Advances in Fiber-Based Wearable Sensors for Personal Digital Health Monitoring
Abstract
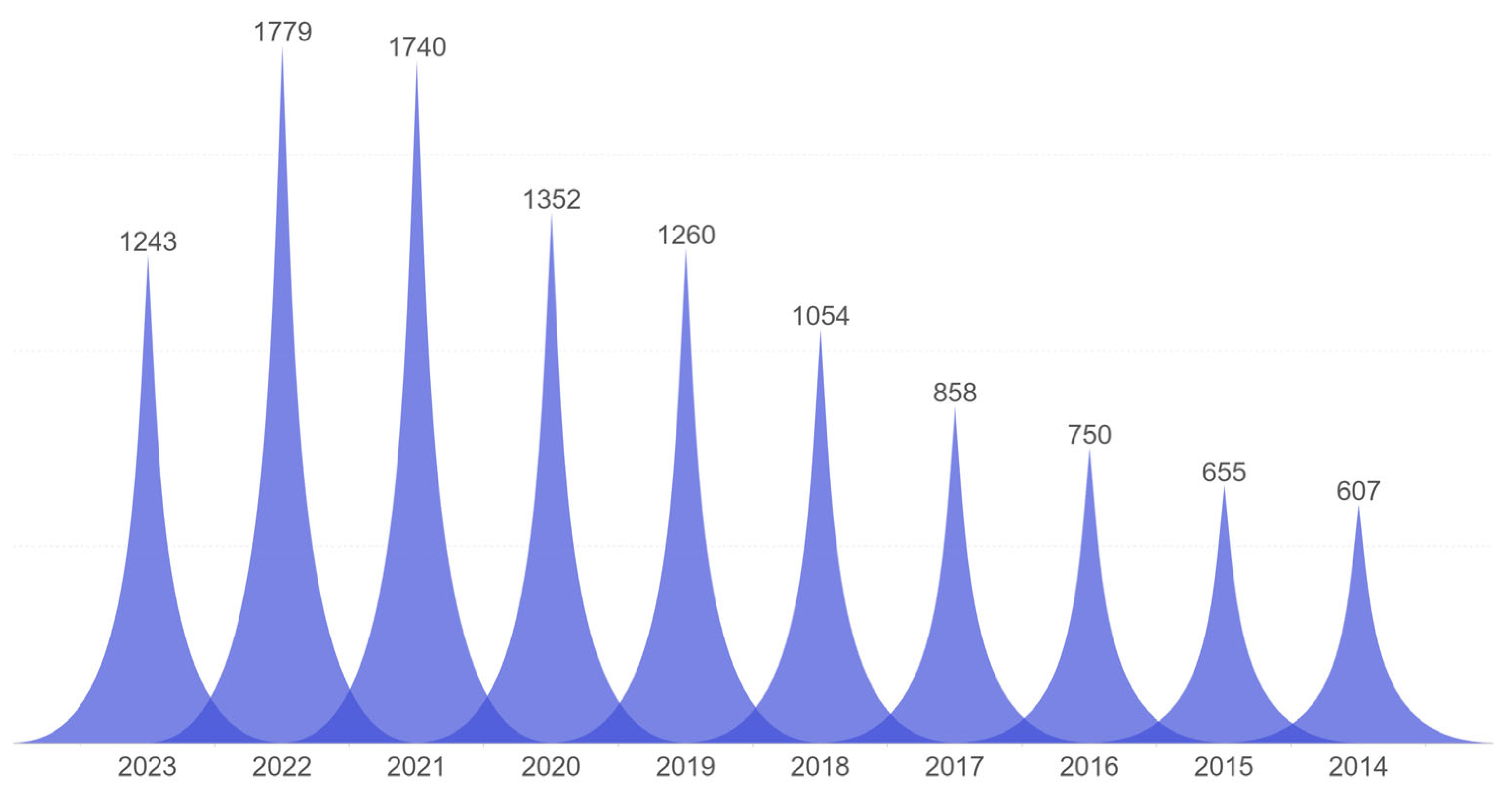
:1. Introduction
2. Fiber-Based Sensors for Monitoring Biophysical Signals
2.1. Fiber-Based Biomechanical Signal Sensors


2.2. Biological Temperature Sensor

3. Fiber-Based Sensors for Monitoring Biochemical Signals
3.1. Fiber-Based Biofluid Signal Sensors
3.2. Fiber-Based Respiratory Gas Sensor

4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Tat, T.; Chen, G.; Zhao, X.; Zhou, Y.; Xu, J.; Chen, J. Smart Textiles for Healthcare and Sustainability. ACS Nano 2022, 16, 13301–13313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.K.; Zhu, T.X.; Wang, J.R.; Zheng, Z.J.; Li, Y.; Li, J.S.; Lai, Y.K. Functionalized Fiber-Based Strain Sensors: Pathway to Next-Generation Wearable Electronics. Nano Micro Lett. 2022, 14, 61–89. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Cui, T.; Li, D.; Jian, J.; Li, Z.; Ji, S.; Cheng, A.; Li, X.; Liu, K.; Liu, H.; et al. Carbon-Based Textile Sensors for Physiological-Signal Monitoring. Materials 2023, 16, 3932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Xia, X.H.; Ma, K.K.; Xia, G.; Wu, M.Q.; Cheung, Y.H.; Yu, H.; Zou, B.S.; Zhang, X.W.; Farha, O.K.; et al. Functional Textiles with Smart Properties: Their Fabrications and Sustainable Applications. Adv. Funct. Mater. 2023, 33, 2301607. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Van Langenhove, L. Integration of Conductive Materials with Textile Structures, an Overview. Sensors 2020, 20, 6910. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, B.K.; Hooper, M.W.; Aklin, W.M.; Jean-Francois, B.; Elwood, W.N.; Belis, D.; Riley, W.T.; Hunter, C.M. Advancing digital health Equity: Directions for behavioral and social science research. Transl. Behav. Med. 2023, 13, 132–139. [Google Scholar] [CrossRef]
- Loke, G.; Khudiyev, T.; Wang, B.; Fu, S.; Payra, S.; Shaoul, Y.; Fung, J.; Chatziveroglou, I.; Chou, P.-W.; Chinn, I.; et al. Digital electronics in fibres enable fabric-based machine-learning inference. Nat. Commun. 2021, 12, 3317. [Google Scholar] [CrossRef]
- Ouyang, Z.; Xu, D.; Yu, H.-Y.; Li, S.; Song, Y.; Tam, K.C. Novel ultrasonic-coating technology to design robust, highly sensitive and wearable textile sensors with conductive nanocelluloses. Chem. Eng. J. 2022, 428, 131289. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Jiang, Y.; Sun, H.; Liu, T.; Li, G.; Yu, W.; Meng, C.; Guo, S. Bioinspired flexible, breathable, waterproof and self-cleaning iontronic tactile sensors for special underwater sensing applications. Nano Energy 2023, 110, 108367. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Zhong, W.; Luo, M.; Wang, W.; Qing, X.; Lu, Y.; Liu, Q.; Liu, K.; Wang, Y.; et al. Large-Area, Wearable, Self-Powered Pressure–Temperature Sensor Based on 3D Thermoelectric Spacer Fabric. ACS Sens. 2020, 5, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Goh, C.H.; Yap, K.Z.; Ramakrishnan, N. One-Step Fabrication of Paper-Based Inkjet-Printed Graphene for Breath Monitor Sensors. Biosensors 2023, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Sun, X.; Liang, C.; Han, Y.; Wu, X.; Wang, Z. All textile-based robust pressure sensors for smart garments. Chem. Eng. J. 2023, 454, 140302. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Xu, J.; Chen, G.; Fang, Y.; Tat, T.; Xiao, X.; Song, Y.; Li, S.; Chen, J. Soft fibers with magnetoelasticity for wearable electronics. Nat. Commun. 