Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials
Abstract
:1. Introduction
2. Basic Principles of Microwave and Ultrasound Treatments (Part I)
2.1. Microwaves Radiation
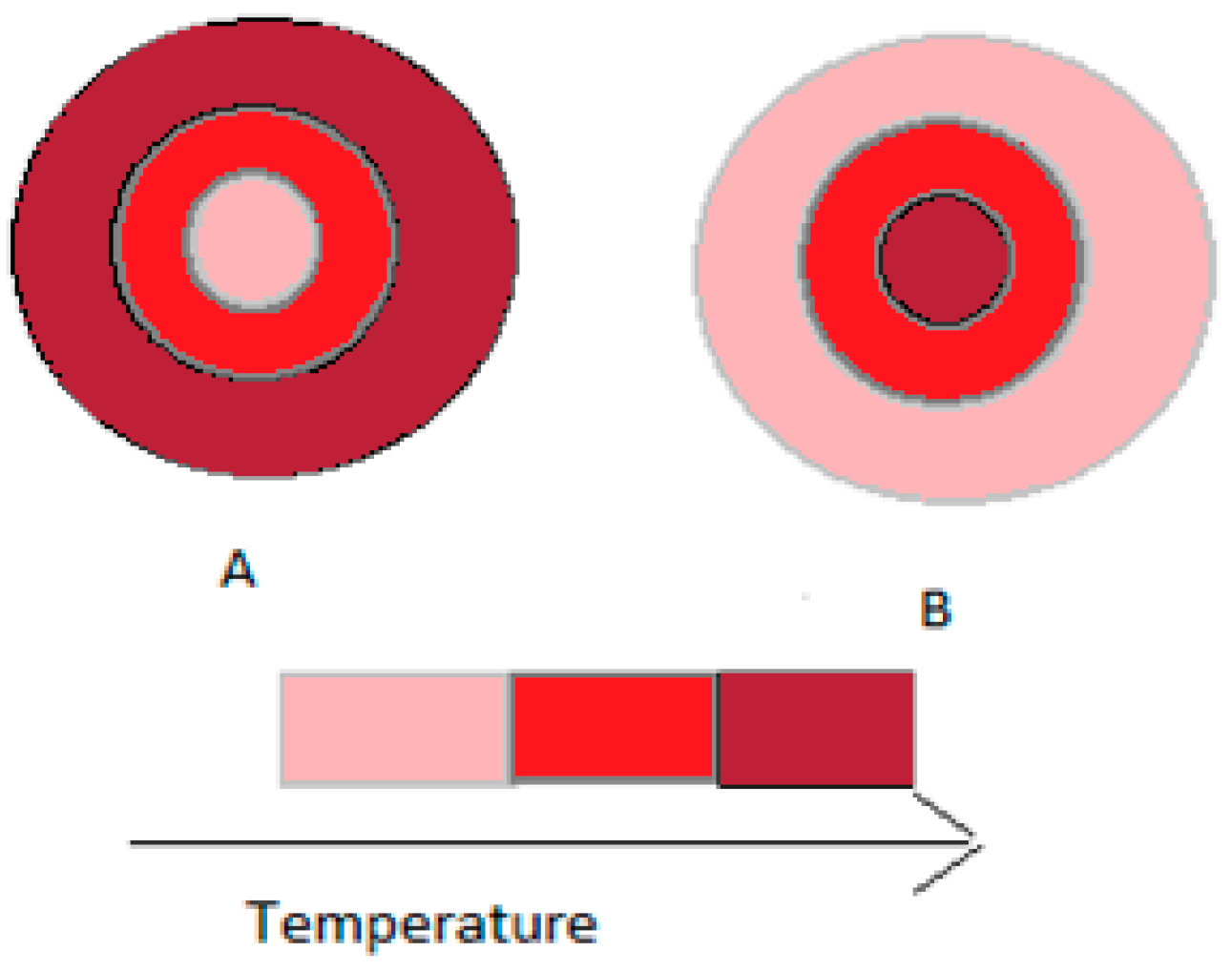
2.1.1. Conventional Heating and Microwave Heating
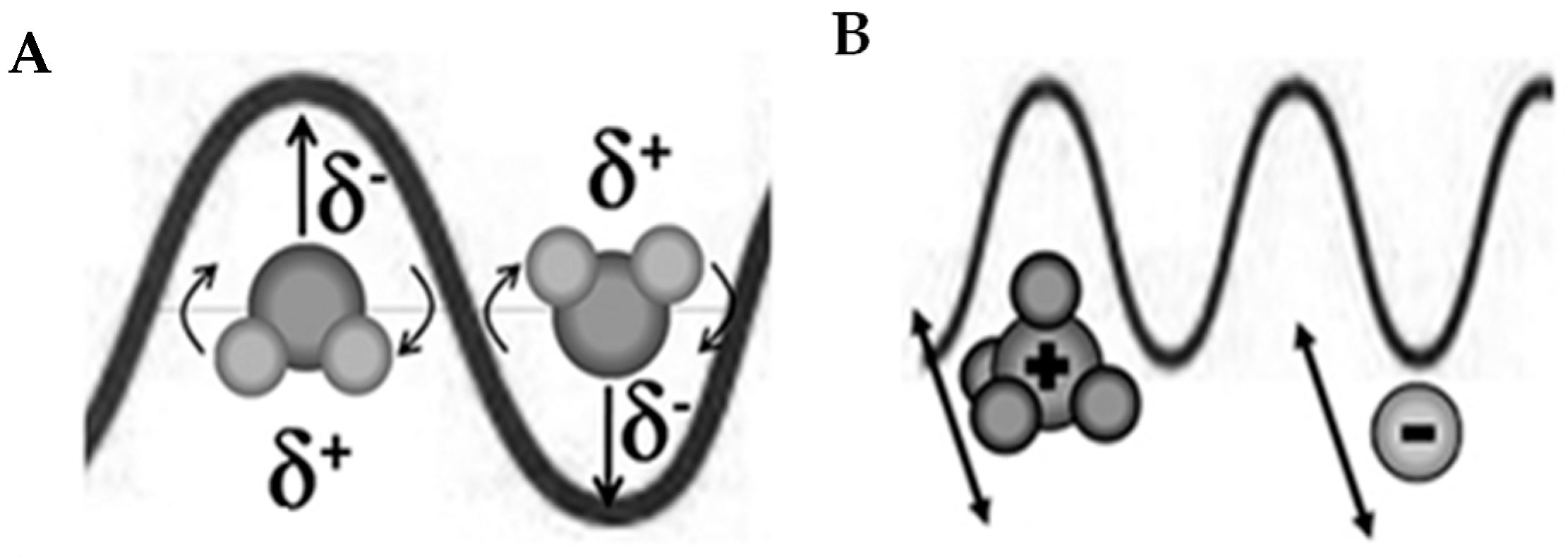
2.1.2. Microwave Heating Mechanisms
2.1.3. Behavior of Materials in Relation to Microwave Radiation
2.1.4. Behavior of lignocellulosic Biomass in Relation to Microwave Radiation
2.1.5. Microwave Absorbing Materials Addition to Lignocellulosic Biomass
2.1.6. Factors to Consider in a Microwave Pretreatment for Lignocellulosic Biomass
2.1.7. The Reasons Justifying Microwave Absorption and Lignocellulosic Biomass Recalcitrance
2.2. Ultrasound and Two Categories of Ultrasound
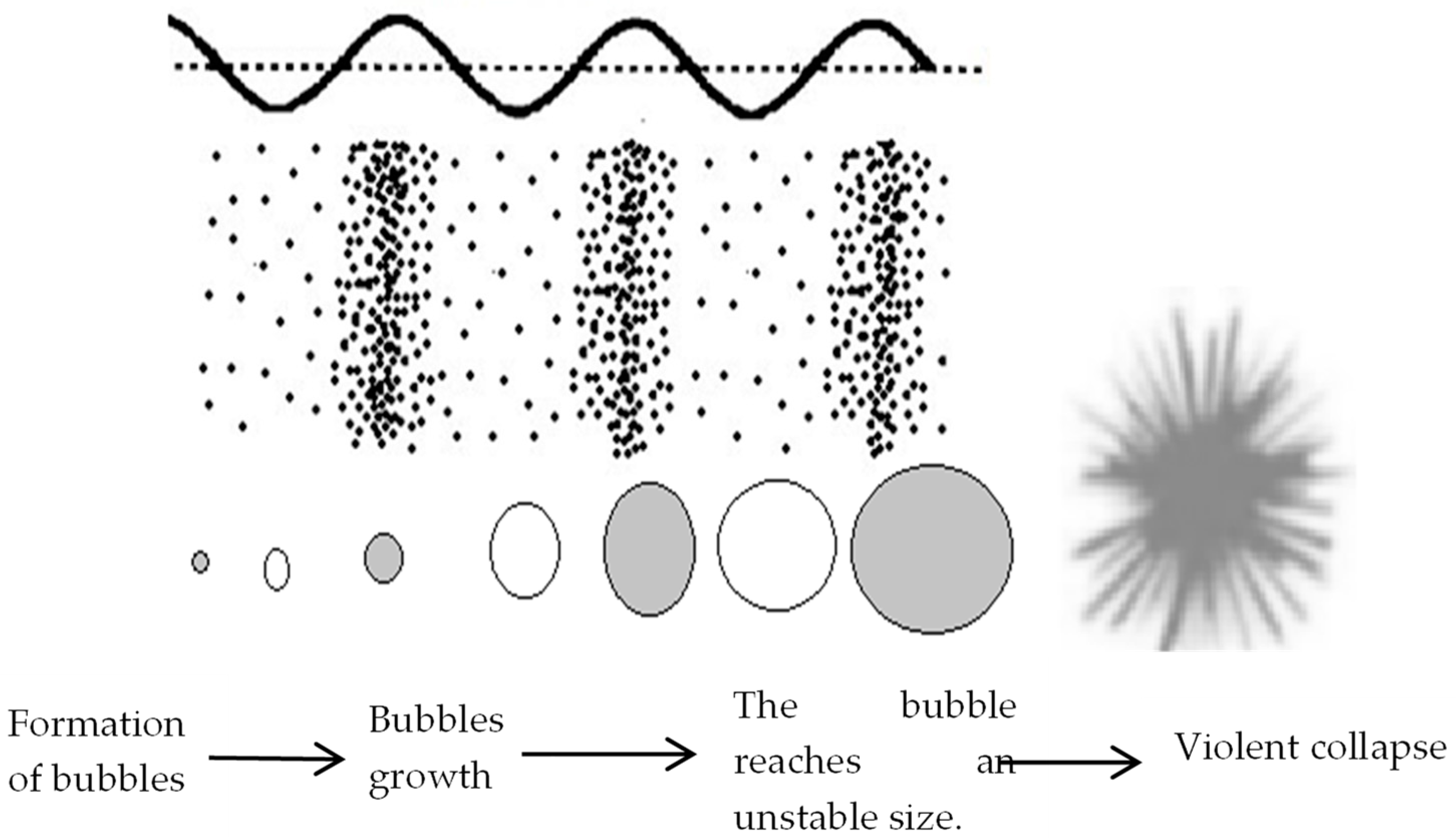
2.2.1. Basic Principles of Cavitation
2.2.2. Factors That Influence the Cavitation of Lignocellulosic Biomass
2.2.3. Physical Effects and Chemical Effects of Ultrasound on Lignocellulosic Biomass
3. Pretreatments with Microwave and/or Ultrasound (Part II)
3.1. The Types of Microwave and/or Ultrasound Pretreatments
3.2. Physical Effects and Chemical Effects of Microwave and Ultrasound on Lignocellulosic Biomass
3.2.1. Effect on Particle Size and Surface Area
| Biomass | Pretreatment | Particle Size ) | Specific Surface Area (m2 g−1) | References |
|---|---|---|---|---|
| Residues from the herb extraction process | No treatment | 197.0 | 0.38 | [70] |
| Alkaline (NaOH) | 187.5 | 0.44 | ||
| Water and MW | 179.8 | 0.51 | ||
| Alkaline (NaOH) + MW | 163.5 | 0.63 | ||
| Hemp stalk | No treatment | ………. | 1.30 | [74] |
| With MEP 1 | ……. | 1.85 | ||
| Microalgae Scenedesmus | No treatment | 7.4 | N. d. | |
| US | 5.1 | N.d. | [73] | |
| Hovenia dulcis and Ampelopsis grossedentata | No treatment | 14.802 | N.d. | [75] |
| Negative pressure and US 2 | 3.719 | N.d. |
3.2.2. Effect on Lignin, Hemicellulose, and Cellulose Content
| Biomass | Pretreatment | Operating Conditions | Initial Composition (%) | Composition after Treatment (%) | References |
|---|---|---|---|---|---|
| Sugarcane bagasse | Acid (H2SO4) + MW | P = 900 W f = 2.45 GHz ∆t = 5 min T = 190 °C | C: 52.45 H: 25.97 L: 12.72 | C: 67.31 H: 0.8 L: 15.67 | [76] |
| Winter wheat (Miscanthus sinensis) | Alkaline (NH4OH) + MW | P = 300 W ∆t = 15 min T = 120 °C | C: 42.7 H: 31.3 L: 17.4 | C: 53.1 H: 32.9 L: 12.2 | [77] |
| Acid (H2SO4) + MW | P = 300 W ∆t = 30 min T = 140 °C | C: 42.7 H: 31.3 L: 17.4 | C: 61.5 H: 20.7 L: 15.8 | ||
| Acid + Alkaline (H2SO4 + NH4OH) + MW | ∆t = 15 min (120 °C) +∆t = 30 min (140 °C) | C: 42.7 H: 31.3 L: 17.4 | C: 69.7 H: 18.4 L: 10.4 | ||
| Sugarcane bagasse | US | P = 400 W f = 24 kHz ∆t = 45min, T = 50 °C | C: 38.0 H: 32.0 L: 27.0 | C: 46.9 H: 29.3 L: 20.7 | [78] |
| Ammonia (10% v/v) | P = 400 W f = 24 kHz ∆t = 30min, T = 80 °C | C: 38.0 H: 32.0 L: 27.0 | C: 50.4 H: 26.8 L: 19.8 | ||
| Ammonia + US (10% v/v) | P = 400 W f = 24 kHz ∆t = 45min, T = 80 °C | C: 38.0 H: 32.0 L: 27.0 | C: 56.1 H: 19.6 L: 18.2 | ||
| Hog plum (Spondias mombin L.) | US | P= 400 W f = 40 kHz ∆t = 60 min, T = 80 °C | C: 53.74 H: 11.35 L: 35.28 | C: 60.19 H: 6.27 L: 13.17 | [79] |
| Nitric acid | P = 400 W f = 40 kHz ∆t = 60 min, T = 80 °C | C: 53.74 H: 11.35 L: 35.28 | C: 55.27 H: 9.73 L: 24.14 | ||
| Nitric acid + US | P = 400 W f = 40 kHz ∆t = 60 min, T = 80 °C | C: 53.74 H: 11.35 L: 35.28 | C: 63.15 H: 3.19 L: 10.18 |
3.2.3. Effect on Cellulose Crystallinity Index
| Biomass | Pretreatment | Operating Conditions | Crystallinity Index After (%) | References |
|---|---|---|---|---|
| Sugarcane bagasse | No treatment | 53.44 | [80] | |
| Sugarcane bagasse | Acid (H2SO4) + MW | P = 450 W f = 2450 MHZ ∆t = 5 min | 58.79 | [80] |
| Alkaline (NaOH) + MW | P = 450 W f = 2450 MHZ ∆t = 5 min | 65.29 | ||
| Alkaline (NaOH) + Acid (H2SO4) + MW | P = 450 W f = 2450 MHZ ∆t = 10 min | 65.55 | ||
| Water hyacinth | No treatment | 16.0 | [81] | |
| Alkaline (NaOH) + MW | P = N.d. 3 ∆t = 10 min T = 190 °C | 13.0 | ||
| Hemp stalk | No treatment | 44.96 | [74] | |
| With MEP 1 | P = 1100 W ∆t = 3 min T = 90 °C | 42.83 | ||
| Black tea residues (Camellia sinensis) | No treatment | 56.86 | [82] | |
| Alkaline bleaching with peroxide | ∆t = 90 min T = 55 °C | 76.86 | ||
| Alkaline bleaching with peroxide +MW 2 | P = 1000 W ∆t = 0.5 min | 88.77 |
| Biomass | Pretreatment | Operating Conditions | Crystallinity Index (%) | References |
|---|---|---|---|---|
| Kenaf powder | No treatment | 49.4 | [84] | |
| Ionic liquid | 38.8 | |||
| Ionic liquid +US | P = 35 W f = 24 kHz ∆t = 15 min T = 25 °C | 31.5 | ||
| Water hyacinth | No treatment | 19.50 | [83] | |
| Ionic liquid | 32.44 | |||
| Ionic liquid + US | P = 100 W f = 20 kHz ∆t = 45 min T = 120 °C | 30.74 | ||
| Ionic liquid +US + SDS 1 | P = 100 W f = 20 kHz ∆t = 45 min T = 120 °C | 28.73 | ||
