Control of the Composition and Morphology of Non-Metallic Inclusions in Superduplex Stainless Steel
Abstract
1. Introduction
This Work
2. Materials and Methods
2.1. Experimental Procedure
2.2. Calculation Method
3. Results
3.1. Analysis of Non-Metallic Inclusions in Experimental Ingots
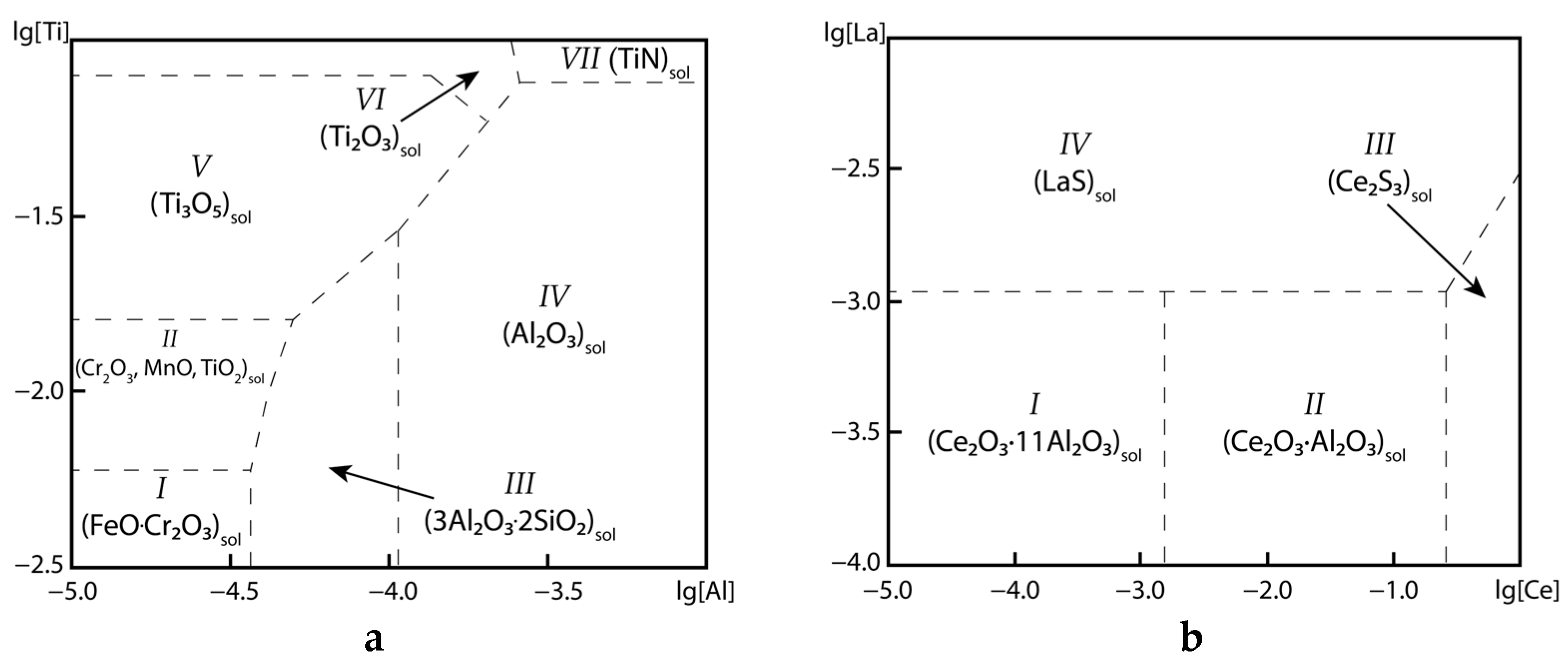
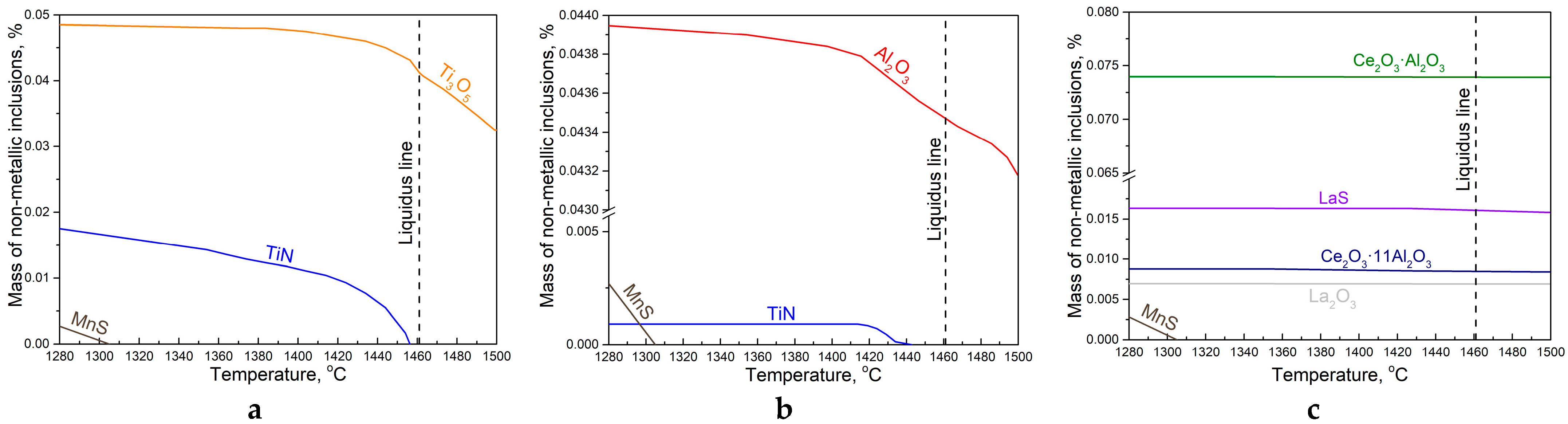
3.2. Thermodynamic Modeling Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reaction | lg(K) | Reaction | lg(K) |
|---|---|---|---|
| (Al2O3)sol = 2[Al] + 3[O] | –64,000/T + 20.48 | (La2O3)sol = 2[La] + 3[O] | –62,050/T + 14.10 |
| (3Al2O3·2SiO2)sol = 3|Al2O3| + 2|SiO2| | –257,700/T + 86.10 | (La2O3·Al2O3)sol = 2[La] + 2[Al] + 6[O] | –124,222/T + 32.49 |
| (SiO2)sol = [Si] + [O] | –31,100/T + 12.00 | (La2O3·11Al2O3)sol = 2[La] + 22[Al] + 36[O] | –740,899/T + 225.98 |
| (MnO)liq = [Mn] + [O] | –15,017/T + 6.77 | (La2S3)sol = 2[La] + 3[S] | –31,380/T + 11.11 |
| (MnO·SiO2)sol = |MnO| + |SiO2| | –44,793/T + 17.42 | (LaS)sol = [La] + [S] | –23,266/T + 7.39 |
| (2MnO·SiO2)sol = 2|MnO| + |SiO2| | –58,811/T + 23.22 | (TiO2)sol = [Ti] + 2[O], Ti < 0,01% | –33,753/T + 11.78 |
| (MnS)sol = [Mn] + [S] | –8279/T + 4.96 | (Ti3O5)sol = 3[Ti] + 5[O], Ti = 0,01…0,2% | –90,752/T + 31.46 |
| (MnO·TiO2)sol = (MnO)sol + (TiO2)sol | –47,729/T + 17.47 | (Ti2O3)sol = 2[Ti] + 3[O], Ti = 0,2…4% | –53,300/T + 18.00 |
| (2MnO·TiO2)sol = 2(MnO)sol + (TiO2)sol | –6317/T + 2.73 | (TiO)sol = [Ti] + [O], Ti > 5% | –17,860/T + 6.55 |
| (CeO2)sol = [Ce] + 2[O] | –43,694/T + 13.55 | (TiN)sol = [Ti] + [N] | –16,452/T + 5.98 |
| (Ce2O3)sol = 2[Ce] + 3[O] | –68,500/T + 19.60 | (TiS)sol = [Ti] + [S] | –8000/T + 4.02 |
| (CeS)sol = [Ce] + [S] | –10,938/T + 3.13 | (TiO2·Al2O3)sol | –96,219/T + 31.09 |
| (Ce2O3·Al2O3)sol = 2[Ce] + 2[Al] + 6[O] | –149,280/T + 43.78 | (Cr3O4)sol = 3[Cr] + 4[O] | –53,352/T + 23.51 |
| (Ce2O3·11Al2O3)sol = 2[Ce] + 22[Al] + 36[O] | –801,740/T + 242.95 | (Cr2O3)sol = 2[Cr] + 3[O] | –43,140/T + 18.63 |
