Novel Cr/Si-Slurry Diffusion Coatings for High Temperatures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cr-Si Slurry Coatings
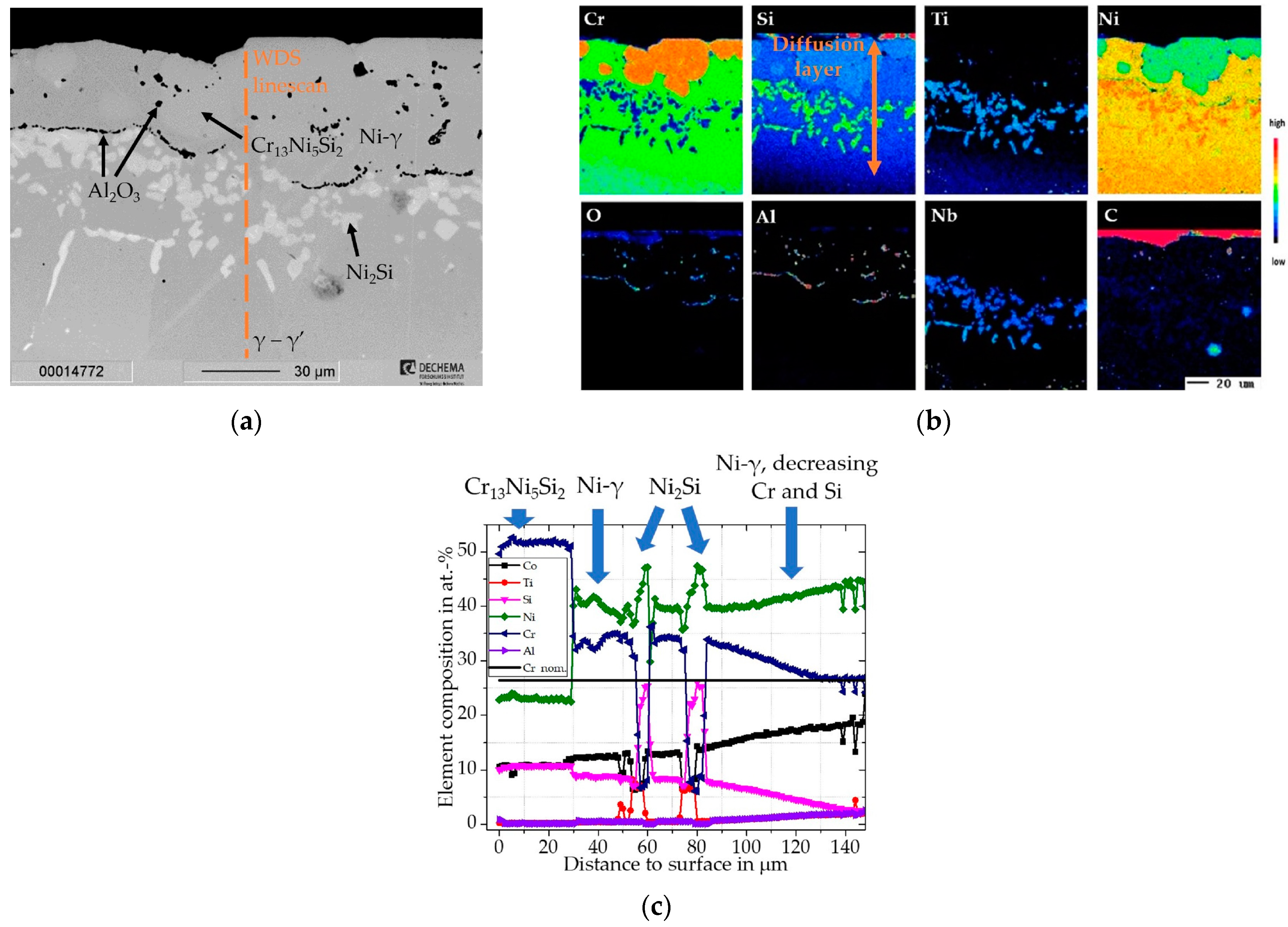
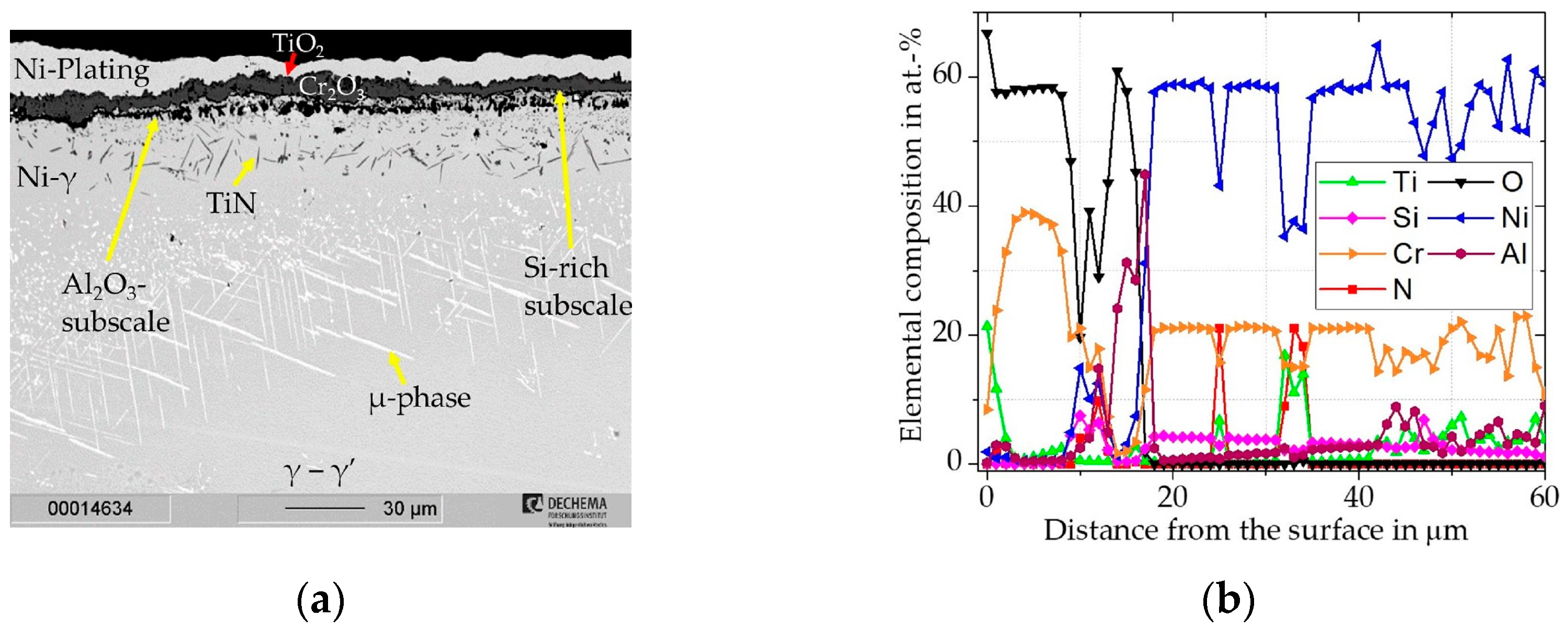
3.1.1. Cr/Si-Coated Rene 80
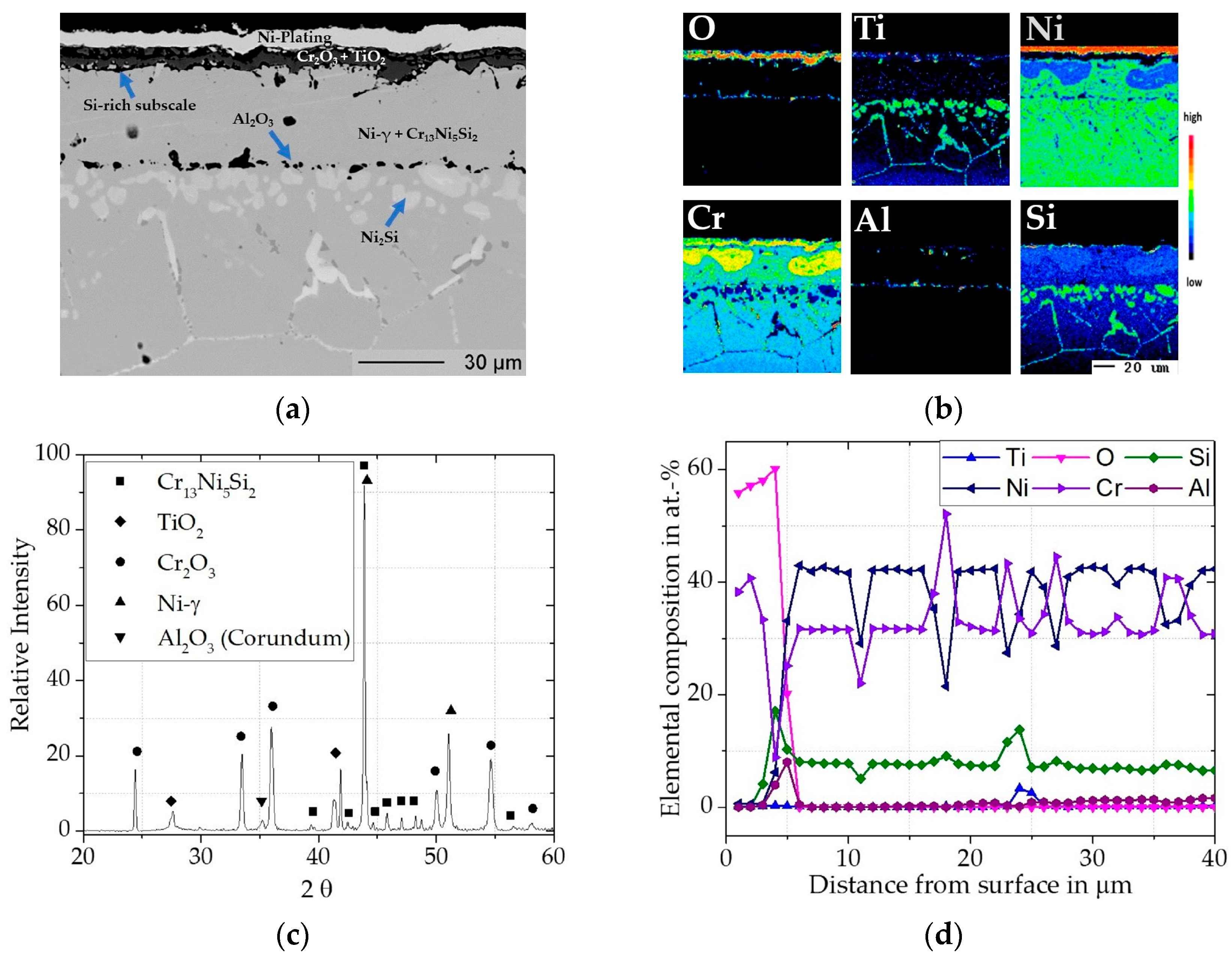
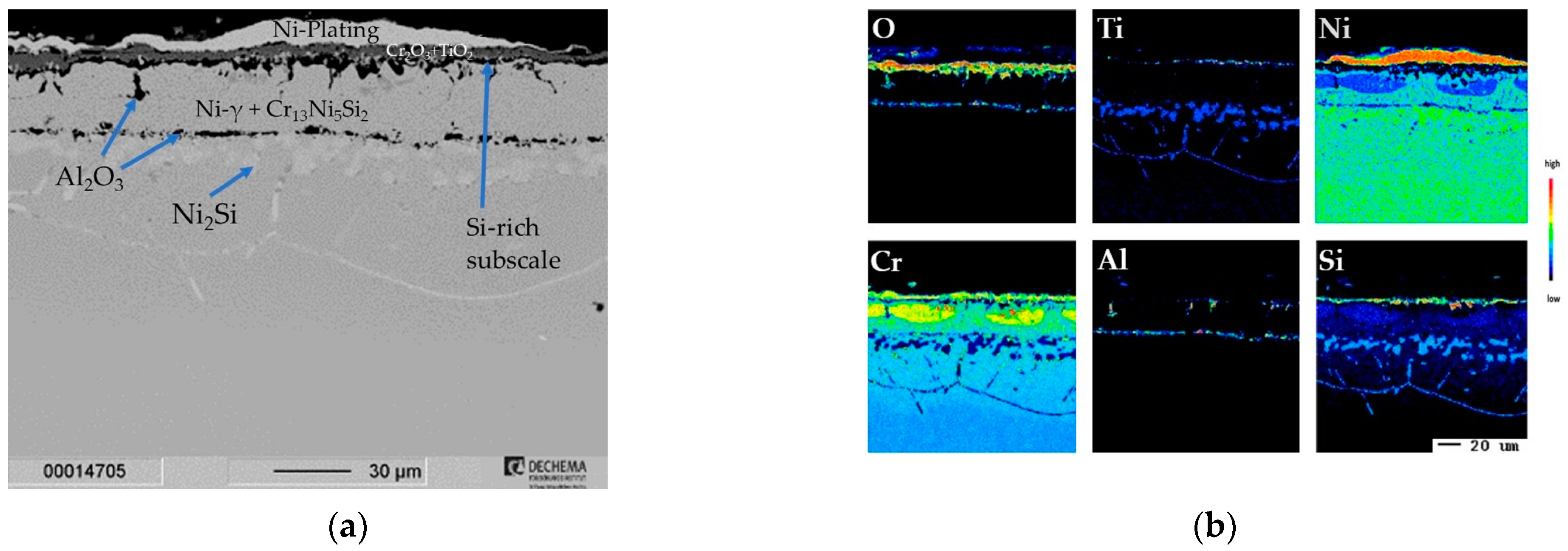
3.1.2. Cr/Si-Coated Inconel 740H
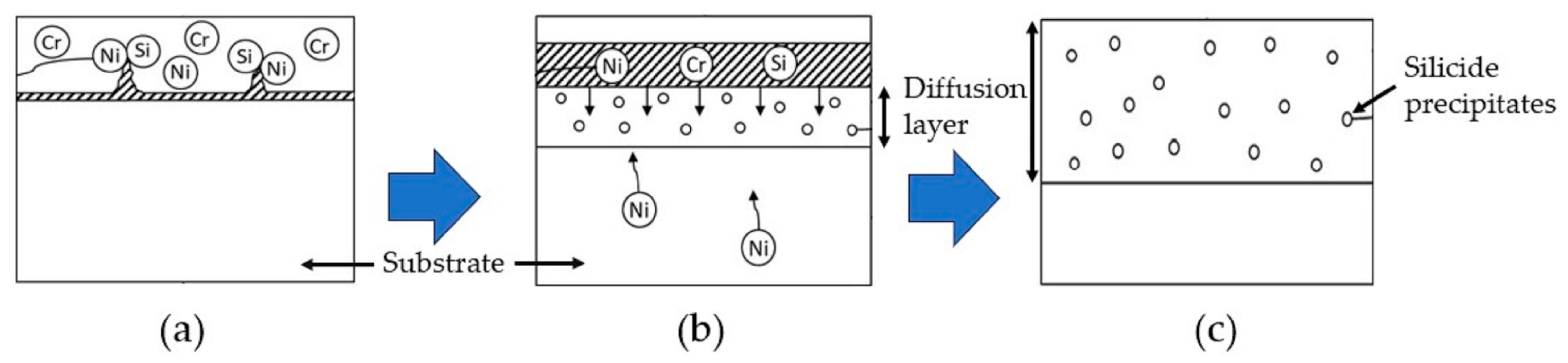
3.1.3. Cr-Si Slurry Coating Process
3.2. Oxidation Behavior: Quasi-Isothermal Oxidation Exposures
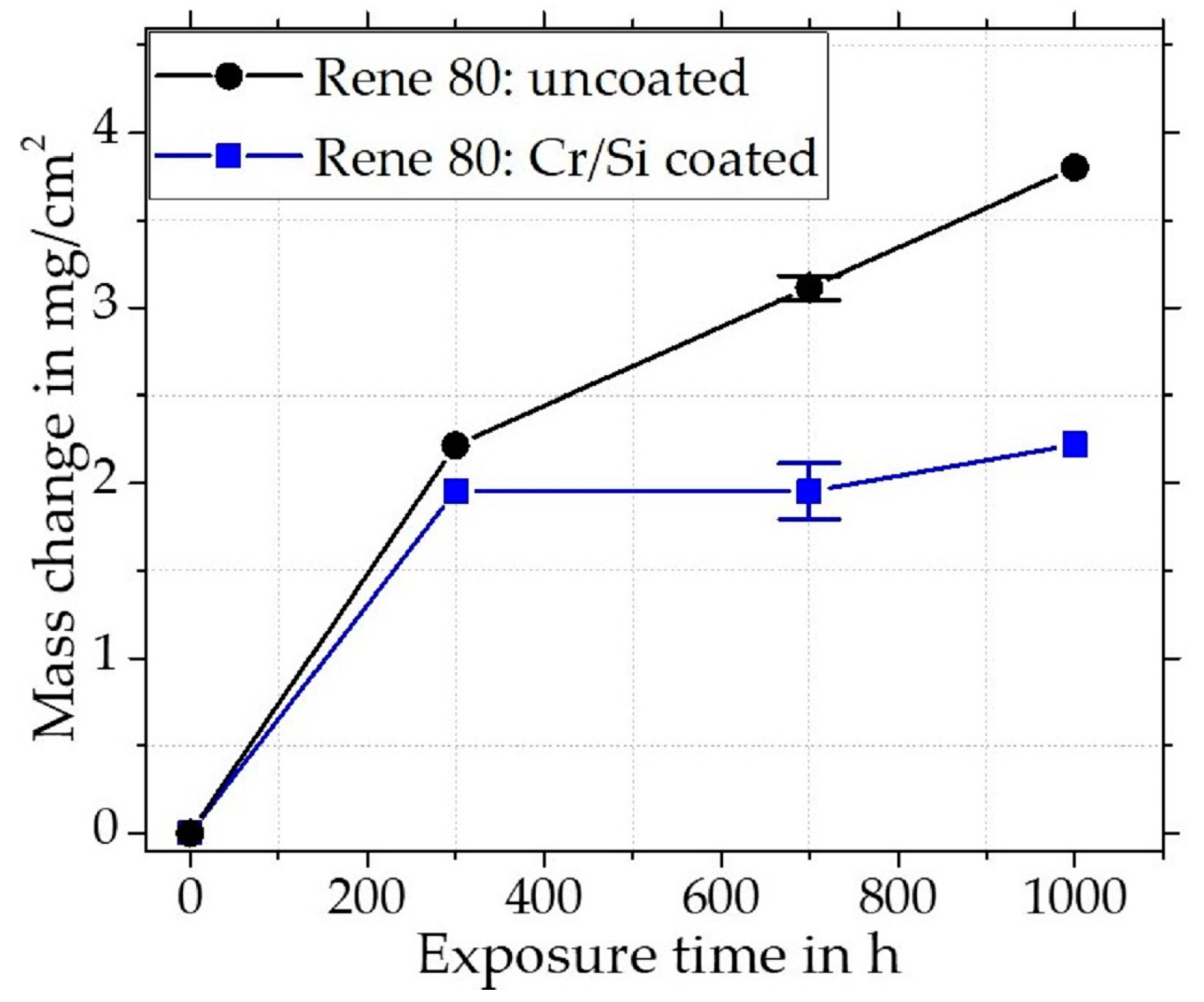
3.2.1. Oxidation of Rene 80
Uncoated Rene 80
Cr/Si-Coated Rene 80
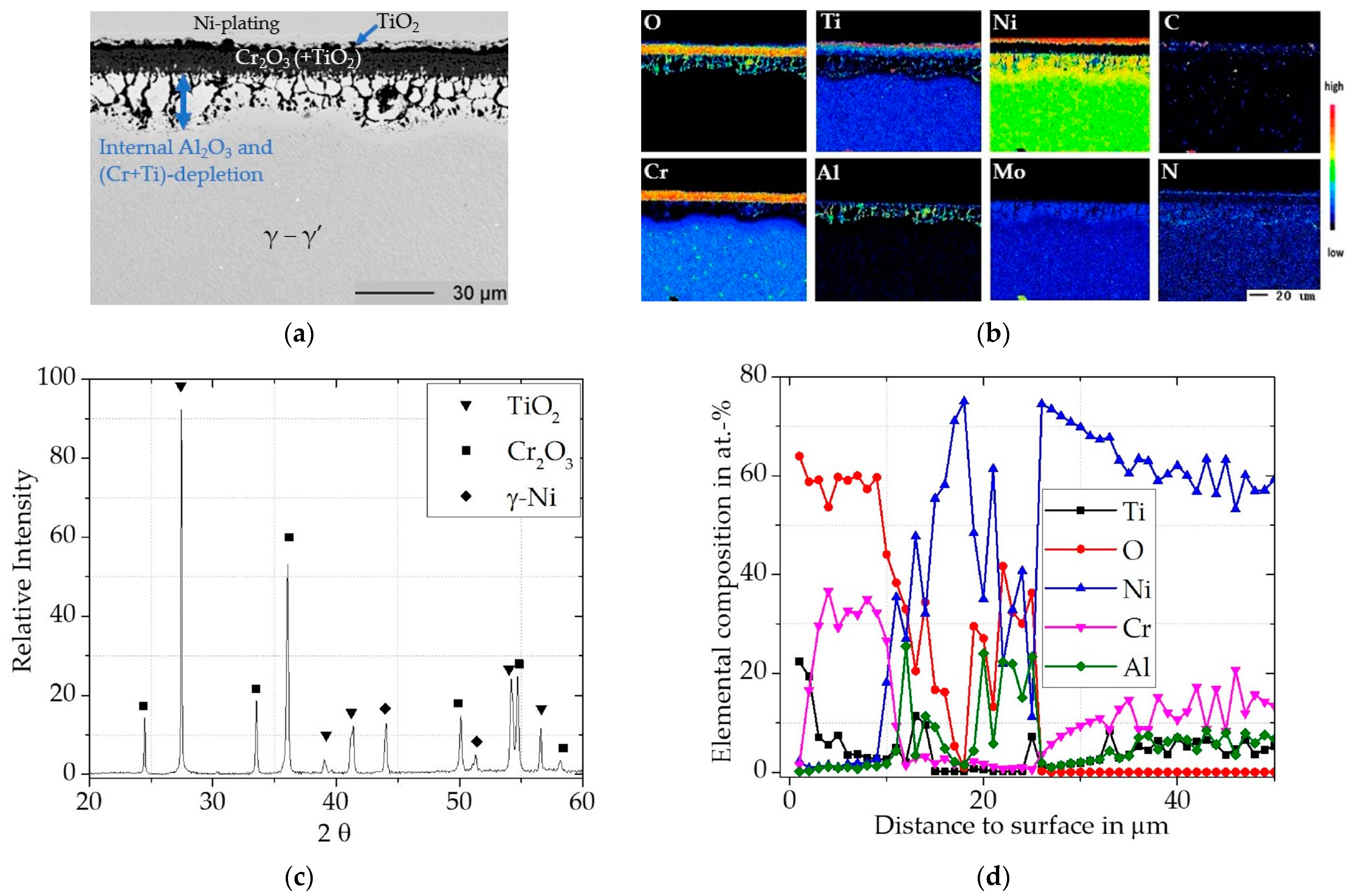
3.2.2. Oxidation of Inconel 740H
Uncoated Inconel 740H
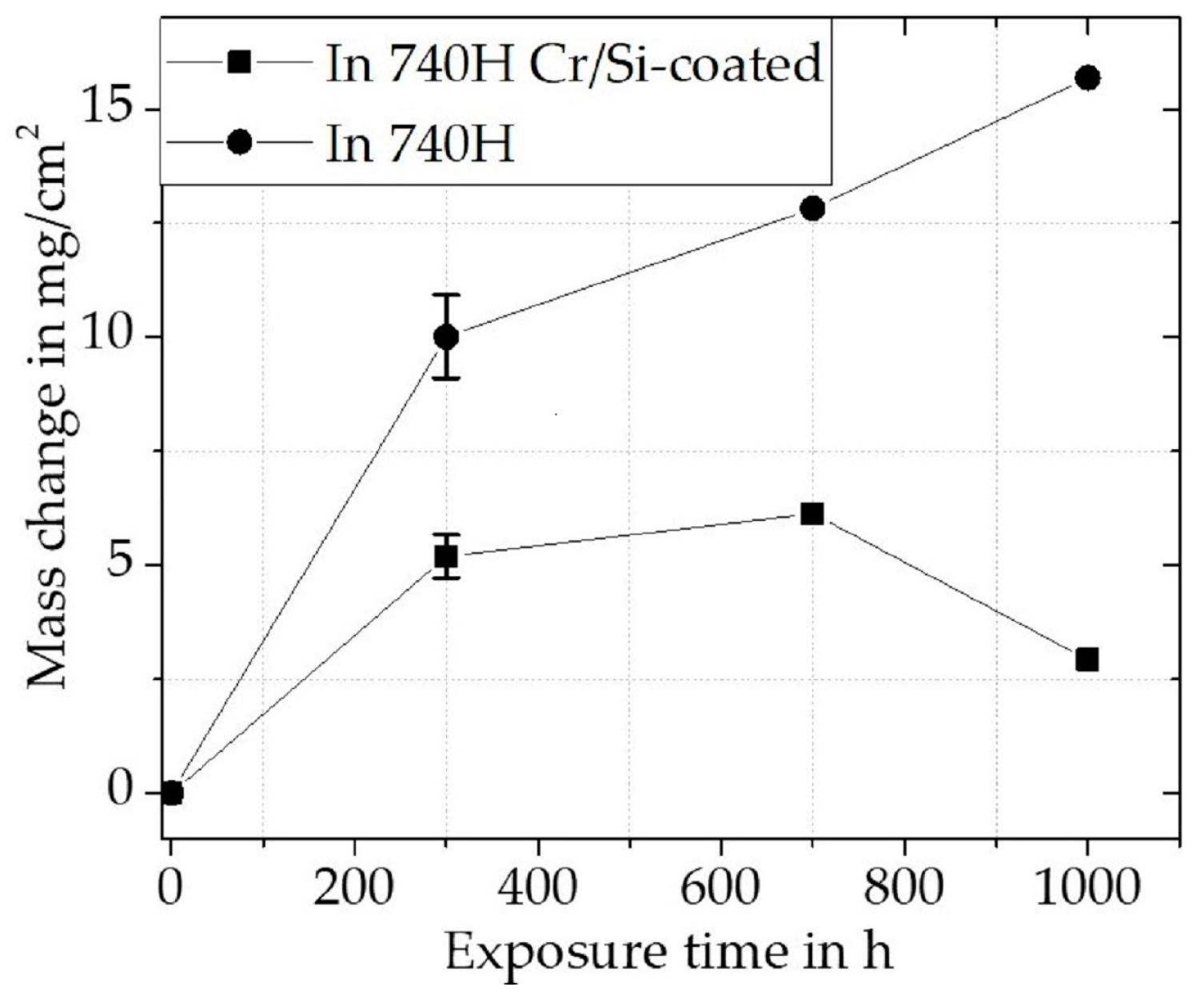
Cr/Si-Coated Inconel 740H
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galetz, M.C. Coatings for Superalloys. In Superalloys; Aliofkhazraei, M., Ed.; InTechOpen: London, UK, 2015; Chapter 12; pp. 277–298. [Google Scholar]