| Eucalyptus powder (Eucalyptus grandis) | No treatment | 31.8 | [85] | |
| Soda solution + US | P = 300 W f = 28 kHz ∆t = 30 min T = 50 °C | 34.7 | ||
| Water + US | P = 300 W f = 28kHz ∆t = 30 min T = 50 °C | 32.6 | ||
| Acetic acid + US | P = 300 W f = 28kHz ∆t = 30 min T = 50 °C | 33.4 | ||
| Cupuaçu husk (Theobroma grandiflorum) | No treatment | 54.3 | [86] | |
| Water + US | P = 100 W f = 40 kHz ∆t = 30 min T = 35 °C | 60.0 | ||
| Acid (HCl) + US | P = 100 W f = 40 kHz ∆t = 30 min T = 35° C | 63.3 | ||
| Alkaline (NaOH) + US | P = 100 W f = 40 kHz ∆t = 30 min T = 35 °C | 57.0 | ||
| Ionic liquid +US | P = 100 W f = 40 kHz ∆t = 30 min T = 35 °C | 58.2 |
3.2.4. Effect on the Solubilization of Organic Matter
3.2.5. Effects on Hydrolysis and Reduction of Sugar Production
| Biomass | Pretreatment | Operating Conditions | Glucose Production | Reference |
|---|---|---|---|---|
| Oil palm trunk | Acid (H2SO4) +MW | P = 450 W ∆t = 7.5 min | 8.95 mg/L | [89] |
| Rice straw | Alkaline (NaOH) + MW | P = 681 W ∆t = 3 min | 255 g/g 1 | [1] |
| Pine chips | No treatment | 77.3 mg/g 1 | [92] | |
| water + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 81.5 mg/g 1 | ||
| NaCs2 + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 107.8 mg/g 1 | ||
| Beech chips | No treatment | 35.0 mg/g 1 | [92] | |
| water + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 278.0 mg/g 1 | ||
| NaCs 2 + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 515.5 mg/g 1 | ||
| Wheat straw | No treatment | 0.0 mg/g 1 | [92] | |
| water + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 435.8 mg/g 1 | ||
| NaCs 2 + MW | P = 600 W ∆t = 60 min Pressure = 117 Psi | 557.3 mg/g 1 | ||
| No treatment | 3.14 g/L | [86] | ||
| Residues of cupuaçu (Theobroma grandiflorum) | water + US | P = 100 W f = 24 kHz ∆t = 30 min | 8.44 g/L | |
| Acid (HCl) + US | P = 100 W f = 24 kHz ∆t = 30 min | 9.90 g/L | ||
| Alkaline (NaOH) + US | P = 100 W f = 24 kHz ∆t = 30 min | 6.08 g/L |
4. Microwaves and Ultrasound on the Route of Value-Added Products (Part III)
4.1. Liquefactions
4.1.1. Ultrasound-Assisted Liquefactions
| Biomass | Type of Liquefaction | Liquefaction time (min) | Liquefaction Yield (%) | Reference |
|---|---|---|---|---|
| Medium density fiberboard (MDF) | Conventional | 90 | 93.8 | [95] |
| US | 10 | 94.9 | ||
| Wheat straw | Conventional | 90 | 94.4 | |
| US | 15 | 95.4 | ||
| Veneered particleboard | Conventional | 120 | 95.0 | |
| US | 20 | 96.0 | ||
| Cork powder | Conventional | 135 | 95.0 | [96] |
| US | 75 | 98.0 | ||
| Poplar sawdust | Conventional | …. | …. | [97] |
| MW | 7 | 100 | ||
| Corn stover and corncob | Conventional | …. | …. | [98] |
| MW | 20 | 95 | ||
| Bamboo wastes | Conventional | …. | …. | [99] |
| MW | 7 | 96.7 | ||
| Bamboo sawdust | Conventional | …. | …. | [100] |
| MW | 8 | 78 | ||
| Fir sawdust | Conventional | 60 | …. | [101] |
| MW + US | 20 | 91 | ||
| Conventional | 60 | …. | [102] | |
| MW + US | …. |
4.1.2. Microwave-Assisted Liquefactions
4.1.3. Liquefactions Assisted Simultaneously by Microwaves and Ultrasound
4.2. Microwave-Assisted and/or Ultrasound-Assisted Extractions
| Biomass | Type of Extraction | Power | Solvent | Extraction Time (min) | Reference |
|---|---|---|---|---|---|
| Eucalyptus robusta * | MW | 600 W | Water | 3 | [104] |
| Eucalyptus robusta * | US | 250 W | Water | 90 | [105] |
| Eucalyptus globulus | Conventional | Medium | Ethanol 56% (V:V) | 225 | [107] |
| US | 90 | ||||
| MW | 7 | ||||
| Lemon peel residues * | US | Amplitude 38% | Ethanol:Water55:45 | 4 | [108] |
| MW | 140 W | 0.75 | |||
| Spatholobus suberectus | Conventional | 100% Methanol | 360 | [109] | |
| US | 30–250 W | 70% Methanol | 60 | ||
| MS | 100–500 W | 70% Methanol | 30 | ||
| MS + US | 100–500 W 30–250 W | Methanol 30–100% + Pure ethanol | 7.5 | ||
| (Coriandrum sativum L.) * | MW | 500 W | 50% Ethanol | 4 | [22] |
4.3. Factors Influencing Microwave- and Ultrasound-Assisted Extraction
4.4. Emerging Routes in Extraction
5. Sonocatalysis of Lignocellulosic Biomass (Part IV)
5.1. Examples of Hydrolysis
5.2. Examples of Hydrogenations
5.3. Examples of Oxidations
| Reaction | Biomass | Operating Conditions | Product | Main Conclusion | Reference |
|---|---|---|---|---|---|
| Hydrolysis | Bamboo (Gigantochloa scortechinii) | Ultrasound 20 kHz, 300 W 10 min, 140 °C Catalyst: ionic liquid CrCl3 | 5-HMF | From 3 h from the conventional route to 10 min. | [114] |
| Soybean straw and corn straw | Ultrasound Bath, 120 min, 70 °C Catalyst: ionic liquid ([HMIM] Cl) | Reducing sugars | Simple and economical approach. | [115] | |
| Cellulose | Ultrasound 20 kHz, 60 min, 30 °C Catalyst: Diluted HNO3 | FF | Simple synthesis, in 60 min, with yield 78%. | [116] | |
| Potato starch waste | Ultrasound 20 kHz and 500 kHz, 120 min, 60 °C Catalyst: H2SO4 | Reducing sugars | 70% yield with 20kHz ultrasound and 84% yield with 500kHz. | [117] | |