| (Ce2S3)sol = 2[Ce] + 3[S] | –53,026/T + 20.43 | (CrO)liq = [Cr] = [O] | –8203/T + 4.51 |
| (FeO)liq = [Fe] + [O] | –6320/T + 4.73 | (2FeO·TiO2)sol | –48,159/T + 23.08 |
| (FeO·Cr2O3)sol | –50,700/T + 21.70 | (FeO·TiO2)sol | –6317/T + 2.73 |
| Elements | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e(i, j) 100 | Al | Si | Mn | Ce | La | S | N | Ni | Mo | Ti | Cr | C | O |
| Al | 4.5 | 5.8 | 0 | −0.33 | −1.16 | 3 | −5.8 | 0.819 | 0.844 | 8 | 2.4 | 9.66 | −198 |
| Si | 5.6 | 14 | 3 | −1.68 | −6.74 | 5.6 | 9.28 | −0.43 | 0.414 | −2.6 | −0.03 | 18.7 | −17.6 |
| Mn | 1.7 | 6 | 0 | 0.4 | 0.31 | −3.6 | −9.1 | −0.07 | 0.45 | 0 | 0.39 | −5.5 | −7.2 |
| Ce | −0.1 | −10.1 | 0.35 | 0.39 | −0.0005 | −102 | −884 | −6.1 | 1.81 | 3.6 | 0.994 | −125 | −455 |
| La | −7.76 | −35.1 | 27.9 | −0.003 | −0.78 | −93 | −689 | −6.67 | 2.64 | 5.20 | 2.16 | −76.3 | −501 |
| S | 3.5 | 6.2 | −2.6 | −23.11 | −19.99 | −2.8 | −4.8 | 0.046 | 0.63 | 0 | −1.2 | 11 | −27 |
| N | −2.8 | 4.7 | −2 | −88.03 | −69.06 | 0.7 | 0 | 0.963 | −1.22 | −45 | −4.7 | 13 | 5 |
| Ni | 14.5 | −2.31 | −0.78 | −2.57 | −2.569 | −0.36 | 3.17 | 0.19 | −0.086 | −3.47 | −0.485 | 4.19 | 1.03 |
| Mo | 1.9 | 0.365 | 0.47 | 1.3761 | 1.9569 | −0.05 | −10.9 | −0.417 | 0.45 | 2.42 | −0.03 | −9.4 | −0.066 |
| Ti | 12.9 | −4.75 | 0 | 1.5249 | 2.0756 | 0 | −180 | −2.752 | 1.42 | 5.6 | 1.7 | −4.9 | −110.8 |
| Cr | 5.2 | −0.06 | 0.3 | 0.642 | 1.0803 | −1.95 | −19 | −0.38 | 0.18 | 1.8 | −0.03 | −10.4 | −13.3 |
| C | 4.3 | 8 | −1.2 | −10.3 | 0.656 | 4.6 | 11 | 1.0129 | −0.83 | −60 | −2.4 | 14 | −34 |
| O | −390 | −10.2 | −2.1 | −55.14 | −57.36 | −13.3 | 5.7 | 0.351 | 0.60 | −37 | −4.1 | −45 | −20.03 |
References
- IMOA. Practical Guidelines for the Fabrication of Duplex Stainless Steels; International Molybdenum Association (IMOA): London, UK, 2009; pp. 1–64. [Google Scholar]