- Harada, H. Superalloys: Present and Future. Current Topics on High Temperature Materials. JSPS Rep. 123rd Comm. Heat Resist. Mater. Al-Loys 2007, 48, 357–363. [Google Scholar]
- Sims, C.T. A History of superalloy metallurgy for superalloy metallurgists. In Proceedings of the Fifth International Symposium on Superalloys, Pittsburgh, PA, USA, 7–11 October 1984; pp. 399–419. [Google Scholar]
- Kofstad, P. Protective Properties of Chromia and Alumina Scales. Mater. Sci. Forum 1994, 154, 99–108. [Google Scholar] [CrossRef]
- Nicholls, J.R. Designing oxidation-resistant coatings. JoM 2000, 52, 28–35. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack cementation diffusion coatings. In Metallurgical and Ceramic Protective Coatings; Stern, K.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 236–260. [Google Scholar]
- Montero, X.; Galetz, M.C.; Schütze, M. A Novel Type of Environmentally Friendly Slurry Coatings. Jom 2015, 67, 77–86. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Development of a new slurry coating design for the surface protection of gas turbine components. Surf. Coat. Technol. 2019, 374, 521–530. [Google Scholar] [CrossRef]
- Bauer, J.T.; Montero, X.; Galetz, M.C. Fast heat treatment methods for al slurry diffusion coatings on alloy 800 prepared in air. Surf. Coat. Technol. 2020, 381, 125–140. [Google Scholar] [CrossRef]
- Omar, H.; Papadopoulos, D.P.; Tsipas, S.A.; Lefakis, H. Aluminizing nickel foam by a slurry coating process. Mater. Lett. 2009, 63, 1387–1389. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Low-activity aluminide coatings for superalloys using a slurry process free of halide activators and chromates. Surf. Coat. Technol. 2013, 222, 9–14. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Slurry coated Ni-plated Fe-base alloys: Investigation of the influence of powder and substrate composition on interdiffusional and structural degradation of aluminides. Surf. Coat. Technol. 2013, 236, 465–475. [Google Scholar] [CrossRef]
- Rapp, R.A.; Otsuka, N. The Role of Chromium in the Hot Corrosion of Metals. ECS Trans. 2009, 16, 271–282. [Google Scholar] [CrossRef]