| Banana peels | Ultrasound 20 kHz, 240 watts Catalyst: H2SO4 | 5-HMF | Production of 50 g/L 5-HMF for 1 h | [23] | |
| Pretreated sugars obtained from cupuaçu husk (Theobroma grandiflorum) | Ultrasound It doesn’t mention power. 60 min, 140 °C Catalyst: ionic liquid | FF 5-HMF | Synthesis with yield of 12.94%, in 5-HMF and 48.84% in FF, in one hour | [86] | |
| Hydrogenation | D- Fructose | Ultrasound 20 kHz, 50 W 20 min, 110 °C Catalysts: Cu/SiO2 Raney-Ni, CuO/ZnO/Al2O 3 | D-mannitol | Cu/SiO2 was the catalyst with best performance. | [118] |
| Lignin from Miscanthus giganteus | Ultrasound 35 kHz, 6 h, 25 °C Catalysts: Fe3O4(NiAlO)x, Fe3O4(NiMgAlO)x, ionic liquid [BMIM]OAc | Low molecular weight compounds | The performances of the catalysts, under ultrasonic conditions, were inferior to those exhibited with conventional heating. | [119] | |
| Gross FAMEs | Ultrasound 40 kHz, 120 W, 35 °C Catalyst: Amorphous alloy of doped nickel boride with La Li-La-B. | hydrogenated FAMEs | Intensification of hydrogenation by catalytic transfer due to the incidence of ultrasound. The same catalyst can be used at least 5 times. | [120] | |
| Oxidation | Cotton pulp | Ultrasound 40 kHz, 300 watts Catalyst: TEMPO (2,2,6,6-tetramethyl-piperidine-N-oxyl) | Nanocellulose with high COOH content | Cellulose nanocrystals stable in water | [122] |
| Hardwood Kraft Pulp | Ultrasound 68 and 170 kHz, 1000 W Catalyst: TEMPO (2,2,6,6-tetramethyl-piperidine-N-oxyl) | Nanocellulose with high COOH content | Selective oxidation of primary hydroxyl groups (C6). | [121] | |
| FF | High-frequency ultrasound 525 to 565 kHz T = 42 °C It uses. H2O2 No catalyst | Maleic acid | Promising route that does not require a catalyst, uses mild temperatures and high-frequency ultrasound | [123] |
5.4. Sonophotocatalysis, the Emerging Area
6. Conclusions
7. Challenges and Perspectives
- Study the dielectric parameters (dielectric constant, dielectric loss, dielectric loss tangent) of the lignocellulosic biomass concerned before subjecting it to pretreatment with microwave radiation;
- Understand how the dielectric parameters of lignocellulosic biomass vary with frequency of incident microwaves, and with operating temperature. There are studies that prove that dielectric parameters vary with frequency and temperature, but so far, it seems to be quite difficult to predict the behavior of biomass in the face of these two factors;
- Develop computational tools to find the best conditions for a given microwave and/or ultrasound pretreatment;
- Study in more detail the changes in the supramolecular structure of cellulose using microwaves or ultrasound in order to better manage these processes;
- Advance the study of liquefaction with microwaves on a pilot scale. This is the recommendation of several researchers who argue that they have already been properly tested on a laboratory scale;
- Expansion of the list of reagents and catalysts for target reactions that use microwaves and/or ultrasound and for reactions that simultaneously use these techniques with other ionizing radiation (for example, ultraviolet light).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jasmine, A.; Rajendran, M.; Thirunavukkarasu, K.; Abinandan, S.; Vaidyanathan, V.K.; Krishnamurthi, T. Microwave-assisted alkali pre-treatment medium for fractionation of rice straw and catalytic conversion to value-added 5-hydroxymethyl furfural and lignin production. Int. J. Biol. Macromol. 2023, 236, 123999. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L., Jr. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, S.; Raynie, D.E. Recent advances of greener pretreatment technologies of lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Kumar, A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Haldar, D.; Sen, D.; Gayen, K. A review on the production of fermentable sugars from lignocellulosic biomass through conventional and enzymatic route—A comparison. Int. J. Green Energy 2016, 13, 1232–1253. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total. Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; Wu, T.Y.; Yang, G.H.; Jahim, J.M.; Teoh, W.H.; Mohammad, A.W. Role of energy irradiation in aiding pretreatment of lignocellulosic biomass for improving reducing sugar recovery. Cellulose 2016, 23, 2761–2789. [Google Scholar] [CrossRef]
- Mehta, S.; Jha, S.; Liang, H. Lignocellulose materials for supercapacitor and battery electrodes: A review. Renew. Sustain. Energy Rev. 2020, 134, 110345. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochemistry 2021, 70, 105293. [Google Scholar] [CrossRef]
- Mathiarasu, A.; Pugazhvadivu, M. Studies on dielectric properties and microwave pyrolysis of karanja seed. Biomass- Convers. Biorefinery 2023, 13, 2895–2905. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanocellulose for Sensing Applications. Adv. Mater. Interfaces 2019, 6, 1900424. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Freidja, M.L.; Silva, A.M.; Cardoso, S.M. Microwave-assisted extraction optimization and conventional extraction of phenolic compounds from coriander leaves: UHPLC characterization and antioxidant activity. North Afr. J. Food Nutr. Res. 2023, 7, 69–83. [Google Scholar] [CrossRef]
