The Influence of Potassium Hexafluorophosphate on the Morphology and Anticorrosive Properties of Conversion Coatings Formed on the AM50 Magnesium Alloy by Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Coatings Preparation
2.2. Coatings Characterization
3. Results and Discussion
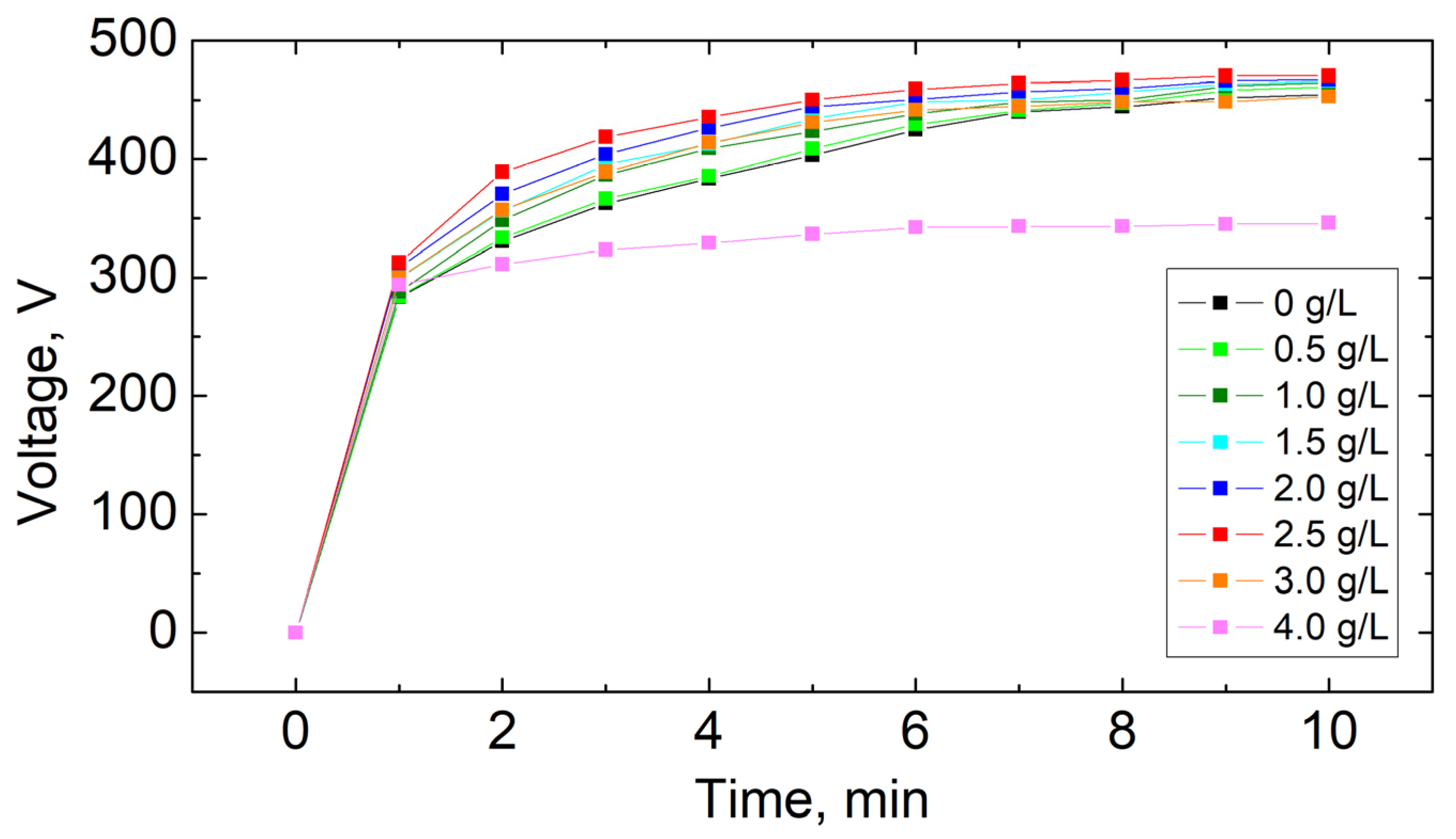
3.1. Plasma Electrolytic