Effect of Aging Treatment on the Strength and Microstructure of 7075-Based Alloys Containing 2% Li and/or 0.12% Sc
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Effect of the Heat Treatments on the Microhardness of Al 7075 Alloy
5. Effect of the Heat Treatments on Microhardness of Al 7075-Sc Alloy
6. Effect of the Heat Treatments on Microhardness of Al 7075-Li-Sc Alloy
7. SEM/EDS Investigations of Al 7075-Li-Sc Alloys
8. Conclusions
- Regardless of the type of aging process, i.e., single or double, the highest aging temperature is the main parameter controlling the alloy hardness/strength;
- Aging at 120 °C for over 24 h provides maximum strength (in the range of 175–200 VHN due to precipitation of the hardening phases in the form of ultra-fine particles;
- Aging at higher temperatures, i.e., 180 °C or 280 °C, whether it is the first aging temperature or the second one in a double aging process, leads to a dramatic decrease in the alloy strength due to coarsening of the precipitated phase particles (65–145 VHN).
- The precipitation of η-phase particles is associated with the formation of precipitate free zones around the pre-existing phases;
- In the T7 condition, the presence of Al and Cu in the alloy would change the composition of the η-phase. In all cases, the Zn/Mg ratios are about unity;
- The coarsening and spheroidization of η-phase particles takes place through the Ostwald ripening mechanism where smaller particles in solution dissolve and deposit on larger particles;
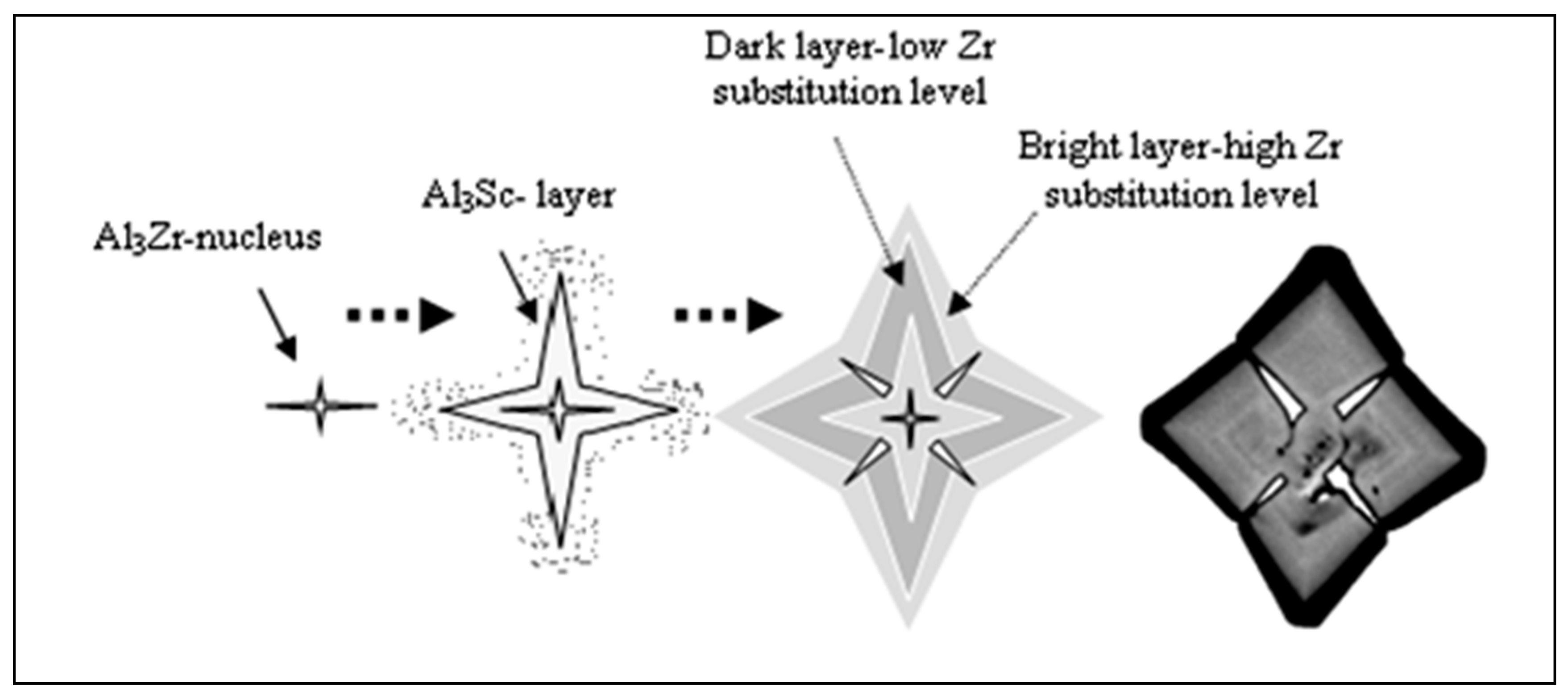
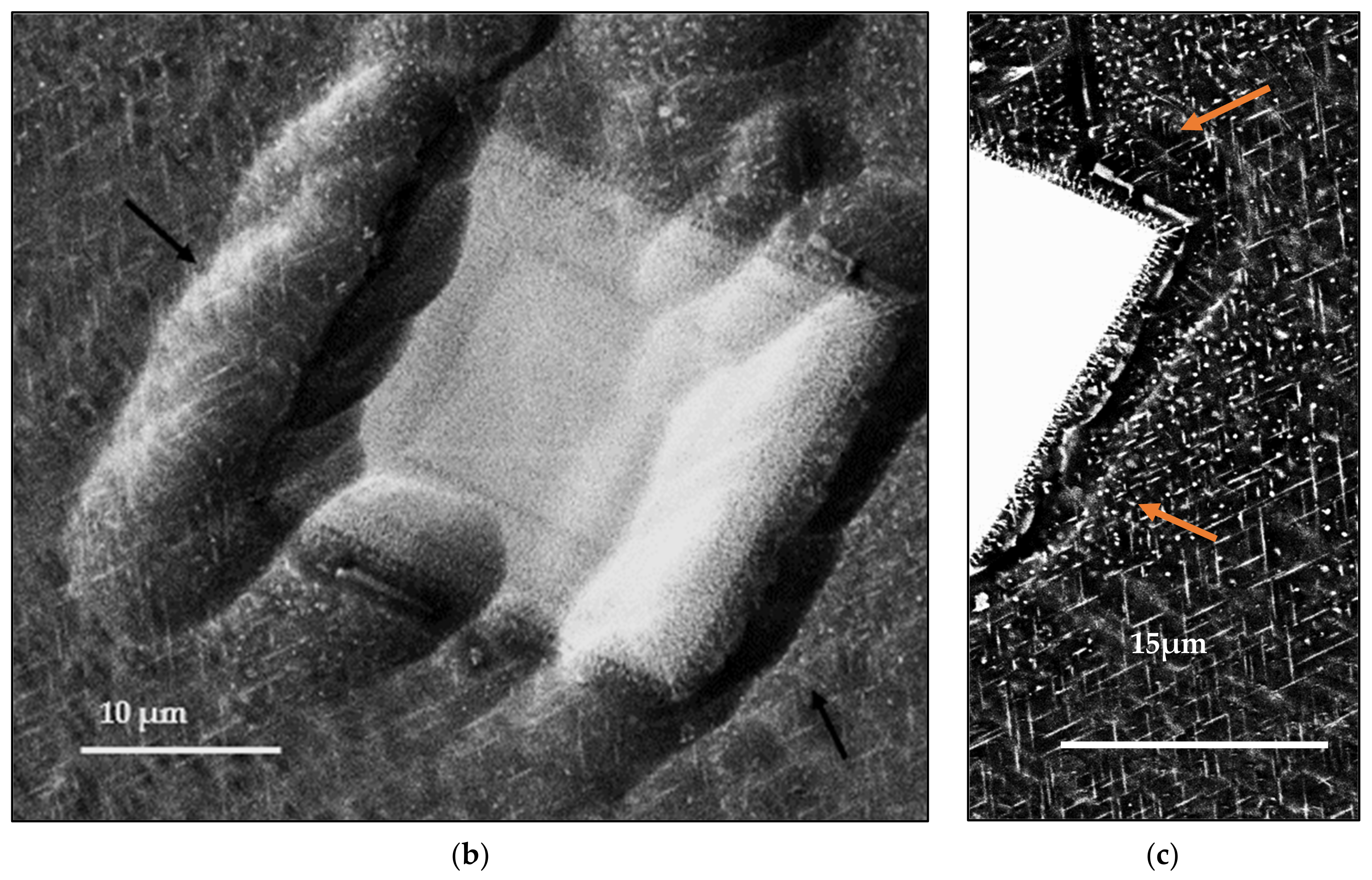