- Fedorov, A.; Zhitenev, A.; Karasev, V.; Alkhimenko, A.; Kovalev, P. Development of a Methodology for the Quality Management of Duplex Stainless Steels. Materials 2022, 15, 6008. [Google Scholar] [CrossRef]
- Fedorov, A.; Zhitenev, A.; Strekalovskaya, D. Effect of heat treatment on the microstructure and corrosion properties of cast duplex stainless steels. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 225, p. 01003. [Google Scholar]
- Jeon, S.H.; Hur, D.H.; Kim, H.J.; Park, Y.S. Influence of oxygen content on the inclusion formation and pitting corrosion resistance of hyper duplex stainless steels. Mater. Trans. 2014, 55, 1872–1877. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S.; Park, Y.S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Dai, M.; Huang, F.; Cheng, F.Y.; Liu, J. Investigation of micro-electrochemical activities of oxide inclusions and microphases in duplex stainless steel and the implication on pitting corrosion. Mater. Corros. 2020, 71, 876–886. [Google Scholar] [CrossRef]
- Shi, W.; Yang, S.; Li, J. Correlation between evolution of inclusions and pitting corrosion in 304 stainless steel with yttrium addition. Sci. Rep. 2018, 8, 4830. [Google Scholar] [CrossRef]
- Yaohua, Z.; Jing, L.; Feng, H.; Qian, H.; Saiwen, L. Effect of Composition and Size of Oxide Inclusions on Pitting Initiation of 2205 Duplex Stainless Steel. J. Corros. Sci. Prot. Technol. 2018, 30, 105–112. [Google Scholar]
- Jeon, S.H.; Kim, S.T.; Choi, M.S.; Kim, J.S.; Kim, K.T.; Park, Y.S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Growth of corrosion pits on stainless steel in chloride solution containing dilute sulphate. Corros. Sci. 1992, 33, 1885–1897. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S.; Park, J.H.; Kim, K.T.; Kim, J.S.; Park, Y.S. Effects of copper addition on the formation of inclusions and the resistance to pitting corrosion of high performance duplex stainless steels. Corros. Sci. 2011, 53, 1408–1416. [Google Scholar] [CrossRef]