Oxidation Process
3.2. Thickness and Roughness of PEO Coatings
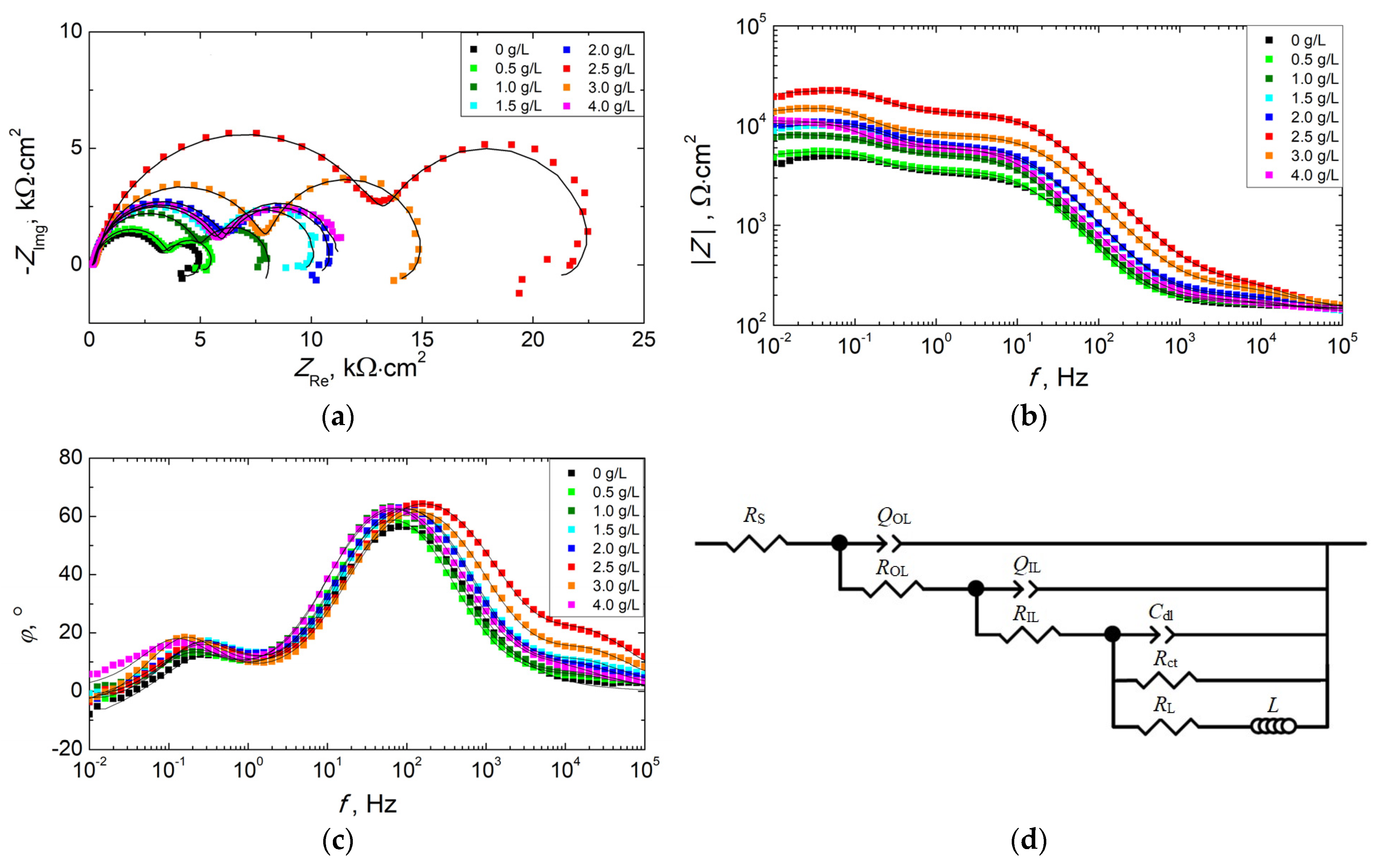