- In Sc-containing alloys, the star phase particle consists of different layers which may be discerned by the variations in brightness across the four arms of the particle;
- The change in the brightness from layer to layer indicates that the Zr and Sc concentrations are varied within the star phase since the atomic number of Zr (40) is higher than the atomic number of Sc (21) so a Zr-rich layer will be brighter than an Sc-rich layer;
- Ultra-fine Al3Li particles were observed in the Al 7075-Sc-Li alloy. However, under T7 temper conditions (treatment #3) the hardening caused by these Al3Li precipitates is compromised by the alloy softening, resulting in a negligible hardening effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eswara Prasad, N.; Gokhale, A.A.; Wanhill, R.J.H. (Eds.) Aluminum-Lithium Alloys: Processing, Properties, and Applications, Butterworth-Heinemann, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Ibrahim, M.F.; Garza-Elizondo, G.H.; Samuel, A.M.; Samuel, F.H. Optimizing the Heat Treatment of High-Strength 7075-Type Wrought Alloys: A Metallographic Study. Int. J. Met. 2016, 10, 264–275. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Garza-Elizondo, G.H.; Samuel, A.M.; Samuel, F.H. Optimizing the heat treatment of high-strength 7075-type wrought alloys: A metallographic study. In Proceedings of the 119th Metalcasting Congress, AFS 2015, Columbus, OH, USA, 21–23 April 2015; American Foundry Society: Schaumburg, IL, USA, 2015. [Google Scholar]
