Synthesis of Manganese Oxide Sorbent for the Extraction of Lithium from Hydromineral Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Study of Conditions for Production of Lithium-Manganese Precursors
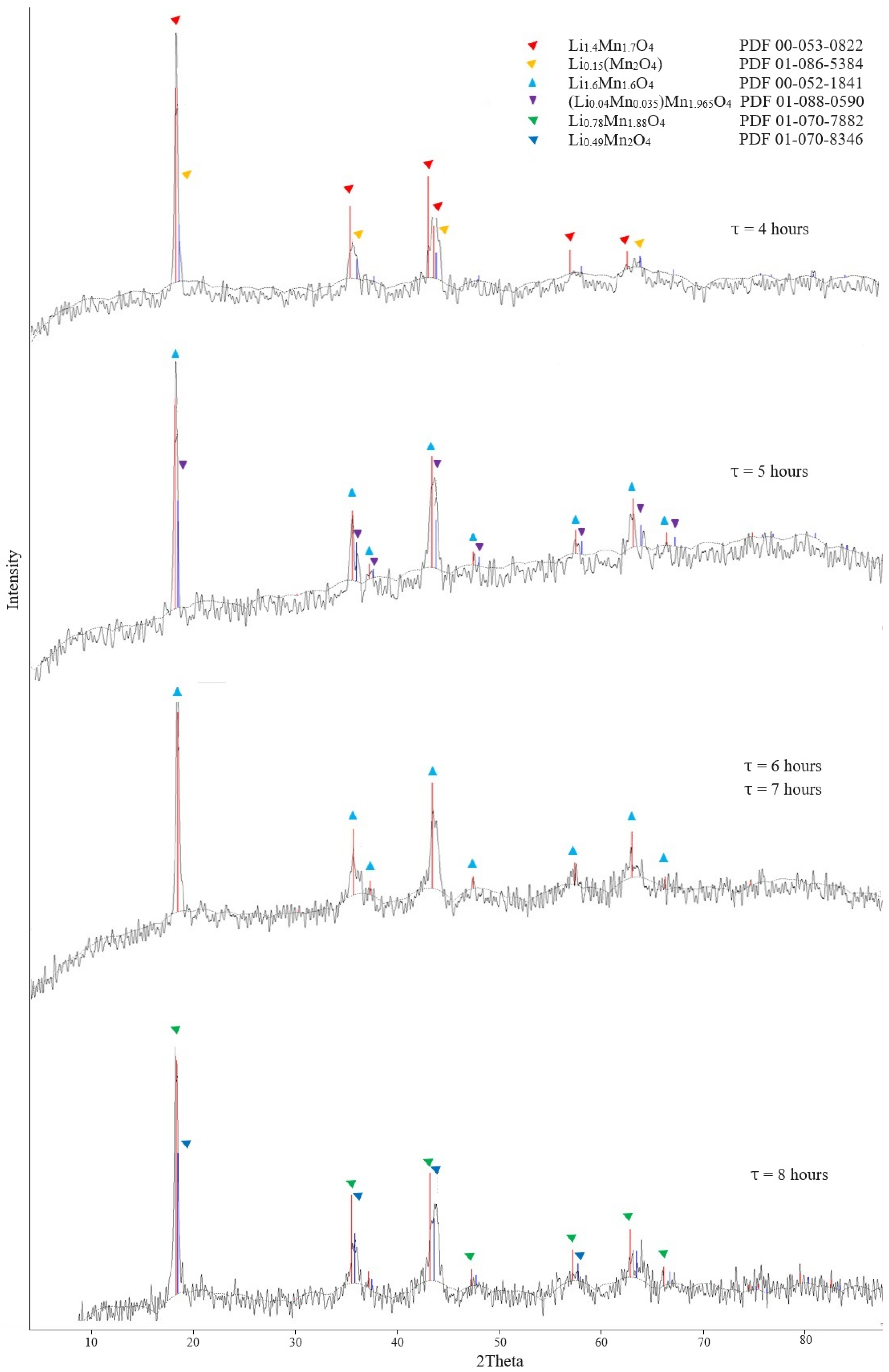
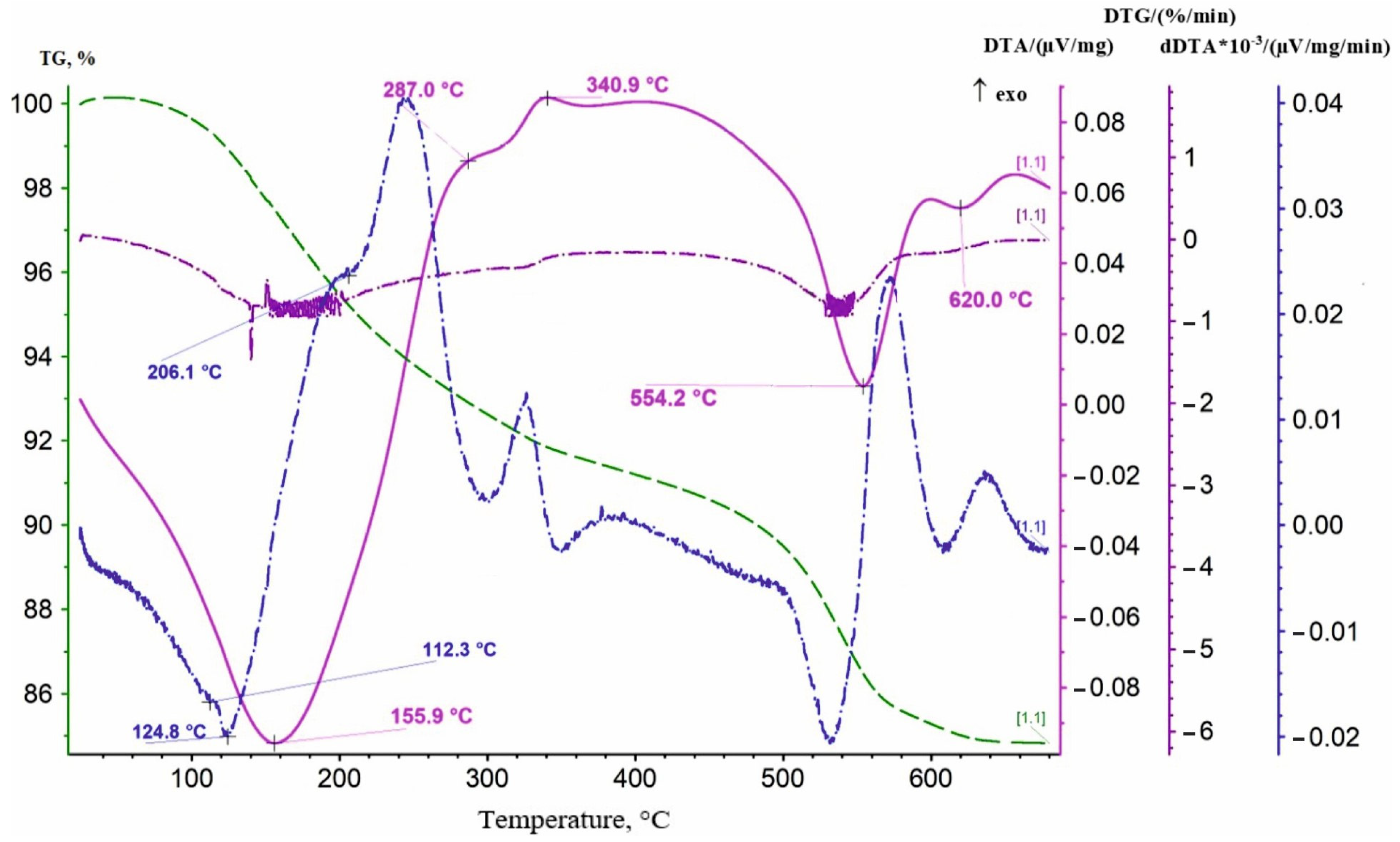
3.1.1. Preparation of Lithium-Manganese Oxides
3.1.2. Obtaining Precursors
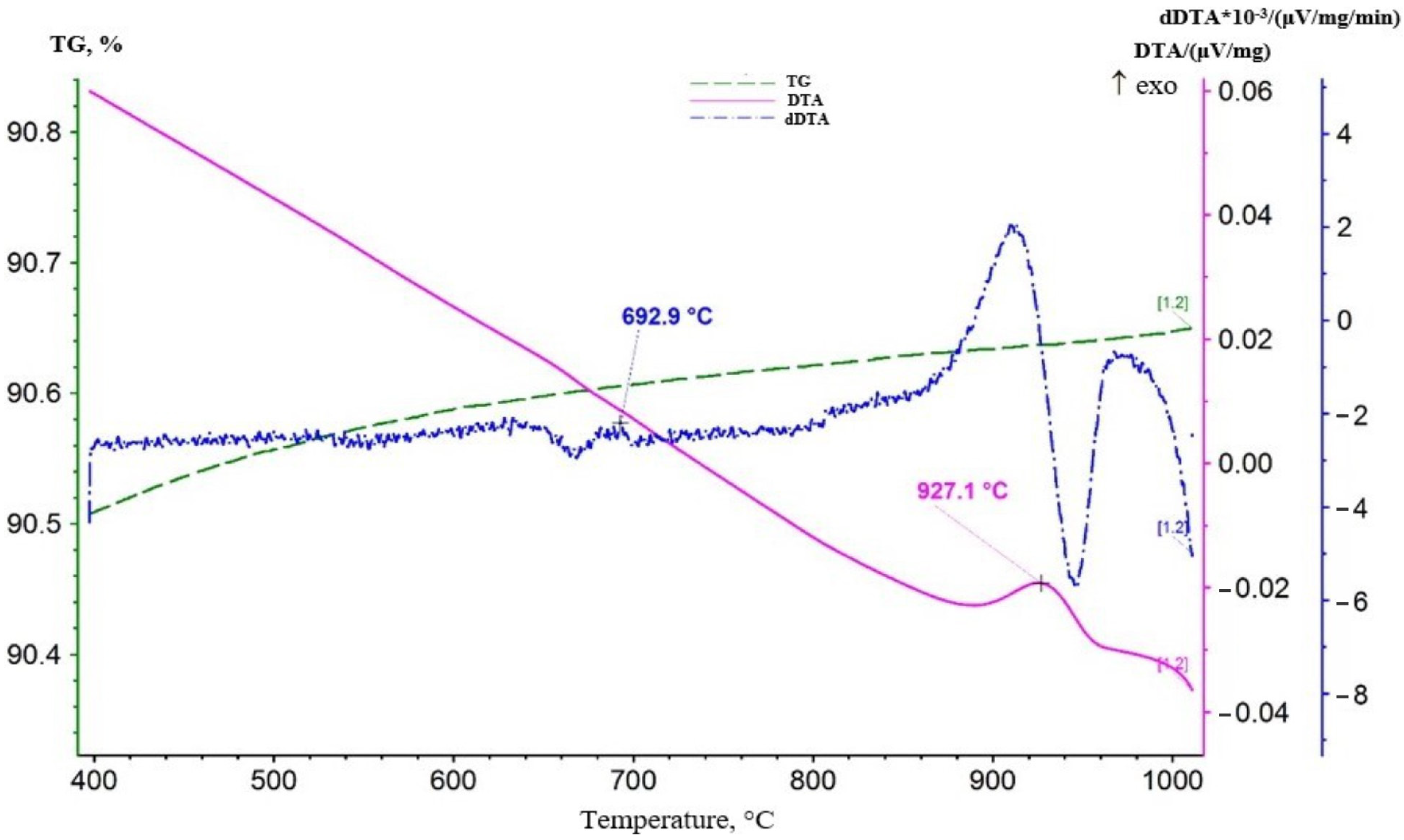
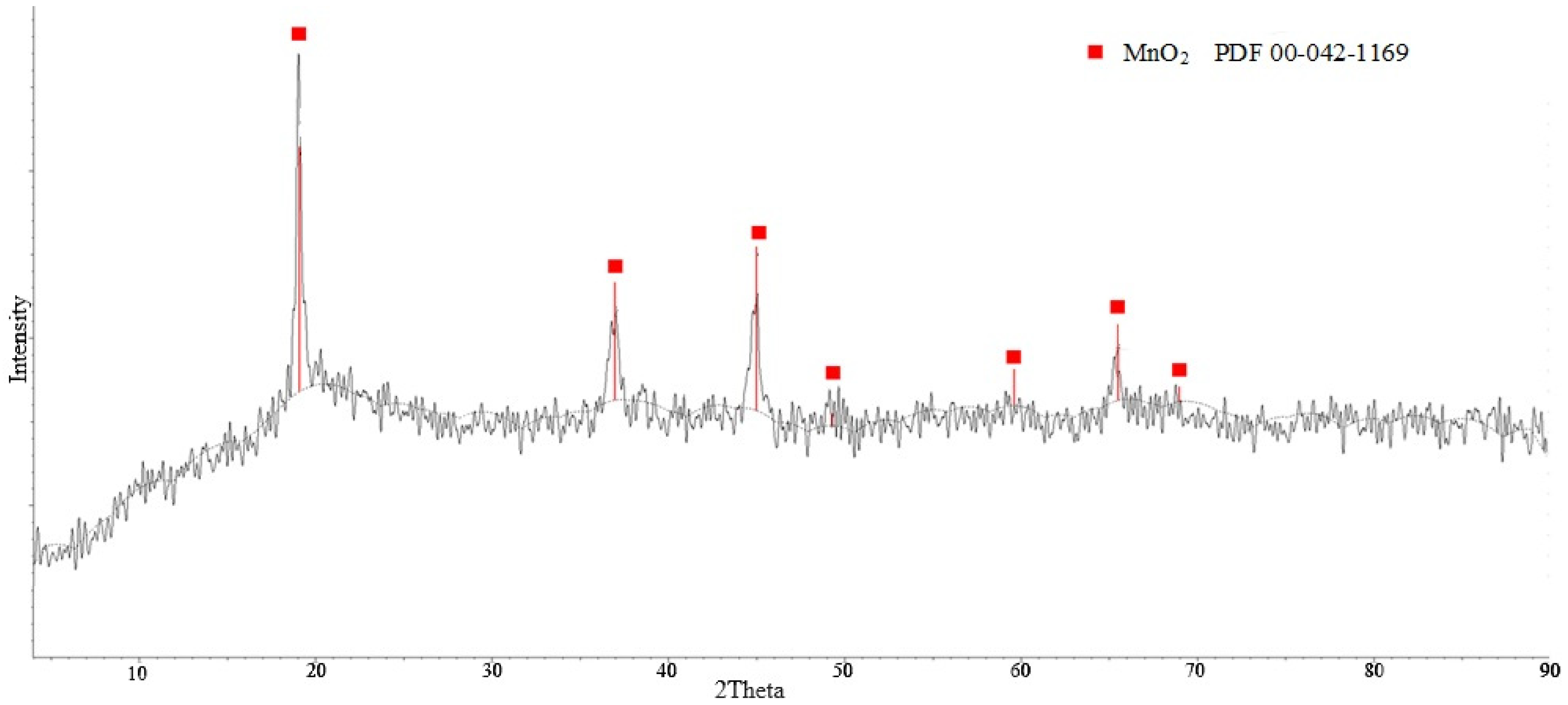
3.2. Study of Acid Treatment Conditions for Lithium-Manganese Precursors
Acid Treatment of Lithium-Manganese Precursors
3.3. Study of the Sorption Characteristics of the Obtained Sorbents
Study of the Process Conditions on the Lithium Sorption Recovery Characteristics
3.4. Determination of the Kinetic Model of the Lithium Sorption Process
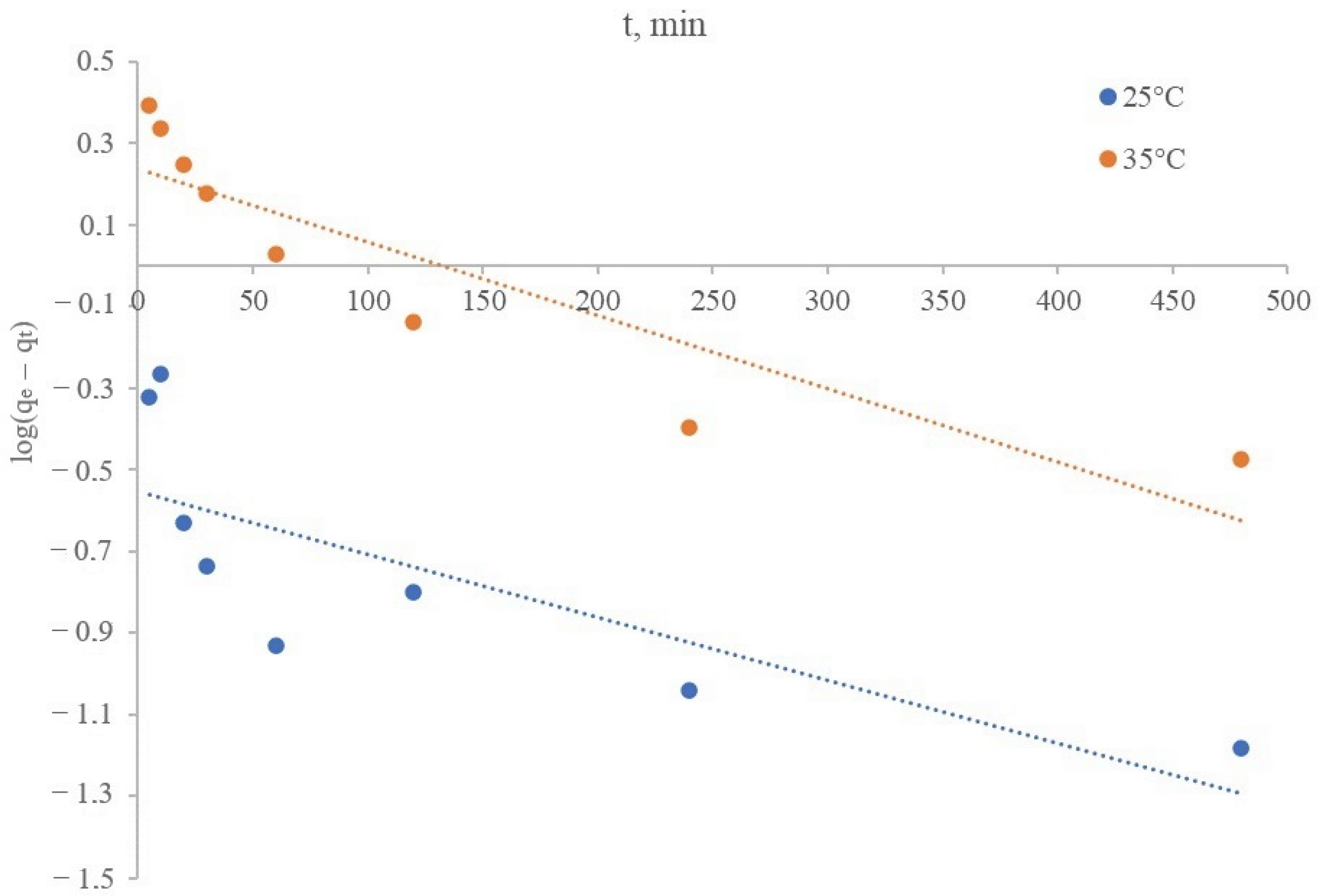
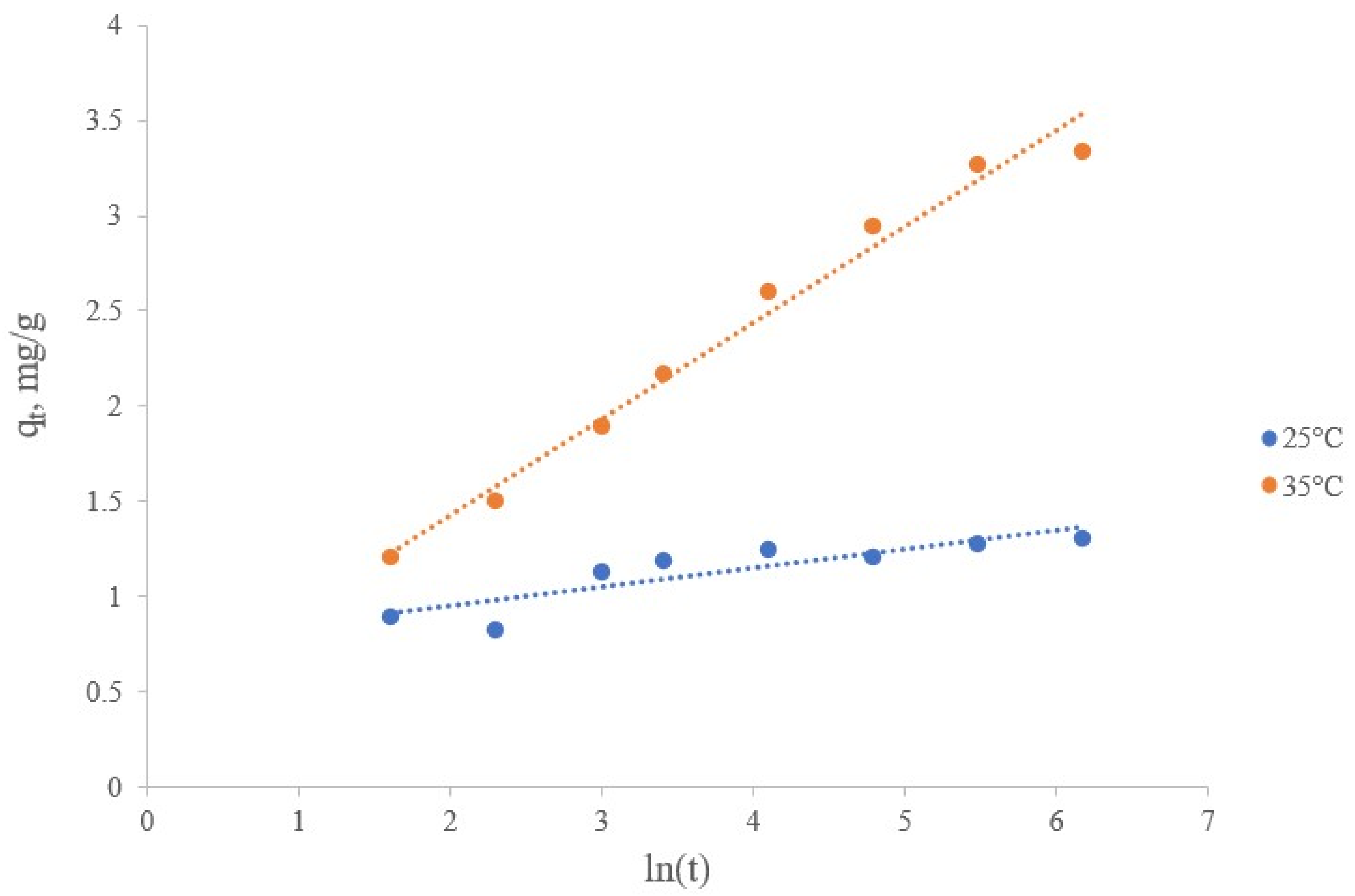
Study of the Lithium Sorption Kinetics on a Synthesized Manganese Oxide Inorganic Sorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choubey, P.K.; Chung, K.S.; Kim, M.S.; Lee, J.C.; Srivastava, R.R. Advance review on the exploitation of the prominent energy-storage element Lithium. Part II: From sea water and spent lithium ion batteries (LIBs). Miner. Eng. 2017, 110, 104–121. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.S.; Srivastava, R.R.; Lee, J.C.; Lee, J.Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Tedjar, F. Challenges for recycling advanced Li-ion batteries. In Proceedings of the International Battery Association (IBA2013), Barcelona, Spain, 11–15 March 2013; Available online: http://congresses.icmab.es/iba2013/images/Oral_26FEB.pdf (accessed on 11 March 2013).