2021, 12, 6755–6765. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shaqeel, A.; Han, S.; Kang, J.; Yun, J.; Lee, M.; Lee, S.; Kim, J.; Noh, S.; Choi, M.; et al. In Situ Formation of Ag Nanoparticles for Fiber Strain Sensors: Toward Textile-Based Wearable Applications. ACS Appl. Mater. Interfaces 2021, 13, 39868–39879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Liu, B.; Pang, Y.N.; Liu, J.; Shi, J.L.; Wan, S.P.; He, X.D.; Yuan, J.H.; Wu, Q. Low-Cost Wearable Sensor Based on a D-Shaped Plastic Optical Fiber for Respiration Monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 4004809. [Google Scholar] [CrossRef]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef]
- Mo, L.; Ma, X.; Fan, L.; Xin, J.H.; Yu, H. Weavable, large-scaled, rapid response, long-term stable electrochemical fabric sensor integrated into clothing for monitoring potassium ions in sweat. Chem. Eng. J. 2023, 454, 140473. [Google Scholar] [CrossRef]
- Kim, S.; Truong, T.; Jang, J.; Kim, J. The Programmable Design of Large-Area Piezoresistive Textile Sensors Using Manufacturing by Jacquard Processing. Polymers 2023, 15, 78. [Google Scholar] [CrossRef]
- Lim, S.J.; Bae, J.H.; Han, J.H.; Jang, S.J.; Oh, H.J.; Lee, W.; Kim, S.H.; Ko, J.H. Foldable and washable fully textile-based pressure sensor. Smart Mater. Struct. 2020, 29, 055010. [Google Scholar] [CrossRef]
- Lin, S.; Hu, S.; Song, W.; Gu, M.; Liu, J.; Song, J.; Liu, Z.; Li, Z.; Huang, K.; Wu, Y.; et al. An ultralight, flexible, and biocompatible all-fiber motion sensor for artificial intelligence wearable electronics. NPJ Flex. Electron. 2022, 6, 27. [Google Scholar] [CrossRef]
- Hao, Y.N.; Yan, Q.Y.; Liu, H.J.; He, X.Y.; Zhang, P.H.; Qin, X.H.; Wang, R.R.; Sun, J.; Wang, L.M.; Cheng, Y. A Stretchable, Breathable, And Self-Adhesive Electronic Skin with Multimodal Sensing Capabilities for Human-Centered Healthcare. Adv. Funct. Mater. 2023, 33, 2303881. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Liu, Z.X.; Li, C.C.; Liu, M.S.; Jiang, L.; Zhou, Y.F.; Zhou, F.L.; Chen, S.J.; Jerrams, S.; Yu, J.Y. A Highly Stretchable and Sensitive Strain Sensor Based on Dopamine Modified Electrospun SEBS Fibers and MWCNTs with Carboxylation. Adv. Electron. Mater. 2021, 7, 2100233. [Google Scholar] [CrossRef]
- Li, Y.; Jia, J.; Yu, H.; Wang, S.; Jin, Z.-Y.; Zhang, Y.-H.; Ma, H.-Z.; Zhang, K.; Ke, K.; Yin, B.; et al. Macromolecule Relaxation Directed 3D Nanofiber Architecture in Stretchable Fibrous Mats for Wearable Multifunctional Sensors. ACS Appl. Mater. Interfaces 2022, 14, 15678–15686. [Google Scholar] [CrossRef] [PubMed]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30, 1910504. [Google Scholar] [CrossRef]
- Ning, C.; Cheng, R.; Jiang, Y.; Sheng, F.; Yi, J.; Shen, S.; Zhang, Y.; Peng, X.; Dong, K.; Wang, Z.L. Helical Fiber Strain Sensors Based on Triboelectric Nanogenerators for Self-Powered Human Respiratory Monitoring. ACS Nano 2022, 16, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.M.; Lee, Y.; Son, Y.; Park, G.; Park, S. Thermally Drawn Multi-material Fibers Based on Polymer Nanocomposite for Continuous Temperature Sensing. Adv. Fiber Mater. 2023, 5, 1712–1724. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Li, S.B.; He, Z.D.; Wu, Y.Z.; Gao, Z.Y.; Hu, C.; Hu, S.Q.; Wang, S.D.; Liu, C.; Shang, J.; et al. Dual Mode Strain-Temperature Sensor with High Stimuli Discriminability and Resolution for Smart Wearables. Adv. Funct. Mater. 2023, 33, 2214907. [Google Scholar] [CrossRef]