- Ibrahim, M.F.; Samuel, A.M.; Alkahtani, S.A.; Samuel, F.H. A novel solution heat treatment of 7075-type alloy. In Proceedings of the Light Metals 2012—TMS 2013 Annual Meeting and Exhibition, San Antonio, TX, USA, 3–7 March 2013; The Minerals, Metals and Materials Society: San Antonio, TX, USA, 2013; pp. 383–390. [Google Scholar]
- Hernandez-Sandoval, J.; Zedan, Y.; Garza-Elizondo, G.H.; Abdelaziz, M.H.; Songmene, V.; Samuel, F.H. Effect of Minor Addition of Ni and Zr on the High-Temperature Performance of Al–Si–Cu–Mg Cast Alloys. Int. J. Met. 2022, 16, 1235–1251. [Google Scholar] [CrossRef]
- Han, N.M.; Zhang, X.M.; Liu, S.; He, D.; Zhang, R. Effect of solution treatment on the strength and fracture toughness of aluminum alloy 7050. J. Alloys Compd. 2011, 509, 4138–4145. [Google Scholar] [CrossRef]
- Ma, W.; Wang, B.; Yang, L.; Tang, X.; Xiao, W.; Zhou, J. Influence of solution heat treatment on mechanical response and fracture behaviour of aluminium alloy sheets: An experimental study. Mater. Des. 2015, 88, 1119–1126. [Google Scholar] [CrossRef]
- Viana, F.; Pinto, A.M.P.; Santos, H.M.C.; Lopes, A.B. Retrogression and re-ageing of 7075 aluminium alloy: Microstructural characterization. J. Mater. Process Technol. 1999, 92–93, 54–59. [Google Scholar] [CrossRef]
- Ozer, G.; Karaaslan, A. Properties of AA7075 aluminum alloy in aging and retrogression and reaging process. Trans. Nonferrous Met. Soc. China 2017, 27, 2357–2362. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H. A Review on the Heat Treatment of Al-Si-Cu/Mg Casting Alloys. In Heat Treatment: Conventional and Novel Applications; IntechOpen: London, UK, 2012; p. 229. [Google Scholar]
