The Biocompatibility and Self-Healing Effect of a Biopolymer’s Coating on Zn Alloy for Biomedical Applications
Abstract
:1. Introduction
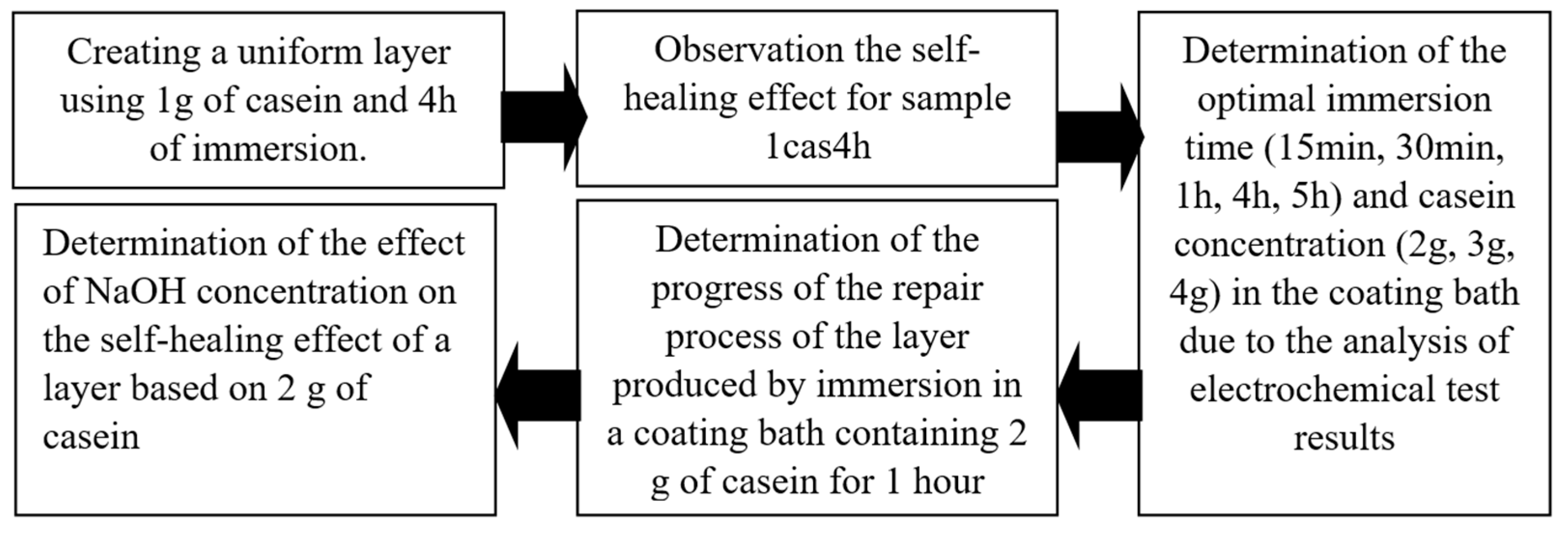
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesz, S.; Hrapkowicz, B.; Gołombek, K.; Karolus, M.; Janiak, P. Synthesis of Mg-based alloys with rare-earth element addition by means of mechanical alloying. Bull. Pol. Ac. Tech. 2021, 69, 1–7. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, D.; Li, X.; Jiang, S. Current status, opportunities and challenges in chemical conversion coatings for zinc. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 221–236. [Google Scholar] [CrossRef]
- Katarivas, G.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Gawecki, J. Proteins in Food and Nutrition; Poznan University of Life Sciences: Poznan, Poland, 2016. [Google Scholar]
- Sikorski, Z.E. Food Chemistry; Scientific and Technical Publishing House: Warsaw, Poland, 2012. [Google Scholar]
- Qin, L.; Dong, H.; Mu, Z.; Zhang, Y.; Dong, G. Preparation and bioactive properties of chitosan and casein phosphopeptides composite coatings for orthopedic implants. Carbohydr. Polym. 2015, 133, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, B.; Kumara, T.; Muthukumara, R.; Deepachitra, R.; Charumathy, T. In-vitro evaluation of biphasic calcium phosphate/casein incorporated with Myristica fragrans for bone tissue engineering. Ceram. Int. 2015, 41, 1725–1734. [Google Scholar] [CrossRef]
- Siek, D.; Czechowska, J.; Mróz, W.; Zima, A.; Burdyńska, S.; Załęczny, R.; Ślósarczyk, A. Bioactivity of cement type bone substitutes. Bull. Pol. Ac. Tech. 2013, 61, 433–439. [Google Scholar] [CrossRef]
- Available online: https://www.marku.com.pl/foldery/nanobone.pdf (accessed on 1 September 2022).
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn–Mg binary alloys. J. Mater. Res. Technol. 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoesu, N.; Benchea, M.; Roman, A.M.; Manole, V.; Alexandru, A.; Cimpoesu, R.; Cazacu, M.M.; Wnuk, I.; Zegan, G. In vitro tests on new biodegradable metallic material based on ZnMgY. Arch. Metall. Mater. 2022, 67, 587–594. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.syntellix.asia/products/technology-and-advantages.html (accessed on 29 November 2023).