- Li, F.; Xue, H.; Lin, X.; Zhao, H.; Zhang, T. Wearable Temperature Sensor with High Resolution for Skin Temperature Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 43844–43852. [Google Scholar] [CrossRef]
- Chen, M.H.; He, Y.C.; Liang, H.H.; Zhou, H.Y.; Wang, X.; Heng, X.B.; Zhang, Z.S.; Gan, J.L.; Yang, Z.M. Stretchable and Strain-Decoupled Fluorescent Optical Fiber Sensor for Body Temperature and Movement Monitoring. ACS Photonics 2022, 9, 1415–1424. [Google Scholar] [CrossRef]
- Xiao, G.; Ju, J.; Li, M.; Wu, H.; Jian, Y.; Sun, W.; Wang, W.; Li, C.M.; Qiao, Y.; Lu, Z. Weavable yarn-shaped supercapacitor in sweat-activated self-charging power textile for wireless sweat biosensing. Biosens. Bioelectron. 2023, 235, 115389. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Deng, Y.; Chen, X.R.; Jiang, P.; Cheung, Y.K.; Yu, H.Y. An ultrafast-response and flexible humidity sensor for human respiration monitoring and noncontact safety warning. Microsyst. Nanoeng. 2021, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, R.; Xu, X.; Iqbal, S.; Hu, J. Fibers/Textiles-Based Flexible Sweat Sensors: A Review. ACS Mater. Lett. 2023, 5, 1420–1440. [Google Scholar] [CrossRef]
- Luo, D.; Sun, H.; Li, Q.; Niu, X.; He, Y.; Liu, H. Flexible Sweat Sensors: From Films to Textiles. ACS Sens. 2023, 8, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Shi, S.; Meng, S.; Wu, H.; Si, Y.; Zhang, K.; Zhang, S.; Hu, J. A biocompatible and antibacterial all-textile structured triboelectric nanogenerator for self-powered tactile sensing. Nano Energy 2023, 115, 108734. [Google Scholar] [CrossRef]
- Pang, Y.N.; Liu, B.; Liu, J.; Wan, S.P.; Wu, T.; He, X.D.; Yuan, J.H.; Zhou, X.; Long, K.P.; Wu, Q. Wearable Optical Fiber Sensor Based on a Bend Singlemode-Multimode-Singlemode Fiber Structure for Respiration Monitoring. IEEE Sens. J. 2021, 21, 4610–4617. [Google Scholar] [CrossRef]
- Dong, K.; Peng, X.; An, J.; Wang, A.C.; Luo, J.; Sun, B.; Wang, J.; Wang, Z.L. Shape adaptable and highly resilient 3D braided triboelectric nanogenerators as e-textiles for power and sensing. Nat. Commun. 2020, 11, 2868–2878. [Google Scholar] [CrossRef]
- Lou, C.G.; Wang, S.; Liang, T.; Pang, C.Y.; Huang, L.; Run, M.T.; Liu, X.L. A Graphene-Based Flexible Pressure Sensor with Applications to Plantar Pressure Measurement and Gait Analysis. Materials 2017, 10, 1068. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.Y.; Jiang, Y.; Zhai, S.Y.; Cheng, R.W.; Liu, D.; Gao, X.P.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef]
- Cai, J.Y.; Du, M.J.; Li, Z.L. Flexible Temperature Sensors Constructed with Fiber Materials. Adv. Mater. Technol. 2022, 7, 2101182. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hao, J.; Huang, Z.Q.; Zheng, G.Q.; Dai, K.; Liu, C.T.; Shen, C.Y. Flexible electrically resistive-type strain sensors based on reduced graphene oxide-decorated electrospun polymer fibrous mats for human motion monitoring. Carbon 2018, 126, 360–371. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Y.; Han, X.-l.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.-L. Graphene Textile Strain Sensor with Negative Resistance Variation for Human Motion Detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef] [PubMed]
