Rice Husk-Based Adsorbents for Removal of Metals from Aqueous Solutions
Abstract
:1. Introduction
2. Rice-Husk-Based Adsorbents’ Production
3. Adsorption of Non-Ferrous Metals
3.1. Adsorption of Lead
3.2. Adsorption of Zinc
3.3. Adsorption of Copper
3.4. Adsorption of Chromium
3.5. Adsorption of Nickel and Manganese
4. Adsorption of Ferrous Metals
Adsorption of Iron
5. Adsorption of Minor Metals
5.1. Adsorption of Mercury
5.2. Adsorption of Cadmium
5.3. Adsorption of Cobalt
5.4. Adsorption of Arsenic
6. Adsorption of Radionuclides
6.1. Adsorption of Cesium and Strontium
6.2. Adsorption of Uranyl Ion
7. Adsorption of Precious Metals
7.1. Adsorption of Silver
7.2. Adsorption of Gold
7.3. Adsorption of Palladium
8. Adsorption of Rare and Rare-Earth Metals
8.1. Adsorption of Lanthanum and Erbium
8.2. Adsorption of Cerium
8.3. Adsorption of Rhenium
9. Assessment of the Effectiveness of Rice-Husk-Based Adsorbents Depending on Production Methods
10. Prerequisites, Current Status, and Tasks for the Future
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| m | Adsorbent dosage, g·dm−3 |
| τ | Adsorbent–solution contact time, min |
| C0 | Adsorbtive initial concentration, mg·dm−3 |
| a | Maximum adsorption capacity, mg·g−1 |
| aL (0.1L, 31.1L, etc.) | Langmuir maximum adsorption capacity, mg·g−1 |
| aO (2.9O, 3.5O, etc.) | Observed maximum adsorption capacity, mg·g−1 |
| α | Removal percentage, % |
| l | Desorption efficiency after one cycle, % |
| IM | Isotherm models |
| L | Langmuir isotherm model |
| F | Freundlich isotherm model |
| R-D | Redlich–Peterson equation |
| l | Linear isotherm |
| S (g):L (cm−3) | Ratio of solid (g):liquid (cm−3) |
| S:L | Ratio of solid:liquid |
| DIW | Deionized water |
| v/v | Volume:volume ratio |
| N/D | Not detected |
| KM | Kinetic models |
| Ps1 | Pseudo-first-order |
| Ps2 | Pseudo-second-order |
| I-PD | Intra-particle diffusion |
| El | Elovich model |
| ΔH | Enthalpy change, kJ·mol−1 |
| ΔS | Entropy change, J·mol−1·K−1 |
| ΔG | Gibbs-free energy change, kJ·mol−1 |
References
- Efremova, S.V. Scientific and Technical Solutions to the Problem of Utilization of Waste from Plant- and Mineral-Based Industries. Russ. J. Gen. Chem. 2012, 82, 963–968. [Google Scholar] [CrossRef]
- Khudyakova, T.M.; Kolesnikov, A.S.; Zhakipbaev, B.E.; Kenzhibaeva, G.S.; Kutzhanova, A.N.; Iztleuov, G.M.; Zhanikulov, N.N.; Kolesnikova, O.G.; Mynbaeva, E. Optimization of Raw Material Mixes in Studying Mixed Cements and Their Physicomechnical Properties. Ref. Ind. Ceram. 2019, 60, 76–81. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Sergeeva, I.V.; Botabaev, N.E.; Al’zhanova, A.Z.; Ashirbaev, K.A. Chemical and Phase Transitions in Oxidized Manganese Ore in the Presence of Carbon. Steel Transl. 2017, 47, 605–609. [Google Scholar] [CrossRef]
- Nadirov, K.S.; Zhantasov, M.K.; Sakybayev, B.A.; Orynbasarov, A.K.; Bimbetova, G.Z.; Sadyrbayeva, A.S.; Kolesnikov, A.S.; Ashirbayev, H.A.; Zhantasova, D.M.; Tuleuov, A.M. The Study of the Gossypol Resin Impact on Adhesive Properties of the Intermediate Layer of the Pipeline Three-Layer Rust Protection Voating. Int. J. Adhes. Adhes. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Swami, D.; Buddhi, D. Removal of Contaminants from Industrial Wastewater Through Various Non-Conventional Technologies: A Review. Int. J. Environ. Pollut. 2006, 27, 324–346. [Google Scholar] [CrossRef]
- Dhir, B. Potential of Biological Materials for Removing Heavy Metals from Wastewater. Environ. Sci. Pollut. Res. 2014, 21, 1614–1627. [Google Scholar] [CrossRef]
- Yu, L.; Lan, T.; Yuan, G.; Duan, C.; Pu, X.; Liu, N. Synthesis and Application of a Novel Metal-Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater. Polymers 2023, 15, 2150. [Google Scholar] [CrossRef]
- Ashfaq, A.; Nadeem, R.; Gong, H.; Rashid, U.; Noreen, S.; Rehman, S.U.; Ahmed, Z.; Adil, M.; Akhtar, N.; Ashfaq, M.Z.; et al. Fabrication of Novel Agrowaste (Banana and Potato Peels)-Based Biochar/TiO2 Nanocomposite for Adsorption of Cr(VI), Statistical Optimization via RSM Approach. Polymers 2022, 14, 2644. [Google Scholar] [CrossRef]
