Effect of Post-Washing on Textural Characteristics of Carbon Materials Derived from Pineapple Peel Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermochemical Property Determination of Pineapple Peel
2.3. Experimental Methods
2.4. Characterization Analysis of Resulting Carbon Materials
3. Results and Discussion
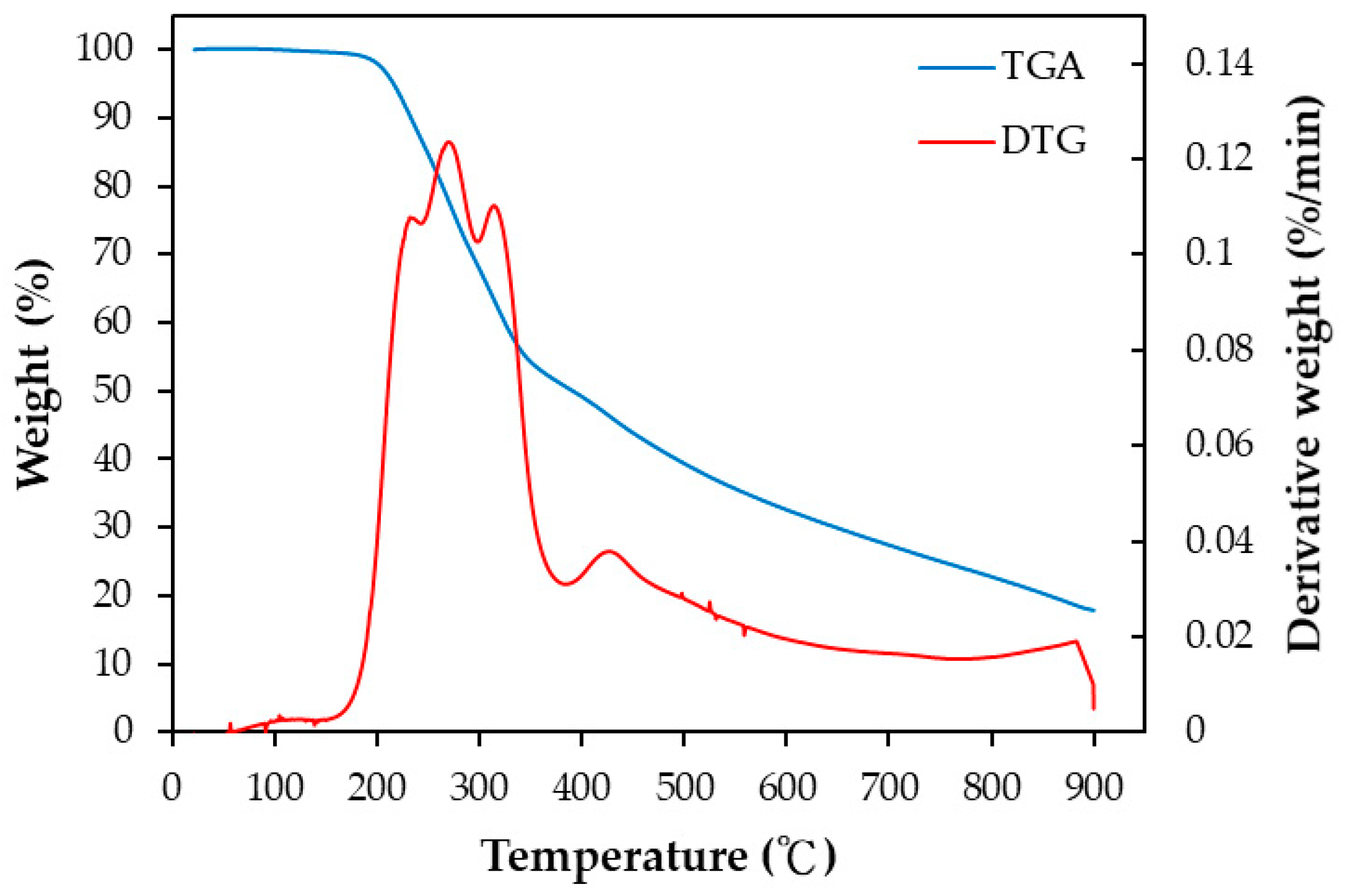
3.1. Thermochemical Characteristics of Pineapple Peel (PP)
3.2. Pore Analysis of Resulting Carbon Materials
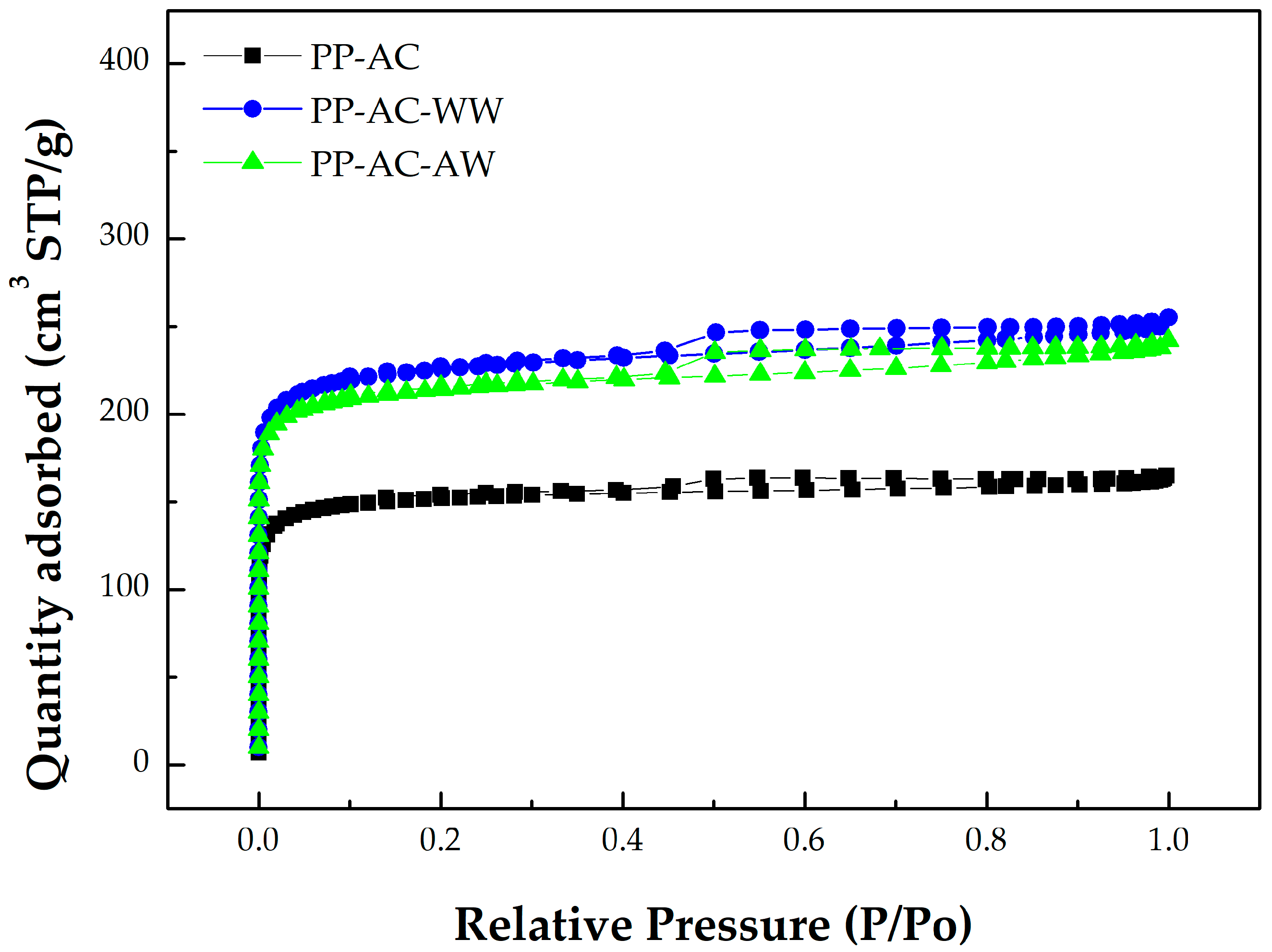
- Based on the classification by the International Union of Pure and Applied Chemistry (IUPAC), these carbon materials are typical of microporous solids, where micropore filling occurs significantly at very low relative pressure (P/P0). In this regard, they feature the Type I isotherms [42,43]. However, the adsorption–desorption isotherms also possess a hysteresis loop from the relative pressure of about 0.45, which should be associated with mesoporous solids. This isotherm shape belongs to the Type IV isotherms, where the capillary condensation occurs [42,43].