- Tang, Z.; Garing, K.E.; Findlay, T.D.; Lewis, T.F., III; Knapp, J.K. Methods of Applying Chromium Diffusion Coatings onto Selective Regions of a Component. U.S. Patent 9,587,302, 25 January 2017. [Google Scholar]
- Lopez Lavernia, N.; Hillen, M.; Pillhöfer, H.; Kliewe, A. Schlicker und Verfahren zur Herstellung einer Oxidations- und Korrosionsbeständigen Diffusionschicht. German Patent DE102014222024A1, 16 June 2016. [Google Scholar]
- Heinrich, W.; Hilser, L. Verfahren zum Versehen einer metallischen Oberfläche mit einer Chrom-Diffusionsschutzschicht. German Patent DE102018215395A1, 8 April 2020. [Google Scholar]
- Kerbstadt, M.; Galetz, M.C. Verfahren zur Diffusionsbeschichtung mit einem Cr-Si-haltigen Schlicker. German Patent Application: 10 2022 112 093.7, (Application Process pending), 13 May 2022. [Google Scholar]
- Cui, S.; Jung, I.-H. Thermodynamic assessments of the Cr-Si and Al-Cr-Si systems. J. Alloys Compd. 2007, 708, 887–902. [Google Scholar] [CrossRef]