- Efremova, S.V. Water Treatment with a Shungite Sorbent and Biosorbents on Its Base. Russ. J. Appl. Chem. 2006, 79, 397–402. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.A.; Reda, M.; Aldawsari, A.M.; Chen, Q.; Kariri, M.A. Chitosan Polymer Functionalized-Activated Carbon/Montmorillonite Composite for the Potential Removal of Lead Ions from Wastewater. Polymers 2023, 15, 2188. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, T.; Wang, X.; Zheng, Y.; Zheng, J.; Song, G.; Liu, S. Recent Progress on Moisture Absorption Aging of Plant Fiber Reinforced Polymer Composites. Polymers 2023, 15, 4121. [Google Scholar] [CrossRef]
- Kailash, D.; Dharmendra, P.; Anil, V. Low Cost Adsorbents for Heavy Metal Removal from Wastewater: A Review. Res. J. Chem. Environ. 2010, 14, 100–103. [Google Scholar]
- Dharsana, M.; Prakash, A.J. Adsorption of Lead from Contaminated Water Using Biosorbent. Mater. Tehnol. 2022, 56, 171–177. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of Gold and Silver from Spent Mobile Phones by Means of Acidothiourea Leaching Followed by Adsorption Using Biosorbent Prepared from Persimmon Tannin. Hydrometallurgy 2013, 133, 84–93. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of Rare Earth Metals: A Review of Recent Literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, A.A. Simple Approach to Fabricate Granular Adsorbent for Adsorption of Rare Elements. Int. J. Biol. Macromol. 2015, 72, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Efremova, S.V. Rice Hull as a Renewable Raw Material and Its Processing Routes. Russ. J. Gen. Chem. 2012, 82, 999–1005. [Google Scholar] [CrossRef]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice Husk Research: From Environmental Pollutant to a Promising Source of Organo-Mineral Raw Materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef]
- Bozęcka, A.; Orlof-Naturalna, M.; Korpalski, A. Removing of Cu2+ Ions from Aqueous Solutions by Using Selected Sorbents. Przem. Chem. 2020, 99, 1501–1504. [Google Scholar] [CrossRef]
- Khokhlov, A.V.; Khokhlova, L.I. Modified Rice Husk Biochar for Binding Cd(II), Cu(II) Ions in Aqueous Solutions. J. Chem. Technol. 2022, 30, 659–666. [Google Scholar] [CrossRef]
- Mirabdolazimi, S.M.; Mohammad-Khah, A.; Ansari, R.; Zanjanchi, M.A. Removal of Chromium (VI) Ion from Aqueous Solutions Using Acid Modified Rice Husk. In Chemistry and Chemical Engineering Research Progress; Haghi, A.K., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 45–54. [Google Scholar]
- Sarma, P.J.; Kumar, R.; Pakshirajan, K. Batch and Continuous Removal of Copper and Lead from Aqueous Solution using Cheaply Available Agricultural Waste Materials. Int. J. Environ. Res. 2015, 9, 635–648. [Google Scholar]
- Ong, S.-T.; Foo, Y.-C.; Hung, Y.-T. Removal of Pb(II) from Aqueous Solution Using Natural Rice Husk. Res. J. Chem. Environ. 2013, 17, 53–57. [Google Scholar]
- Wong, K.K.; Lee, C.K.; Low, K.S.; Haron, M.J. Removal of Cu and Pb by Tartaric Acid Modified Rice Husk from Aqueous Solutions. Chemosphere 2003, 50, 23–28. [Google Scholar] [CrossRef]
- Wong, K.K.; Lee, C.K.; Low, K.S.; Haron, M.J. Removal of Cu and Pb from Electroplating Wastewater Using Tartaric Acid Modified Rice Husk. Process Biochem. 2003, 39, 437–445. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Thomas Choong, S.Y. Rice Husk as a Potentially Low-Cost Biosorbent for Heavy Metal and Dye Removal: An Overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.; Al-Ahmed, A. Removal of Lead Ions (Pb2+) from Water and Wastewater: A Review on the Low-Cost Adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef]
- Singha, B.; Das, S.K. Removal of Pb(II) Ions from Aqueous Solution and Industrial Effluent Using Natural Biosorbents. Environ. Sci. Pollut. Res. 2012, 19, 2212–2226. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, D.; Gaur, J.P. Kinetic and Isotherm Modeling of Lead (II) Sorption onto Some Waste Plant Materials. Chem. Eng. J. 2009, 148, 226–233. [Google Scholar] [CrossRef]
