Effect of Composition on the Physicochemical Properties of Cross-Linked Poly(sodium acrylate)/Sodium Silicate Hydrogels
Abstract
:1. Introduction
- lowering the temperature at which fire-resistant pumice begins to form—sodium phosphate;
- extending the time during which the protective layer is modified at a constant temperature—glycerin, ethylene glycol, starch;
- reduction of the gradient of the time/temperature curve after which the swelling of the fire protection layer ends—glucose, colloidal silica, borax, boric acid, sodium phosphate, sodium aluminate, aluminum phosphate;
2. Materials and Methods
2.1. Materials
- source of sodium silicate species—sodium water glass R-150 (WG) with a molar modulus of M = 2.05 and a density of 1.51 g/cm3 (Rudniki Chemical Plant, Rudniki, Poland);
- vinyl monomer—aqueous solution of sodium acrylate (ANa) with a percentage concentration of 15 wt.% and 20 wt.%, synthesized in the laboratory. It was prepared using acrylic acid (stabilized, 99.5%, density of 1.05 g/cm3 (Acros Organics, Geel, Belgium)) and sodium hydroxide microgranules (Avantor Performance Materials, Gliwice, Poland);
- polymer additive—a mixture of sodium polyacrylates(R-100) of various molecular weights with a density of 1.29 g/cm3 called Midafen R-100 (Lubrina S.A., Łódź, Poland);
- redox initiators of polymerization reaction—potassium persulfate (KPS; Acros Organics, Geel, Belgium) and sodium thiosulfate (NTS; Acros Organics, Geel, Belgium);
- cross-linking monomer—N,N′-methylenebisacrylamide (NNMBA; Acros Organics, Geel, Belgium)