- Verma, L.; Karnawal, I.; Chaudhary, V.; Kumar, P.; Singh, D.; Kumar, R.; Sapra, G.; Cleveland, B.; Afazov, S.; Felton, P.; et al. Design and Fabrication of Flexible Carbon Fabric PDMS-Based Strain Sensor for Human Motion Monitoring. IEEE Sens. J. 2023, 23, 16729–16735. [Google Scholar] [CrossRef]
- Sadi, M.S.; Kumpikaitė, E. Highly conductive composites using polypyrrole and carbon nanotubes on polydopamine functionalized cotton fabric for wearable sensing and heating applications. Cellulose 2023, 30, 7981–7999. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Liu, J.; Zhao, Z.Y.; Sun, W.W.; Zhao, G.J.; Liu, J.G.; Xu, J.C.; Li, Y.L.; Liu, Z.K.; Li, Y.; et al. Calotropis gigantea Fiber-Based Sensitivity-Tunable Strain Sensors with Insensitive Response to Wearable Microclimate Changes. Adv. Fiber Mater. 2023, 5, 1378–1391. [Google Scholar] [CrossRef]
- Liu, Z.K.; Li, Z.H.; Zhai, H.; Jin, L.; Chen, K.L.; Yi, Y.P.; Gao, Y.; Xu, L.L.; Zheng, Y.; Yao, S.R.; et al. A highly sensitive stretchable strain sensor based on multi-functionalized fabric for respiration monitoring and identification. Chem. Eng. J. 2021, 426, 130869. [Google Scholar] [CrossRef]
- Zhao, K.; Niu, W.; Zhang, S. Highly stretchable, breathable and negative resistance variation textile strain sensor with excellent mechanical stability for wearable electronics. J. Mater. Sci. 2020, 55, 2439–2453. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhao, Z.; Liu, Y.; Tam, W.C.; Zheng, Z.; Wang, X.; Li, Y.; Liu, Z.; Li, Y.; et al. A negative-response strain sensor towards wearable microclimate changes for body area sensing networks. Chem. Eng. J. 2023, 459, 141628. [Google Scholar] [CrossRef]
- Lu, D.; Liao, S.; Chu, Y.; Cai, Y.; Wei, Q.; Chen, K.; Wang, Q. Highly Durable and Fast Response Fabric Strain Sensor for Movement Monitoring Under Extreme Conditions. Adv. Fiber Mater. 2023, 5, 223–234. [Google Scholar] [CrossRef]
- Pan, H.; Chen, G.; Chen, Y.; Di Carlo, A.; Mayer, M.A.; Shen, S.; Chen, C.; Li, W.; Subramaniam, S.; Huang, H.; et al. Biodegradable cotton fiber-based piezoresistive textiles for wearable biomonitoring. Biosens. Bioelectron. 2023, 222, 114999. [Google Scholar] [CrossRef]
- Hou, N.; Zhao, Y.; Jiang, R.; Nie, L.; Yang, J.; Wang, Y.; Li, L.; Li, X.; Zhang, W. Flexible piezoresistive sensor based on surface modified dishcloth fibers for wearable electronics device. Colloids Surf. A 2022, 650, 129638. [Google Scholar] [CrossRef]
- Choi, H.; Sun, J.; Ren, B.; Cha, S.; Lee, J.; Lee, B.-M.; Park, J.-J.; Choi, J.-H.; Park, J.-J. 3D textile structure-induced local strain for a highly amplified piezoresistive performance of carbonized cellulose fabric based pressure sensor for human healthcare monitoring. Chem. Eng. J. 2022, 450, 138193. [Google Scholar] [CrossRef]
- Atalay, O. Textile-Based, Interdigital, Capacitive, Soft-Strain Sensor for Wearable Applications. Materials 2018, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Si, Y.; Zhao, C.; Yu, D.; Wang, W.; Sun, G. Flexible and Washable Poly(Ionic Liquid) Nanofibrous Membrane with Moisture Proof Pressure Sensing for Real-Life Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 27200–27209. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chhetry, A.; Zhang, S.; Yoon, H.; Park, C.; Kim, H.; Sharifuzzaman, M.; Hui, X.; Park, J.Y. Hydrogen-Bond-Triggered Hybrid Nanofibrous Membrane-Based Wearable Pressure Sensor with Ultrahigh Sensitivity over a Broad Pressure Range. ACS Nano 2021, 15, 4380–4393. [Google Scholar] [CrossRef]