- Samoilova, V.I.; Zelenin, V.I.; Saduakasova, A.T.; Kulenova, N.A. Uranium sorption from lake water by natural sorbents and products of their modification. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2016, 3, 91–96. [Google Scholar]
- Kenzhaliyev, B. Innovative technologies providing enhancement of non-ferrous, precious, rare and rare earth metals extraction. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2019, 310, 64–75. [Google Scholar] [CrossRef]
- Abdikerim, B.E.; Kenzhaliyev, B.K.; Surkova, T.Y.; Didik, N.; Berkinbayeva, A.N.; Dosymbayeva, Z.D.; Umirbekova, N.S. Uranium extraction with modified sorbents. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2020, 3, 84–90. [Google Scholar] [CrossRef]
- Guo, T.; Wang, S.; Ye, X.; Liu, H.; Gao, X.; Li, Q.; Guo, M.; Wu, Z. Competitive adsorption of Li, K, Rb, and Cs ions onto three ion-exchange resins. Desalination Water Treat. 2013, 51, 3954–3959. [Google Scholar] [CrossRef]
- Guijosa, A.N.; Casas, R.N.; Calahorro, C.V.; Gonzalez, J.D.L.; Rodríguez, A.G. Lithium adsorption by acid and sodium amberlite. J. Colloid Interface Sci. 2003, 264, 60–66. [Google Scholar] [CrossRef]
- Poluektov, N.S.; Meshkova, S.B.; Poluektova, E.N. Analytical Chemistry of Lithium; Nauka: Moscow, Russia, 1975; p. 204. [Google Scholar]
- Shi, X.; Zhou, D.; Zhang, Z.; Yu, L.; Xu, H.; Chen, B.; Yang, X. Synthesis and properties of Li1.6Mn1.6O4 and its adsorption application. Hydrometallurgy 2011, 110, 99–106. [Google Scholar] [CrossRef]
- Kan, S.M.; Berstenev, S.V. To the technology extraction of lithium from the formation waters of oil and gas fields of southern Mangyshlak. News Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2017, 5, 149–155. [Google Scholar]