- All reagents were used without further purification.
2.2. Samples Preparation Procedure
2.3. Characterization Methods
- -
- G′—storage modulus [Pa],
- -
- G″—loss modulus [Pa],
- -
- tanφ—tangent of the phase shift angle [−].
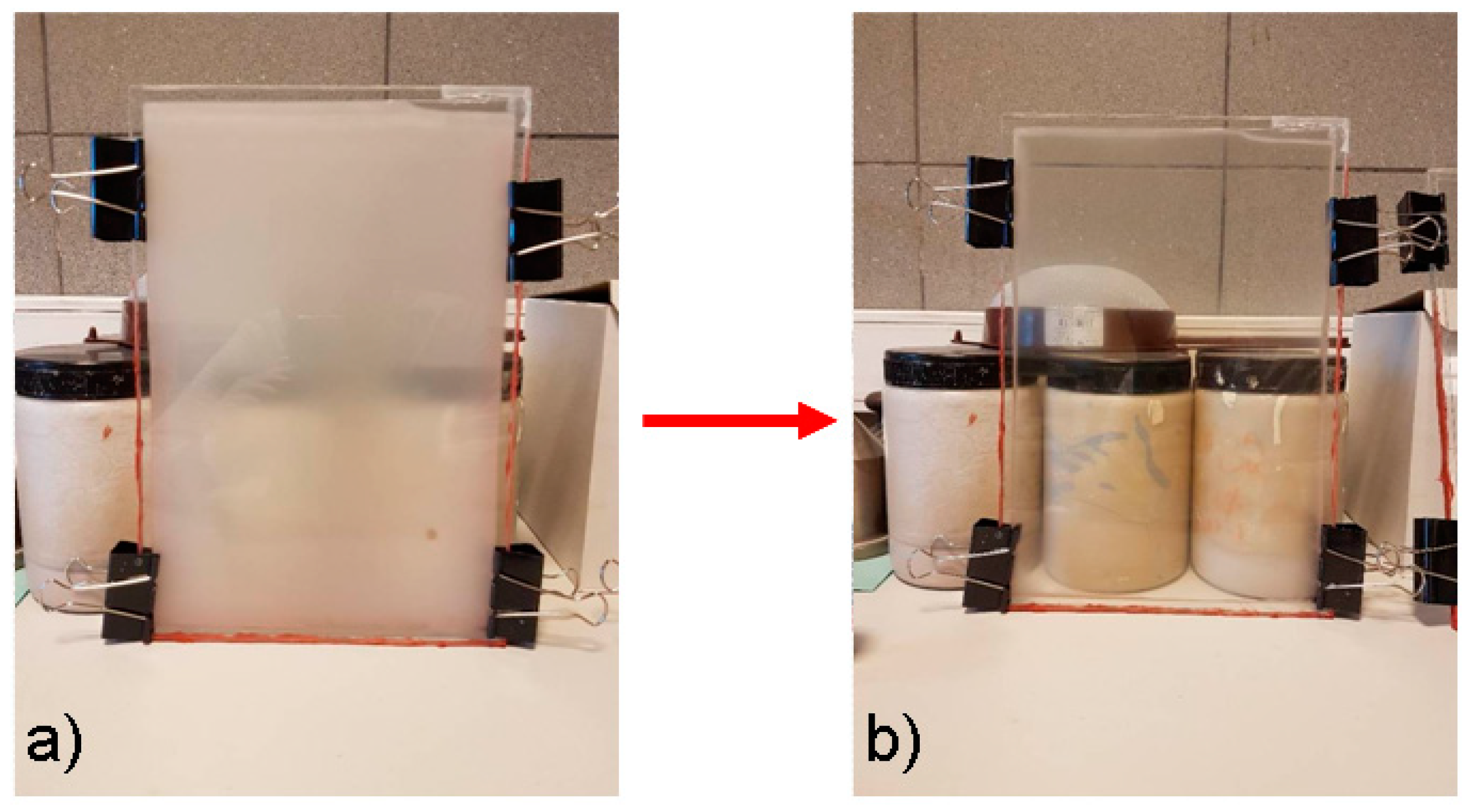
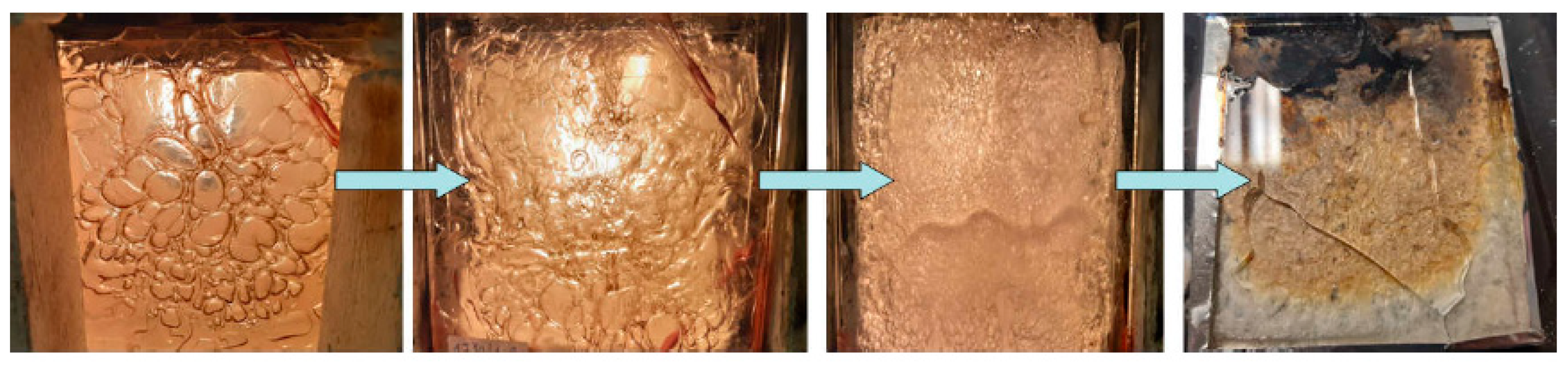
- glass forms were prepared from two glass panes with dimensions of 20 cm × 30 cm × 0.4 cm connected with a 1 mm thick silicone seal. The use of a silicone gasket allowed for the creation of a gap between the glass panes, in which the fire-resistant hydrogel was placed. The edges of the prepared forms were additionally protected with a high-temperature silicone to prevent the gel from flowing out during the test;
- immediately after the preparation of the hydrogel, it was introduced into the prepared forms, which were additionally sealed with aluminum tape at the infusion point (Figure 1);