- Dutta, A.; Kininge, M.M.; Priya; Gogate, P.R. Intensification of delignification and subsequent hydrolysis of sustainable waste as banana peels for the HMF production using ultrasonic irradiation. Chem. Eng. Process. Process. Intensif. 2023, 183, 109247. [Google Scholar] [CrossRef]
- Zhang, X.; Rajagopalan, K.; Lei, H.; Ruan, R.; Sharma, B.K. An overview of a novel concept in biomass pyrolysis: Microwave irradiation. Sustain. Energy Fuels 2017, 1, 1664–1699. [Google Scholar] [CrossRef]
- Fia, A.; Amorim, J. Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process. Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Al-Raggad, M.; Shareef, N. Production of activated carbon derived from agricultural by-products via microwave-induced chemical activation: A review. Carbon Lett. 2021, 31, 957–971. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, P.; Labidi, J. Influence of the heating mechanism during the aqueous processing of vine shoots for the obtaining of hemicellulosic oligosaccharides. Waste Manag. 2021, 120, 146–155. [Google Scholar] [CrossRef]
- del Río, P.G.; Pérez-Pérez, A.; Garrote, G.; Gullón, B. Manufacturing of hemicellulosic oligosaccharides from fast-growing Paulownia wood via autohydrolysis: Microwave versus conventional heating. Ind. Crop. Prod. 2022, 187, 115313. [Google Scholar] [CrossRef]
- Filho, P.C.d.S.; Serra, O.A. Liquid phase synthesis methodologies for the obtainment of rare earth-based inorganic nanomaterials. Quimica Nova 2015, 38, 679–696. [Google Scholar] [CrossRef]
- Salema, A.A.; Yeow, Y.K.; Ishaque, K.; Ani, F.N.; Afzal, M.T.; Hassan, A. Dielectric properties and microwave heating of oil palm biomass and biochar. Ind. Crop. Prod. 2013, 50, 366–374. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N.; Mouris, J.; Hutcheon, R. Microwave dielectric properties of Malaysian palm oil and agricultural industrial biomass and biochar during pyrolysis process. Fuel Process. Technol. 2017, 166, 164–173. [Google Scholar] [CrossRef]
- Farag, S.; Sobhy, A.; Akyel, C.; Doucet, J.; Chaouki, J. Temperature profile prediction within selected materials heated by microwaves at 2.45 GHz. Appl. Therm. Eng. 2012, 36, 360–369. [Google Scholar] [CrossRef]
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Isma, M.A. Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 2011, 90, 1536–1544. [Google Scholar] [CrossRef]
- Bossou, O.V.; Mosig, J.R.; Zurcher, J.-F. Dielectric measurements of tropical wood. Measurement 2010, 43, 400–405. [Google Scholar] [CrossRef]
- El-Meligy, M.G.; Mohamed, S.H.; Mahani, R.M. Study mechanical, swelling and dielectric properties of prehydrolysed banana fiber—Waste polyurethane foam composites. Carbohydr. Polym. 2010, 80, 366–372. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Degs, Y.S.; Mashal, K.; Al Bakain, R.Z. Fast activation of natural biomasses by microwave heating. J. Ind. Eng. Chem. 2015, 21, 230–238. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T.; Salema, A.A. Microwave dielectric characterization of hay during pyrolysis. Ind. Crop. Prod. 2014, 61, 492–498. [Google Scholar] [CrossRef]
- Fares, O.; Al-Oqla, F.M.; Hayajneh, M.T. Dielectric relaxation of mediterranean lignocellulosic fibers for sustainable functional biomaterials. Mater. Chem. Phys. 2019, 229, 174–182. [Google Scholar] [CrossRef]
- Bichot, A.; Lerosty, M.; Radoiu, M.; Méchin, V.; Bernet, N.; Delgenès, J.-P.; García-Bernet, D. Decoupling thermal and non-thermal effects of the microwaves for lignocellulosic biomass pretreatment. Energy Convers. Manag. 2020, 203, 112220. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Smith, M.W.; Link, D.; Stiegman, A.E. Microwave-assisted pyrolysis of Mississippi coal: A comparative study with conventional pyrolysis. Fuel 2018, 217, 656–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Wang, X.; Zhang, Q. Microwave-assisted pyrolysis of sewage sludge: A review. Fuel Process. Technol. 2019, 187, 84–104. [Google Scholar] [CrossRef]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef]