- Shaoju, B.; Dongdong, L.; Dandan, G.; Jiaoyu, P.; Yaping, D.; Wu, L. Hydrometallurgical processing of lithium, potassium, and boron for the comprehensive utilization of Da Qaidam lake brine via natural evaporation and freezing. Hydrometallurgy 2017, 173, 80–83. [Google Scholar]
- Yoshinaga, T.; Kawano, K.; Imoto, H. Basic study on lithium recovery from lithium containing solution. Bull. Chem. Soc. Jpn. 1986, 59, 1207–1213. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent Advances in Magnesium/Lithium Separation and Lithium Extraction Technologies from Salt Lake Brine. Sep. Purif. Technol. 2020, 256, 117807. [Google Scholar] [CrossRef]
- Amer, A.M. The hydrometallurgical extraction of lithium from Egyptian montmorillonite-type clay. J. Met. 2008, 60, 55–57. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 2012, 117–118, 64–70. [Google Scholar] [CrossRef]
- Ho, P.C.; Nelson, F.; Kraus, K.A. Adsorption on inorganic materials: VII. Hydrous tin oxide and SnO2 filled carbon. J. Chromatogr. A 1978, 147, 263–269. [Google Scholar] [CrossRef]
- Bukowsky, H.; Uhlemann, E.; Steinborn, D. The recovery of pure lithium chloride from “brines” containing higher contents of calcium chloride and magnesium chloride. Hydrometallurgy 1991, 27, 317–325. [Google Scholar] [CrossRef]
- Kitajo, A.; Suzuki, T.; Nishihama, S.; Yoshizuka, K. Selective recovery of lithium from seawater using a novel MnO2 type adsorbent II—Enhancement of lithium ion selectivity of the adsorbent. Ars Separatoria Acta 2003, 2, 97–106. [Google Scholar]
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of Li+ insertion in spinel-type manganese oxide. Redox and ion-exchange reactions. Langmuir 1991, 7, 1167–1171. [Google Scholar] [CrossRef]