- the samples prepared in this way were seasoned at room temperature for 7 days before testing.
3. Results and Discussion
3.1. Rheological Measurements
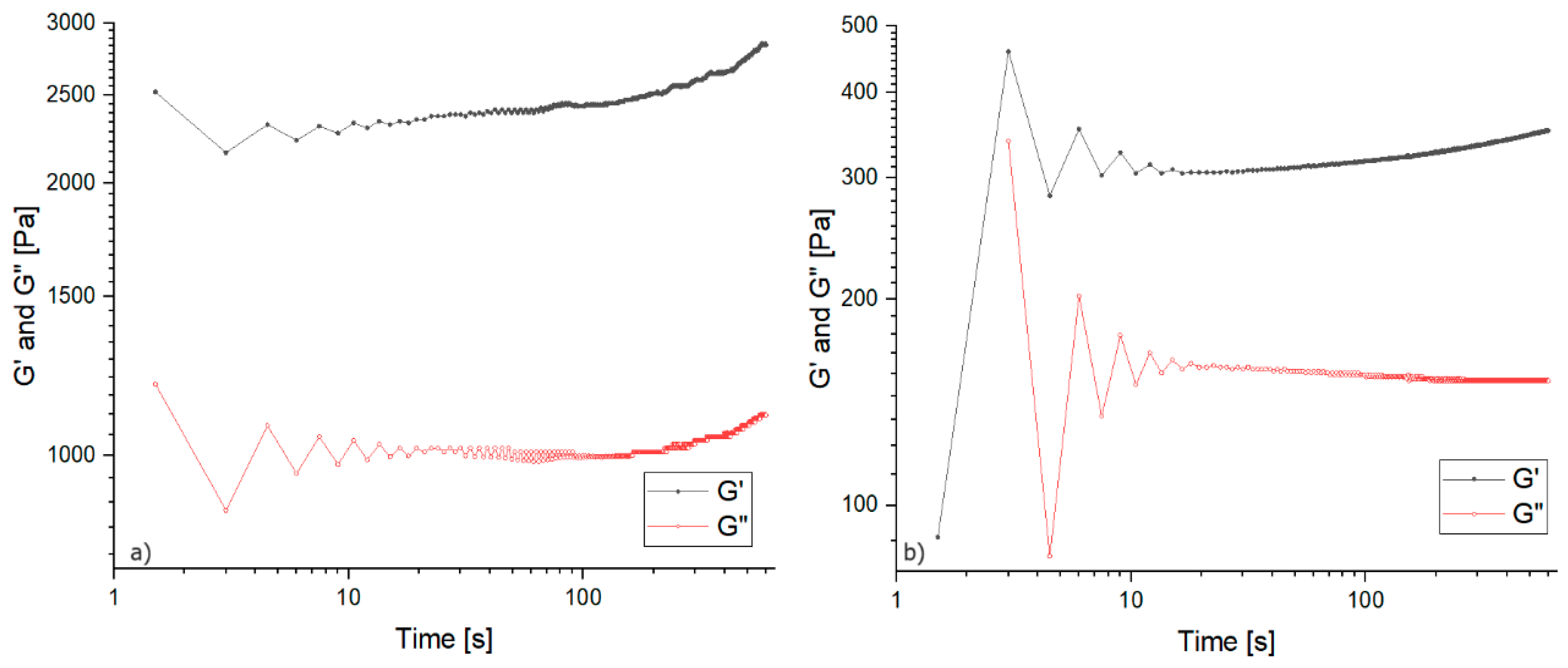
3.1.1. Gelation Kinetics
3.1.2. Oscillation Measurements
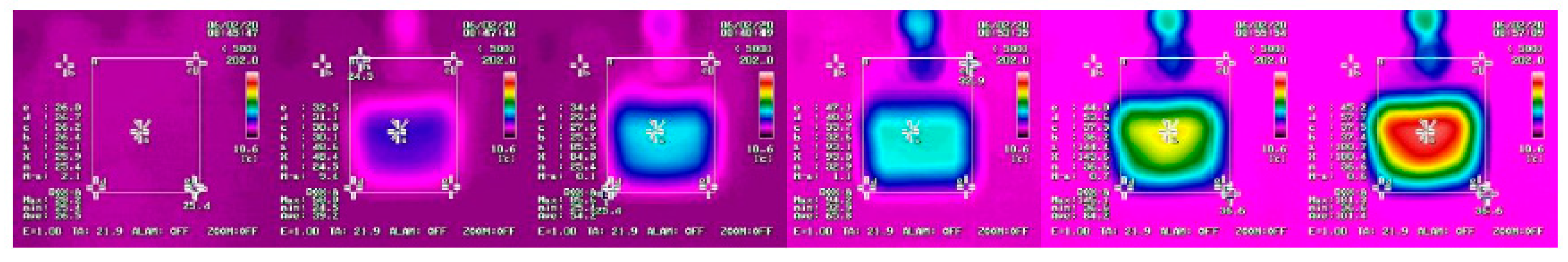
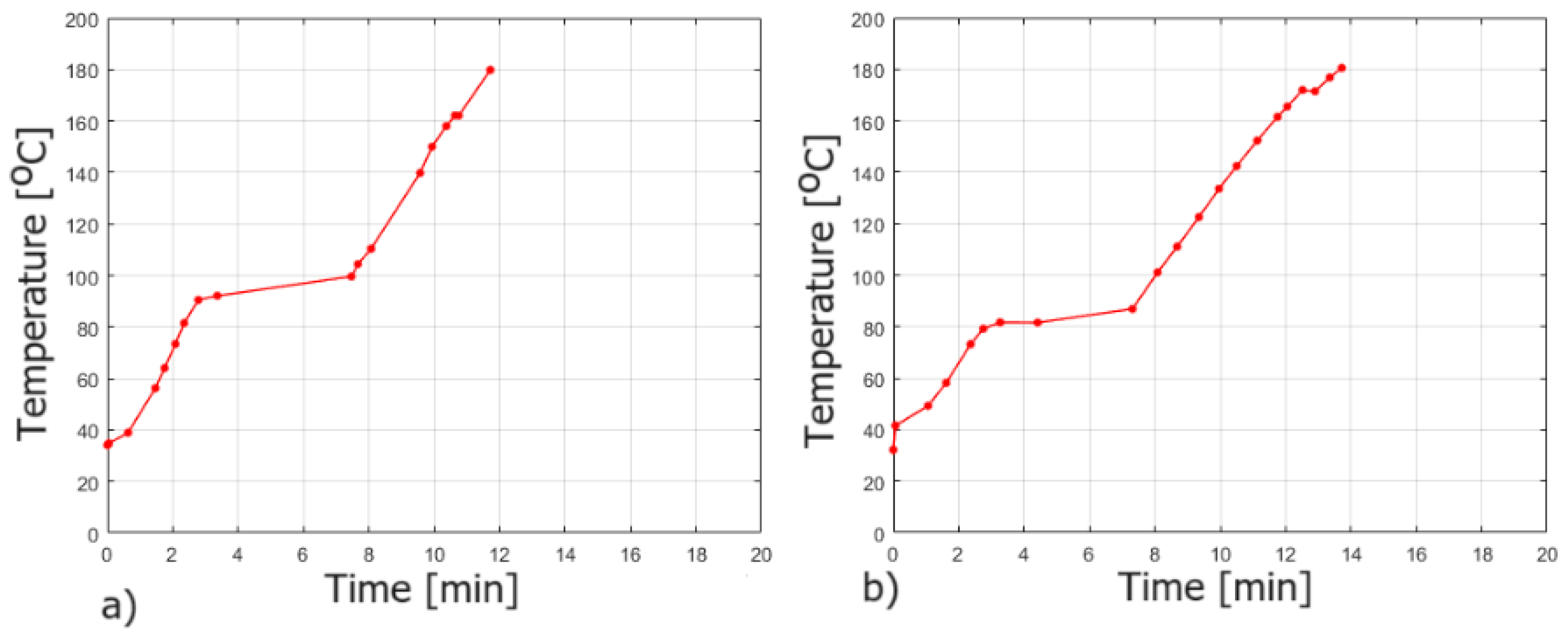
3.2. Fire Tests
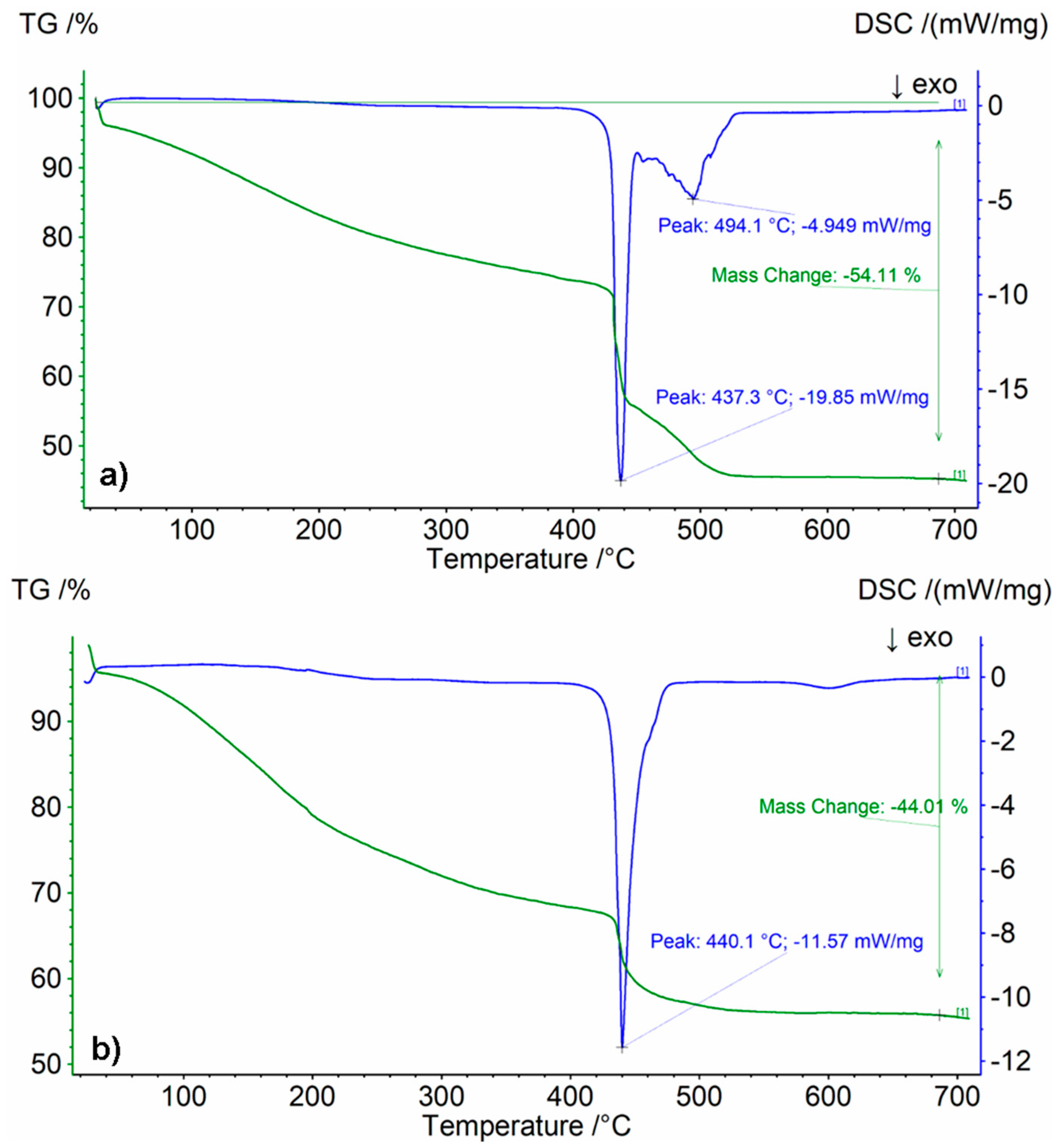
3.3. TG/DSC Analysis
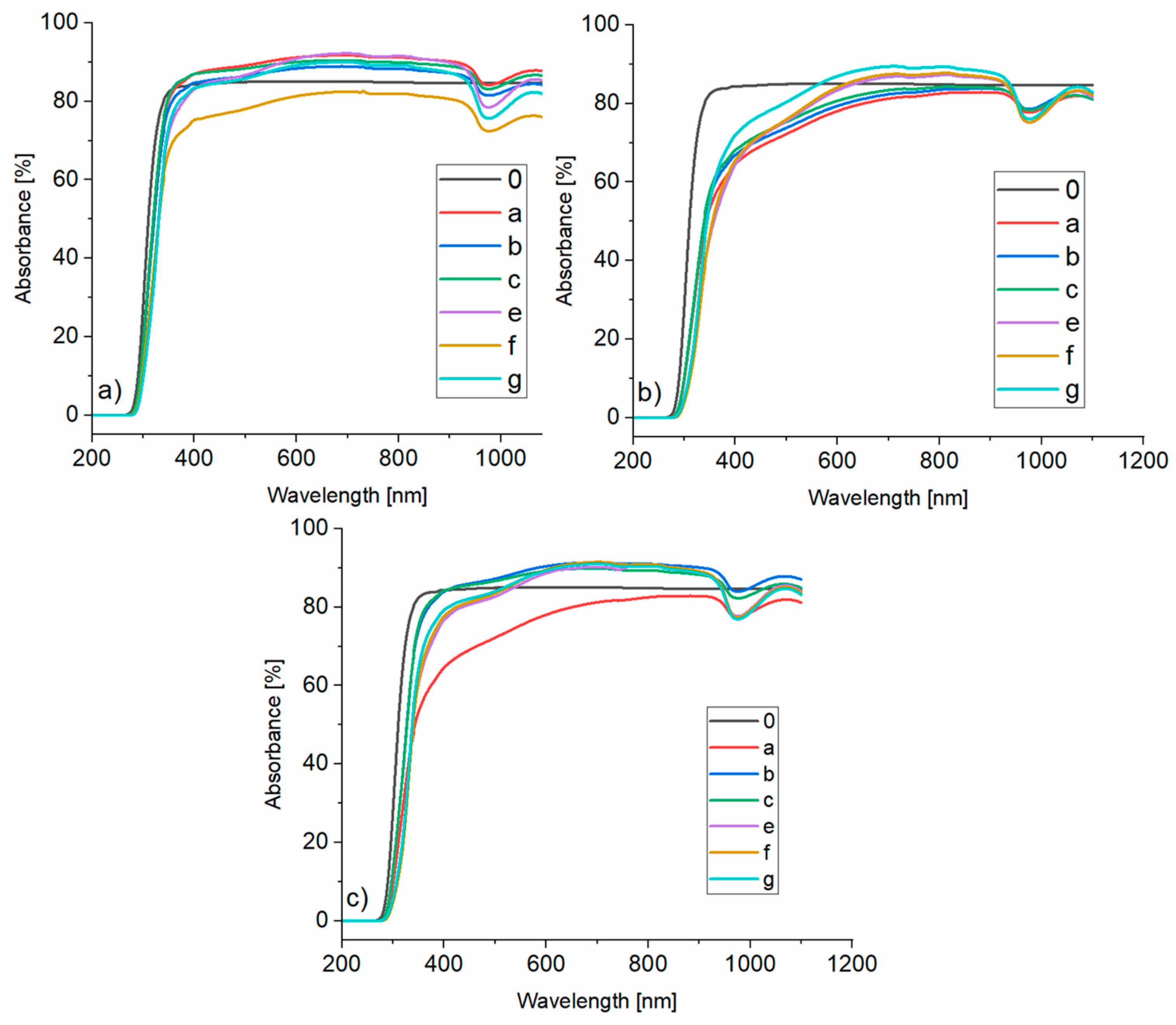
3.4. UV-Vis Analysis
- (a)
- composition 5%/1:9 with 20 wt.% aqueous solution of sodium acrylate,
- (b)
- composition 20%/1:1 with 15 wt.% aqueous solution of sodium acrylate,
- (c)
- composition 5%/1:9 with 15 wt.% aqueous solution of sodium acrylate.