- Gupta, N.; Amritphale, S.S.; Chandra, N. Removal of Lead from Aqueous Solution by Hybrid Precursor Prepared by Rice Hull. J. Hazard. Mater. 2009, 163, 1194–1198. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Oboh, I.O.; Obahiagbon, K.O. Equilibrium Sorption Isotherm for Lead (Pb) Ions on Hydrogen Peroxide Modified Rice Hulls. Int. J. Phys. Sci. 2009, 4, 423–427. [Google Scholar]
- Masoumi, A.; Hemmati, K.; Ghaemy, M. Low-Cost Nanoparticles Sorbent from Modified Rice Husk and a Copolymer for Efficient Removal of Pb(II) and Crystal Violet from Water. Chemosphere 2016, 146, 253–262. [Google Scholar] [CrossRef]
- Fan, Y.; Zhai, S.; Liu, N.; Lv, J.; Lei, Z.; An, Q. Adsorption Equilibrium, Kinetics and Mechanism of Pb(II) over Carbon–Silica Composite Biosorbent with Designed Surface Oxygen Groups. Res. Chem. Intermed. 2016, 42, 869–891. [Google Scholar] [CrossRef]
- Ullah, S.; Assiri, M.A.; Al-Sehemi, A.G.; Bustam, M.A.; Sagir, M.; Abdulkareem, F.A.; Raza, M.R.; Ayoub, M.; Irfan, A. Characteristically Insights, Artificial Neural Network (ANN), Equilibrium, and Kinetic Studies of Pb (II) Ion Adsorption on Rice Husks Treated with Nitric Acid. Int. J. Environ. Res. 2020, 14, 43–60. [Google Scholar] [CrossRef]
- Zharmenov, A.; Yefremova, S.; Sukharnikov, Y.; Bunchuk, L.; Kablanbekov, A.; Anarbekov, K.; Murtazayeva, D.; Yessengarayev, E. Carbonaceous Materials from Rice Husk: Production and Application in Industry and Agriculture. J. Pol. Miner. Eng. Soc. 2018, 1, 263–274. [Google Scholar]
- Roha, B.; Yao, J.; Batool, A.; Hameed, R.; Ghufran, M.A.; Hayat, M.T.; Sunahara, G. Model Sorption of Industrial Wastewater Containing Cu2+, Cd2+, And Pb2+ Using Individual and Mixed Rice Husk Biochar. Environ. Technol. Innov. 2021, 24, 101900. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of Rice Husk- and Dairy Manure-Derived Biochars for Simultaneously Removing Heavy Metals from Aqueous Solutions: Role of Mineral Components in Biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Akhtar, M.; Iqbal, S.; Kausar, A.; Bhanger, M.I.; Shaheen, M.A. An Economically Viable Method for the Removal of Selected Divalent Metal Ions from Aqueous Solutions Using Activated Rice Husk. Colloids Surf. 2010, 75, 149–155. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption Mechanism of Nine Different Heavy Metals onto Biomatrix from Rice Husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef]
- Sheveleva, I.V.; Kholomeidik, A.N.; Voit, A.V.; Morgun, N.P.; Zemnukhova, L.A. Removal of Metal Ions with Rice Husk-Based Sorbents. Russ. J. Appl. Chem. 2009, 82, 1840–1844. [Google Scholar] [CrossRef]
- Lattuada, R.M.; Peralba, M.C.R.; DosSantos, J.H.Z. Peat, Rice Husk and Rice Husk Carbon as Low-Cost Adsorbents for Metals from Acidic Aqueous Solutions. Sep. Sci. Technol. 2014, 49, 101–111. [Google Scholar] [CrossRef]
- Ong, K.K.; Tarmizi, A.F.A.; WanYunus, W.M.Z.; Safidin, K.M.; Fitrianto, A.; Hussin, A.G.A.; Azmi, F.M. Sorption Kinetics of Zn(II) Ion by Thermally Treated Rice Husk. In Proceedings of the International Conference on Mathematics, Engineering and Industrial Applications, Penang, Malaysia, 28–30 May 2014; Volume 1660. Issue 1. [Google Scholar] [CrossRef]
- Meretin, R.N.; Nikiforova, T.E. Investigation of the Reactivity of the Surface of a Carbon-Containing Silicate Sorbent of Plant Origin. Chem. Chem. Tech. 2021, 64, 117–125. [Google Scholar] [CrossRef]
- Meretin, R.N. Sorption Properties of Coal-Mineral Sorbent Based on Rice Husk in Relation to Heavy Metal Ions. Sorbt. Hromatogr. Protcessy Sorpt. Chromatogr. Process. 2019, 19, 703–710. [Google Scholar] [CrossRef]
- El-Shafey, E.I. Removal of Zn(II) and Hg(II) from Aqueous Solution on a Carbonaceous Sorbent Chemically Prepared from Rice Husk. J. Hazard. Mater. 2010, 175, 319–327. [Google Scholar] [CrossRef]