- As compared to PP-based activated carbons produced by chemical activation [22,23,25,26,27,28,29], it was clearly shown that the resulting activated carbons produced by physical activation in this work had slightly lower pore properties (e.g., BET surface area), as shown in Table 2. For example, the BET surface area of PP-based activated carbon produced by KOH activation was 1160 m2/g [29], which was higher than the optimal value (843 m2/g) in Table 2.
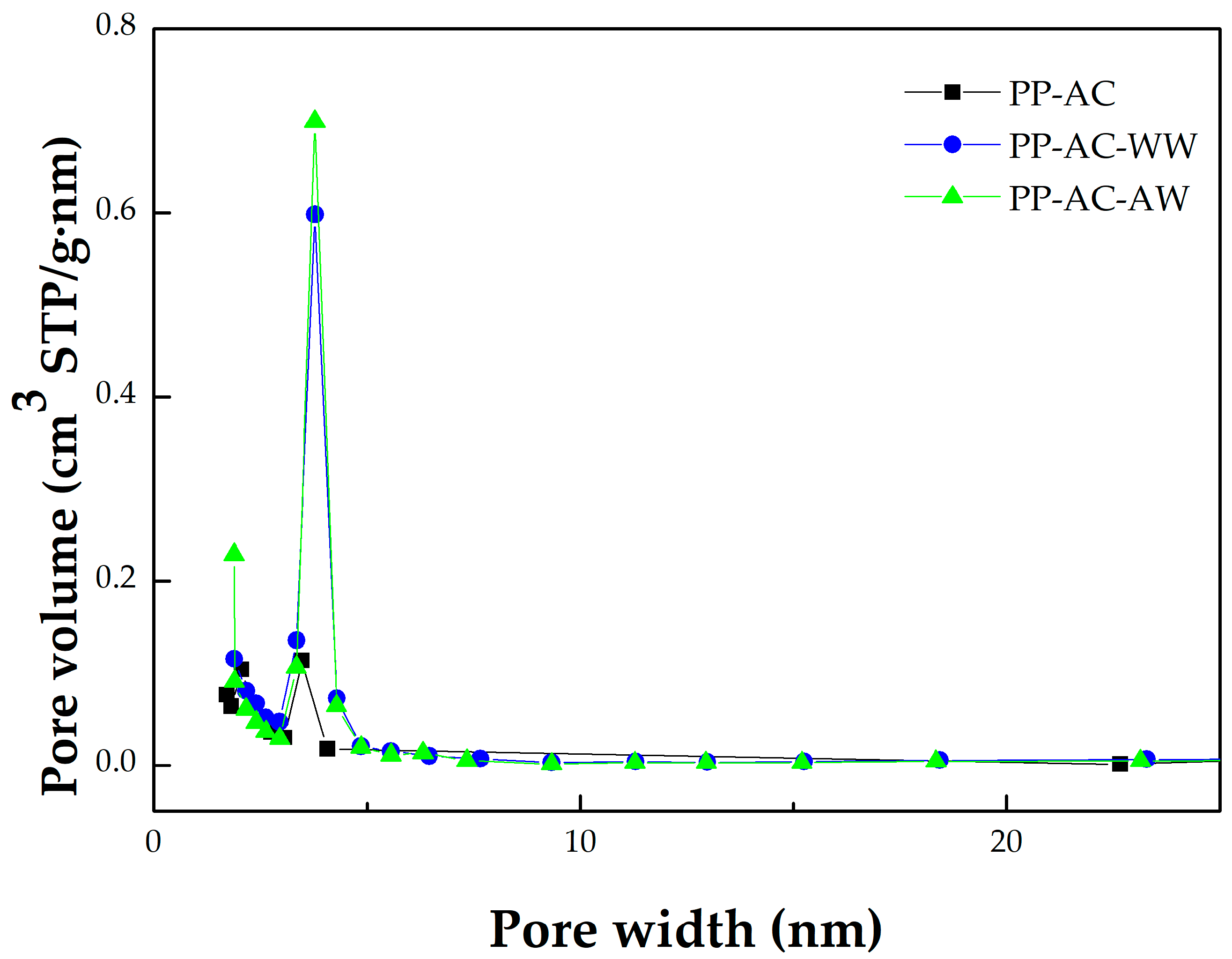
- Using the Harrett–Joyner–Halenda (BJH) method and the data on desorption isotherms for the textural characteristics of the mesoporous solids, it was found that the significant peaks of mesopore size distribution occurred at about 3.5 nm. In addition, these peaks were more obvious for the resulting activated carbon materials (i.e., PP-AC-WW and PP-AC-AW) by post-washing in comparison with the crude product (i.e., PP-AC), thus leading to higher pore properties, as listed in Table 1.