- Umeno, A.; Miyai, Y.; Takagi, N.; Chitrakar, R.; Sakane, K.; Ooi, K. Preparation and adsorptive properties of membrane-type adsorbents for lithium recovery from seawater. Ind. Eng. Chem. Res. 2002, 41, 4281–4287. [Google Scholar] [CrossRef]
- Yoshizuka, K.; Fukui, K.; Inoue, K. Selective recovery of lithium from seawater using a novel MnO2 type adsorbent. Ars Separatoria Acta 2002, 1, 79–86. [Google Scholar]
- Menzheres, L.T.; Ryabcev, A.D.; Mamylova, E.V.; Kocupalo, N.P. Method of Producing a Sorbent for the Extraction of Lithium from Brines. Patent RF 2223142, IPC B01J 20/02, C01D 15/00, 10 February 2004. [Google Scholar]
- Menzherez, L.T.; Kotsupalo, N.P. Granular sorbents based on LiCl·2Al(OH)3·mH2O. Appl. Chem. J. 1999, 10, 1623–1627. [Google Scholar]
- Ryabtsev, A.D.; Menzheres, L.T.; Kotsupalo, N.P.; Serikova, L.A. Obtainment of a granular sorbent based on LiCl·2Al(OH)3·mH2O by the non-waste method. Chem. Sustain. Dev. 1999, 7, 343–349. [Google Scholar]
- Menzheres, L.T.; Kocupalo, N.P.; Orlova, L.B. Method of Obtaining Granular Sorbent. Patent RF 2050184, IPC B01J 20/00, 20/30, 20 December 1995. [Google Scholar]
- Kotsupalo, N.P.; Ryabcev, A.D. Chemistry and Technology for Producing Lithium Compounds from Lithium-Bearing Hydromineral Raw Materials; Geo: Novosibirsk, Russia, 2008; p. 291. [Google Scholar]
- Zhang, L.; Zhou, D.; Yao, Q.; Zhou, J. Preparation of H2TiO3-lithium adsorbent by the sol–gel process and its adsorption performance. Appl. Surf. Sci. 2016, 368, 82–87. [Google Scholar] [CrossRef]
- Moazeni, M.; Hajipour, H.; Askari, M.; Nusheh, M. Hydrothermal synthesis and characterization of titanium dioxide nanotubes as novel lithium adsorbents. Mater. Res. Bull. 2014, 61, 70–75. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, D.; He, G.; Wang, F.; Zhou, J. Effect of crystal phases of titanium dioxide on adsorption performance of H2TiO3-lithium adsorbent. Mater. Lett. 2014, 135, 206–209. [Google Scholar] [CrossRef]