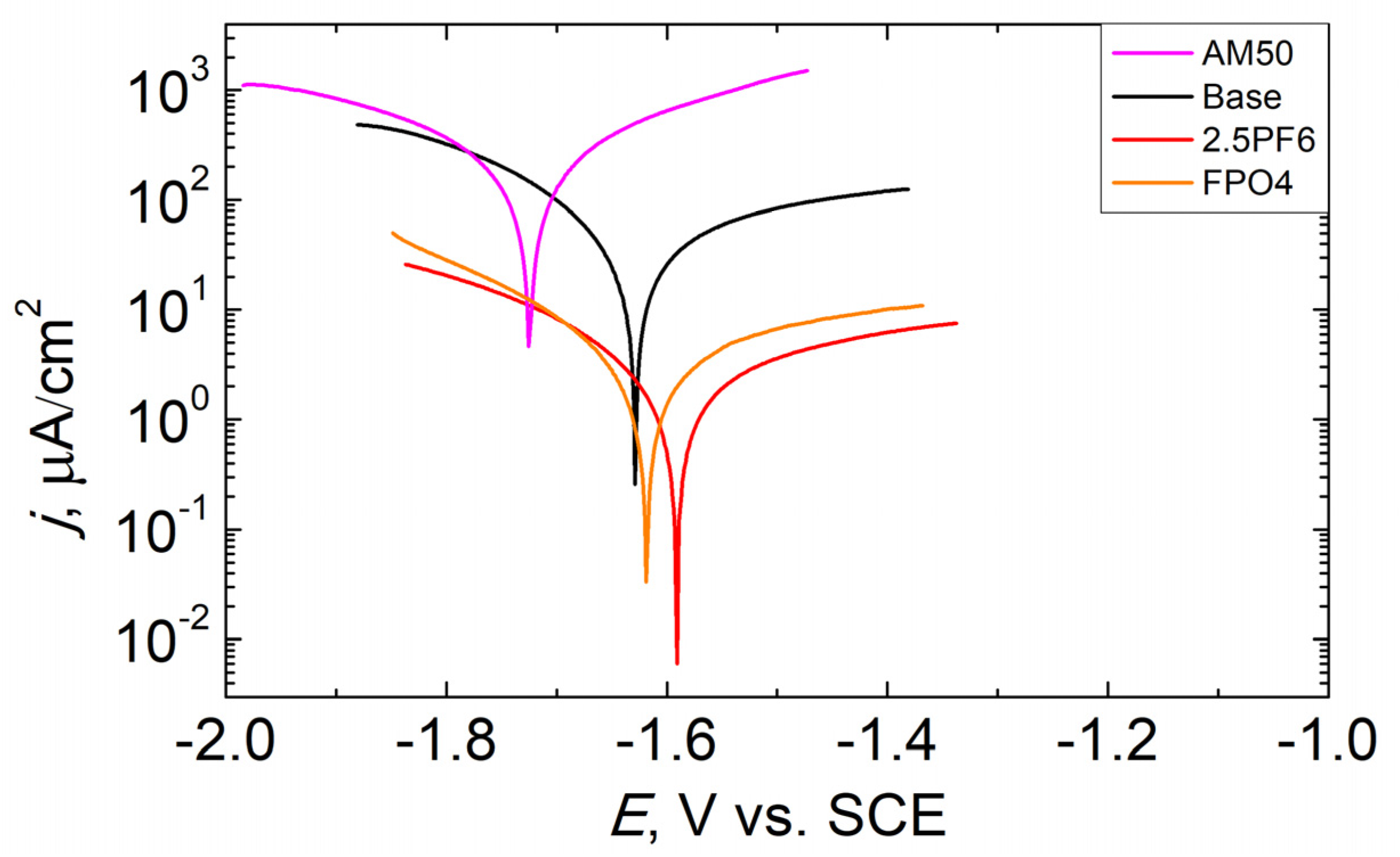
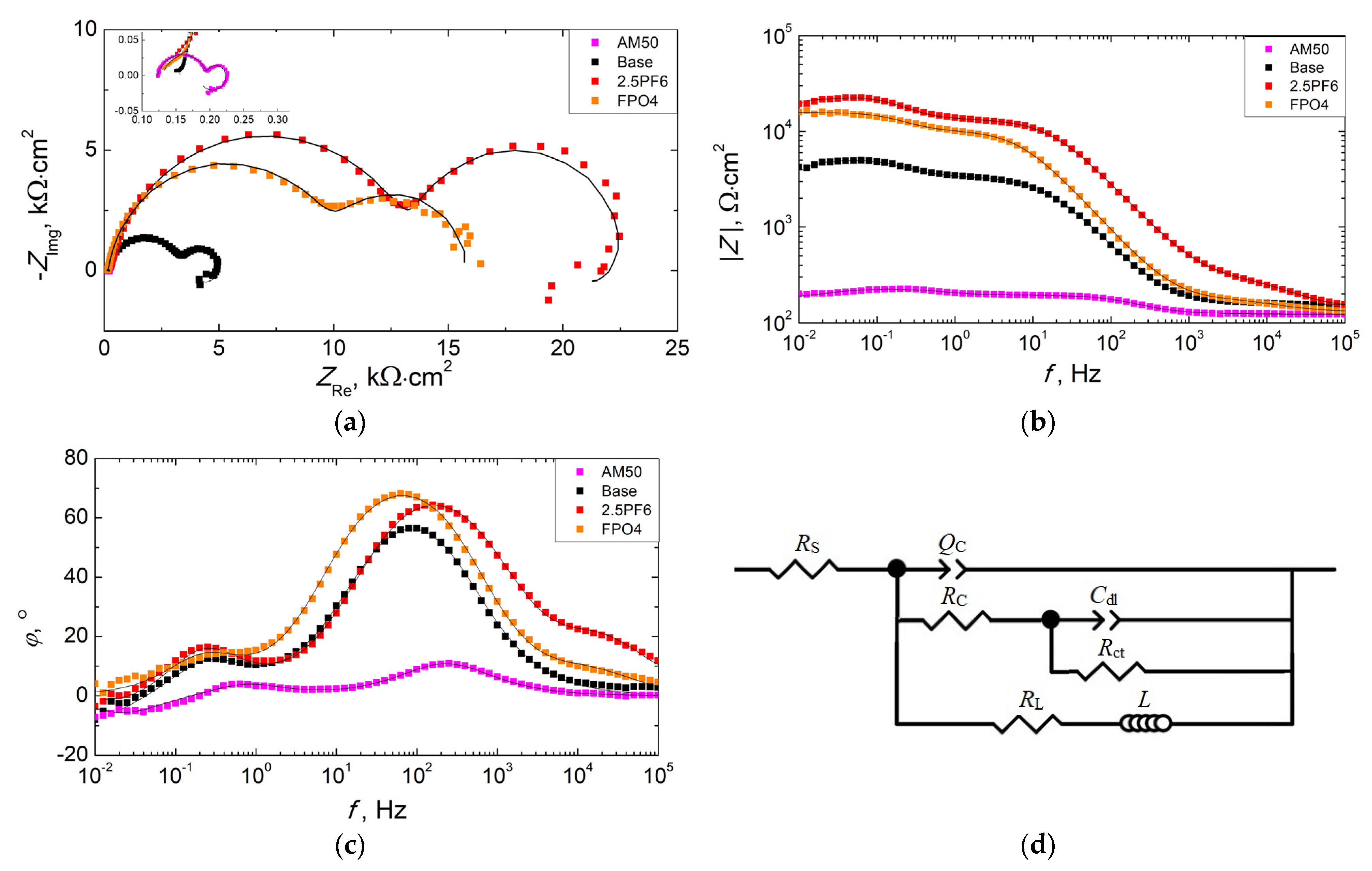
3.3. Corrosion Resistance of PEO Coatings