- Ha, H.Y.; Park, C.J.; Kwon, H.S. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scr. Mater. 2006, 55, 991–994. [Google Scholar] [CrossRef]
- Ha, H.Y.; Park, C.J.; Kwon, H.S. Effects of non-metallic inclusions on the initiation of pitting corrosion in 11% Cr ferritic stainless steel examined by micro-droplet cell. Corros. Sci. 2007, 49, 1266–1275. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Heintz, O.; Oltra, R.; Chauveau, E. Dissolution of Chromium-Enriched Inclusions and Pitting Corrosion of Resulfurized Stainless Steels. Metall. Mater. Trans. A 2006, 37, 1541–1549. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, Y. Inclusions in stainless steels—A review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.M. Effect of Rare Earth on the Inclusions and Pitting Resistance of duplex Stainless Steel. Adv. Mater. Res. 2012, 528, 130–134. [Google Scholar]
- Wang, H.; Wang, A.; Li, C.; Yu, X.; Xie, J.; Liang, T.; Liu, C. Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel. Rev. Adv. Mater. Sci. 2022, 61, 873–887. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of Adding Cerium on Microstructure And Morphology of Ce-Based Inclusions Formed in Low-Carbon Steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Zhang, T.; Jiang, M.; Peng, C. Inclusions modification in heat resistant steel containing rare earth elements. Ironmak. Steelmak. 2016, 45, 76–82. [Google Scholar] [CrossRef]
- Smirnov, L.A.; Rovnushkin, V.A.; Oryshchenko, A.S.; Kalinin, G.Y.; Milyuts, V.G. Modification of Steel and Alloys with Rare-Earth Elements. Part 1. Metallurgist 2016, 59, 1053–1061. [Google Scholar] [CrossRef]
- Lindskog, N.; Kjellberg, B. Removal of oxide inclusions resulting from strong deoxidizers. Scand. J. Metall. 1977, 6, 49–55. [Google Scholar]
- Wang, X.; Hao, K.; Zhou, G.; Wu, H.; Wu, R. Effect of rare-earth on sulfides morphology and abrasive resistance of high sulfur steel. Mater. Mech. Eng. 2012, 36, 33–37. [Google Scholar] [CrossRef]