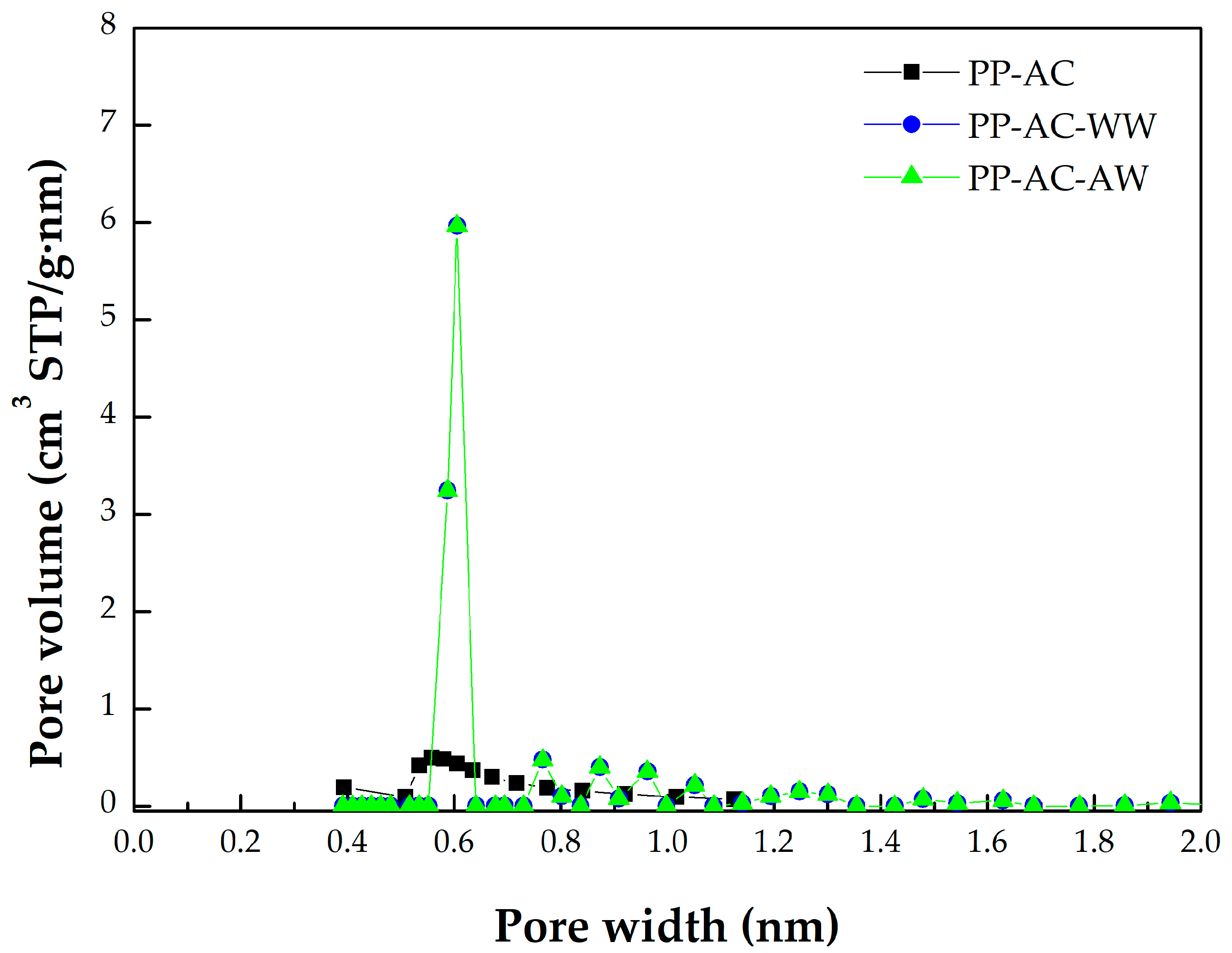
- As mentioned above, the micropore size distributions of the resulting activated carbon materials can be verified in Figure 3, which was obtained by the 2D-NLDFT-HS model. The micropore peak was observed at about 0.6 nm, where it was in the range of less than 2.0 nm. Assuming the cylindrical geometry for all pores, the data on the average pore diameter (or width) were slightly smaller than 2.0 nm (1.6–1.9 nm). Therefore, other significant peaks in the pore size distribution curves were observed at the left side (less than 2.0 nm), indicating micropores are present in all activated carbon products.