- a—without aging, gel thickness 2 mm,
- b—aged for 1 h, gel thickness 2 mm,
- c—aged for 24 h, gel thickness 2 mm,
- e—no aging, gel thickness 4 mm,
- f—aged for 1 h, gel thickness 4 mm,
- g—aged for 24 h, gel thickness 4 mm.
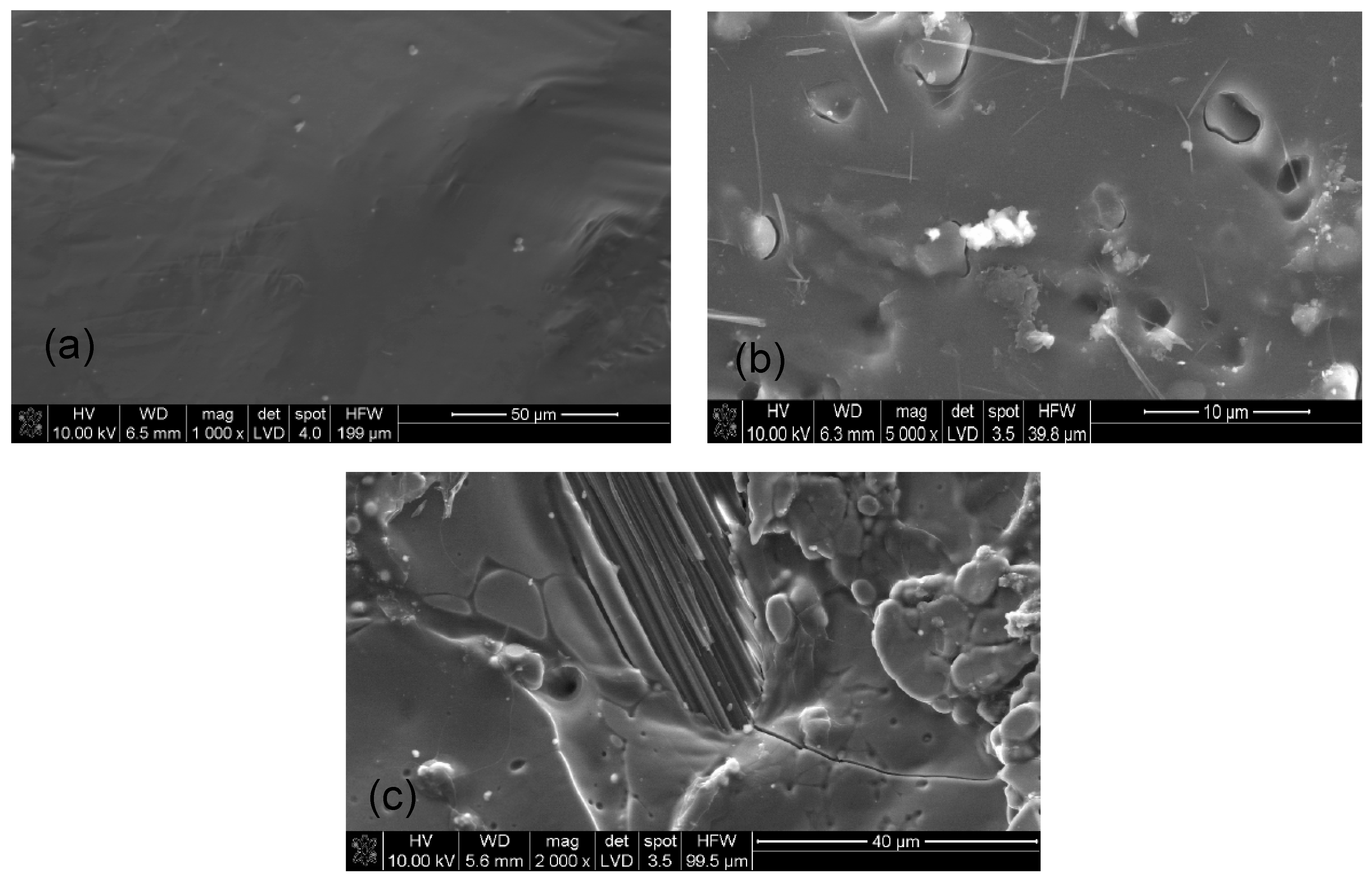
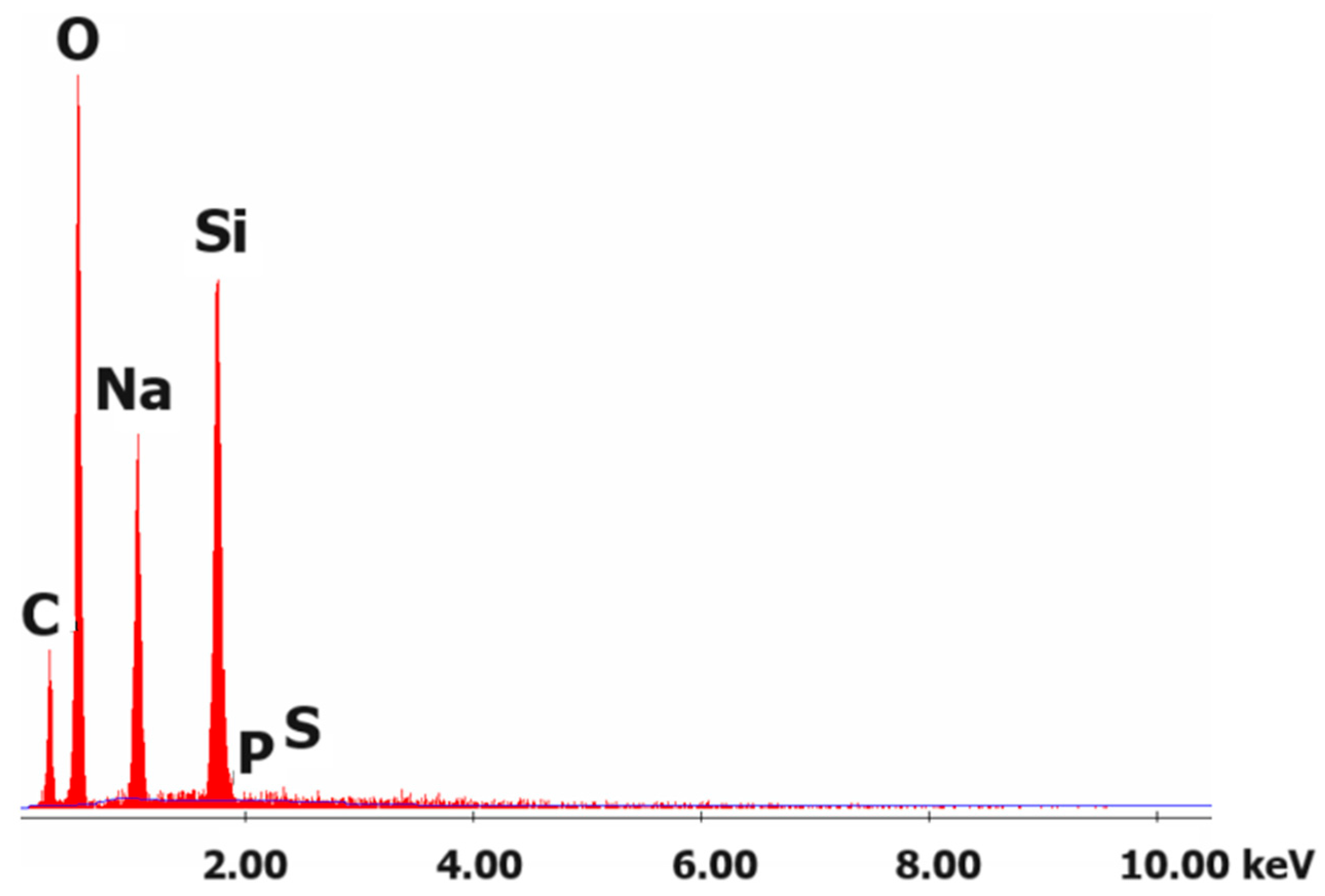
3.5. SEM Analysis
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaeth, R.; Stastny, F.; Breu, R.; Gaertner, F. Thermally Insulating Transparent Laminated Glass with Alkali Metal Silicate Interlayer. U.S. Patent 3 640 837, 8 February 1972. [Google Scholar]
- Jacquemin, F.; Terneu, R.; Voiturier, J.-P. Fireproof Glasswork. U.S. Patent 3 974 316, 10 August 1976. [Google Scholar]
- Nolte, H.-H.; De Boel, M.; Baudin, P. Laminated Light-Transmitting Fire-Screening Panel. U.S. Patent 4 104 427, 20 November 1979. [Google Scholar]
- Schaar, J.L.; Ellard, J.A.; Butler, J.M. Intumescent Compositions and Substrates Coated Therewith. U.S. Patent 3 955 987, 11 May 1976. [Google Scholar]
- Gandini, G.; Frignoli, L. Fireproofing Materials. U.S. Patent 4 102 794, 20 July 1977. [Google Scholar]
- Hahn, F.J.; Vandersall, H.L. Intumescent Coating Compositions. U.S. Patent 3 513 114 A, 19 May 1970. [Google Scholar]
- De Boel, M.; Baudin, P. Light-Transmitting Fire Screening Panel. U.S. Patent 4 190 698, 21 November 1977. [Google Scholar]
- Zhang, X.; Mu, J.; Chu, D.; Zhao, Y. Synthesis of fire retardants based on N and P and poly(sodium silicate-aluminum dihydrogen phosphate) (PSADP) and testing the flame-retardant properties of PSADP impregnated poplar wood. Wood Res. Tech. 2015, 70, 341–350. [Google Scholar] [CrossRef]