- Nazri, G.A.; Pistoia, G. Lithium Batteries-Science and Technology; Kluwer Academic Publishers: Boston, MA, USA, 2003; p. 708. [Google Scholar]
- Chitrakar, R.; Sakane, K.; Umeno, A.; Kasaishi, S.; Takagi, N.; Ooi, K. Synthesis of orthorhombic LiMnO2 by solid-phase reaction under steam atmosphere and a study of its heat and acid-treated phases. J. Solid State Chem. 2002, 169, 66–74. [Google Scholar] [CrossRef]
- Chitrakar, R.; Kahon, H.; Miyai, Y.; Ooi, K. Recovery of Lithium from Seawater Using Manganese Oxide Adsorbent (H1.6Mn1.6O4) Derived from Li1.6Mn1.6O4. Ind. Eng. Chem. Res. 2001, 40, 2054–2058. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Magnesium-doped manganese oxide with lithium ion-sieve property: Lithium adsorption from salt lake brine. Bull. Chem. Soc. Jpn. 2013, 86, 850–855. [Google Scholar] [CrossRef]
- Sorour, M.H.; Hani, H.A.; El-Sayed, M.M.H.; Mostafa, A.A.; Shaalan, H.F. Synthesis, Characterization and Performance Evaluation of Lithium Manganese Oxide Spinels for Lithium Adsorption. Egypt. J. Chem. 2017, 60, 697–710. [Google Scholar]
- Lukman, N.; Gita, A.S.; Diah, S.; Amien, W. Synthesis and Characterization of Lithium Manganese Oxide with Different Ratio of Mole on Lithium Recovery Process from Geothermal Fluid of Lumpur Sidoarjo. J. Mater. Sci. Chem. Eng. 2015, 3, 56–62. [Google Scholar]
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313. [Google Scholar] [CrossRef]
- Zandevakili, S.; Ranjbar, M. Synthesis of Lithium Ion Sieve Nanoparticles and Optimizing Uptake Capacity by Taguchi Method. Iran. J. Chem. Chem. Eng. 2014, 33, 15–24. [Google Scholar]