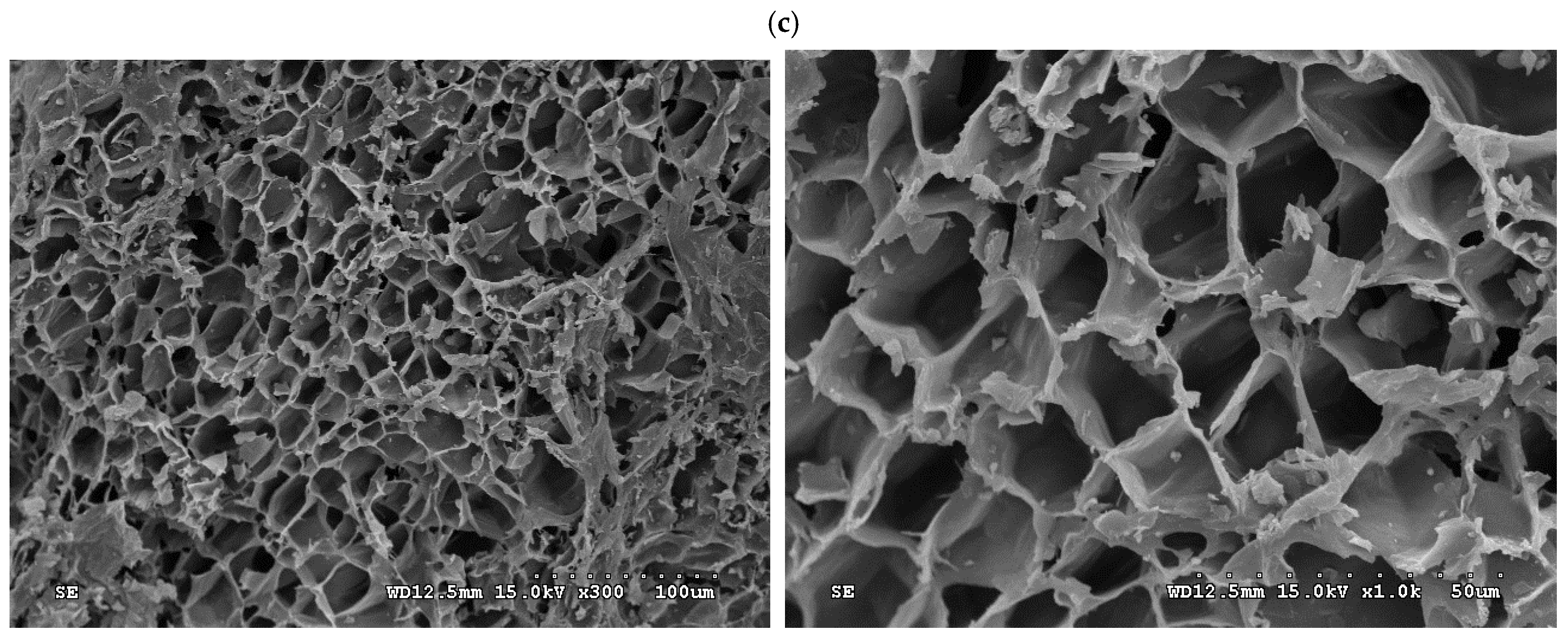
- In order to see the porous textures on the surface of the resulting carbon materials, Figure 5 shows the scanning electron microscopy (SEM) images (i.e., ×300 and ×1000) for the resulting activated carbon samples (i.e., PP-AC, PP-AC-WW and PP-AC-AW). Obviously, the resulting activated carbon products displayed a porous texture on the rigid surface without significant difference. However, post-washing removed the residual impurities and/or particles, thus producing a cleaner surface and greater pore properties, as listed in Table 1.