- Lugscheider, E.; Knotek, O.; Klöhn, K. Melting behaviour of nickel—Chromium—Silicon alloys. Thermochim. Acta 1979, 29, 323–326. [Google Scholar] [CrossRef]
- Nash, P.; Nash, A. The Ni-Si (nickel-silicon) system. Bull. Alloy Phase Diagr. 1987, 8, 6–14. [Google Scholar] [CrossRef]
- Liu, X.; Lin, M.; Yang, S.; Ruan, J.; Wang, C. Experimental investigation of phase equilibria in the Ni-Cr-Si ternary system. J. Phase Equilibria Diffus. 2014, 35, 334–342. [Google Scholar] [CrossRef]
- Gupta, K.P. The Cr-Ni-Si (chromium-nickel-silicon) system. J. Phase Equilibria Diffus. 2006, 27, 523–528. [Google Scholar] [CrossRef]
- Harper, M.A.; Rapp, R.A. Chromized/siliconized pack cementation diffusion coatings for heat-resistant alloys. In Proceedings of the First International Conference on Heat Resistant Materials; Natesan, K., Tillack, D.J., Eds.; The American Society for Metals: Detroit, MI, USA, 1991; pp. 379–386. [Google Scholar]
- Harper, M.A.; Rapp, R.A. Codeposited chromium and silicon diffusion coatings for Fe-Base alloys via pack cementation. Oxid. Met. 1994, 42, 303–333. [Google Scholar] [CrossRef]
- Grünling, H.W.; Bauer, R. The role of silicon in corrosion-resistant high temperature coatings. Thin Solid Film. 1982, 1, 3–20. [Google Scholar] [CrossRef]
- Douglass, D.L.; Armijo, J.S. The effect of silicon and manganese on the oxidation mechanism of Ni-20 Cr. Oxid. Met. 1970, 2, 207–231. [Google Scholar] [CrossRef]
- Lowell, C.E. Cyclic and isothermal oxidation behavior on some Ni-Cr alloys. Oxid. Met. 1973, 2, 95–115. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, H.M. Hot corrosion behaviors of a Cr13Ni5Si2-based metal silicide alloy in Na2SO4 + 25wt.% K2SO4 and Na2SO4 + 25wt.% NaCl molten salts. Intermetallics 2010, 18, 324–329. [Google Scholar] [CrossRef]
- Fang, Y.L.; Wang, H.M. High-temperature sliding wear resistance of a ductile metal-toughened Cr13Ni5Si2 ternary metal silicide alloy. J. Alloys Compd. 2007, 433, 114–119. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wang, H.M. High-temperature sliding wear resistance of a Cr3Si/Cr13Ni5Si2 multiphase intermetallic alloy. Mater. Lett. 2003, 57, 2710–2715. [Google Scholar] [CrossRef]
- Galiullin, T.; Gobereit, B.; Naumenko, D.; Buck, R.; Amsbeck, L.; Neises-von Puttkamer, M.; Quadakkers, W.J. High temperature oxidation and erosion of candidate materials for particle receivers of concentrated solar power tower systems. Sol. Energy 2019, 188, 883–889. [Google Scholar] [CrossRef]
- PCC Energy Group. Inconcel Alloy 740H—A Superalloy Specifically Designed for Advanced Ultra Supercritical Power Generation. Available online: https://www.specialmetals.com/documents/technical-bulletins/inconel/inconel-alloy-740h.pdf (accessed on 3 November 2023).
- ISO 21608; Corrosion of Metals and Alloys—Test Method for Isothermal-Exposure Oxidation Testing under High-Temperature Corrosion Conditions for Metallic Materials 2012, ISO TC156 WG 13 (High Temperature Corrosion). International Organization for Standardization: Geneva, Switzerland, 2012.