- Bozęcka, A.; Orlof-Naturalna, M.; Korpalski, A. Comparison of Copper and Cobalt Ions Sorption from Aqueous Solutions on Selected Sorbents. J. Ecol. Eng. 2020, 21, 84–90. [Google Scholar] [CrossRef]
- Liang, X.; Luo, X.G.; Lin, X.Y. Adsorption Mechanism of Copper(II) Ions on Expansion-Treated Rice Husk. Mater. Sci. Forum 2011, 695, 13–16. [Google Scholar] [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, N.; Wijaya, L. Adsorption-Reduction Performance of Tea Waste and Rice Husk Biochars for Cr(VI) Elimination from Wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, R.; Lei, Z.; Liu, N.; Lv, J.; Zhai, S.; Zhai, B.; Wang, L. Removal of Cr(VI) from Aqueous Solution by Rice Husk Derived Magnetic Sorbents. Korean J. Chem. Eng. 2016, 33, 1416–1424. [Google Scholar] [CrossRef]
- Pourfadakari, S.; Jorfi, S.; Ahmadi, M.; Takdastan, A. Experimental Data on Adsorption of Cr(VI) from Aqueous Solution Using Nanosized Cellulose Fibers Obtained from Rice Husk. Data Brief 2017, 15, 887–895. [Google Scholar] [CrossRef]
- Maliki, S.; Rosnelly, C.M.; Adisalamun, A.; Husin, H.; Bilqis, N. Removal of Fe(II) in Groundwater using Rice Husk-Sourced Biosorbent in Continuous Column Adsorption. J. Phys. 2019, 1402, 055007. [Google Scholar] [CrossRef]
- Rocha, L.S.; Lopes, I.; Lopes, C.B.; Lopes, C.B.; Henriques, B.; Soares, A.M.V.M.; Duarte, A.C.; Pereira, E. Efficiency of a Cleanup Technology to Remove Mercury from Natural Waters by Means of Rice Husk Biowaste: Ecotoxicological and Chemical Approach. Environ. Sci. Pollut. Res. 2014, 21, 8146–8156. [Google Scholar] [CrossRef]
- Rocha, L.S.; Lopes, C.B.; Borges, J.A.; Duarte, A.C.; Pereira, E. Valuation of Unmodified Rice Husk Waste as an Eco-Friendly Sorbent to Remove Mercury: A Study Using Environmental Realistic Concentrations. Water Air Soil Pollut. 2013, 224, 1599. [Google Scholar] [CrossRef]
- Rocha, L.S.; Lopes, C.B.; Henriques, B.; Tavares, D.S.; Borgesb, J.A.; Duarte, A.C.; Pereira, E. Competitive Effects on Mercury Removal by an Agricultural Waste: Application to Synthetic and Natural Spiked Waters. Environ. Technol. 2014, 35, 661–673. [Google Scholar] [CrossRef]
- Kumar, S.P.; Ramakrishnan, K.; Kirupha, D.S.; Sivanesan, S. Thermodynamic and Kinetic Studies of Cadmium Adsorption from Aqueous Solution onto Rice Husk. Braz. J. Chem. Eng. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Liu, J.F.; Gao, X.Q.; Wu, X.S.; Zhang, Z.Y.; Zhang, X.R. Sorption of Cadmium by Rice Husk Char, Bamboo Char, and Coconut Shell Char in Aqueous Solutions. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012109. [Google Scholar] [CrossRef]
- Qu, J.; Meng, X.; You, H.; Ye, X.; Du, Z. Utilization of Rice Husks Functionalized with Xanthates as Cost-Effective Biosorbents for Optimal Cd(II) Removal from Aqueous Solution Via Response Surface Methodology. Bioresour. Technol. 2017, 241, 1036–1042. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Azlina, W.; Abd, W.K.G.; Idris, A.; Ahmad, M.B. Hydrogel Biochar Composite for Arsenic Removal from Wastewater. Desalination Water Treat. 2016, 57, 3674–3688. [Google Scholar] [CrossRef]
- Singh, P.; Sarswat, A.; Pittman, C.U., Jr.; Mlsna, T.; Mohan, D. Sustainable Low-Concentration Arsenite [As(III)] Removal in Single and Multicomponent Systems Using Hybrid Iron Oxide-Biochar Nanocomposite Adsorbents—A Mechanistic Study. ACS Omega 2020, 5, 2575–2593. [Google Scholar] [CrossRef]
- Zhuravlev, I. Titanium Silicates Precipitated on the Rice Husk Biochar as Adsorbents for the Extraction of Cesium and Strontium Radioisotope Ions. Colloids Interfaces 2019, 3, 36. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Luo, X.G.; Huang, S.T.; Wang, J.; Zong, Y.L.; Zhou, J.; Ou, M.H. A Sorbent of Expanded Rice Husk Powder for Removal of Uranyl Ion from Aqueous Solution. Rare Met. 2016, 35, 425–432. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Luo, X.; Luo, A.; Liang, X. Adsorption of Ag+ from Aqueous Solutions by Rice Husk. Mater. Sci. Forum 2010, 658, 45–48. [Google Scholar] [CrossRef]