- Mushtaq, F.; Mat, R.; Ani, F.N. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Chen, M.-Q.; Wang, J.; Zhang, M.-X.; Zhu, X.-F.; Min, F.-F.; Tan, Z.-C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Domı́nguez, A.; Menéndez, J.A.; Inguanzo, M.; Bernad, P.L.; Pis, J.J. Gas chromatographic–mass spectrometric study of the oil fractions produced by microwave-assisted pyrolysis of different sewage sludges. J. Chromatogr. A 2003, 1012, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, Z.; Liu, H.; Li, Z.; Li, L.; Ma, C. Microwave pyrolysis of corn stalk bale: A promising method for direct utilization of large-sized biomass and syngas production. J. Anal. Appl. Pyrolysis 2010, 89, 87–94. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Joel, J.; Gomez, A.; Patel, A.K.; Singhania, R.R.; Haldar, D. Environment friendly emerging techniques for the treatment of waste biomass: A focus on microwave and ultrasonication processes. Environ. Sci. Pollut. Res. 2023, 30, 79706–79723. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.H.H.; Burheim, O.S.; Pollet, B.G. Sonochemical and sonoelectrochemical production of hydrogen. Ultrason. Sonochemistry 2019, 51, 533–555. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.-L.; Appels, L.; Dewil, R. Ultrasonic Treatment of Waste Sludge: A Review on Mechanisms and Applications. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1220–1288. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochemistry 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Karimi, M.; Jenkins, B.; Stroeve, P. Ultrasound irradiation in the production of ethanol from biomass. Renew. Sustain. Energy Rev. 2014, 40, 400–421. [Google Scholar] [CrossRef]
- Le, N.T.; Julcour-Lebigue, C.; Delmas, H. An executive review of sludge pretreatment by sonication. J. Environ. Sci. 2015, 37, 139–153. [Google Scholar] [CrossRef]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A Potential Sustainable Pathway for the Valorization Of Lignocellulosic Biomass and Derivatives. In Chemistry and Chemical Technologies in Waste Valorization; Lin, C.S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Ong, V.Z.; Wu, T.Y. An application of ultrasonication in lignocellulosic biomass valorisation into bio-energy and bio-based products. Renew. Sustain. Energy Rev. 2020, 132, 109924. [Google Scholar] [CrossRef]
- Wang, D.; Yan, L.; Ma, X.; Wang, W.; Zou, M.; Zhong, J.; Ding, T.; Ye, X.; Liu, D. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018, 119, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical technologies in the catalytic conversion of biomass. Chem. Soc. Rev. 2020, 50, 1785–1812. [Google Scholar] [CrossRef]
- Bundhoo, Z.M. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, S.; Narale, B.A.; Shanmugam, A.; Mettu, S.; Ashokkumar, M. Ultrasonic Processing of Food Waste to Generate Value-Added Products. Foods 2022, 11, 2035. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-Y.; Liu, C.-Z. Enhanced biogas production from herbal-extraction process residues by microwave-assisted alkaline pretreatment. J. Chem. Technol. Biotechnol. 2010, 85, 127–131. [Google Scholar] [CrossRef]
- Peng, H.; Chen, H.; Qu, Y.; Li, H.; Xu, J. Bioconversion of different sizes of microcrystalline cellulose pretreated by microwave irradiation with/without NaOH. Appl. Energy 2014, 117, 142–148. [Google Scholar] [CrossRef]
- Khanal, S.K.; Montalbo, M.; van Leeuwen, J.; Srinivasan, G.; Grewell, D. Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnol. Bioeng. 2007, 98, 978–985. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Li, K.; An, L.; Shang, Z.; Yang, Q.; Hu, J.; Liu, Y.; Chen, S. Microwave-assisted separation hemicellulose from hemp stalk: Extracting performance, extracting mechanism and mass transfer model. Ind. Crop. Prod. 2023, 197, 116619. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J.-H. Development of an ultrasound-negative pressure cavitation fractional precipitation for the purification of (+)-dihydromyricetin from biomass. Korean J. Chem. Eng. 2023, 40, 1133–1140. [Google Scholar] [CrossRef]
- Chen, W.-H.; Tu, Y.-J.; Sheen, H.-K. Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl. Energy 2011, 88, 2726–2734. [Google Scholar] [CrossRef]
- Boonmanumsin, P.; Treeboobpha, S.; Jeamjumnunja, K.; Luengnaruemitchai, A.; Chaisuwan, T.; Wongkasemjit, S. Release of monomeric sugars from Miscanthus sinensis by microwave-assisted ammonia and phosphoric acid treatments. Bioresour. Technol. 2012, 103, 425–431. [Google Scholar] [CrossRef]
- Ramadoss, G.; Muthukumar, K. Ultrasound assisted ammonia pretreatment of sugarcane bagasse for fermentable sugar production. Biochem. Eng. J. 2014, 83, 33–41. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Olawuyi, I.K.; Afolabi, T.A. Synergistic Effect of Ultrasound and Citric Acid Treatment on Functional, Structural and Storage Properties of Hog Plum ((Spondias mombin L) bagasse. Food Sci. Technol. Int. 2023, 10820132231176579. [Google Scholar] [CrossRef]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Song, W.; Ding, L.; Xie, B.; Zhou, J.; Cen, K. Characterisation of water hyacinth with microwave-heated alkali pretreatment for enhanced enzymatic digestibility and hydrogen/methane fermentation. Bioresour. Technol. 2015, 182, 1–7. [Google Scholar] [CrossRef]
- Debnath, B.; Duarah, P.; Purkait, M.K. Microwave-assisted quick synthesis of microcrystalline cellulose from black tea waste (Camellia sinensis) and characterization. Int. J. Biol. Macromol. 2023, 244, 125354. [Google Scholar] [CrossRef]