- Yu, P.; Li, X.; Li, H.; Fan, Y.; Cao, J.; Wang, H.; Guo, Z.; Zhao, X.; Wang, Z.; Zhu, G. All-Fabric Ultrathin Capacitive Sensor with High Pressure Sensitivity and Broad Detection Range for Electronic Skin. ACS Appl. Mater. Interfaces 2021, 13, 24062–24069. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, J.; Oh, J.; Lim, S.; Sim, J.Y.; Jeon, J.S.; No, K.; Park, S.; Hong, S. Intrinsically stretchable multi-functional fiber with energy harvesting and strain sensing capability. Nano Energy 2019, 55, 348–353. [Google Scholar] [CrossRef]
- Kim, J.; Byun, S.; Lee, S.; Ryu, J.; Cho, S.; Oh, C.; Kim, H.; No, K.; Ryu, S.; Lee, Y.M.; et al. Cost-effective and strongly integrated fabric-based wearable piezoelectric energy harvester. Nano Energy 2020, 75, 104992. [Google Scholar] [CrossRef]
- Meng, K.Y.; Xiao, X.; Wei, W.X.; Chen, G.R.; Nashalian, A.; Shen, S.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Su, Y.J.; Chen, C.X.; Pan, H.; Yang, Y.; Chen, G.R.; Zhao, X.; Li, W.X.; Gong, Q.C.; Xie, G.Z.; Zhou, Y.H.; et al. Muscle Fibers Inspired High-Performance Piezoelectric Textiles for Wearable Physiological Monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Q.; Yan, C.; Liu, Y.; Zeng, X.; Wei, X.; Hu, Y.; Zheng, Z. Machine-washable and breathable pressure sensors based on triboelectric nanogenerators enabled by textile technologies. Nano Energy 2020, 70, 104528. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Li, J.C.; Yin, J.; Wee, M.G.V.; Chinnappan, A.; Ramakrishna, S. A Self-Powered Piezoelectric Nanofibrous Membrane as Wearable Tactile Sensor for Human Body Motion Monitoring and Recognition. Adv. Fiber Mater. 2023, 5, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, F.; Cui, X.; Zhu, Y. Recent progress in textile-based triboelectric force sensors for wearable electronics. Adv. Compos. Hybrid Mater. 2023, 6, 70. [Google Scholar] [CrossRef]
- Luo, J.J.; Gao, W.C.; Wang, Z.L. The Triboelectric Nanogenerator as an Innovative Technology toward Intelligent Sports. Adv. Mater. 2021, 33, 2004178. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Dong, K.; Shen, S.; Jiang, Y.; Peng, X.; Ye, C.; Wang, Z.L. Fully Fabric-Based Triboelectric Nanogenerators as Self-Powered Human–Machine Interactive Keyboards. Nano Micro Lett. 2021, 13, 103. [Google Scholar] [CrossRef]
- Jiang, D.W.; Lian, M.Y.; Xu, M.J.; Sun, Q.; Xu, B.B.; Thabet, H.K.; El-Bahy, S.M.; Ibrahim, M.M.; Huang, M.A.; Guo, Z.H. Advances in triboelectric nanogenerator technology-applications in self-powered sensors, Internet of things, biomedicine, and blue energy. Adv. Compos. Hybrid Mater. 2023, 6, 57. [Google Scholar] [CrossRef]
- Zhi, C.W.; Shi, S.; Zhang, S.; Si, Y.F.; Yang, J.Q.; Meng, S.; Fei, B.; Hu, J.L. Bioinspired All-Fibrous Directional Moisture-Wicking Electronic Skins for Biomechanical Energy Harvesting and All-Range Health Sensing. Nano Micro Lett. 2023, 15, 60. [Google Scholar] [CrossRef]
- Chen, G.R.; Zhao, X.; Andalib, S.; Xu, J.; Zhou, Y.H.; Tat, T.; Lin, K.; Chen, J. Discovering giant magnetoelasticity in soft matter for electronic textiles. Matter 2021, 4, 3725–3740. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Wu, Z.; Guo, H.; Yu, W.; Du, Z.; Wang, Z.L. 3D double-faced interlock fabric triboelectric nanogenerator for bio-motion energy harvesting and as self-powered stretching and 3D tactile sensors. Mater. Today 2020, 32, 84–93. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, B.Q.; Yang, C.X.; Dai, Q.H.; Kong, L.J. Stretchable and Temperature-Sensitive Polymer Optical Fibers for Wearable Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902898. [Google Scholar] [CrossRef]