3.3. Chemical Characteristics of Resulting Carbon Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upadhyay, A.; Lama, J.P.; Tawata, S. Utilization of pineapple waste: A review. J. Food Sci. Technol. Nepal 2013, 6, 10–18. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Aili Hamzah, A.F.; Hamzah, M.H.; Che Man, H.; Jamali, N.S.; Siajam, S.I.; Ismail, M.H. Recent updates on the conversion of pineapple waste (Ananas comosus) to value-added products, future perspectives and challenges. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- Hikal, W.M.; Mahmoud, A.A.; Ahl, H.A.H.S.-A.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), waste streams, characterisation and valorisation: An overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Tywabi-Ngeva, Z. Application of pineapple waste to the removal of toxic contaminants: A review. Toxics 2022, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Nguyen, D.T.C.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Jalil, A.A.; Nabgan, W.; Lee, T. A critical review on pineapple (Ananas comosus) wastes for water treatment, challenges and future prospects towards circular economy. Sci. Total Environ. 2023, 856 Pt 1, 158817. [Google Scholar] [CrossRef] [PubMed]
- Mubarik, S.; Qureshi, N.; Sattar, Z.; Shaheen, A.; Kalsoom, A.; Imran, M.; Hanif, F. Synthetic approach to rice waste-derived carbon-based nanomaterials and their applications. Nanomanufacturing 2021, 1, 109–159. [Google Scholar] [CrossRef]
- Krishni, R.R.; Foo, K.Y.; Hameed, B.H. Food cannery effluent, pineapple peel as an effective low-cost biosorbent for removing cationic dye from aqueous solutions. Desalin. Water Treat. 2014, 52, 6093–6103. [Google Scholar] [CrossRef]
- Ahmad, A.; Khatoon, A.; Mohd-Setapar, S.H.; Kumar, R.; Rafatulah, M. Chemically oxidized pineapple fruit peel for the biosorption of heavy metals from aqueous solutions. Desalin. Water Treat. 2016, 57, 6432–6442. [Google Scholar] [CrossRef]
- Koc, S.N.T.; Kipcak, A.S.; Derun, E.M.; Tugrul, N. Removal of zinc from wastewater using orange, pineapple and pomegranate peels. Int. J. Environ. Sci. Technol. 2021, 18, 2781–2792. [Google Scholar]
- Gómez-Aguilar, D.L.; Rodríguez-Miranda, J.P.; Salcedo-Parra, O.J. Fruit peels as a sustainable waste for the biosorption of heavy metals in wastewater: A review. Molecules 2022, 27, 2124. [Google Scholar] [CrossRef]
- Yılmaz, O.; Tugrul, N. Zinc adsorption from aqueous solution using lemon, orange, watermelon, melon, pineapple, and banana rinds. Water Pract. Technol. 2022, 17, 318–328. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Fu, B.; Ge, C.; Yue, L.; Luo, J.; Deng, H.; Yu, H. Characterization of biochar derived from pineapple peel waste and its application for sorption of oxytetracycline from aqueous solution. BioResources 2016, 11, 9017–9035. [Google Scholar] [CrossRef]
- Wang, C.; Gu, L.; Liu, X.; Zhang, X.; Cao, L.; Hu, X. Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int. Biodeterior. Biodegrad. 2016, 111, 78–84. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef] [PubMed]
- Mahmuda, K.N.; Wen, T.H.; Zakaria, Z.A. Activated carbon and biochar from pineapple waste biomass for the removal of methylene blue. Environ. Toxicol. Manag. 2021, 1, 30–36. [Google Scholar] [CrossRef]