- Chu, F.; Qiu, S.; Zhou, Y.; Zhou, X.; Cai, W.; Zhu, Y.; He, L.; Song, L.; Hu, W. Novel glycerol-based polymerized flame retardants with combined phosphorus structures for preparation of high performance unsaturated polyester resin composites. Compos. B Eng. 2022, 233, 109647. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; White, R.H.; Zhao, Q.; Yao, F.; Cintron, M.S. Effect of urea additive on the thermal decomposition kinetics of flame retardant greige cotton nonwoven fabric. Polym. Degrad. Stab. 2012, 97, 738–746. [Google Scholar] [CrossRef]
- Ortmans, G.; Hassiepen, M. Fire-Resistant Glazing and Method of Making Same. U.S. Patent 4 830 913, 16 May 1989. [Google Scholar]
- Litovchenko, D.; Burmistrov, I.; Panova, L.; Godymchuk, A.; Kosova, N. Acrylate hydrogel modification using a cross-linking agent for increasing multilayer glazing flame resistance. Adv. Maters. Res. 2015, 1085, 265–269. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Wang, H.; Shen, J.; Liu, J. Study on transparent and fireproof sandwich gel in the thin type fireproof glass. New Build. Maters. 2007, 34, 4. [Google Scholar]
- Zhang, C.S.; Can, Y.; Wang, H.T. Clarity and fireproof gel in the thin type fireproof glass. App. Chem. Ind. 2007, 36, 4. [Google Scholar]
- Liu, W.; Ge, X.; Zhang, Z. Study of the relationship between thermal insulation behavior and microstructure of a fire-resistant gel containing silica during heating. Fire Mater. 2018, 42, 44–49. [Google Scholar] [CrossRef]
- Liu, W.; Ge, X.; Zhou, X.; Tang, Y. Thermal intumescent behavior of a gel containing silica. RSC Adv. 2015, 5, 33208–33211. [Google Scholar] [CrossRef]
- Sugiura, K.; Koyama, A.; Yoshii, T. Fireproof Sheet Glass. U.S. Patent 6 054 401, 20 July 2000. [Google Scholar]
- Itoh, H.; Abe, T.; Yamashita, H.; Yoshimura, T.; Hisanaga, T.; Takebayashi, T.; Nakata, K.; Hashimoto, C. Fire-Resistant Glass and Process for Production Thereof. U.S. Patent 5 437 902, 1 August 1995. [Google Scholar]