- Liang, X.; Luo, X.; Lin, X.; Mei, Q. The Behaviors of Removing Silver Ions by Low-Cost Expanded Rice Husk, Nature Diatomite and Nature Bentonite as Sorbents. Mater. Sci. Forum 2012, 724, 472–475. [Google Scholar] [CrossRef]
- Aktas, S.; Morcali, M.H. Gold Uptake from Dilute Chloride Solutions by a Lewatit TP 214 and Activated Rice Husk. Int. J. Miner. Process. 2011, 101, 63–70. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Ozlem, E.; Aktas, S. Studies of Gold Adsorption from Chloride Media. Mater. Res. 2015, 18, 660–667. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Supiyeva, Z.A.; Yeleuov, M.A.; Taurbekov, A.T.; Pavlenko, V.V.; Smagulova, G.T. Experimental Determination of Electrochemical Sorption/Desorption Properties of Gold(III) Ions. Russ. J. Non-Ferr. Met. 2021, 62, 257–264. [Google Scholar] [CrossRef]
- Supiyeva, Z.; Avchukir, K.; Pavlenko, V.; Yeleuov, M.; Taurbekov, A.; Smagulova, G.; Mansurov, Z. The Investigation of Electroreduction of AuCl4- in the Case of Gold Electrosorption Using Activated Carbon. Mater. Today Proc. 2020, 25, 33–38. [Google Scholar] [CrossRef]
- Nabieh, K.A.; Mortada, W.I.; Helmy, T.E.; Kenawy, I.M.M.; El-Reash, A.Y.G. Chemically Modified Rice Husk as an Effective Adsorbent for Removal of Palladium Ions. Heliyon 2021, 7, e06062. [Google Scholar] [CrossRef]
- Awwad, N.S.; Gad, H.M.H.; Ahmad, M.I.; Aly, H.F. Sorption of Lanthanum and Erbium from Aqueous Solution by Activated Carbon Prepared from Rice Husk. Colloids Surf. B Biointerfaces 2010, 81, 593–599. [Google Scholar] [CrossRef]
- Zafar, S.; Khan, M.I.; Khraisheh, M.; Shahida, S.; Javed, T.; Mirza, M.L.; Khalid, N. Use of Rice Husk as an Effective Sorbent for the Removal of Cerium Ions from Aqueous Solution: Kinetic, Equilibrium and Thermodynamic Studies. Desalination Water Treat. 2019, 150, 124–135. [Google Scholar] [CrossRef]
- Kablanbekov, A.; Yefremova, S.; Berdikulova, F.; Satbaev, S.; Yermishin, S.; Shalabaev, N.; Satbaev, B.; Terlikbayeva, A.; Zharmenov, A. Rice Husk Cellulose-Based Adsorbent to Extract Rare Metals: Preparing and Properties. Materials 2023, 16, 6277. [Google Scholar] [CrossRef]
- Zharmenov, A.; Yefremova, S.; Suicharnikov, Y.; Korabayev, A. The Preparing of Carbon- and Siliceous Materials from Vegetable Wastes and Using of New Materials in Sorptive and Catalytic Processes. In Proceedings of the 14th Conference on Environment and Mineral Processing, Ostrava, Czech Republic, 3–5 June 2010; Volume 2, pp. 191–195. [Google Scholar]
- Bozęcka, A.; Surdek, A.; Bozęcki, P. Assessment of Suitability of Selected Sorbents for Removal of Co2+ Ions from Aqueous Solutions. Przem. Chem. 2018, 97, 1565–1568. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Gilmanov, M.K. Nanostructural Carbon Sorbents for Different Functional Application. In Sorbents: Properties, Materials and Applications; Willis, T.P., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 217–284. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption: Part XI. A System of Classification of Solution Adsorption Isotherms and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Area Solids. J. Chem. Soc. 1960, 14, 3973–3993. [Google Scholar] [CrossRef]
- Yefremova, S.; Zharmenov, A.; Sukharnikov, Y.; Bunchuk, L.; Kablanbekov, A.; Anarbekov, K.; Kulik, T.; Nikolaichuk, A.; Palianytsia, B. Rice Husk Hydrolytic Lignin Transformation in Carbonization Process. Molecules 2019, 24, 3075. [Google Scholar] [CrossRef]
- Bugaenko, L.T.; Ryabykh, S.M.; Bugaenko, A.L. A Near Total System of the Average Ionic Crystallo-Graphic Radius and Their Usage for Ionization Potentials Determination. Vestn. Mosk. Univ. Khimiya 2008, 49, 363–384. [Google Scholar]
- Rahman, D.Z.; Vijayaraghavan, J.; Thivya, J. A Comprehensive Review on Zinc(II) Sequestration from Wastewater Using Various Natural/Modified Low-Cost Agro-Waste Sorbents. Biomass. Conv. Bioref. 2023, 13, 5469–5499. [Google Scholar] [CrossRef]