- Godard, D.; Archambault, P.; Aeby-Gautier, E.; Lapasset, G. Precipitation sequences during quenching of the AA 7010 alloy. Acta Mater. 2002, 50, 2319–2329. [Google Scholar] [CrossRef]
- Oskouei, R.H.; Ibrahim, R.N. The effect of typical flight temperatures on the fatigue behaviour of Al 7075-T6 clamped plates. Mater. Sci. Eng. A 2011, 528, 1527–1533. [Google Scholar] [CrossRef]
- Tekeli, S.; Simsek, I.; Simsek, D.; Ozyurek, D. Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment. High Temp. Mater. Process 2019, 38, 892–896. [Google Scholar] [CrossRef]
- Tai, C.-L.; Pua, Y.-M.; Chung, T.-F.; Yang, Y.-L.; Chen, H.-R.; Chen, C.-Y.; Wang, S.-H.; Yu, C.-Y.; Yang, J.-R. The effect of minor addition of Mn in AA7075 Al–Zn–Mg–Cu aluminum alloys on microstructural evolution and mechanical properties in warm forming and paint baking processes. Int. J. Light Mater. Manuf. 2023, 6, 521–533. [Google Scholar] [CrossRef]
- Ye, J.; Pan, Q.; Liu, B.; Hu, Q.; Qu, L.; Wang, W.; Wang, X. Influences of small addition of Sc and Zr on grain structure and quenching sensitivity of Al-Zn-Mg-Cu alloys. Mater. Today Commun. 2023, 35, 105943. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Songmene, V.; Samuel, F.H. A Review on the Analysis of Thermal and Thermodynami Aspects of Grain Refinement of Aluminum-Silicon-Based Alloys. Materials 2023, 16, 5639. [Google Scholar] [CrossRef] [PubMed]