- Su, Y.; Ma, C.; Chen, J.; Wu, H.; Luo, W.; Peng, Y.; Luo, Z.; Li, L.; Tan, Y.; Omisore, O.M.; et al. Printable, Highly Sensitive Flexible Temperature Sensors for Human Body Temperature Monitoring: A Review. Nanoscale Res. Lett. 2020, 15, 200–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qin, Y.; Wang, Z.; Yu, T.; Ge, Z. Recent Advances in the Development of Flexible Sensors: Mechanisms, Materials, Performance Optimization, and Applications. J. Electron. Mater. 2022, 51, 6735–6769. [Google Scholar] [CrossRef]
- Komatsu, N.; Ichinose, Y.; Dewey, O.S.; Taylor, L.W.; Trafford, M.A.; Yomogida, Y.; Wehmeyer, G.; Pasquali, M.; Yanagi, K.; Kono, J. Macroscopic weavable fibers of carbon nanotubes with giant thermoelectric power factor. Nat. Commun. 2021, 12, 4931. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Zhu, G.; Wang, C.; Cui, X.; Xi, M.; Chang, X.; Zhu, Y. Breathable Strain/Temperature Sensor Based on Fibrous Networks of Ionogels Capable of Monitoring Human Motion, Respiration, and Proximity. ACS Appl. Mater. Interfaces 2021, 13, 51567–51577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.K.; Li, T.T.; Hussain, B.; Zhou, S.B.; Wang, Z.S.; Peng, Y.; Hu, J.C.; Zhang, K.Q. Facile Fabrication of Cotton-Based Thermoelectric Yarns for the Construction of Textile Generator with High Performance in Human Heat Harvesting. Adv. Fiber Mater. 2023, 5, 1725–1736. [Google Scholar] [CrossRef]
- Li, Y.P.; Zeng, J.Y.; Zhao, Y.; Wang, C.R.; Zhang, C.Y.; Cheng, T.T.; Tao, J.H.; Li, J.; Wang, C.H.; Zhang, L.; et al. Fabric-based flexible thermoelectric generators: Design methods and prospects. Front. Mater. 2022, 9, 1046883. [Google Scholar] [CrossRef]
- Kwon, C.; Lee, S.; Won, C.; Lee, K.H.; Kim, M.; Lee, J.; Yang, S.J.; Lee, M.; Lee, S.; Yoon, K.; et al. Multi-Functional and Stretchable Thermoelectric Bi2Te3 Fabric for Strain, Pressure, and Temperature-Sensing. Adv. Funct. Mater. 2023, 33, 2300092–2300098. [Google Scholar] [CrossRef]
- Yang, P.; Wei, G.; Liu, A.; Huo, F.; Zhang, Z. A review of sampling, energy supply and intelligent monitoring for long-term sweat sensors. NPJ Flex. Electron. 2022, 6, 33. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.D.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, F.; Patella, B.; D’Agostino, C.; Bruno, M.G.; Carbone, S.; Lopresti, F.; Aiello, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; et al. PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors 2021, 9, 169. [Google Scholar] [CrossRef]
- Clark, K.M.; Ray, T.R. Recent Advances in Skin-Interfaced Wearable Sweat Sensors: Opportunities for Equitable Personalized Medicine and Global Health Diagnostics. ACS Sens. 2023, 8, 3606–3622. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Possanzini, L.; Scavetta, E.; Fraboni, B. Textile Chemical Sensors Based on Conductive Polymers for the Analysis of Sweat. Polymers 2021, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, F.; Patella, B.; Divita, V.; Zanca, C.; Botta, L.; Radacsi, N.; O’Riordan, A.; Aiello, G.; Kersaudy-Kerhoas, M.; Inguanta, R.; et al. Green and Integrated Wearable Electrochemical Sensor for Chloride Detection in Sweat. Sensors 2022, 22, 8223. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.L.; Yang, D.Z.; Hua, T.J.; Li, S.; Wang, B.Y.; Shao, Y.L. Multifunctional Fiber for Synchronous Bio-Sensing and Power Supply in Sweat Environment. Adv. Funct. Mater. 2023, 33, 2301174. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L.; Deng, T.B.; Li, C.Z. High-performance sensing, breathable, and biodegradable integrated wearable sweat biosensors for a wireless glucose early warning system. J. Mater. Chem. A 2023, 11, 12395–12404. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Liu, J.; Li, Y. Wearable, nanofiber-based microfluidic systems with integrated electrochemical and colorimetric sensing arrays for multiplex sweat analysis. Chem. Eng. J. 2023, 454, 140248. [Google Scholar] [CrossRef]
- Lei, Y.; Gong, Z.; Li, Y.; Zhang, J.; Liu, Z.; Sun, Z.; Ouyang, X.; Tang, Y.; Chan, C.C. Highly Sensitive Physiological Sensor Based on Tapered Fiber-Optic Interferometer for Sweat pH Detection. IEEE Sens. J. 2023, 23, 11627–11634. [Google Scholar] [CrossRef]
- Wu, J.; Sato, Y.; Guo, Y. Microelectronic fibers for multiplexed sweat sensing. Anal. Bioanal. Chem. 2023, 415, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.-M.; Curto, V.F.; Braendlein, M.; Strakosas, X.; Donahue, M.J.; Fiocchi, M.; Malliaras, G.G.; Owens, R.M. Organic Transistor Arrays Integrated with Finger-Powered Microfluidics for Multianalyte Saliva Testing. Adv. Healthc. Mater. 2016, 5, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Santana-Jiménez, L.A.; Márquez-Lucero, A.; Osuna, V.; Estrada-Moreno, I.; Dominguez, R.B. Naked-Eye Detection of Glucose in Saliva with Bienzymatic Paper-Based Sensor. Sensors 2018, 18, 1071. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an alternative specimen to plasma for drug bioanalysis: A review. TrAC Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Xu, T.; Jin, W.; Wang, Z.Z.; Cheng, H.Y.; Huang, X.H.; Guo, X.Y.; Ying, Y.; Wu, Y.P.; Wang, F.; Wen, Y.; et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Naderi, L.; Shahrokhian, S. Metal-organic framework-assisted Co3O4/CuO@CoMnP with core-shell nanostructured architecture on Cu fibers for fabrication of flexible wire-typed enzyme-free micro-sensors. Chem. Eng. J. 2023, 456, 141088. [Google Scholar] [CrossRef]
- Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, W.-J.; Kim, S.D.; Park, S.; Kim, J.H. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors 2022, 12, 1020. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Su, H.; Sun, F.; Lu, Z.; Su, A. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sens. Actuators B 2021, 341, 130046. [Google Scholar] [CrossRef]
- Li, X.; Zhan, C.; Huang, Q.; He, M.; Yang, C.; Yang, C.; Huang, X.; Chen, M.; Xie, X.; Chen, H.-J. Smart Diaper Based on Integrated Multiplex Carbon Nanotube-Coated Electrode Array Sensors for In Situ Urine Monitoring. ACS Appl. Nano Mater. 2022, 5, 4767–4778. [Google Scholar] [CrossRef]
- Zhao, J.L.; Shen, W.F.; Lv, D.W.; Yin, J.Q.; Liang, T.X.; Song, W.J. Gas-Sensing Technology for Human Breath Detection. Prog. Chem. 2023, 35, 302–317. [Google Scholar] [CrossRef]
- Ansari, H.R.; Mirzaei, A.; Shokrollahi, H.; Kumar, R.; Kim, J.Y.; Kim, H.W.; Kumar, M.; Kim, S.S. Flexible/wearable resistive gas sensors based on 2D materials. J. Mater. Chem. C 2023, 11, 6528–6549. [Google Scholar] [CrossRef]