- Otieno, A.O.; Home, P.G.; Raude, J.M.; Murunga, S.I.; Ngumba, E.; Ojwang, D.O.; Tuhkanen, T. Pineapple peel biochar and lateritic soil as adsorbents for recovery of ammonium nitrogen from human urine. J. Environ. Manag. 2021, 293, 112794. [Google Scholar] [CrossRef]
- Tsai, W.T.; Ayestas, R.; Tsai, C.H.; Lin, Y.Q. Preparation and characterization of porous materials from pineapple peel at elevated pyrolysis Temperatures. Materials 2022, 15, 4686. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Porous structure and adsorptive properties of pineapple peel based activated carbons prepared via microwave assisted KOH and K2CO3 activation. Microporous Mesoporous Mater. 2012, 148, 191–195. [Google Scholar] [CrossRef]
- Yacob, A.R.; Azmi, A.; Mustajab, M.K.A.A. Physical and chemical activation effect on activated carbon prepared from local pineapple waste. Appl. Mech. Mater. 2014, 699, 87–92. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Onia, B.A.; Abatana, O.G.; Busarib, A.; Odunlamia, O.; Nweke, C. Production and characterization of activated carbon from pineapple waste for treatment of kitchen wastewater. Desalin. Water Treat. 2020, 183, 413–424. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Singh, M.; Maheshwari, P.H.; Sundriyal, S.; Dhakate, S.R. Electrolytic study of pineapple peel derived porous carbon for all-solid-state supercapacitors. ChemistrySelect 2021, 6, 11736–11746. [Google Scholar] [CrossRef]
- Veeramalai, S.; Ramlee, N.N.; Mahdi, H.I.; Manas, N.H.A.; Ramli, A.N.M.; Illias, R.M.; Azelee, N.I.W. Development of organic porous material from pineapple waste as a support for enzyme and dye adsorption. Ind. Crops Prod. 2022, 181, 114823. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, M.A.; Noh, T.U.; Ahmad, N.A. Pineapple peel-derived carbon for adsorptive removal of dyes. Mater. Chem. Phys. 2023, 306, 128094. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, M.A.; Noh, T.U. Unleashing the potential of pineapple peel-based activated carbon: Response surface methodology optimization and regeneration for methylene blue and methyl red dyes adsorption. Inorg. Chem. Commun. 2023, 155, 111041. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Devi, N.S.; Hariram, M.; Vivekanandhan, S. Modification techniques to improve the capacitive performance of biocarbon materials. J. Energy Storage 2021, 33, 101870. [Google Scholar] [CrossRef]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on production and surface modifications of biochar materials via biomass pyrolysis process for supercapacitor applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Tsai, W.T.; Hsu, C.H.; Lin, Y.Q.; Tsai, C.H.; Chen, W.S.; Chang, Y.-T. Enhancing the pore properties and adsorption performance of cocoa pod husk (CPH)-derived biochars via post-acid treatment. Processes 2020, 8, 144. [Google Scholar] [CrossRef]
- Sierra, I.; Iriarte-Velasco, U.; Cepeda, E.A.; Gamero, M.; Aguayo, A.T. Preparation of carbon-based adsorbents from the pyrolysis of sewage sludge with CO2. Investigation of the acid washing procedure. Desalin. Water Treat. 2016, 57, 16053–16065. [Google Scholar] [CrossRef]