- Girgis, A.; Samuel, E.; Samuel, A.M.; Songmene, V.; Samuel, F.H. Effect of Cu Content on the Alloy Tensile Properties of Al-Cu Based Alloys Tested at 25 °C and 250 °C: Application of the Concept of Quality Index. Materials 2023, 16, 1400. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Todorova, T.Z.; Zwanziger, J.W. Temperature dependent lattice misfit and coherency of Al3X (X = Sc, Zr, Ti and Nb) particles in an Al matrix. Acta Mater. 2015, 89, 109–115. [Google Scholar] [CrossRef]
- Bharti, P.; Singh, R.; Sahoo, J.R.; Tripathi, A.; Mishra, S. Yield strength modeling of an Al-Cu-Li alloy through circle rolling and flow stress superposition approach. J. Alloys Compd. 2023, 964, 171343. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Zhang, L.; Guo, Y.; Wang, C.; Li, L.; Xiong, X. Enhanced strength and ductility in sand-cast Al-Li-Cu-Mg-Zr alloy via synergistic microalloying with Sc and Ti. J. Alloys Compd. 2023, 962, 170954. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Ma, P.; He, J.; Chen, L. Enhanced age-hardening response at elevated temperature by natural-ageing-modified precipitation in an Al-Cu-Li-Mg alloy. Mater. Charact. 2023, 199, 112791. [Google Scholar] [CrossRef]
- Samuel, A.M.; Samuel, F.H.; Abdelaziz, M.H.; Doty, H.W. Hardening of Al–Si–Cu–Mg Cast Alloys: Role of Ag and Zn addition. Int. J. Met. 2022, 16, 3–19. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Cao, L.; Tong, X.; Zou, Y.; Zhu, Q.; Tang, S.; Song, H.; Guo, M. Effect of Ag on aging precipitation behavior and mechanical properties of aluminum alloy 7075. Mater. Sci. Eng. A 2020, 804, 140515. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effect of Extended Thermal Exposure and Alloying Elements on the Morphology of Eutectic Si in Al–Si Cast Alloys. Int. J. Met. 2020, 14, 1013–1024. [Google Scholar] [CrossRef]