- Iitani, K.; Chien, P.J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Fiber-Optic Bio-sniffer (Biochemical Gas Sensor) Using Reverse Reaction of Alcohol Dehydrogenase for Exhaled Acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.J.; Suzuki, T.; Ye, M.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Ultra-Sensitive Isopropanol Biochemical Gas Sensor (Bio-Sniffer) for Monitoring of Human Volatiles. Sensors 2020, 20, 6827. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Tai, H.; Liu, B.; Duan, Z.; Yuan, Z.; Pan, H.; Xie, G.; Du, X.; Su, Y. An integrated flexible self-powered wearable respiration sensor. Nano Energy 2019, 63, 103829. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review-Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Ti@MoS2 incorporated Polypropylene/Nylon fabric-based porous, breathable triboelectric nanogenerator as respiration sensor and ammonia gas sensor applications. Sens. Actuators B 2023, 380, 133346. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Wu, Y.; Xing, H.; An, Z.; Shi, Z.; Lv, J.; Zhang, F.; Jiang, J.; Wang, D.; et al. MXene-based wireless facemask enabled wearable breath acetone detection for lipid metabolic monitoring. Biosens. Bioelectron. 2023, 222, 114945. [Google Scholar] [CrossRef]
- Xu, L.; Zhai, H.; Chen, X.; Liu, Y.; Wang, M.; Liu, Z.; Umar, M.; Ji, C.; Chen, Z.; Jin, L.; et al. Coolmax/graphene-oxide functionalized textile humidity sensor with ultrafast response for human activities monitoring. Chem. Eng. J. 2021, 412, 128639. [Google Scholar] [CrossRef]
- Zhao, Q.N.; Jiang, Y.D.; Duan, Z.H.; Yuan, Z.; Zha, J.J.; Wu, Z.K.; Huang, Q.; Zhou, Z.; Li, H.; He, F.; et al. A Nb2CTx/sodium alginate-based composite film with neuron-like network for self-powered humidity sensing. Chem. Eng. J. 2022, 438, 135588. [Google Scholar] [CrossRef]
- Xu, D.; Ouyang, Z.; Dong, Y.; Yu, H.-Y.; Zheng, S.; Li, S.; Tam, K.C. Robust, Breathable and Flexible Smart Textiles as Multifunctional Sensor and Heater for Personal Health Management. Adv. Fiber Mater. 2023, 5, 282–295. [Google Scholar] [CrossRef]
- Maity, D.; Fussenegger, M. An Efficient Ambient-Moisture-Driven Wearable Electrical Power Generator. Adv. Sci. 2023, 10, e2300750. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Lou, J.; Yao, H.; Zhao, C. Fast response humidity sensor based on hyperbranched zwitterionic polymer for respiratory monitoring and non-contact human machine interface. Chem. Eng. J. 2023, 471, 144582. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, J.; Liu, J.; Sun, W.; Li, W.; Shen, H.; Wang, L.; Li, G. Advances in Fiber-Based Wearable Sensors for Personal Digital Health Monitoring. Materials 2023, 16, 7428. https://doi.org/10.3390/ma16237428
Liu J, Zhang J, Liu J, Sun W, Li W, Shen H, Wang L, Li G. Advances in Fiber-Based Wearable Sensors for Personal Digital Health Monitoring. Materials. 2023; 16(23):7428. https://doi.org/10.3390/ma16237428
Chicago/Turabian StyleLiu, Jingge, Junze Zhang, Jing Liu, Weiwei Sun, Weiqiang Li, Hongqiang Shen, Lingxiao Wang, and Gang Li. 2023. "Advances in Fiber-Based Wearable Sensors for Personal Digital Health Monitoring" Materials 16, no. 23: 7428. https://doi.org/10.3390/ma16237428
APA StyleLiu, J., Zhang, J., Liu, J., Sun, W., Li, W., Shen, H., Wang, L., & Li, G. (2023). Advances in Fiber-Based Wearable Sensors for Personal Digital Health Monitoring. Materials, 16(23), 7428. https://doi.org/10.3390/ma16237428