- Liang, Z.; Guo, S.; Dong, H.; Li, Z.; Liu, X.; Li, X.; Kang, H.; Zhang, L.; Yuan, L.; Zhao, L. Modification of activated carbon and-its application in selective hydrogenation of naphthalene. ACS Omega 2022, 7, 38550–38560. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S. Effects of washing pretreatment on properties and pyrolysis biochars of penicillin mycelial residues. Biomass Bioenergy 2022, 161, 106477. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Shen, Y.H. Production of mesoporous magnetic carbon materials from oily sludge by combining thermal activation and post-washing. Materials 2022, 15, 5794. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Lin, Y.Q. Preparation and characterization of porous carbon composites from oil-containing sludge by a pyrolysis-activation process. Processes 2022, 10, 834. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tsai, W.T. Optimization of physical activation process by CO2 for activated carbon preparation from Honduras Mahogany pod husk. Materials 2023, 16, 6558. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Suzuki, M. Adsorption Engineering; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Li, L.; Yao, X.; Li, H.; Liu, Z.; Ma, W.; Liang, X. Thermal stability of oxygen-containing functional groups on activated carbon surfaces in a thermal oxidative environment. J. Chem. Eng. Jpn. 2004, 47, 21–27. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Gharehkhani, S.; Afifi, A.B.M. Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Johnston, C.T. Biochar analysis by Fourier-Transform Infra-Red Spectroscopy. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 199–228. [Google Scholar]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]


| Pore Property | PP-BC | PP-BC-WW | PP-BC-AW | PP-AC | PP-AC-WW | PP-AC-AW |
|---|---|---|---|---|---|---|
| BET surface area a | 100.20 | 243.60 | 269.94 | 569.56 | 843.09 | 799.25 |
| t-plot micropore area b | 92.15 | 213.14 | 237.60 | 498.59 | 717.90 | 689.26 |
| External surface area | 8.05 | 30.46 | 31.34 | 70.97 | 125.19 | 109.99 |
| Total pore volume c | 0.042 | 0.110 e | 0.121 | 0.253 | 0.391 | 0.371 |
| t-plot micropore area b | 0.038 | 0.089 | 0.097 | 0.204 | 0.294 | 0.283 |
| Average pore width d | 1.661 | 1.806 | 1.802 | 1.778 | 1.854 | 1.856 |
| Elemental Content (wt%) | PP | PP-BC | PP-BC-WW | PP-BC-AW | PP-AC | PP-AC-WW | PP-AC-AW |
|---|---|---|---|---|---|---|---|
| Carbon (C) | 54.072 | 81.826 | 88.391 | 85.916 | 71.120 | 78.835 | 74.921 |
| Oxygen (O) | 40.773 | 14.451 | 10.186 | 12.402 | 20.466 | 15.819 | 14.406 |
| Sodium (Na) | 0.071 | 0.018 | 0.007 | 0.029 | 0.000 | 0.000 | 0.000 |
| Magnesium (Mg) | 0.229 | 0.493 | 0.075 | 0.309 | 0.775 | 1.132 | 0.207 |
| Aluminum (Al) | 4.470 | 0.123 | 0.067 | 0.150 | 0.006 | 0.111 | 2.555 |
| Silicon (Si) | 0.227 | 0.287 | 0.254 | 0.283 | 1.050 | 1.371 | 2.530 |
| Phosphorus (P) | 0.101 | 2.421 | 0.370 | 0.131 | 3.719 | 1.076 | 0.409 |
| Sulfur (S) | 0.058 | 0.345 | 0.544 | 0.460 | 1.738 | 0.632 | 2.913 |
| Calcium (Ca) | 0.000 | 0.035 | 0.106 | 0.319 | 1.126 | 1.024 | 2.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Tsai, W.-T.; Kuo, L.-A. Effect of Post-Washing on Textural Characteristics of Carbon Materials Derived from Pineapple Peel Biomass. Materials 2023, 16, 7529. https://doi.org/10.3390/ma16247529
Tsai C-H, Tsai W-T, Kuo L-A. Effect of Post-Washing on Textural Characteristics of Carbon Materials Derived from Pineapple Peel Biomass. Materials. 2023; 16(24):7529. https://doi.org/10.3390/ma16247529
Chicago/Turabian StyleTsai, Chi-Hung, Wen-Tien Tsai, and Li-An Kuo. 2023. "Effect of Post-Washing on Textural Characteristics of Carbon Materials Derived from Pineapple Peel Biomass" Materials 16, no. 24: 7529. https://doi.org/10.3390/ma16247529