- Lagergren, S. About the theory of so called adsorption of soluble substance. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 2, 189–232. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Trans. IChemE 1998, 76 Pt B, 332–340. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chitrakar, R.; Kanoh, H.; Miyai, Y.; Ooi, K. A New Type of Manganese Oxide (MnO2 ∙ 0.5H2O) Derived from Li1.6Mn1.6O4 and Its Lithium Ion-Sieve Properties. Chem. Mater. 2000, 12, 3151–3157. [Google Scholar] [CrossRef]
- Javadian, H. Application of kinetic, isotherm and thermodynamic models for the adsorption of Co(II) ions on polyamidine/polypyrrole copolymer nanofibers from aqueous solution. J. Ind. Eng. Chem. 2014, 6, 4233–4241. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar] [CrossRef]
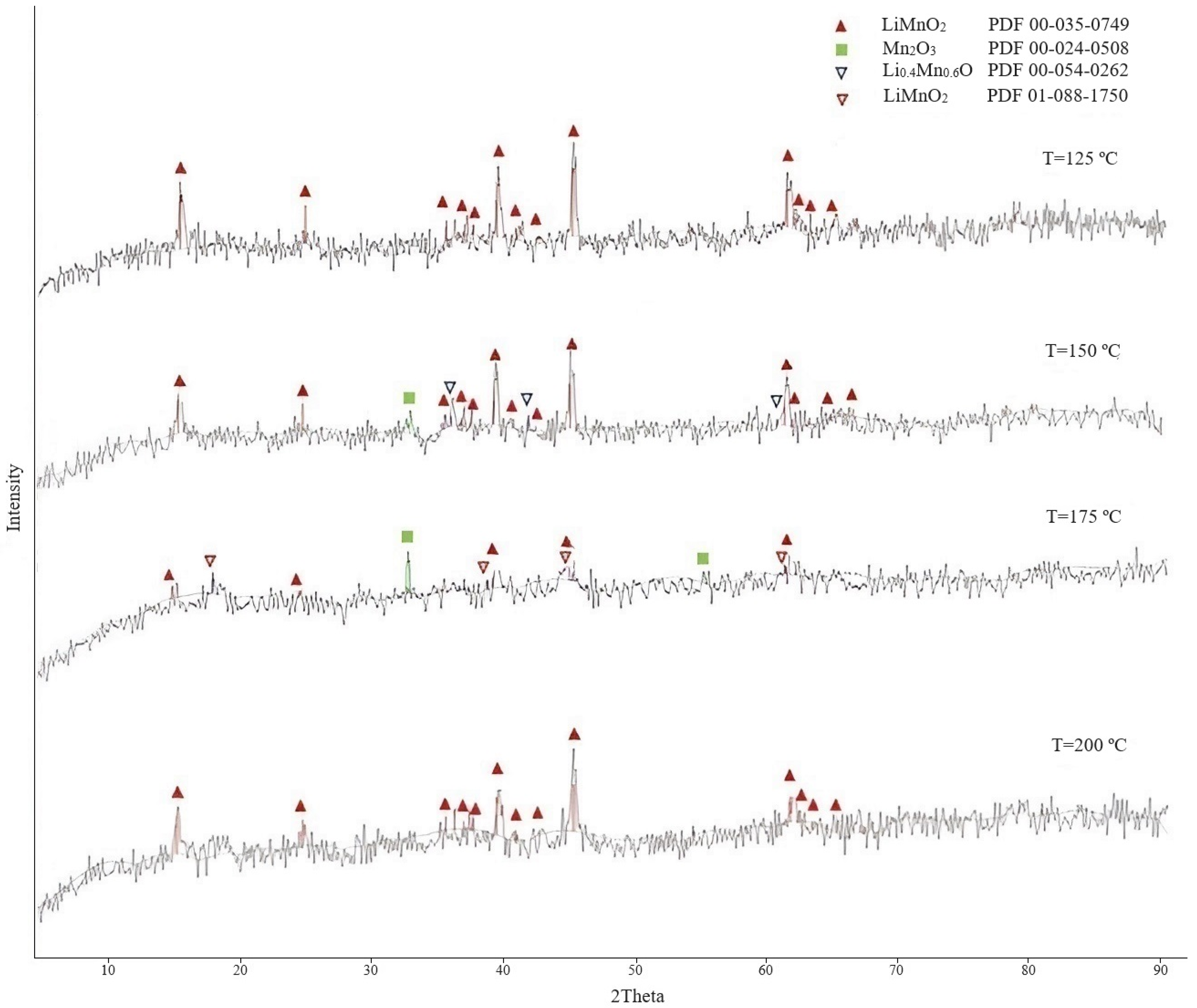
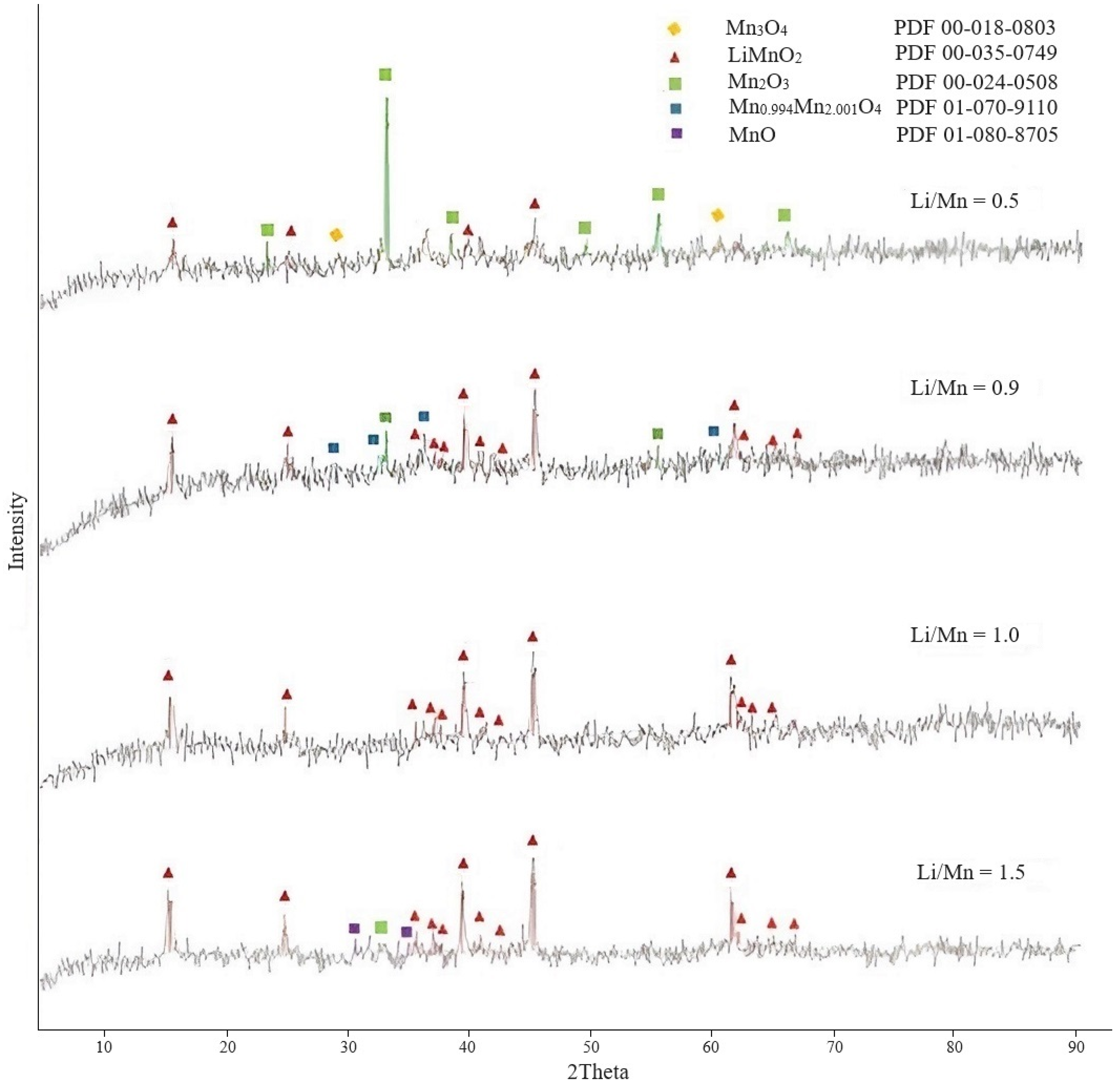
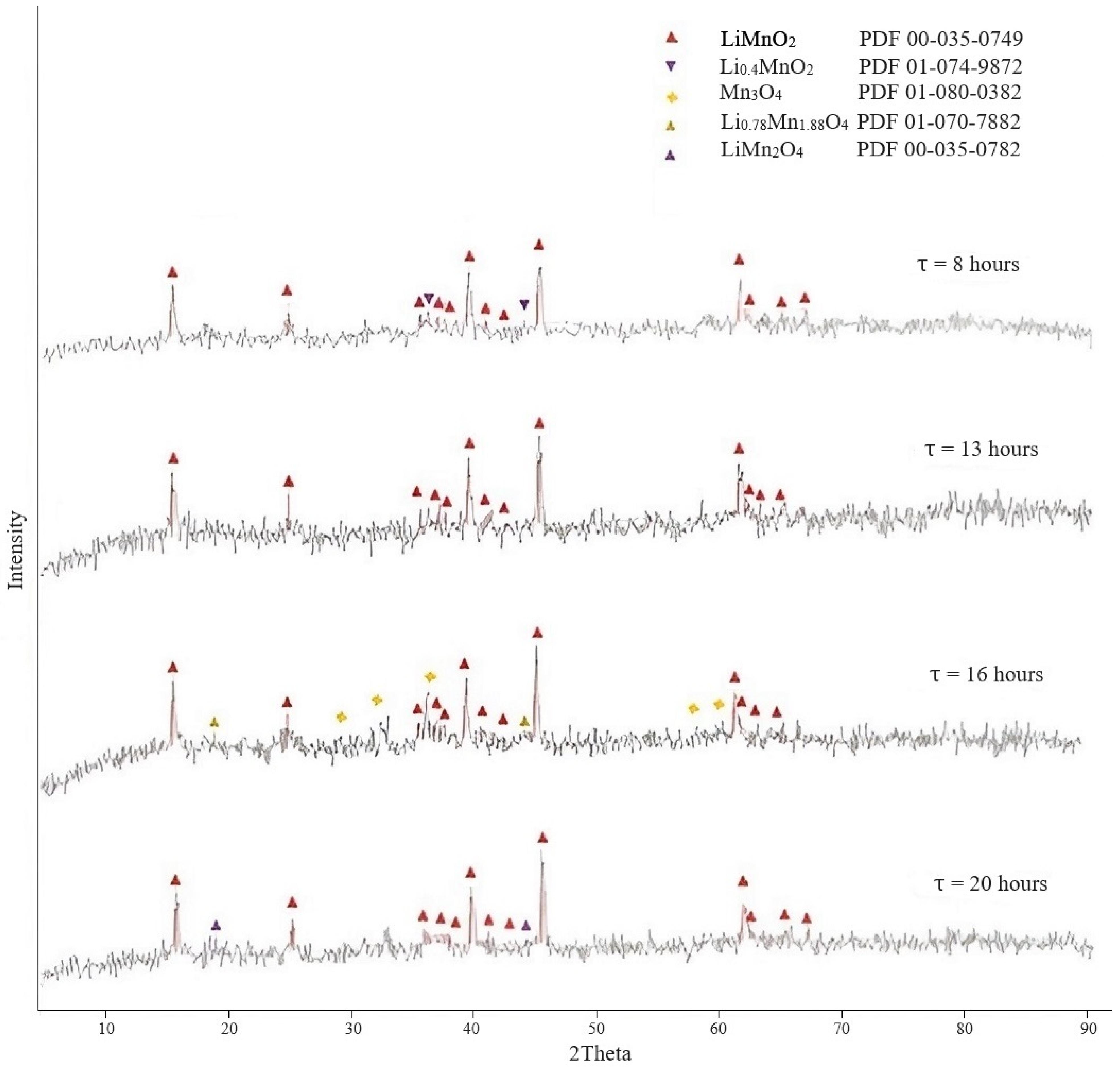

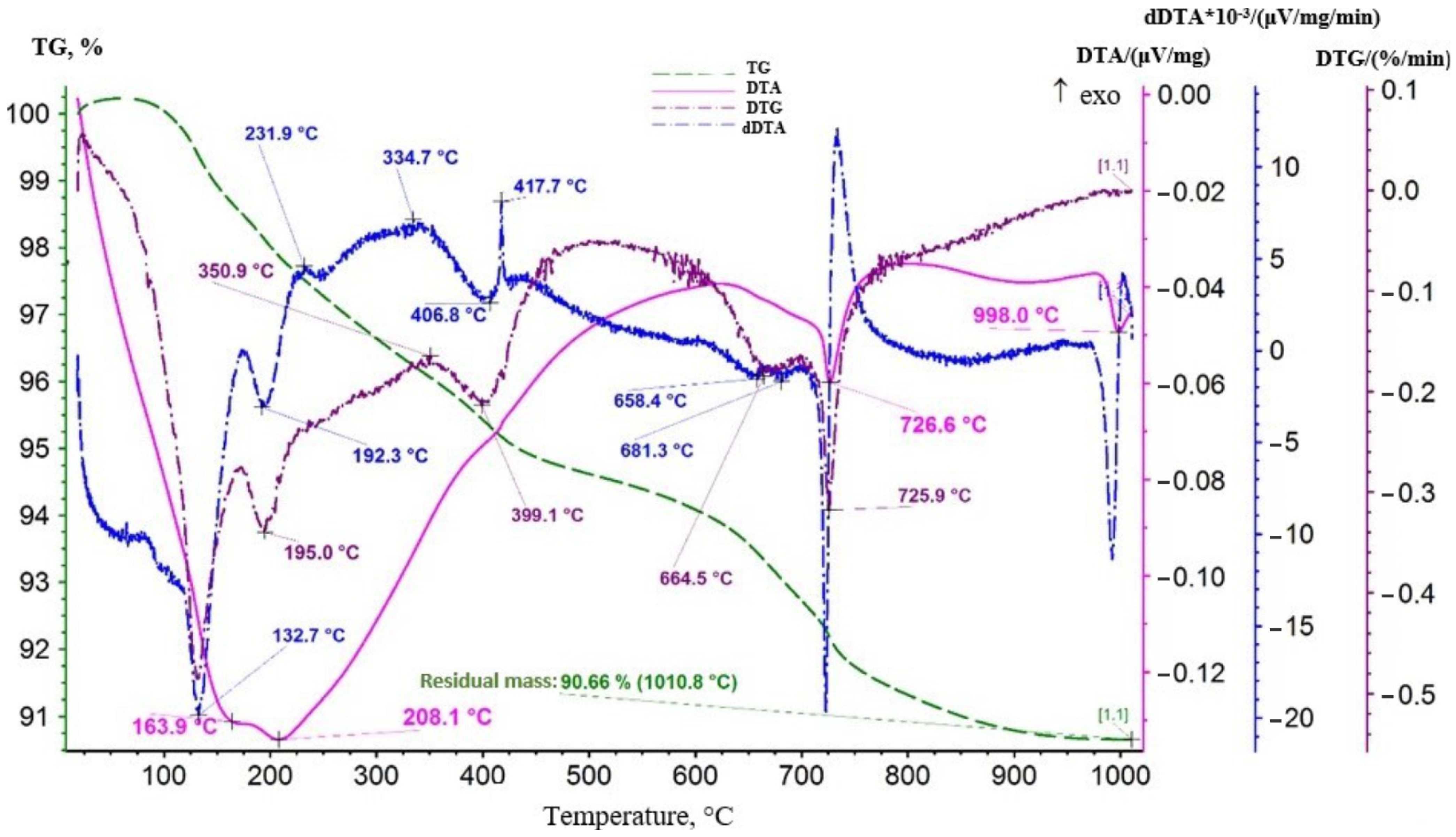



| Temperature, °C | Lithium Content in the Sorbent, % | Lithium Extraction into Solution, % | Loss of Manganese in Solution, % |
|---|---|---|---|
| 30 | 1.698 | 83.77 | 12.51 |
| 40 | 1.041 | 91.02 | 12.95 |
| 50 | 1.003 | 97.49 | 13.38 |
| 60 | 0.692 | 98.00 | 13.94 |
| Ratio S:L | Lithium Content in the Sorbent, % | Lithium Extraction into Solution, % | Loss of Manganese in Solution, % |
|---|---|---|---|
| 1:600 | 1.117 | 88.90 | 11.34 |
| 1:700 | 1.033 | 90.38 | 12.28 |
| 1:800 | 1.041 | 91.02 | 12.95 |
| 1:900 | 1.150 | 88.33 | 12.72 |
| Duration, h | Lithium Content in the Sorbent, % | Lithium Extraction into Solution, % | Loss of Manganese in Solution, % |
|---|---|---|---|