- Birkhahn, M. Modifizierte, transparene, wassrige Alkalisilicat-Losung. Verfahren zu Deren Herstellung und deren Verwendung zur Herstellung von Transparenten Hydrogelen. EP 0542022 A1, 27 October 1992. [Google Scholar]
- Gelderie, U.; Frommelt, S.; Groteklaes- Broring, M.; Michels, D. Fireproof Window Panel. PL 189700 B1, 22 July 1998. (In Polish). [Google Scholar]
- Ma, Y. Fire Resistant Polymer Sheets. U.S. Patent 7 238 427 B2, 3 July 2007. [Google Scholar]
- Cheung, A.; Varma, K.; Holden, D.; Holland, J.; Bond, S. Fire Resistant Glazing. GB 20090017905, 13 October 2009. [Google Scholar]
- Mastalska-Popławska, J.; Izak, P.; Wójcik, Ł.; Stempkowska, A.; Góral, Z.; Krzyżak, A.T.; Habina, I. Synthesis and characterization of cross-linked poly(sodium acrylate)/sodium silicate hydrogels. Polym. Eng. Sci. 2019, 59, 1279–1287. [Google Scholar] [CrossRef]
- Mastalska-Popławska, J.; Izak, P.; Wójcik, Ł.; Stempkowska, A. Rheology of cross-linked poly(sodium acrylate)/sodium silicate hydrogels. Arab. J. Sci. Eng. 2016, 41, 2221–2228. [Google Scholar] [CrossRef]
- Mastalska-Popławska, J.; Stempkowska, A.; Habina-Skrzyniarz, I.; Krzyżak, A.T.; Rutkowski, P.; Izak, P.; Rudny, J.; Gawenda, T. Water interactions in hybrid polyacrylate-silicate hydrogel systems. Materials 2020, 13, 4092. [Google Scholar] [CrossRef] [PubMed]
- Bertalan, A.; Csanda, F.; Czerny, G.; Engel, T.; Nagy, G.; Szekely, T. Way of Production of Hydrogels, Especially to Increase Strength and Impermeability of Soil and Engineering. Structures. Patent Description 146456, 22 April 1985. [Google Scholar]
- PN-EN 13501-1:2019-02; Fire Classification of Construction Products and Building Elements—Part 1: Classification Based on Reaction to Fire Tests. British Standards Institution: London, UK, 2002.