- Pruthvi, H.M.; Shenoy, H.G. Effect of Zn, Mg and heat treatment on Al base alloy on hardness using Taguchi Technique. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1258, 012043. [Google Scholar] [CrossRef]
- Li, W.; Pan, Q.; Zou, L.; Liang, W.; He, Y.; Liu, J. Effects of minor Sc on the microstructure and mechanical properties of Al-Zn-Mg-Cu-Zr based alloys. Rare Met. 2009, 28, 102–106. [Google Scholar] [CrossRef]
- Darsono, F.B.; Koin, S.T. The Effect of T6 Heat Treatment on 7075 Aluminum on its Hardness and Tensile Strength. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1096, 012042. [Google Scholar] [CrossRef]
- Din, S.U.; Bin Awais, H.; Tariq, N.U.H.; Mehmood, M. Effect of Li addition on microstructure and mechanical properties of Al–Mg–Si alloy. Int. J. Mater. Res. 2014, 105, 770–777. [Google Scholar] [CrossRef]
- Ganiev, N.N.; Nazarov, K.M.; Badalov, M.D. Interaction of Lithium with Rare-Earth Metals. Russ. Metall. 1998, 6, 132–136. [Google Scholar]
- Xia, P.; Wang, S.; Huang, H.; Zhou, N.; Song, D.; Jia, Y. Effect of Sc and Zr Additions on Recrystallization Behavior and Intergranular Corrosion Resistance of Al-Zn-Mg-Cu Alloys. Materials 2021, 14, 5516. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Intermetallics Formation, Hardness and Toughness of A413.1 Type Alloys: Role of Melt and Aging Treatments. Int. J. Met. 2023, 17, 1095–1113. [Google Scholar] [CrossRef]