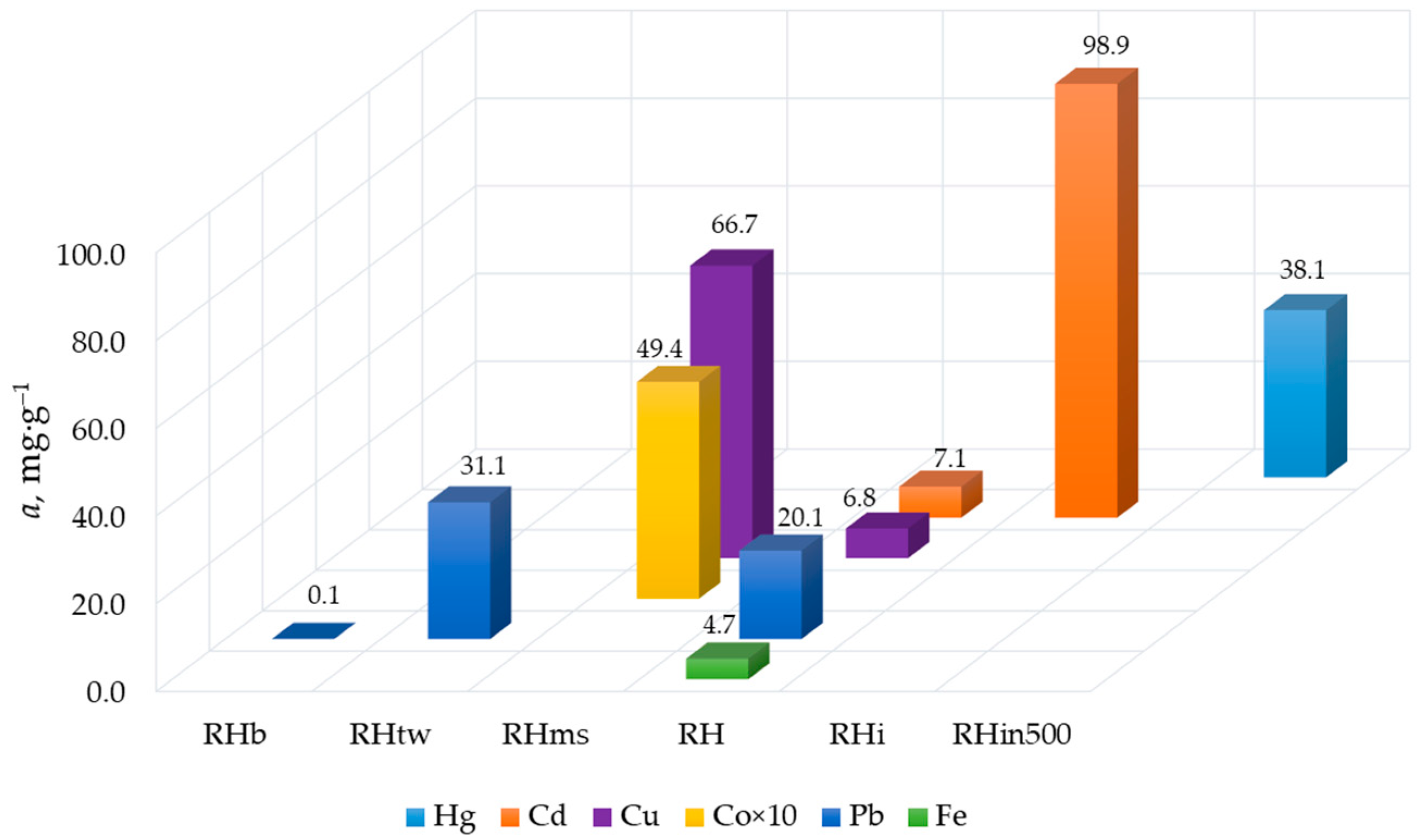
| Sorbent | Production Conditions | Ref. |
|---|---|---|
| RHb | RH, boiled for 6 h, washed with distilled water, dried at 105 °C, sieved to a particle size of 250–350 μm. | [28] |
| RHtw | RH, washed with tap water, then with Milli-Q water, dried at 80 °C overnight, ground, and sieved. Particles of 100–200 μm in size were used. | [29] |
| RHg | RH, washed with distilled water, and refluxed in 3, 6, 9, and 12 M NaOH solutions with the addition of ethylene glycol for 4 h at 198–200 °C. Unreacted ethylene glycol was removed by distillation. Then, 25% NH4NO3 solution was added to pH 8.0. The prepared gel was filtered and dried in air at room temperature. | [30] |
| RHHP | RH, dried, ground into 20–30 mesh, treated with 0.1 N NaOH at 23 °C for 1 h with stirring, washed with distilled water, mixed with 0.1, 0.3, 0.6, 0.9, 1.2, and 1.5 M H2O2 at a 1.0 (g):7.0 (cm3) ratio, then washed, and dried overnight. | [31] |
| RHTA | RH, modified with C4H6O6 at a 1:12.5 weight ratio for 24 h at 50 °C, heated at 180 °C for 15 min, dried under vacuum for 48 h, and mixed with poly(MMA-co-MA) at a weight ratio of 0.5, 0.75, and 1. The mixture was refluxed for 3 h using inert gas and stirring. The prepared crosslinked nanoparticles were filtered, washed with tetrahydrofuran, and dried at 80 °C in a vacuum oven for 12 h. | [32] |
| RHC-OX | RH, washed with distilled water, dried at 100 °C overnight, ground into 60 mesh, mixed with 70% H2SO4 at a 1.0 (g):10.0 (cm3) ratio for 10 min, then poured into a stainless-steel autoclave, and carbonized at 100 °C for 6 h. The residue was filtered, washed with distilled water, dried at 100 °C for 24 h, and treated with (NH4)2S2O8 in 1 M H2SO4 at a 1:10 solid-to-liquid ratio for 12 h at room temperature. The solid residue was filtered, washed with distilled water, dried at 100 °C for 12 h, and ground into a powder. | [33] |
| RHC-Si-400 | RH, washed with distilled water, ground into 63 μm, soaked in 0.1 M HNO3 for 24 h, filtered, washed with distilled water, dried at room temperature, and carbonized in a Fixed-Bed Reactor Unit under an air-free atmosphere at 400 °C. | [34] |
| RHC-Si-600 | RH, washed with distilled water, ground into 63 μm, soaked in 0.1 M HNO3 for 24 h, filtered, washed with distilled water, dried at room temperature, and carbonized in a Fixed-Bed Reactor Unit under an air-free atmosphere at 600 °C. | |
| RHC-Si-800 | RH, washed with distilled water, ground into 63 μm, soaked in 0.1 M HNO3 for 24 h, filtered, washed with distilled water, dried at room temperature, and carbonized in a Fixed-Bed Reactor Unit under an air-free atmosphere at 800 °C. | |
| SCActA | RH, heated in a rotary furnace at 400 °C under an off-gas atmosphere for 30 min, cooled without air to room temperature, activated with water vapor at 850 °C for 30 min, and treated with 70 g·dm−3 of NaOH at 70–80 °C for 2 h. | [35] |
| RH1 | RH, washed with fresh and then distilled water, air-dried for 5 days, and sieved (2 mm). | [36] |
| RHB | RH1, carbonized at 500 °C for 2 h under an O2-limited atmosphere. | |
| EDTA-RHB | RHB, soaked by 0.05 M EDTA (1 g:7 cm3), dried at 50 °C overnight, washed with 20 cm3 of distilled water, and dried at 50 °C for 6 h. | |
| MB | RH:RHB:EDTA-RHB ratio of 1:1:1. | |
| RHBC | RH, heated under an O2-limited atmosphere at 350 °C for 4 h, ground, and sieved (0.5 mm). | [37] |
| RHw | RH, crushed, sieved (100–1000 μm), washed with tap water, then washed and boiled in double-distilled DIW, and oven-dried at 110 °C for 24 h. | [38] |
| RHwN | RHw, soaked in 0.1 M HNO3 for 2 h, and oven-dried at 110 °C for 2 h. | |
| RHwNc | RHwN, impregnated with 1 M K2CO3, and oven-dried at 110 °C for 24 h. | |
| RHwNcT | RHwNc, heated at 100–200 °C at a heating rate of 10 °C·min−1 for 8 h under an N2 flow of 500 cm3·min−1. | |
| RHa | RH, subjected to 1.5% alkali treatment (300 g in 1 dm3), autoclaved at 121 °C for 30 min, washed with deionized water, and dried at 50 °C. | [39] |