- Mei, Z.; Wan, T.; Lou, D. Influence of RE modifier on as-cast grain refinement of super-low carbon cast steel. Spec. Cast. Nonferrous Alloy 2002, 2, 3–4. [Google Scholar]
- Yan, H.H.; Hu, Y.; Zhao, D.W. Microstructure and properties of as-cast 30Mn steel. AIP Adv. 2018, 8, 125128. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, J.; Chen, H.-L.; Su, Y.-H.; Hwang, W.-S. Thermodynamic Calculation among Cerium, Oxygen, and Sulfur in Liquid Iron. Sci. Rep. 2016, 6, 35843. [Google Scholar] [CrossRef]
- Suler, B.; Burja, J.; Medved, J. Modification of non-metallic inclusions with rare-earth metals in 50CrMoV13-1 steel. Mater. Technol. 2019, 53, 441–447. [Google Scholar]
- Kazakov, A.A.; Zhitenev, A.I.; Ishpaev, P.A.; Fomina, O.V.; Melnikov, P.V. Hot physical simulation of δ-ferrite behavior at production and welding of high-nitrogen corrosion-resistant steels. CIS Iron Steel Rev. 2020, 19, 48–55. [Google Scholar] [CrossRef]
- Kazakov, A.A.; Kiselev, D. Industrial application of thixomet image analyzer for quantitative description of steel and alloy’s microstructure. Metallogr. Microstruct. Anal. 2016, 5, 294–301. [Google Scholar] [CrossRef]
- ASTM E 1245; Standard Practice for Determining the Inclusion or Second-Phase Constituent Content of Metals by Automatic Image Analysis. ASTM International: West Conshohocken, PA, USA, 2016; 8 p.
- Mikhailov, G.G. Thermodynamics of Metallurgical Processes and Systems; MISIS Publishing House: Moscow, Russia, 2009; p. 520. [Google Scholar]
- Mikhailov, G.G.; Makrovets, L.A.; Smirnov, L.A. Thermodynamic modeling of lanthanum interaction processes using iron-based metallic melts. Izv. Ferr. Metall. 2015, 58, 877–883. [Google Scholar] [CrossRef][Green Version]
- Mikhailov, G.G.; Makrovets, L.A.; Smirnov, L.A. Thermodynamics of the processes of interaction of liquid metal components in Fe—Mg—Al—La—O system. Izv. Ferr. Metall. 2018, 61, 460–465. [Google Scholar] [CrossRef]