- Champier, G.; Samuel, F.H. Microstructures of Al-Li-Si alloys rapidly solidified. In Aluminium-Lithium Alloys III: Proceedings of the Third International Aluminium-Lithium Conference, University of Oxford, 8–11 July 1985; Oxford University: Oxford, UK, 1986; pp. 131–136. [Google Scholar]
- Samuel, F.H.; Champier, G. Ageing characteristics of Al-2.5% Li rapidly solidified alloy. J. Mater. Sci. 1987, 22, 3851–3863. [Google Scholar] [CrossRef]
- Huang, Z.W.; Loretto, M.H.; White, J. Influence of lithium additions on precipitation and age hardening of 7075 alloy. Mater. Sci. Technol. 1993, 9, 967–980. [Google Scholar] [CrossRef]
- Toropova, L.S.; Eskin, D.G.; Kharaktrova, M.L.; Dobakina, T.V. Advanced Aluminum Alloys Containing Scandium: Structure and Properties; Gordon and Breach Science Publishers: Philadelphia, PN, USA, 1998; p. 175. [Google Scholar]
- Krug, M.E.; Mao, Z.; Seidman, D.N.; Dunand, D.C. Comparison between dislocation dynamics model predictions and experiments in precipitation strengthened Al-Li-Sc alloys. Acta Mater. 2014, 79, 382–395. [Google Scholar] [CrossRef]
- Samuel, E.; Nabawy, A.M.; Samuel, A.M.; Doty, H.W.; Songmene, V.; Samuel, F.H. Effect of Zr and Ti Addition and Aging Treatment on the Microstructure and Tensile Properties of Al-2%Cu-Based Alloys. Materials 2022, 15, 4511. [Google Scholar] [CrossRef] [PubMed]
- Marquis, E.A.; Seidman, D.N. Nanoscale structural evolution of Al3Sc precipitates in Al (Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef]



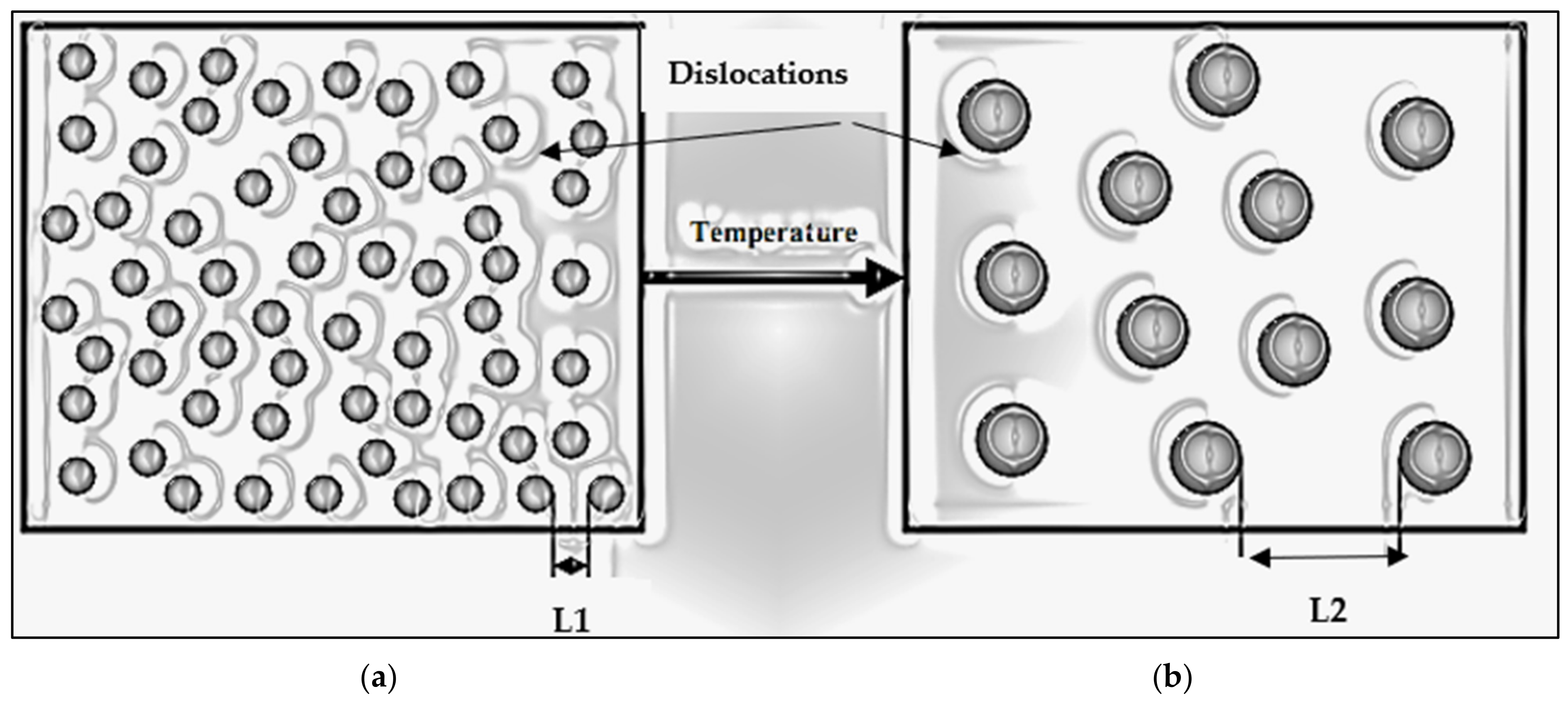




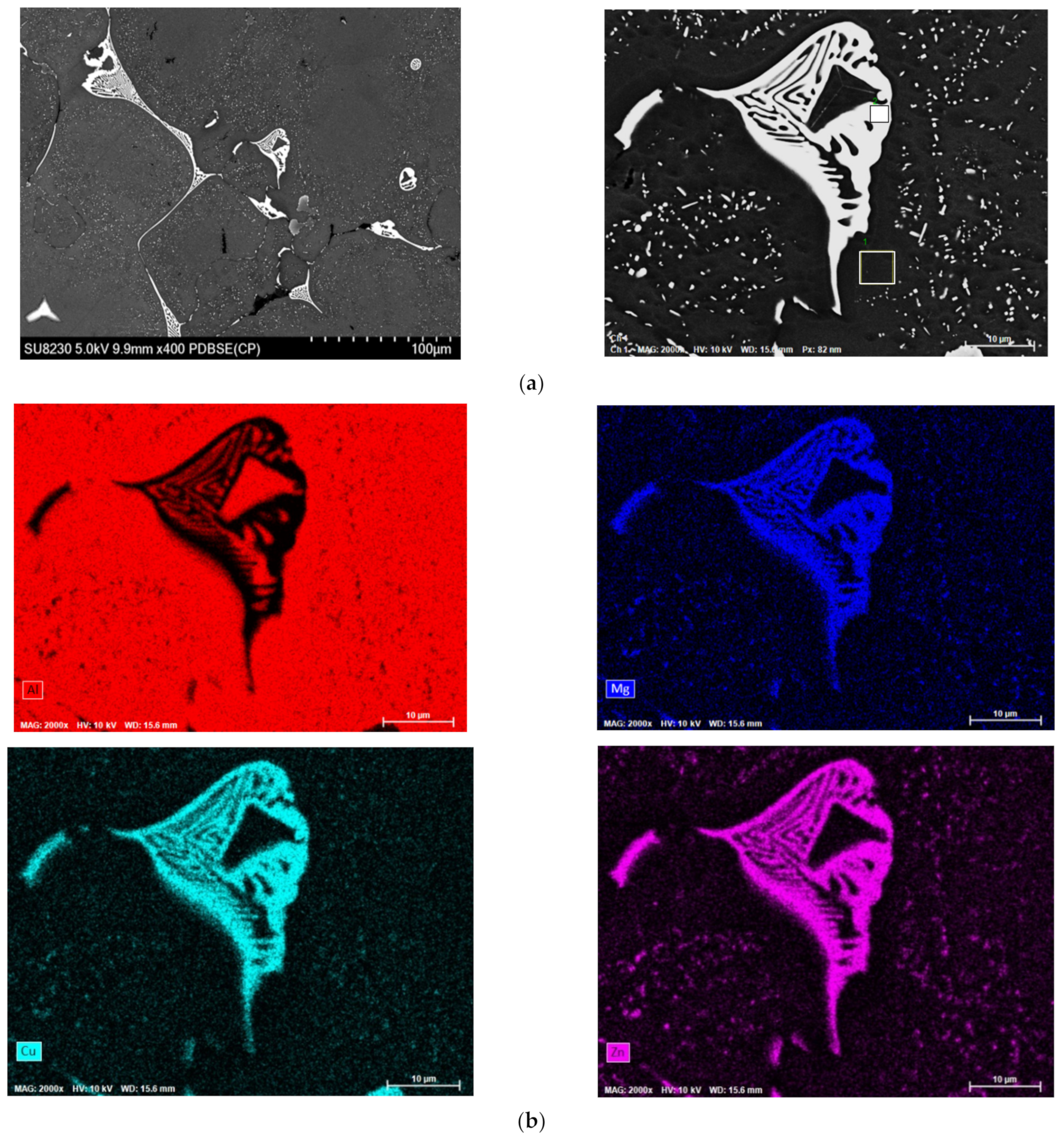
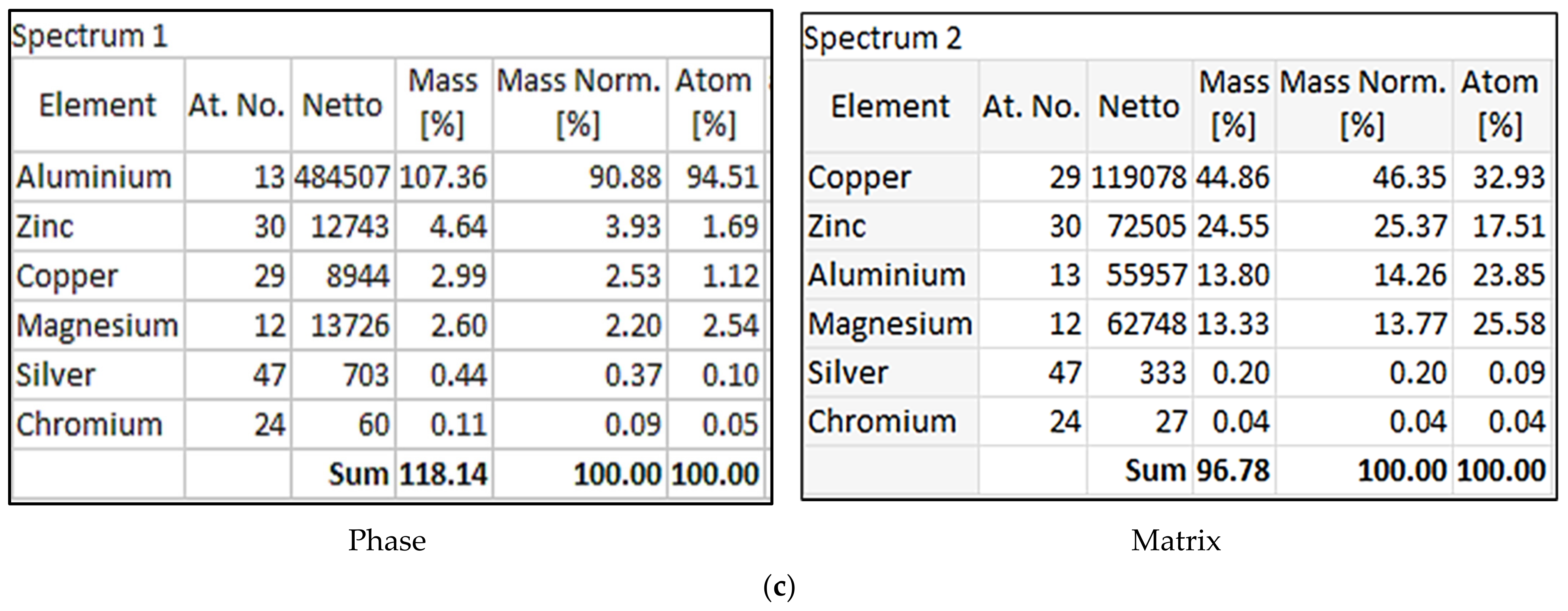
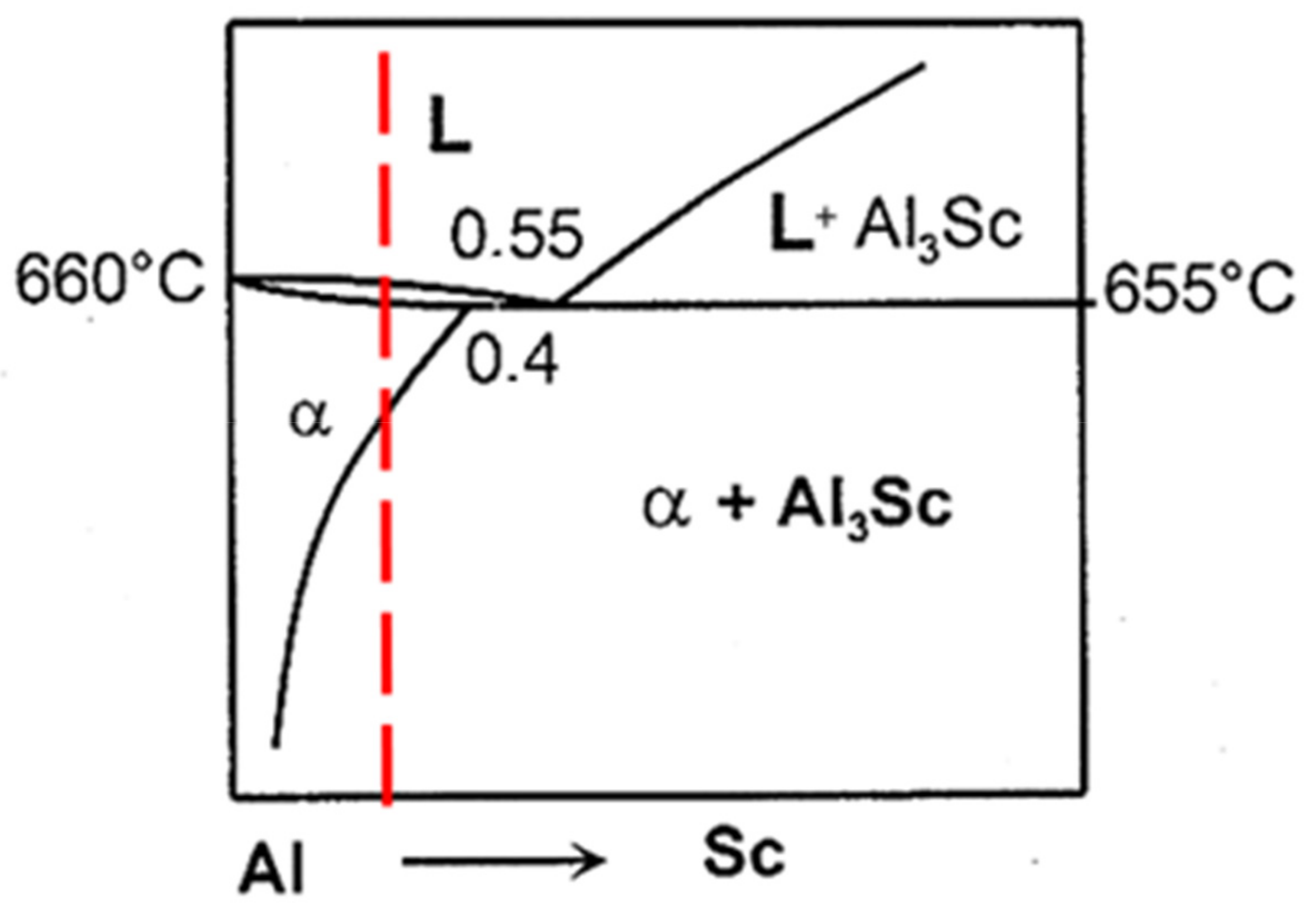