| 2 | 2.095 | 75.58 | 12.35 |
| 6 | 1.693 | 81.55 | 12.31 |
| 12 | 1.041 | 91.02 | 12.95 |
| 18 | 0.332 | 89.13 | 12.51 |
| 24 | 0.277 | 93.26 | 12.91 |
| pH of Initial Brine | Concentration in Initial Brine, g/L | SEC, mg/g | ||||
|---|---|---|---|---|---|---|
| Li, mg/L | Ca | Mg | Na | K | ||
| 7.32 | 6.389 | 2.329 | 0.759 | 19.74 | 0.376 | 10.00 |
| 8.08 | 8.094 | 2.204 | 0.721 | 17.88 | 0.348 | 9.39 |
| 9.08 | 8.392 | 2.317 | 0.756 | 18.83 | 0.449 | 12.39 |
| 10.04 | 8.129 | 2.298 | 0.690 | 18.59 | 0.408 | 18.948 |
| 11.08 | 7.838 | 2.185 | 0.032 | 21.45 | 0.387 | 12.408 |
| 12.06 | 7.661 | 2.047 | 0.001 | 21.86 | 0.373 | 21.204 |
| pH of Initial Brine | Distribution Coefficient, Kd | Partition Coefficient, Ks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Li | Ca | Mg | Na | K | Li/Ca | Li/Mg | Li/Na | Li/K | |
| 7.32 | 2116 | 224 | 221 | 0 | 0 | 9.4 | 9.6 | - | - |
| 8.08 | 1438 | 71 | 67 | 0 | 0 | 20 | 21 | - | - |
| 9.08 | 1958 | 73 | 0 | 0 | 207 | 27 | - | - | 9.4 |
| 10.04 | 3811 | 0 | 0 | 0 | 0 | - | - | - | - |
| 11.08 | 2150 | 72 | 0 | 318 | 0 | 30 | - | 6.8 | - |
| 12.06 | 5138 | 110 | 0 | 398 | 0 | 46 | - | 13 | - |
| Ratio msorbent to Vsolvent | Lithium Recovery onto Sorbent, % | SEC, mg/g | Distribution Coefficient, Kd |
|---|---|---|---|
| 1:650 | 85.9 | 3.66 | 3952 |
| 1:1000 | 66.7 | 4.15 | 2003 |
| 1:2000 | 53.2 | 6.62 | 2273 |
| 1:3000 | 39.2 | 7.33 | 1937 |
| Duration, h | Extraction of Lithium onto Sorbent, % | SEC, mg/g | Distribution Coefficient, Kd |
|---|---|---|---|
| 8 | 78.3 | 3.33 | 2340 |
| 16 | 83.7 | 3.57 | 3348 |
| 24 | 85.9 | 3.66 | 3952 |
| 48 | 86.1 | 3.67 | 4038 |
| Kinetic Model | Parameters | Temperature of the Sorption Process, °C | |
|---|---|---|---|
| 35 | 25 | ||