- Mikhailov, G.G.; Makrovets, L.A.; Samoilova, O.V. Thermodynamic analysis of liquid steel refining by complex alloy containing La—Ce—Al. Izv. Ferr. Metall. 2020, 63, 238–247. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, X.; Li, J.; Chen, C.; Li, C.; Zhuang, C. Study on the thermodynamic stability and evolution of inclusions in Al–Ti deoxidized steel. High Temp. Mater. Process. 2022, 41, 206–215. [Google Scholar] [CrossRef]
- Kulikov, I.S. Deoxidation of Metals; Metallurgiya (Metallurgy): Moscow, Russia, 1975; p. 504. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High-Temperature Processes; Metallurgiya (Metallurgy): Moscow, Russia, 1985; p. 344. [Google Scholar]
- Grigoryan, V.A. Physical and Chemical Calculations of Electric Steelmaking Processes; Textbook for Universities; Metallurgiya (Metallurgy): Barnaul, Russia, 1989; p. 288. [Google Scholar]
- Zhitenev, A.; Salynova, M.; Shamshurin, A.; Ryaboshuk, S.; Kolnyshenko, V. Database Clustering after Automatic Feature Analysis of Nonmetallic Inclusions in Steel. Metals 2021, 11, 1650. [Google Scholar] [CrossRef]
- Kazakov, A.A.; Urazgil’deev, A.K.; Gusev, A.A. Algorithmic model of formation of nonmetallic inclusions in liquid and solidifying steel. Izv. Akad. Nauk. SSSR Met. 1989, 3, 60–65. [Google Scholar]
- Kazakov, A.A.; Kovalev, P.V.; Ryaboshuk, S.V.; Mileikovskii, A.B.; Malakhov, N.V. Study of Thermal Time Nature of Non-Metallic Inclusions in Order to Improve Metallurgical Quality of High-Strength Tube Steels. 2009. Available online: https://rudmet.ru/journal/634/article/7869/ (accessed on 20 November 2023).
- Kurz, W.; Fisher, D.; Rappaz, M. Fundamentals of Solidification; CRC Press: Boca Raton, FL, USA; Metallurgiya: Moscow, Russia, 1980; 175p. [Google Scholar]
- Yavoiskii, V.I.; Rubenchik, Y.I.; Okenko, A.P. Nonmetallic inclusions and properties of steel. Mosc. Metall 1980, 173, 169–172. [Google Scholar]