- Xu, Z.; Jiang, L.; Dong, J.; Li, Z.; Zhou, X. The effect of silicon on precipitation and decomposition behaviors of M6C carbide in a Ni–Mo–Cr superalloy. J. Alloys Compd. 2015, 620, 197–203. [Google Scholar] [CrossRef]
- Jena, A.K.; Chaturvedi, M.C. The role of alloying elements in the design of nickel-base superalloys. J. Mater. Sci. 1984, 19, 3121–3139. [Google Scholar] [CrossRef]
- Oskay, C.; Meißner, T.M.; Dobler, C.; Grégoire, B.; Galetz, M.C. Scale formation and degradation of diffusion coatings deposited on 9% Cr steel in molten solar salt. Coatings 2019, 10, 687. [Google Scholar] [CrossRef]
- Fähsing, D.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion testing of diffusion-coated steel in molten salt for concentrated solar power tower systems. Surf. Coat. Technol. 2018, 354, 46–55. [Google Scholar] [CrossRef]
- Essuman, E.; Meier, G.H.; Zurek, J.; Hänsel, M.; Singheiser, L.; Norby, T.; Quadakkers, W.J. Effect of oxygen partial pressure on the oxidation behaviour of an yttria dispersion strengthened NiCr-base alloy. J. Mater. Sci. 2008, 43, 5591–5598. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Shemet, P.-A.J.V.; Singheiser, L. Metallic interconnectors for solid oxide fuel cells–a review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 315–356. [Google Scholar]
- Cruchley, S.; Evans, H.; Taylor, M. An overview of the oxidation of Ni-based superalloys for turbine disc applications: Surface condition, applied load and mechanical performance. Mater. High Temp. 2016, 33, 465–475. [Google Scholar] [CrossRef]
- Jalowicka, A.; Nowak, W.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Effect of nickel base superalloy composition on oxidation resistance in SO2 containing high pO2 environments. Mater. Corros. 2014, 65, 178–187. [Google Scholar] [CrossRef]
- Maier, H.J.; Niendorf, T.; Bürgel, R. Handbuch Hochtemperatur-Werkstofftechnik, 5th ed.; Springer: Wiesbaden, Germany, 2015; pp. 245–273. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1000 °C and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782–1790. [Google Scholar] [CrossRef]
- Wagner, C. Formation of composite scales consisting of oxides of different metals. J. Electrochem. Soc. 1956, 103, 627. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der Oxydation von Legierungen. Z. Für Elektrochem. Berichte Der Bunsenges. Für Phys. Chem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Geers, C.; Babic, V.; Mortazavi, N.; Halvarsson, M.; Jönsson, B.; Johansson, L.G.; Svensson, J.E. Properties of alumina/chromia scales in N2-containing low oxygen activity environment investigated by experiment and theory. Oxid. Met. 2017, 87, 321–332. [Google Scholar] [CrossRef]
- Jönsson, B.; Svedberg, C. Limiting Factors for Fe-Cr-Al and NiCr in Controlled Industrial Atrmospheres. Mater. Sci. Forum 1997, 251, 551–558. [Google Scholar] [CrossRef]
- Cruchley, S.; Evans, H.E.; Taylor, M.P.; Hardy, M.C.; Stekovic, S. Chromia layer growth on a Ni-based superalloy: Sub-parabolic kinetics and the role of titanium. Corros. Sci. 2013, 75, 58–66. [Google Scholar] [CrossRef]
- Qin, X.Z.; Guo, J.T.; Yuan, C.; Yang, G.X.; Zhou, L.Z.; Ye, H.Q. µ-Phase behavior in a cast Ni-base superalloy. J. Mater. Sci. 2009, 44, 4840–4847. [Google Scholar] [CrossRef]
- Gore, P.; Gosain, O.P.; Singh, M.P.; Basu, B.; Chattopadhyay, K. Towards Understanding the Oxide Evolution in Inconel 740H and Haynes 282 in Ambient Pressure Steam Oxidation. Oxid. Met. 2022, 97, 509–525. [Google Scholar] [CrossRef]





| Alloying Elements | Rene 80 | Inconel 740H |
|---|---|---|
| Cr | 14 | 24.5 |
| Co | 9.5 | 20 |
| Al | 3 | 1.35 |
| Ti | 5 | 1.35 |
| Nb | - | 1.5 |
| Mo | 4 | 0.1 |
| Si | - | 0.15 |
| W | 4 | - |
| C | 0.17 | 0.03 |
| Hf | 0.03 | - |
| B | 0.02 | - |
| Zr | 0.03 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerbstadt, M.; White, E.M.H.; Galetz, M.C. Novel Cr/Si-Slurry Diffusion Coatings for High Temperatures. Materials 2023, 16, 7480. https://doi.org/10.3390/ma16237480
Kerbstadt M, White EMH, Galetz MC. Novel Cr/Si-Slurry Diffusion Coatings for High Temperatures. Materials. 2023; 16(23):7480. https://doi.org/10.3390/ma16237480
Chicago/Turabian StyleKerbstadt, Michael, Emma Marie Hamilton White, and Mathias Christian Galetz. 2023. "Novel Cr/Si-Slurry Diffusion Coatings for High Temperatures" Materials 16, no. 23: 7480. https://doi.org/10.3390/ma16237480
APA StyleKerbstadt, M., White, E. M. H., & Galetz, M. C. (2023). Novel Cr/Si-Slurry Diffusion Coatings for High Temperatures. Materials, 16(23), 7480. https://doi.org/10.3390/ma16237480