| RH | RH, washed with water, and dried at 105 °C. | [40] |
| RHHCl | RH, treated with 0.1 M HCl at 90 °C for 1 h, and washed with distilled water. | |
| RHs | RHHCl, oxidized at 300 °C and then at 600 °C. | [40] |
| RHNaOH | RH, treated with 1 M NaOH at 90 °C for 1 h, and washed with distilled water. | |
| RHUn | RH, unmodified (information about preparation is absent). | [41] |
| RHCa-CO2 | RH, carbonized at 300 °C for 1 h, soaked in a KOH solution at S:L ratio of 1, and activated at 780 °C for 1 h while CO2 was poured in for 0, 15, 30, and 60 min. | |
| RH-500 | RH, crushed, sieved (2 mm), washed with ultra-pure water several times, oven-dried at 105 °C for 24 h, and heated at 500 °C for 2 h. | [42] |
| RHCAA | RH, washed with water, dried at 105 °C, heated at 450–500 °C for 8–10 min, cooled in distilled water (S:L = 1:5), filtered out, and treated with 2% CH3CHOH. Solid residue washed with double-distilled water, dried at 100–150 °C, and crushed. | [43,44] |
| RHH2SO4wet | RH, washed with distilled water, air-dried, treated with 13 M sulfuric acid at S (g):L (cm3) of 20:100 at 175–180 °C for 20 min with occasional stirring, cooled, washed, stored under acidic conditions (pH 1.5–2), and washed with a stream of distilled water between two sieves of 16 and 60 mesh before experiments. | [45] |
| RHH2SO4dry | RHH2SO4wet, dried at 120 °C. | |
| RHms | RH, milled and sieved (0.5 mm). | [46] |
| ERH | RH, expansion-treated, and treated with an alkaline solution. | [47] |
| RHP450 | RH, dried in the sun for 48 h, oven-dried at 65 °C for 72 h, ground in a mechanical grinder, sieved with a pulverized sieve of size < 250 µm, pyrolyzed at 450 °C for 2 h, cooled, and sieved (<250 µm). | [48] |
| RHCf | RH, powder, mixed with 2 M NaOH at S:L = 1:7, heated to 100 °C for 4 h, washed with DIW, and dried at 120 °C overnight. | [49] |
| RHCf-Mag-2 | RHCf (5 g), mixed with 10.0 g of FeCl3·6H2O in 50 cm3 of ethanol, stirred for 2 h, kept in a water bath at 50 °C to evaporate ethanol, oven-dried at 100 °C for 24 h, heated at 800 °C under N2 atmosphere for 2 h, ground, washed with DIW, and dried in a vacuum oven at 80 °C. | |
| RHCf-Mag-0.5 | Ratio of FeCl3·6H2O/RHCf = 0.5:1. | |
| RHCf-Mag-1 | Ratio of FeCl3·6H2O/RHCf = 1:1. | |
| RH-NCFs | RH, washed with distilled water, oven-dried at 40 °C overnight, crushed (5–10 mm), and passed through a 60-mesh screen. Soaked by a 2:1 (v/v) toluene/ethanol mixture (S:L = 30 g:450 cm3) for 20 h, and dried at 55 °C for 24 h. Treated with sodium chlorite solution (pH 4) at 50 °C for 1 h, and washed with distilled water. Treated with 600 cm3 of 5% KOH for 24 h, dried at 90 °C for 2 h, and washed with distilled water. Hydrolyzed by a mixture of (40 cm3 DIW + 20 cm3 12.1 N HCl + 40 cm3 36 N H2SO4) at 70 °C for 3 h. Sonicated at 50 KHZ at 80 °C for 3 h, and dried. | [50] |
| RHC-400 | RH, washed and dried at 80 °C for 24 h, carbonized at 400 °C for 1.5 h, and crushed (20–80 mesh). | [51] |
| RHC-400-A650 | RH, washed and dried at 80 °C for 24 h, carbonized at 400 °C for 0.5 h, activated by the pyrolysis technique at 650 °C for 1.5 h, and crushed (20–80 mesh). | |
| RHin200–500 | RH, washed by distilled water, dried at 60 °C, ground, and sieved (200–500 µm). | [52] |
| RHin500 | RH, washed by distilled water, dried at 60 °C, ground, and sieved (<500 µm). | [53] |
| RHin500 | RH, washed by distilled water, dried at 60 °C, ground, and sieved (<500 µm). | [54] |
| RHi | RH, crushed, sieved, washed with distilled water, and dried at 100 °C. | [55] |
| RHC | RH, pyrolyzed at 350 °C for 30 min, crushed, and sieved (2 mm). | [56] |
| RH-X | RH, washed with distilled water and dried at 80 °C for 24 h, crushed and sieved (0.15 mm), treated with concentrated H2SO4 (S (g):L (cm3) = 1:3) with stirring, washed and dried overnight at room temperature, mixed with 4 M NaOH (S (g):L (cm3) = 1:3, contact time—90 min) with stirring, and treated with CS2 under optimum conditions (S (g):L (cm3) = 1.00, contact time—60 min, and xanthation temperature—18.5 °C). | [57] |