- Chang, K.-L.; Han, Y.-J.; Wang, X.-Q.; Chen, X.-M.; Leu, S.-Y.; Liu, J.-Y.; Peng, Y.-P.; Liao, Y.-L.; Potprommanee, L. The effect of surfactant-assisted ultrasound-ionic liquid pretreatment on the structure and fermentable sugar production of a water hyacinth. Bioresour. Technol. 2017, 237, 27–30. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kamide, K.; Takahashi, K.; Shimizu, N. Enhanced enzymatic saccharification of kenaf powder after ultrasonic pretreatment in ionic liquids at room temperature. Bioresour. Technol. 2012, 103, 259–265. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason. Sonochemistry 2017, 34, 136–141. [Google Scholar] [CrossRef]
- Marasca, N.; Cardoso, I.; Rambo, M.; Bertuol, D.; Rambo, M.; Guarda, E.; Scapin, E. Ultrasound Assisted Pretreatments Applied to Cupuaçu Husk (Theobroma grandiflorum) from Brazilian Legal Amazon for Biorefinery Concept. J. Braz. Chem. Soc. 2022, 33, 906–915. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Paul, R.; Silkina, A.; Melville, L.; Suhartini, S.; Sulu, M. Optimisation of Ultrasound Pretreatment of Microalgal Biomass for Effective Biogas Production through Anaerobic Digestion Process. Energies 2023, 16, 553. [Google Scholar] [CrossRef]
- Khamtib, S.; Plangklang, P.; Reungsang, A. Optimization of fermentative hydrogen production from hydrolysate of microwave assisted sulfuric acid pretreated oil palm trunk by hot spring enriched culture. Int. J. Hydrogen Energy 2011, 36, 14204–14216. [Google Scholar] [CrossRef]
- Zhu, Z.; Macquarrie, D.J.; Simister, R.; Gomez, L.D.; McQueen-Mason, S.J. Microwave assisted chemical pretreatment of Miscanthus under different temperature regimes. Sustain. Chem. Process. 2015, 3, 15. [Google Scholar] [CrossRef]
- Eblaghi, M.; Niakousari, M.; Sarshar, M.; Mesbahi, G.R. Combining Ultrasound with Mild Alkaline Solutions as an Effective Pretreatment to Boost the Release of Sugar Trapped in Sugarcane Bagasse for Bioethanol Production. J. Food Process. Eng. 2016, 39, 273–282. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Cellulose hydrolysis and bioethanol production from various types of lignocellulosic biomass after microwave-assisted hydrotropic pretreatment. Renew. Energy 2023, 206, 168–179. [Google Scholar] [CrossRef]
- Sawhney, D.; Vaid, S.; Bangotra, R.; Sharma, S.; Dutt, H.C.; Kapoor, N.; Mahajan, R.; Bajaj, B.K. Proficient bioconversion of rice straw biomass to bioethanol using a novel combinatorial pretreatment approach based on deep eutectic solvent, microwave irradiation and laccase. Bioresour. Technol. 2023, 375, 128791. [Google Scholar] [CrossRef] [PubMed]
- Domingos, I.; Ferreira, J.; Cruz-Lopes, L.P.; Esteves, B. Liquefaction and chemical composition of walnut shells. Open Agric. 2022, 7, 249–256. [Google Scholar] [CrossRef]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically assisted liquefaction of lignocellulosic materials. Bioresour. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef]
- Mateus, M.M.; Acero, N.F.; Bordado, J.C.; dos Santos, R.G. Sonication as a foremost tool to improve cork liquefaction. Ind. Crop. Prod. 2015, 74, 9–13. [Google Scholar] [CrossRef]
- Kržan, A.; Žagar, E. Microwave driven wood liquefaction with glycols. Bioresour. Technol. 2009, 100, 3143–3146. [Google Scholar] [CrossRef]
- Xiao, W.; Niu, W.; Yi, F.; Liu, X.; Han, L. Influence of Crop Residue Types on Microwave-Assisted Liquefaction Performance and Products. Energy Fuels 2013, 27, 3204–3208. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Shupe, T.F.; Hu, T. Influence of solvent type on microwave-assisted liquefaction of bamboo. Eur. J. Wood Wood Prod. 2016, 74, 249–254. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ha, T.M.N.; Nguyen, B.T.; Ha, D.; Vo, T.V.; Nguyen, D.M.; Vo, D.K.; Nguyen, N.T.; Nguyen, T.V.; Hoang, D. Microwave-assisted polyol liquefication from bamboo for bio-polyurethane foams fabrication. J. Environ. Chem. Eng. 2023, 11, 109605. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, Z.; Fan, L.; Zhang, H.; Liao, Y.; Zheng, D.; Wang, S. Rapid and solvent-saving liquefaction of woody biomass using microwave–ultrasonic assisted technology. Bioresour. Technol. 2016, 199, 423–426. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, J.; Yang, Y.; Li, K.; Zhu, H.; Lu, Z. Intensification Effect of Ultrasonic-microwave on Liquefaction of Fir Sawdust. Chem. Ind. For. Prod. 2022, 42, 87–94. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, H.; Xie, J.; Qi, J.; Shupe, T.F. Agricultural and Forest Residues towards Renewable Chemicals and Materials Using Microwave Liquefaction. Int. J. Polym. Sci. 2019, 2019, e7231263. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crop. Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Development of the ultrasonic conditions as an advanced technique for extraction of phenolic compounds from Eucalyptus robusta. Sep. Sci. Technol. 2017, 52, 100–112. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Green approaches for the extraction of antioxidants from eucalyptus leaves. Ind. Crop. Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Wan, J.-Y.; Li, P.; Qi, L.-W. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography–quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in Spatholobus suberectus. J. Chromatogr. A 2011, 1218, 5774–5786. [Google Scholar] [CrossRef]