- Katta, P.; Nalliyan, R. Corrosion resistance with self-healing behavior and biocompatibility of Ce incorporated niobium oxide coated 316L SS for orthopedic applications. Surf. Coat. Technol. 2019, 375, 715–726. [Google Scholar] [CrossRef]
- Safoora, F.; Kharaziha, M.; Atapour, M. A self-healing and bioactive coating based on duplex plasmaelectrolytic oxidation/polydopamine on AZ91 alloy for bone implants. J. Magnes. Alloy. 2023, 11, 592–606. [Google Scholar] [CrossRef]
- Kalaiyarasan, M.; Pugalmani, S.; Rajendran, N. Fabrication of chitosan/silica hybrid coating on AZ31 Mg alloy for orthopaedic applications. J. Magnes. Alloy. 2023, 11, 614–628. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, Z.; Zhang, F.; Li, S.; Cui, H.; Han, E. Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. J. Mater. Chem. 2014, 32, 1010. [Google Scholar] [CrossRef]
- Junaid Anjum, M.; Zhao, J.; Ali, H.; Tabish, M.; Murtaza, H.; Yasin, G.; Uzair Malik, M.; Khan, W.Q. A review of self–healing coatings applied to Mg Alloys and their electrochemical evaluation techniques. Int. J. Electrochem. 2020, 15, 3040–3053. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Roy, I. Doxorubicin-loaded casein nanoparticles for drug delivery: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2019, 121, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.; Mohan-Raj, R.; Vasanthakumara, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. COCIS 2010, 15, 73–83. [Google Scholar] [CrossRef]










| Coating Bath Chemical Composition | Immersion Time | Designation of Sample | |||
|---|---|---|---|---|---|
| Casein (g) | NaOH (g) | nanoHA | Rest | ||
| 1 g | 6 g | 0.1 g | distilled water | 4 h | 1cas4h |
| 2 g | 15 min | 2cas15 | |||
| 30 min | 2cas30 | ||||
| 1 h | 2cas1h | ||||
| 5 h | 2cas5h | ||||
| 3 g | 1 h | 3cas1h | |||
| 4 g | 1 h | 4cas1h | |||
| 2 g | 2 g | 1 h | 2naoh2cas | ||
| 2 g | 4 g | 1 h | 4naoh2cas | ||
| Sample (2 g Casein in Coating Bath) | Ecorr (V) | jcorr (µA/cm2) | Rp (kΩ) | Vcorr (mm/year) |
| 15 min | −1.02 | 9 | 2.5 | 0.22 |
| 30 min | −1.01 | 18 | 0.8 | 0.43 |
| 1 h | −0.98 | 6 | 2.5 | 0.15 |
| 5 h | −1.26 | 32 | 1.08 | 0.75 |
| ZnMg3.2 | −1.03 | 40 | 2.2 | 0.93 |
| Sample (1 h of Immersion in Coating Bath) | Ecorr (V) | jcorr (µA/cm2) | Rp (kΩ) | Vcorr(mm/year) |
| 2 g casein | −0.98 | 6 | 2.5 | 0.15 |
| 3 g casein | −1.01 | 62 | 0.3 | 1.46 |
| 4 g casein | −1.02 | 8 | 3.2 | 0.20 |
| ZnMg3.2 | −1.03 | 40 | 2.2 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesarz-Andraczke, K.; Tuncay, B.; Pakieła, W.; Brytan, Z.; Skonieczna, M.; Bidulská, J.; Bidulsky, R. The Biocompatibility and Self-Healing Effect of a Biopolymer’s Coating on Zn Alloy for Biomedical Applications. Materials 2023, 16, 7486. https://doi.org/10.3390/ma16237486
Cesarz-Andraczke K, Tuncay B, Pakieła W, Brytan Z, Skonieczna M, Bidulská J, Bidulsky R. The Biocompatibility and Self-Healing Effect of a Biopolymer’s Coating on Zn Alloy for Biomedical Applications. Materials. 2023; 16(23):7486. https://doi.org/10.3390/ma16237486
Chicago/Turabian StyleCesarz-Andraczke, Katarzyna, Badegül Tuncay, Wojciech Pakieła, Zbigniew Brytan, Magdalena Skonieczna, Jana Bidulská, and Robert Bidulsky. 2023. "The Biocompatibility and Self-Healing Effect of a Biopolymer’s Coating on Zn Alloy for Biomedical Applications" Materials 16, no. 23: 7486. https://doi.org/10.3390/ma16237486
APA StyleCesarz-Andraczke, K., Tuncay, B., Pakieła, W., Brytan, Z., Skonieczna, M., Bidulská, J., & Bidulsky, R. (2023). The Biocompatibility and Self-Healing Effect of a Biopolymer’s Coating on Zn Alloy for Biomedical Applications. Materials, 16(23), 7486. https://doi.org/10.3390/ma16237486










