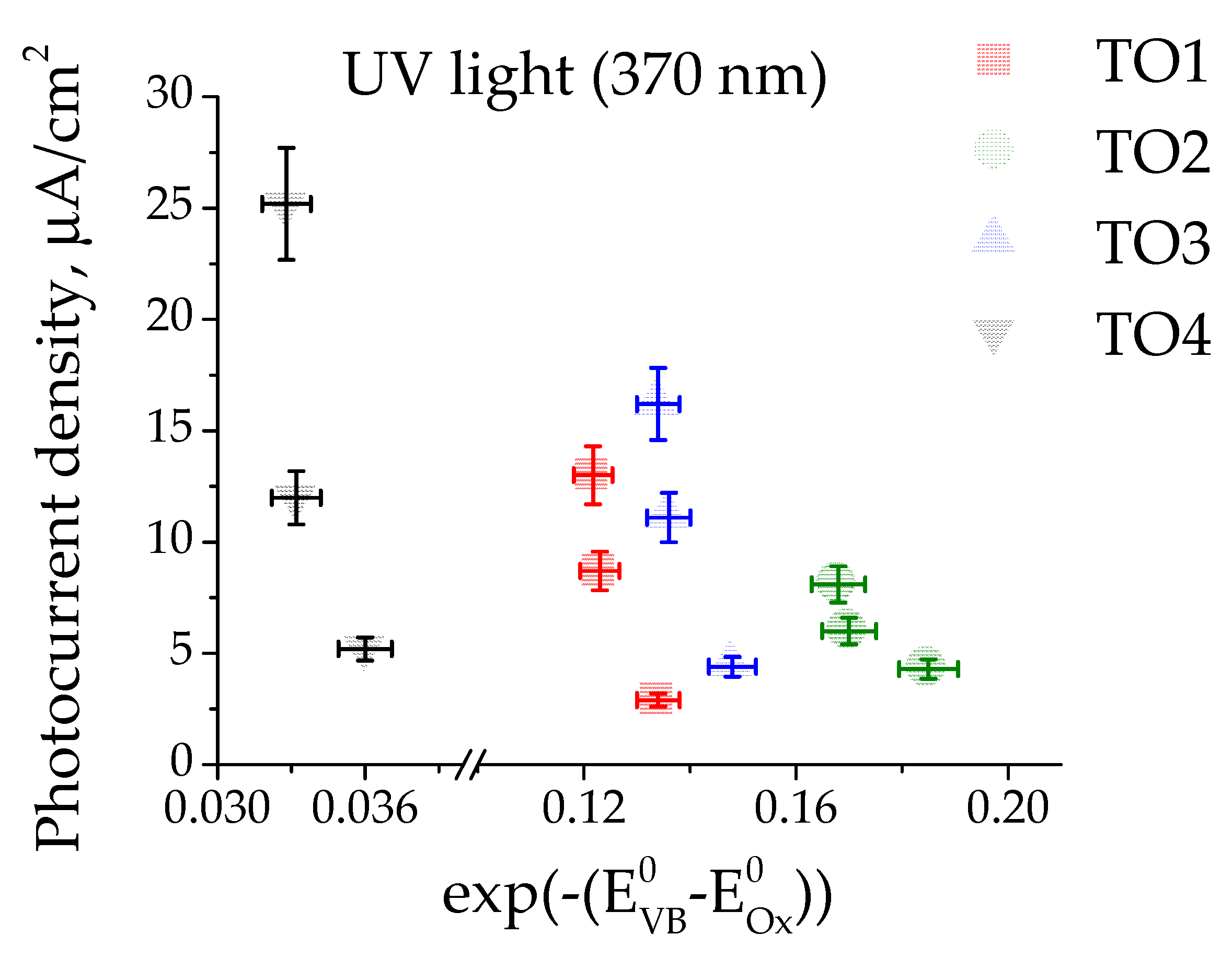
| Sample | Sn2+, % 1 | Sn4+, % 1 | O1, % 2 | O2, % 2 | Surface Composition |
|---|---|---|---|---|---|
| TO1 | 54 | 46 | 81 | 19 | SnO1.25 |
| TO2 | 38 | 62 | 94 | 6 | SnO1.3 |
| TO3 | 28 | 72 | 91 | 9 | SnO1.43 |
| TO4 | 0 | 100 | 81 3 | 19 4 | SnO1.88 |
| Sample | CSR 1, nm | Phase Composition, % | Band-Gap Width, eV | SBET 2, m2/g | Vpore 3, cm3/g | ||
|---|---|---|---|---|---|---|---|
| Sn3O4 | SnO2 | Sn3O4 | SnO2 | ||||
| TO1 | 27 | - | 100 | 0 | 2.94 | 38.5 | 0.077 |
| TO2 | 24 | 9 | 46 | 54 | 2.64 | 63 | 0.131 |
| TO3 | 12 | 5.5 | 38 | 62 | 2.86 | 95.9 | 0.098 |
| TO4 | - | 3 | 0 | 100 | 3.98 | 2.05 | 0.008 |
| Sample | EMS, V | EPOP, V | EOCP, V | EFB, V |
|---|---|---|---|---|
| TO1 | −0.06 ± 0.02 | −0.38 | 0.127 | −0.42 |
| TO1 + EtOH | −0.04 ± 0.02 | −0.42 | −0.133 | |
| TO2 | −0.04 ± 0.04 | −0.39 | 0.163 | −0.44 |
| TO2 + EtOH | −0.01 ± 0.03 | −0.44 | −0.155 | |
| TO3 | 0.04 ± 0.03 | −0.14 | 0.191 | −0.44 |
| TO3 + EtOH | 0.01 ± 0.03 | −0.44 | −0.131 | |
| TO4 | −0.11 ± 0.03 | −0.05 | 0.295 | −0.15 |
| TO4 + EtOH | −0.26 ± 0.04 | −0.15 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribov, E.; Koshevoy, E.; Kuznetsov, A.; Mikhnenko, M.; Losev, E.; Lyulyukin, M. Flat-Band Potential Determination and Catalytical Properties of Sn3O4/SnO2 Heterostructures in the Photo-Electrooxidation of Small Organic Molecules under Ultraviolet (370 nm) and Blue (450 nm) Light. Materials 2023, 16, 7300. https://doi.org/10.3390/ma16237300
Gribov E, Koshevoy E, Kuznetsov A, Mikhnenko M, Losev E, Lyulyukin M. Flat-Band Potential Determination and Catalytical Properties of Sn3O4/SnO2 Heterostructures in the Photo-Electrooxidation of Small Organic Molecules under Ultraviolet (370 nm) and Blue (450 nm) Light. Materials. 2023; 16(23):7300. https://doi.org/10.3390/ma16237300
Chicago/Turabian StyleGribov, Evgeny, Evgeny Koshevoy, Aleksey Kuznetsov, Maxim Mikhnenko, Evgeniy Losev, and Mikhail Lyulyukin. 2023. "Flat-Band Potential Determination and Catalytical Properties of Sn3O4/SnO2 Heterostructures in the Photo-Electrooxidation of Small Organic Molecules under Ultraviolet (370 nm) and Blue (450 nm) Light" Materials 16, no. 23: 7300. https://doi.org/10.3390/ma16237300