- Walayat, N.; Yurdunuseven-Yıldız, A.; Kumar, M.; Goksen, G.; Öztekin, S.; Lorenzo, J.M. Oxidative stability, quality, and bioactive compounds of oils obtained by ultrasound and microwave-assisted oil extraction. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and sonoprocessing: The link, the trends and (probably) the future. Ultrason. Sonochemistry 2003, 10, 175–179. [Google Scholar] [CrossRef]
- Chatel, G.; Vigier, K.D.O.; Jérôme, F. Sonochemistry: What Potential for Conversion of Lignocellulosic Biomass into Platform Chemicals? ChemSusChem 2014, 7, 2774–2787. [Google Scholar] [CrossRef]
- Sarwono, A.; Man, Z.; Muhammad, N.; Khan, A.S.; Hamzah, W.S.W.; Rahim, A.H.A.; Ullah, Z.; Wilfred, C.D. A new approach of probe sonication assisted ionic liquid conversion of glucose, cellulose and biomass into 5-hydroxymethylfurfural. Ultrason. Sonochemistry 2017, 37, 310–319. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, Y.; Niu, K.; Zhao, Y.; Zhang, B.; Hu, B. Functional ionic liquids for hydrolysis of lignocellulose. Carbohydr. Polym. 2013, 97, 172–176. [Google Scholar] [CrossRef]
- Santos, D.; Silva, U.F.; Duarte, F.A.; Bizzi, C.A.; Flores, E.M.M.; Mello, P.A. Ultrasound-assisted acid hydrolysis of cellulose to chemical building blocks: Application to furfural synthesis. Ultrason. Sonochem. 2018, 40, 81–88. [Google Scholar] [CrossRef]
- Hernoux, A.; Lévêque, J.-M.; Lassi, U.; Molina-Boisseau, S.; Marais, M.-F. Conversion of a non-water soluble potato starch waste into reducing sugars under non-conventional technologies. Carbohydr. Polym. 2013, 92, 2065–2074. [Google Scholar] [CrossRef]
- Toukoniitty, B.; Kuusisto, J.; Mikkola, J.-P.; Salmi, T.; Murzin, D.Y. Effect of Ultrasound on Catalytic Hydrogenation of d-Fructose to d-Mannitol. Ind. Eng. Chem. Res. 2005, 44, 9370–9375. [Google Scholar] [CrossRef]
- Finch, K.B.; Richards, R.M.; Richel, A.; Medvedovici, A.V.; Gheorghe, N.G.; Verziu, M.; Coman, S.M.; Parvulescu, V.I. Catalytic hydroprocessing of lignin under thermal and ultrasound conditions. Catal. Today 2012, 196, 3–10. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, K.; Wei, G.; Gao, L.; Xin, Z.; Li, J. Intensification of catalytic transfer hydrogenation of fatty acid methyl esters by using ultrasound. Chem. Eng. Process. Process. Intensif. 2021, 170, 108645. [Google Scholar] [CrossRef]
- Mishra, S.P.; Thirree, J.; Manent, A.S.; Chabot, B.; Daneault, C. Ultrasound-catalyzed TEMPO-mediated oxidation of native cellulose for the production of nanocellulose: Effect of process variables. BioResources 2011, 6, 121–143. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Tong, G.; Chin, Y.F.; Zhou, J.C. Preparation of ultrasonic-assisted high carboxylate content cellulose nanocrystals by TEMPO oxidation. BioResources 2011, 6, 1136–1146. [Google Scholar] [CrossRef]
- Ayoub, N.; Toufaily, J.; Guénin, E.; Enderlin, G. Catalyst-free process for oxidation of furfural to maleic acid by high frequency ultrasonic activation. Green Chem. 2022, 24, 4164–4173. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D. Nanotechnology Applied to Cellulosic Materials. Materials 2023, 16, 3104. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Valange, S.; Behling, R.; Colmenares, J.C. A Combined Approach using Sonochemistry and Photocatalysis: How to Apply Sonophotocatalysis for Biomass Conversion? ChemCatChem 2017, 9, 2615–2621. [Google Scholar] [CrossRef]
- Djellabi, R.; Aboagye, D.; Galloni, M.G.; Andhalkar, V.V.; Nouacer, S.; Nabgan, W.; Rtimi, S.; Constantí, M.; Cabello, F.M.; Contreras, S. Combined conversion of lignocellulosic biomass into high-value products with ultrasonic cavitation and photocatalytic produced reactive oxygen species—A review. Bioresour. Technol. 2023, 368, 128333. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Teja, V.; Vinu, R. Sonophotocatalytic degradation of lignin: Production of valuable chemicals and kinetic analysis. J. Environ. Chem. Eng. 2020, 8, 104286. [Google Scholar] [CrossRef]
| Advantage of Microwave Heating | |
|---|---|
| Non-contact heating | In microwave heating, there is no physical contact between the material to be heated and the heat source. This prevents overheating of the material surfaces. |
| Lower energy consumption | In conventional heating, the energy consumption is higher since part of the energy is used to heat the container. |
| Fast heating | In conventional heating, heating is slower. |