- Kazakov, A.A.; Zhitenev, A.I.; Kolpishon, E.Y.; Salynova, M.A. Nonmetallic Inclusions Quantitative Assessment for Forgings Made from Super Large Steel Ingot. 2018. Available online: https://rudmet.ru/journal/1781/article/30434/?language=en (accessed on 20 November 2023).
- Yavoiskii, V.I.; Bliznyukov, S.A.; Vishkarev, A.F.; Gorokhov, L.S.; Khokhlov, S.F.; Yavoiskii, A.V. Inclusions and Gases in Steels; Metallurgiya (Metallurgy): Moscow, Russia, 1979; p. 272. [Google Scholar]
- You, D.L.; Michelic, S.K.; Bernhard, C. Formation of Multi-Type Inclusions during the Cooling and Solidification of Steel: A Trend Model. Metals 2018, 8, 452. [Google Scholar] [CrossRef]
- Smirnov, L.A.; Rovnushkin, V.A.; Oryshchenko, A.S.; Kalinin, G.Y.; Milyuts, V.G. Modification of Steel and Alloys with Rare-Earth Elements. Part 2. Metallurgist 2016, 60, 38–46. [Google Scholar] [CrossRef]
- Silva, A.C. Non-metallic inclusions in steels—Origin and control. J. Mater. Res. Technol. 2018, 7, 283–299. [Google Scholar] [CrossRef]

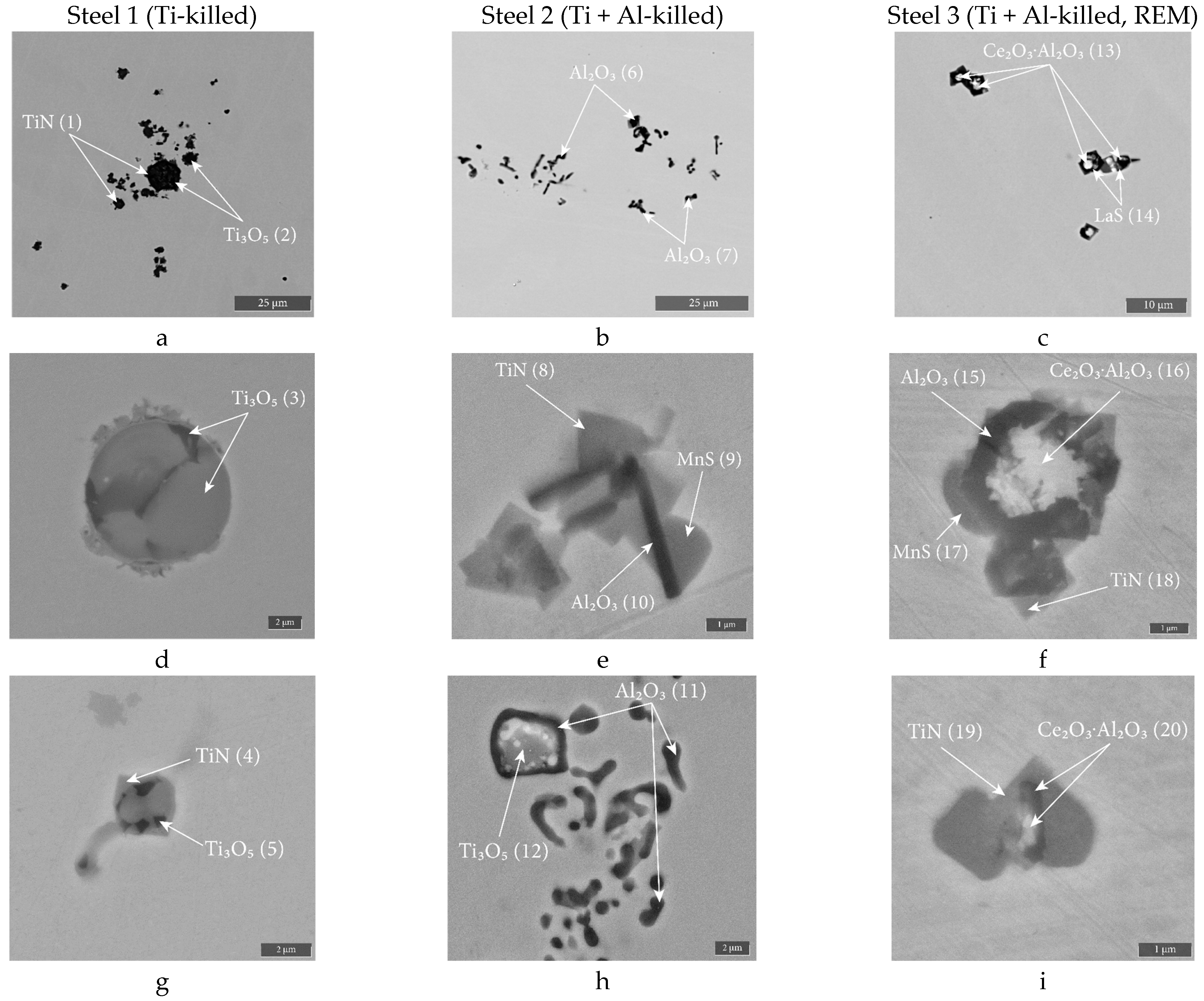
| Steel | [R] | Element, wt.% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Si | Mn | Ni | Mo | N | Cu | S | Ti | Al | REMs | ||
| 1 | Ti | 0.04 | 26.0 | 0.7 | 0.1 | 7.0 | 4.0 | 0.10 | 0.6 | 0.02 | 0.07 | – | – |
| 2 | Al | 0.03 | 0.05 | – | |||||||||
| 3 | REMs | 0.1 | |||||||||||
| [R] | Single Inclusions | Clusters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V, % | d, µm | N, 1/mm2 | dmax, µm | Shape Factor | V, % | d, µm | N, 1/mm2 | dmax, µm | Shape Factor | |
| Ti-killed | 0.023 | 5 | 20 | 15 | 0.65 | 0.066 | 84 | 0.58 | 226 | 0.12 |
| Ti + Al-killed | 0.014 | 5 | 11 | 24 | 0.66 | 0.010 | 68 | 0.17 | 128 | 0.08 |
| Ti + Al-killed, REM | 0.016 | 5 | 17 | 9 | 0.75 | – | – | – | - | - |
| [R] | Element, wt.% | Spectrum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | O | Al | Ti | S | Mn | La | Ce | ||
| Ti-killed | 21.3 | 7.7 | 1.0 | 70.0 | – | – | – | – | 1 |
| – | 53.0 | 8.5 | 38.5 | – | – | – | – | 2 | |
| – | 48.4 | 1.8 | 49.8 | – | – | – | – | 3 | |