| HBC-RHs | Mix of rice husk biochar, acrylamide, N,N’-methylenebisacrylamide, and ammonium persulfate, poured into polyvinyl chloride straws (3 mm diameter), kept in an oven at 40 °C for 30 min, and left at room temperature (30 °C) for 24 h, then crushed, washed, and dried in air and then in a vacuum oven at 40 °C for 24 h. | [58] |
| RHIOB | RH, soaked by FeCl3·6H2O for 24 h, dried for 2 h at 80 °C, and pyrolyzed at 600 °C for 1 h under N2. | [59] |
| RH-Ti | RH, filled with a titanyl sulphate solution, heated in a water bath for 10 h, and neutralized with a potassium alkali solution or potassium liquid glass. | [60] |
| Powder-TiSi | RH-Ti, heated in an autoclave at ≥150 °C for 10 h, washed with distilled water, and dried at 120 °C. | |
| Carbon-TiSi | RH-Ti, heated separately in the tube furnace at 400, 500, 600, 700, and 800 °C for 2–3 h with water vapor blowing, washed with distilled water, and dried at 120 °C. | |
| ERH-CO3 | RH, mixed with 3–5% carbonate, passed through the extruder at the exit temperature of 250–300 °C, washed with DIW, and dried at 45 °C. | [61] |
| Rice husk powder | RH, produced by Haitian High-Tech Material Co., Ltd., without added purification. | [62] |
| Expansion-treated rice husk powder | Expansion-treated rice husk powder produced by Haitian High-Tech Material Co., Ltd., without added purification. | [63] |
| ARH | RH, heated at 1000 °C for 3 h, cooled overnight under an Ar atmosphere, and homogenized using a three-dimensional shaker for 1 h. | [64] |
| ARH-250 | RH, ground (size of end product = 250 μm), washed with distilled water, dried at 105 °C, heated at 1000 °C for 3 h, cooled in a desiccator, and homogenized using a three-dimensional shaker for 1 h. | [65] |
| CA-RH | RH, washed and dried, heated at 500 °C under an Ar atmosphere, mixed with KOH at a weight ratio of 1:5, activated at 850 °C under an Ar atmosphere, and washed with distilled water. | [66,67] |
| RH@MCM-41@ARS | RH, mixed with Mobil Composition of Matter No. 41 adsorbent (MCM-41) and modified by alizarin red S. | [68] |
| RH- H3PO4-C | RH, activated by H3PO4, and then carbonized at 700 °C for 2.5 h. | [69] |
| RHwd | RH, washed and dried without other treatment. | [70] |
| KHC4 | RH, treated for 1 h with 25 cm3 of Kürschner and Hoffer reagent 4 times, filtered on a glass filter, washed with a fresh portion of Kürschner and Hoffer reagent and hot distilled water, and dried at 105 °C. | [71] |
| KHC4–600VA | KHC4, heated at 600 °C for 30 min, activated by water vapor at 850 °C for 30 min, treated with 70 g·dm−3 of NaOH at a S:L ratio of 1:10, boiled for 90 min, and washed with distilled water. | |
| RHNaOH-S-gr | RH, carbonized at 650 °C at a heating rate of 15 °C·min for 30 min, boiled with 70 g·dm−3 NaOH for 2 h at a S (g):L (cm3) ratio of 1:15, washed with distilled water, dried at 105–110 °C for 2 h, milled (0.25–0.04 mm), mixed with a 35% aqueous solution of sugar at a S (g):L (cm3) ratio of 1:0.35, granulated on the plate granulator for 60 min up to 0.63–2.5 mm, dried at 105–110 °C for 2 h, and carbonized at 650 °C for 30 min with concurrent activation by water vapor for 30 min at 850–900 °C. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yefremova, S.; Kablanbekov, A.; Satbaev, B.; Zharmenov, A. Rice Husk-Based Adsorbents for Removal of Metals from Aqueous Solutions. Materials 2023, 16, 7353. https://doi.org/10.3390/ma16237353
Yefremova S, Kablanbekov A, Satbaev B, Zharmenov A. Rice Husk-Based Adsorbents for Removal of Metals from Aqueous Solutions. Materials. 2023; 16(23):7353. https://doi.org/10.3390/ma16237353
Chicago/Turabian StyleYefremova, Svetlana, Askhat Kablanbekov, Baimakhan Satbaev, and Abdurassul Zharmenov. 2023. "Rice Husk-Based Adsorbents for Removal of Metals from Aqueous Solutions" Materials 16, no. 23: 7353. https://doi.org/10.3390/ma16237353