| Lower heat losses | The microwave heating container is non-conductive. |
| Shorter reaction times | In conventional heating, reaction times are longer. |
| Volumetric heating | Microwave heating has a uniform heating where the whole material is heated simultaneously, while in conventional heating, there is superficial heating and then a transfer of energy via convection and/or conduction |
| Better level of control | Microwave heating can be turned off immediately. |
| Better product yield | In microwave heating, there is low formation of collateral products. |
| Allows overheating of the material | In microwave heating, the maximum temperature reached is not limited by the boiling point of the substance to be heated. |
| More selective | Microwave radiation is a more selective than conventional heating because it is only absorbed by the biomass and the reaction media |
| Improved moisture reduction | In microwave heating, moisture loss occurs first from the surface of the material. |
| Materials | Characteristics | Examples | |
|---|---|---|---|
| Conductive | Microwaves cannot penetrate them. They reflect the microwaves. | tan | Metals |
| Non-conductive | They are microwave-transparent and have low or zero dielectric loss. They are the materials for the construction of containers for microwave heating. | an | Glass Teflon Ceramics Quartz Air |
| Dielectric (or absorbers) | They absorb microwaves. They are ideal to be heated by microwave | tan | Water Methanol Carbon |
| Biomass | Dp (cm) | Frequencies and Temperature | Reference | |||
|---|---|---|---|---|---|---|
| Tropical wood | 2.08 | 0.1849 | 0.0954 | ---- | 8.2 to 12.4 GHz | [36] |
| Banana fibers with polyurethane 30% | 137 | 26 | ---- | ---- | 1 kHz | [37] |
| Empty fruit bunch (18 wt% moisture) | 6.4 | 1.9 | 0.3 | 3.5 | 2.45 GHz, 27 °C | [35] |
| Empty fruit bunch char | 3.5 | 0.47 | 0.13 | ---- | 2.45 GHz, 500 °C | [35] |
| Pinewood | 2.7 | 0.53 | ---- | 59 | 2.45 GHZ, 17 °C | [34] |
| Oil palm fiber | 1.99 | 0.16 | 0.08 | 24.8 | 2.45 GHZ, 500 °C | [32] |
| Oil palm shell | 2.76 | 0.35 | 0.12 | 13.4 | 2.45 GHz, 500 °C | [32] |
| Oil palm char | 2.83 | 0.23 | 0.08 | 20.6 | 2.45 GHz, 500 °C | [32] |
| Hay | ---- | ---- | ---- | 0.02 | 2.45 GHz, 700 °C | [39] |
| Pinewood | 13.4 | 0.08 | 0.006 | 0.2 | 2.45 GHZ, 25 °C | [38] |
| Arabica coffee | 26.8 | 3.14 | 0.117 | 0.5 | 2.45 GHZ, 25 °C | [38] |
| Wood | ---- | ---- | 0.11 | ---- | ---- | [20] |
| Fir plywood | ---- | ---- | 0.01–0.05 | ---- | ---- | [20] |
| Karanja seeds | ---- | ---- | 1.3 | 1.26 | 0.1 to 3.0 GHz at room temperature | [20] |
| Solvent | tan δ | ||
|---|---|---|---|
| Water | 80.4 | 0.123 | 9.889 |
| Ethylene glycol | 37.0 | 6.079 | 0.161 |
| Methanol | 32.6 | 21.483 | 0.856 |
| Ethanol | 24.3 | 22.866 | 0.941 |
| Cross Linkages | Types of Bonds | Polymers Involved |
|---|---|---|
| Intra-polymer | Ether | Lignin, cellulose, hemicellulose |
| Ester | Hemicellulose | |
| Hydrogen | Cellulose | |
| C-C | Lignin | |
| Inter-polymer | Ether | Lignin-hemicellulose |
| Ester | Lignin-hemicellulose | |
| Hydrogen | Cellulose-hemicellulose | |
| Hydrogen | Lignin- cellulose | |
| Hydrogen | Lignin- hemicellulose |
| Low- and Medium-Frequency Waves | High-Frequency Waves |
|---|---|
| 20 kHz100 kHz | 3 MHz10 MHz |
| Have high power | Have low power |
| Suffer cavitation | Do not suffer cavitation |
| They influence the environment in which they propagate | They do not influence the environment in which they propagate |
| Applications: Sonochemistry (Part IV- of this review) and industry | Applications: Medical diagnostics and non-destructive control of materials (for example) |
| Pretreatment | Operating Conditions | Increase in Soluble Organic Matter | Increase in Soluble Proteins | Increase in Soluble Carbohydrates | Increase in soluble Lipids |
|---|---|---|---|---|---|
| MW | P = 900 W f = 2450 MHz ∆t = 3 min | 8× | 18× | 12× | 2× |
| US | P = 70 W f = 20 kHz ∆t = 30 min | 7× | 12× | 9× | 3× |
| Biomass | Pretreatment | Operating Conditions | Increased sCOD 1 Efficiency (%) |
|---|---|---|---|
| Tetraselmis suecica | US | P = 500 W f = 20 kHz ∆t = 5 s T = 19.1 °C | 5.13 |
| Nannochloropsis oceanica | US | P = 500 W f = 20 kHz ∆t = 54 s T = 21.6 °C | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. https://doi.org/10.3390/ma16237351
Fernandes A, Cruz-Lopes L, Esteves B, Evtuguin DV. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials. 2023; 16(23):7351. https://doi.org/10.3390/ma16237351
Chicago/Turabian StyleFernandes, Ana, Luísa Cruz-Lopes, Bruno Esteves, and Dmitry V. Evtuguin. 2023. "Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials" Materials 16, no. 23: 7351. https://doi.org/10.3390/ma16237351
APA StyleFernandes, A., Cruz-Lopes, L., Esteves, B., & Evtuguin, D. V. (2023). Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials, 16(23), 7351. https://doi.org/10.3390/ma16237351