| Pseudo-first order | qe, mg/g | 0.28 | 1.73 |
| k1, 1/min | 3.454 × 10−3 | 4.145 × 10−3 | |
| R2 | 0.6175 | 0.8123 | |
| Pseudo-second order | qe, mg/g | 1.31 | 3.44 |
| k2, g/(mg min) | 0.170 | 0.019 | |
| h, g/(mg min) | 0.292 | 0.224 | |
| R2 | 0.9997 | 0.9995 | |
| Elovich model | α, mg/(g min) | 203.626 | 1.162 |
| βt, g/mg | 10.111 | 1.982 | |
| R2 | 0.7604 | 0.9818 | |
| Intraparticle diffusion model | kid, mg/(g min1/2) | 0.0194 | 0.1068 |
| C, mg/g | 0.9625 | 1.4125 | |
| R2 | 0.5516 | 0.8290 | |
| Experimental sorbent capacity | qexp., mg/g | 1.37 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karshyga, Z.; Yersaiynova, A.; Yessengaziyev, A.; Orynbayev, B.; Kvyatkovskaya, M.; Silachyov, I. Synthesis of Manganese Oxide Sorbent for the Extraction of Lithium from Hydromineral Raw Materials. Materials 2023, 16, 7548. https://doi.org/10.3390/ma16247548
Karshyga Z, Yersaiynova A, Yessengaziyev A, Orynbayev B, Kvyatkovskaya M, Silachyov I. Synthesis of Manganese Oxide Sorbent for the Extraction of Lithium from Hydromineral Raw Materials. Materials. 2023; 16(24):7548. https://doi.org/10.3390/ma16247548
Chicago/Turabian StyleKarshyga, Zaure, Albina Yersaiynova, Azamat Yessengaziyev, Bauyrzhan Orynbayev, Marina Kvyatkovskaya, and Igor Silachyov. 2023. "Synthesis of Manganese Oxide Sorbent for the Extraction of Lithium from Hydromineral Raw Materials" Materials 16, no. 24: 7548. https://doi.org/10.3390/ma16247548
APA StyleKarshyga, Z., Yersaiynova, A., Yessengaziyev, A., Orynbayev, B., Kvyatkovskaya, M., & Silachyov, I. (2023). Synthesis of Manganese Oxide Sorbent for the Extraction of Lithium from Hydromineral Raw Materials. Materials, 16(24), 7548. https://doi.org/10.3390/ma16247548