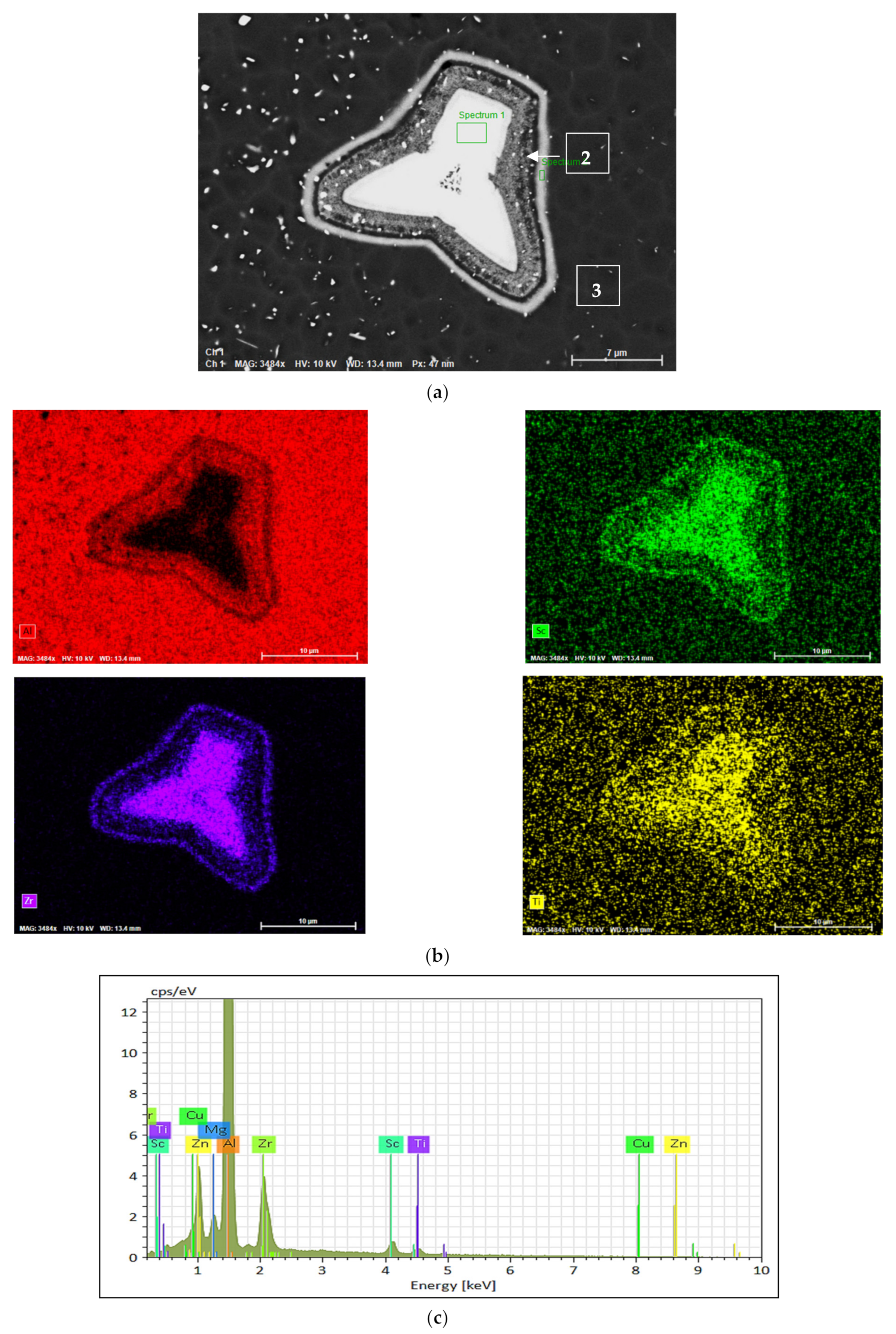
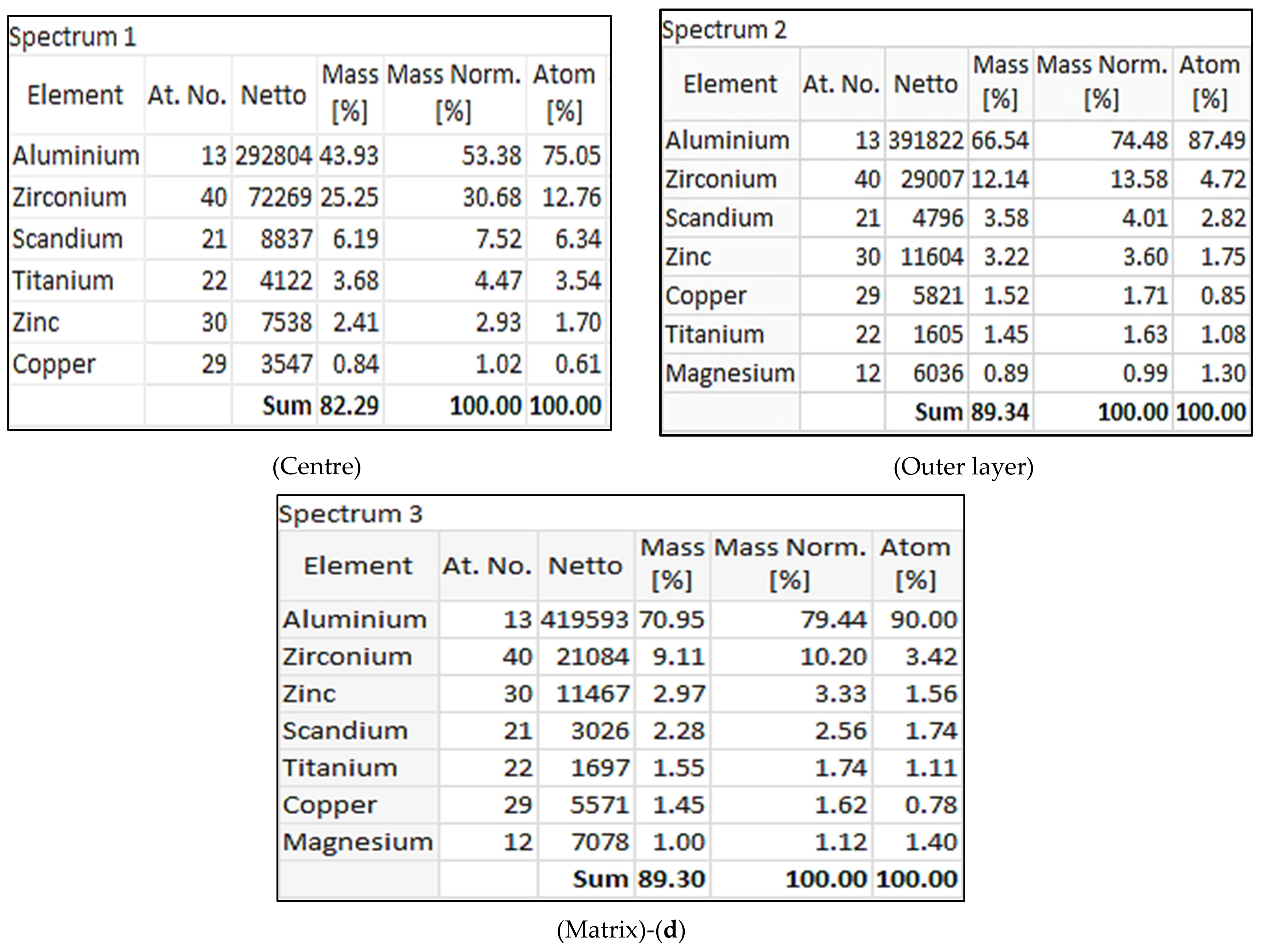

| Alloy Code | Alloy | Si | Mg | Cr | Mn | Fe | Cu | Zn | Zr | Ti | Ag | Li | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * A | Al 7075 | 0.21 | 2.15 | 0.19 | 0.035 | 0.16 | 1.45 | 5.62 | 0.3 | 0.027 | 0.23 | - | - |
| B | Al 7075-Sc | 0.16 | 2.2 | 0.14 | <0.003 | 0.099 | 1.3 | 6.5 | 0.3 | 0.080 | 0.23 | 0.06 | 0.12 |
| C | Al 7075-Li-Sc | 0.11 | 2.8 | 0.16 | <0.003 | 0.053 | 1.5 | 6.6 | 0.3 | 0.094 | 0.23 | 2.2 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahmasbi, A.; Samuel, A.M.; Zedan, Y.; Songmene, V.; Samuel, F.H. Effect of Aging Treatment on the Strength and Microstructure of 7075-Based Alloys Containing 2% Li and/or 0.12% Sc. Materials 2023, 16, 7375. https://doi.org/10.3390/ma16237375
Tahmasbi A, Samuel AM, Zedan Y, Songmene V, Samuel FH. Effect of Aging Treatment on the Strength and Microstructure of 7075-Based Alloys Containing 2% Li and/or 0.12% Sc. Materials. 2023; 16(23):7375. https://doi.org/10.3390/ma16237375
Chicago/Turabian StyleTahmasbi, Ali, Agnes M. Samuel, Yasser Zedan, Victor Songmene, and Fawzy H. Samuel. 2023. "Effect of Aging Treatment on the Strength and Microstructure of 7075-Based Alloys Containing 2% Li and/or 0.12% Sc" Materials 16, no. 23: 7375. https://doi.org/10.3390/ma16237375
APA StyleTahmasbi, A., Samuel, A. M., Zedan, Y., Songmene, V., & Samuel, F. H. (2023). Effect of Aging Treatment on the Strength and Microstructure of 7075-Based Alloys Containing 2% Li and/or 0.12% Sc. Materials, 16(23), 7375. https://doi.org/10.3390/ma16237375