- Bortel, E. Handbook of Thermoplastics; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Mori, H.; Muller, A.H.E. New polymeric architectures with (meth)acrylic acid segments. Prog. Polym. Sci. 2003, 28, 1403–1439. [Google Scholar] [CrossRef]
- Blauer, G. Polymerization of methacrylic acid at pH 4 to 11. Trans. Faraday Soc. 1960, 56, 606–612. [Google Scholar] [CrossRef]
- Mayoux, C.; Dandurand, J.; Ricard, A.; Lacabanne, C. Inverse suspension polymerization of sodium acrylate: Synthesis and characterization. J. App. Polym. Sci. 2000, 77, 2621–2630. [Google Scholar] [CrossRef]
- Rabek, J.F. Contemporary Knowledge about Polymers; PWN: Warsaw, Poland, 2009. (In Polish) [Google Scholar]
- Barth, J.; Buback, M. Termination and transfer kinetics of sodium acrylate polymerization in aqueous solution. Macromolecules 2012, 45, 4152–4157. [Google Scholar] [CrossRef]
- Cicha-Szot, R.; Walkowicz, S. The influence of modifier on the viscoelastic properties of silicate gels. Nafta-Gaz 2010, 56, 1–12. (In Polish) [Google Scholar]
- Kowalski, D. Glass materials in lightweight housing. Part 3. Technical and utility features of glass. Builder 2017, 3, 3–4. (In Polish) [Google Scholar]
- Burmistrov, I.; Vikulova, M.; Panova, L.; Yudintseva, T. Development of acrylate-based polymeric layers for fireproof laminated glass. AIP Conf. Proc. 2017, 1899, 020003. [Google Scholar]
- Pallikari-Viras, F.; Li, X.; King, T.A. Thermal analysis of PMMA/gel silica glass composites. J. Sol-Gel Sci. Tech. 1996, 7, 203–209. [Google Scholar] [CrossRef]
- Schmid, R.L.; Felsche, J. Thermal studies on sodium silicate hydrates. I. Trisodium hydrogensilicate pentahydrate, Na3HSiO4 · 5 H2O; Thermal stability and thermal decomposition reactions. Therm. Acta 1983, 71, 359–364. [Google Scholar] [CrossRef]
- Liufu, S.-C.; Xiao, H.-N.; Li, Y.-P. Thermal analysis and degradation mechanism of polyacrylate/ZnO nanocomposites. Polym. Deg. Stabil. 2005, 87, 103–110. [Google Scholar] [CrossRef]
- Xiang, Y.; Qi, X.; Cai, E.; Zhang, C.; Wang, J.; Lan, Y.; Deng, H.; Shen, J.; Hu, R. Highly efficient bacteria-infected diabetic wound healing employing a melanin-reinforced biopolymer hydrogel. Chem. Eng. J. 2023, 460, 141852. [Google Scholar] [CrossRef]
- Qi, X.; Cai, E.; Xiang, Y.; Zhang, C.; Ge, X.; Wang, J.; Lan, Y.; Xu, H.; Hu, R.; Shen, J. An Immunomodulatory Hydrogel by Hyperthermia-Assisted Self-Cascade Glucose Depletion and ROS Scavenging for Diabetic Foot Ulcer Wound Therapeutics. Adv. Mater. 2023, 2306632. [Google Scholar] [CrossRef] [PubMed]





| Sample Symbol * | R-100 [wt.%] | WG [wt.%] | ANa aq.** [wt.%] | NNMBA [wt.%] | NTS [wt.%] | KPS [wt.%] |
|---|---|---|---|---|---|---|
| 5%/1:9 | 1 | 9 | 90 | 0.08 | 0.04 | 0.04 |
| 20%/1:9 | 2 | 8 | 90 | |||
| 5%/1:1 | 2.5 | 47.5 | 50 | |||
| 20%/1:1 | 10 | 40 | 50 | |||
| 5%/9:1 | 4.5 | 85.5 | 10 |
| Sample Symbol | G′ [Pa] | G″ [Pa] | φ [°] |
|---|---|---|---|
| 20 wt.% sodium acrylate solution | |||
| 5%/1:9 | 9300 | 3322 | 19.66 |
| 20%/1:9 | 13,582 | 4979 | 20.13 |
| 5%/1:1 | 14,103.5 | 3888 | 15.41 |
| 20%/1:1 | 2631.8 | 1047.2 | 21.7 |
| 5%/9:1 | 2800.8 | 1245.8 | 23.98 |
| 15 wt.% sodium acrylate solution | |||
| 5%/1:9 | 1617 | 533 | 18.24 |
| 20%/1:9 | 1757.7 | 469 | 14.94 |
| 5%/1:1 | 321.7 | 157.8 | 26.13 |
| 20%/1:1 | 1522.7 | 482 | 17.56 |
| 5%/9:1 | 335 | 152.75 | 24.51 |
| Type of Sodium Acrylate Solution | Sample Symbol | (I) (180 °C) [min] |
|---|---|---|
| 20 wt.% | 5%/1:9 | 11:43 |
| 20%/1:9 | 11:22 | |
| 5%/1:1 | 19:11 | |
| 20%/1:1 | 15:19 | |
| 5%/9:1 | 13:43 | |
| 15 wt.% | 5%/1:9 | 12:44 |
| 20%/1:9 | 12:04 | |
| 5%/1:1 | 15:25 | |
| 20%/1:1 | 15:03 | |
| 5%/9:1 | 13:06 |
| Type of Sodium Acrylate Solution | Sample Symbol | Mass Loss [wt.%] | Tmax [°C] |
|---|---|---|---|
| 20 wt.% | 5%/1:9 | 54.11 | 437 |
| 20%/1:9 | 53.98 | 440 | |
| 5%/1:1 | 48.04 | 438 | |
| 20%/1:1 | 46.86 | 435 | |
| 5%/9:1 | 46.70 | 444 | |
| 15 wt.% | 5%/1:9 | 54.26 | 438 |
| 20%/1:9 | 52.70 | 437 | |
| 5%/1:1 | 42.50 | 436 | |
| 20%/1:1 | 47.18 | 436 | |
| 5%/9:1 | 44.01 | 440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalska-Popławska, J.; Wójcik, Ł.; Izak, P.; Konaszewski, D. Effect of Composition on the Physicochemical Properties of Cross-Linked Poly(sodium acrylate)/Sodium Silicate Hydrogels. Materials 2023, 16, 7422. https://doi.org/10.3390/ma16237422
Mastalska-Popławska J, Wójcik Ł, Izak P, Konaszewski D. Effect of Composition on the Physicochemical Properties of Cross-Linked Poly(sodium acrylate)/Sodium Silicate Hydrogels. Materials. 2023; 16(23):7422. https://doi.org/10.3390/ma16237422
Chicago/Turabian StyleMastalska-Popławska, Joanna, Łukasz Wójcik, Piotr Izak, and Damian Konaszewski. 2023. "Effect of Composition on the Physicochemical Properties of Cross-Linked Poly(sodium acrylate)/Sodium Silicate Hydrogels" Materials 16, no. 23: 7422. https://doi.org/10.3390/ma16237422