| 18.9 | 37.9 | 1.3 | 41.9 | – | – | – | – | 4 | |
| – | 67.6 | 2.1 | 30.3 | – | – | – | – | 5 | |
| Ti + Al-killed | 2.0 | 46.6 | 45.7 | 5.6 | – | – | – | – | 6 |
| – | 46.3 | 53.7 | – | – | – | – | – | 7 | |
| 22.7 | 24.3 | 11.7 | 41.3 | – | – | – | – | 8 | |
| – | 10.3 | 7.1 | 2.3 | 35.7 | 44.5 | – | – | 9 | |
| – | 54.3 | 40.2 | 5.4 | – | – | – | – | 10 | |
| – | 53.1 | 46.9 | – | – | – | – | – | 11 | |
| – | 43.8 | 1.9 | 54.4 | – | – | – | – | 12 | |
| Ti + Al-killed, REM | – | 37.3 | 23.3 | – | – | – | 7.6 | 31.8 | 13 |
| – | 28.2 | 37.6 | – | 2.5 | – | 5.2 | 21.5 | 14 | |
| – | 47.8 | 30.4 | 5.1 | – | – | 3.2 | 13.5 | 15 | |
| – | 37.5 | 19.3 | – | – | – | 3.3 | 39.9 | 16 | |
| – | 13.0 | 6.0 | 2.2 | 30.5 | 40.1 | 5.3 | 2.9 | 17 | |
| 32.6 | 21.2 | 4.7 | 41.6 | – | – | – | – | 18 | |
| 12.5 | 29.9 | 8.4 | 39.6 | – | – | – | 9.6 | 19 | |
| – | 41.0 | 20.7 | 10.9 | – | – | 2.53 | 24.8 | 20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhitenev, A.; Karasev, V.; Fedorov, A.; Ryaboshuk, S.; Alkhimenko, A. Control of the Composition and Morphology of Non-Metallic Inclusions in Superduplex Stainless Steel. Materials 2023, 16, 7337. https://doi.org/10.3390/ma16237337
Zhitenev A, Karasev V, Fedorov A, Ryaboshuk S, Alkhimenko A. Control of the Composition and Morphology of Non-Metallic Inclusions in Superduplex Stainless Steel. Materials. 2023; 16(23):7337. https://doi.org/10.3390/ma16237337
Chicago/Turabian StyleZhitenev, Andrey, Vladimir Karasev, Aleksandr Fedorov, Sergey Ryaboshuk, and Alexey Alkhimenko. 2023. "Control of the Composition and Morphology of Non-Metallic Inclusions in Superduplex Stainless Steel" Materials 16, no. 23: 7337. https://doi.org/10.3390/ma16237337
APA StyleZhitenev, A., Karasev, V., Fedorov, A., Ryaboshuk, S., & Alkhimenko, A. (2023). Control of the Composition and Morphology of Non-Metallic Inclusions in Superduplex Stainless Steel. Materials, 16(23), 7337. https://doi.org/10.3390/ma16237337