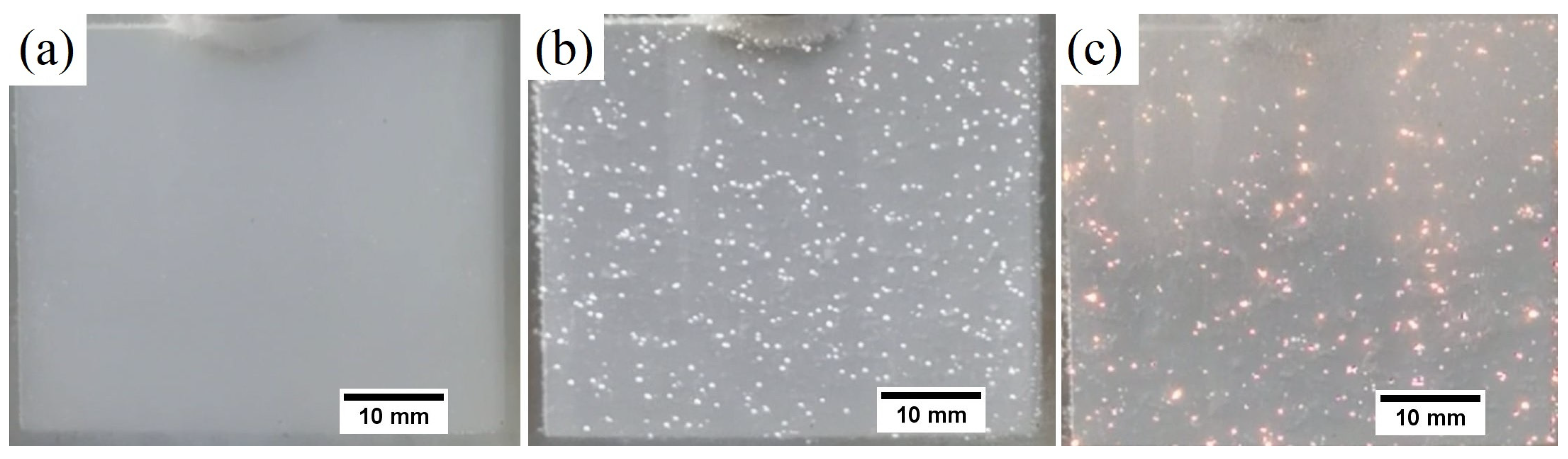
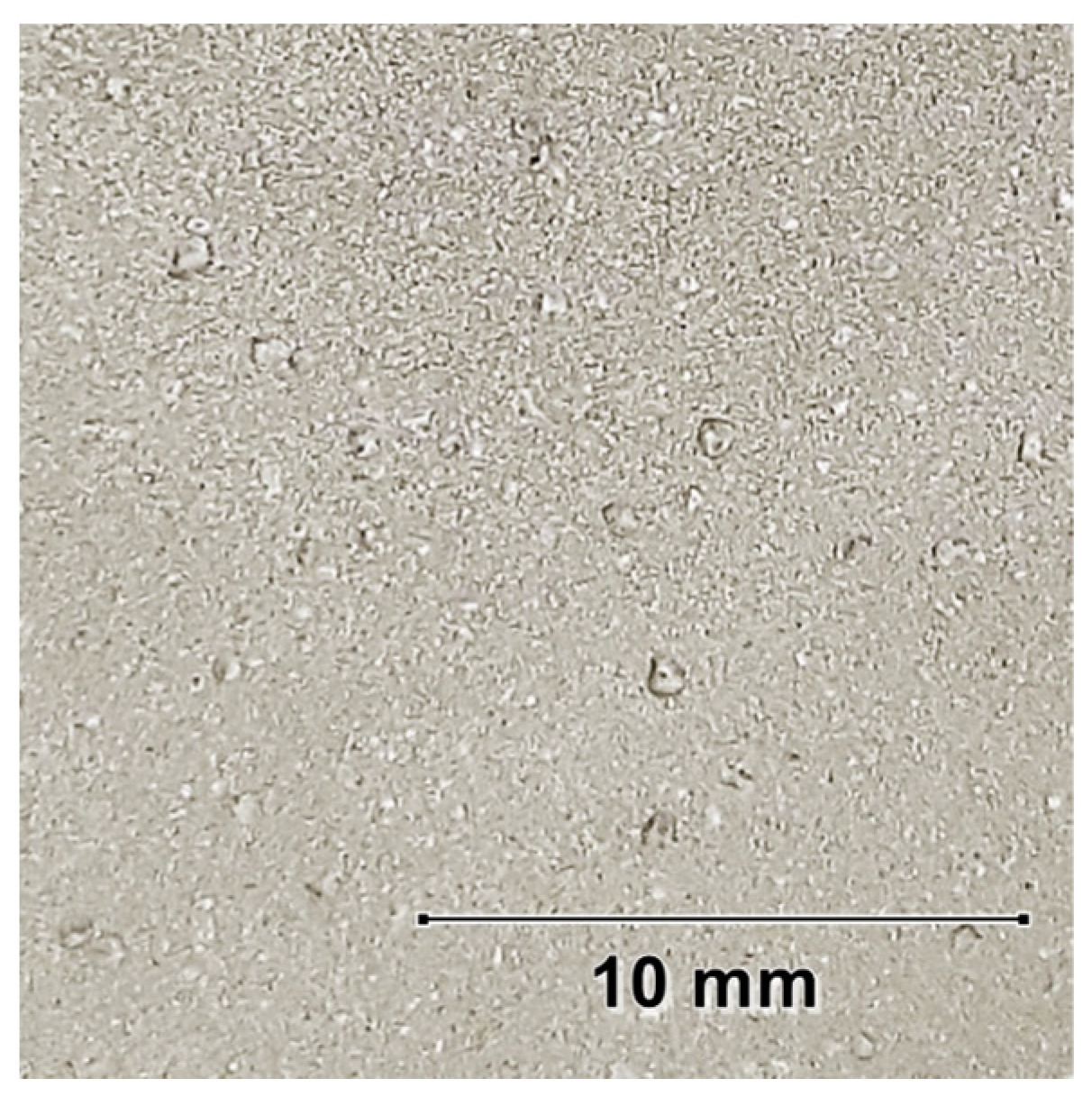
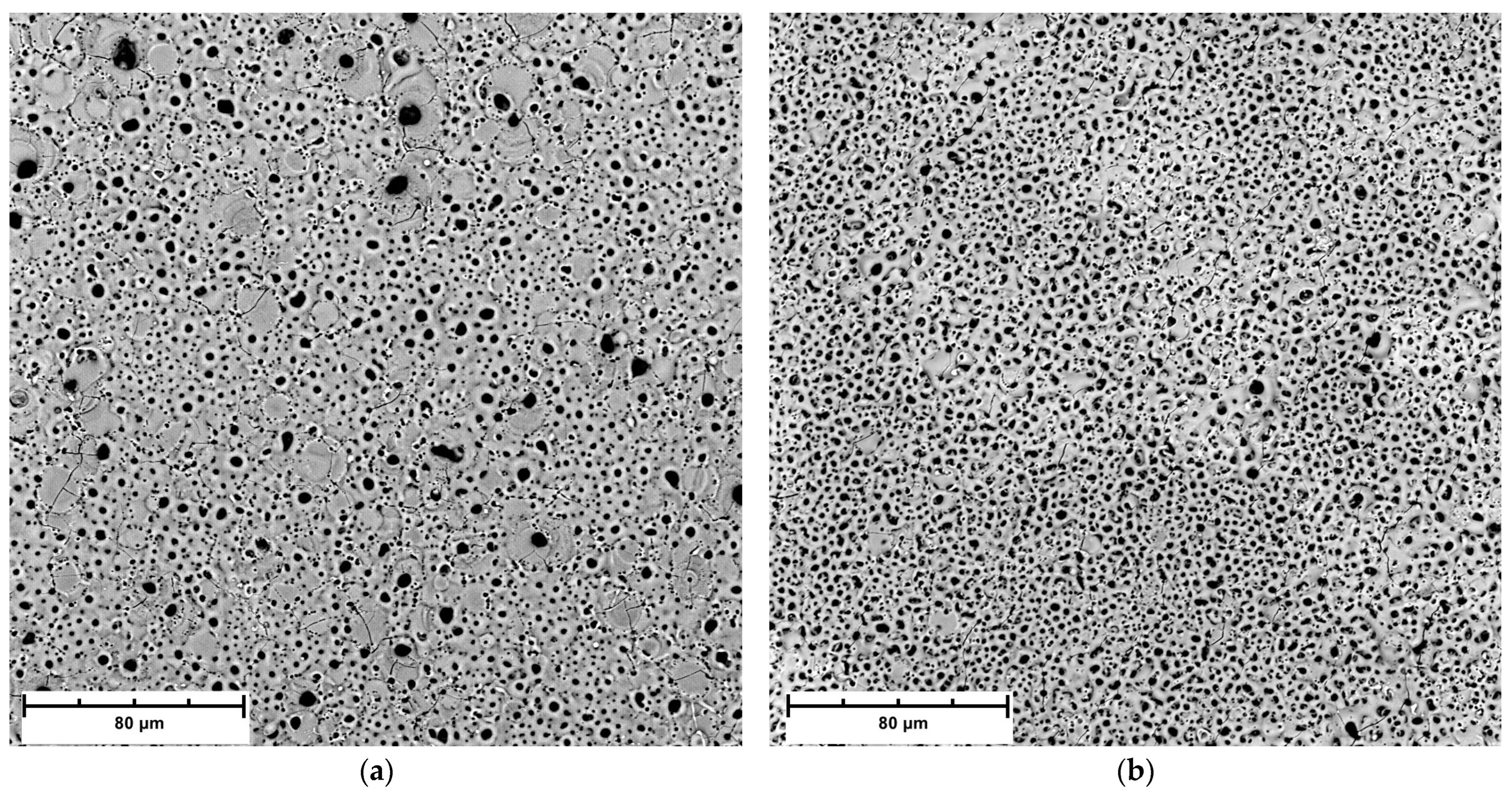
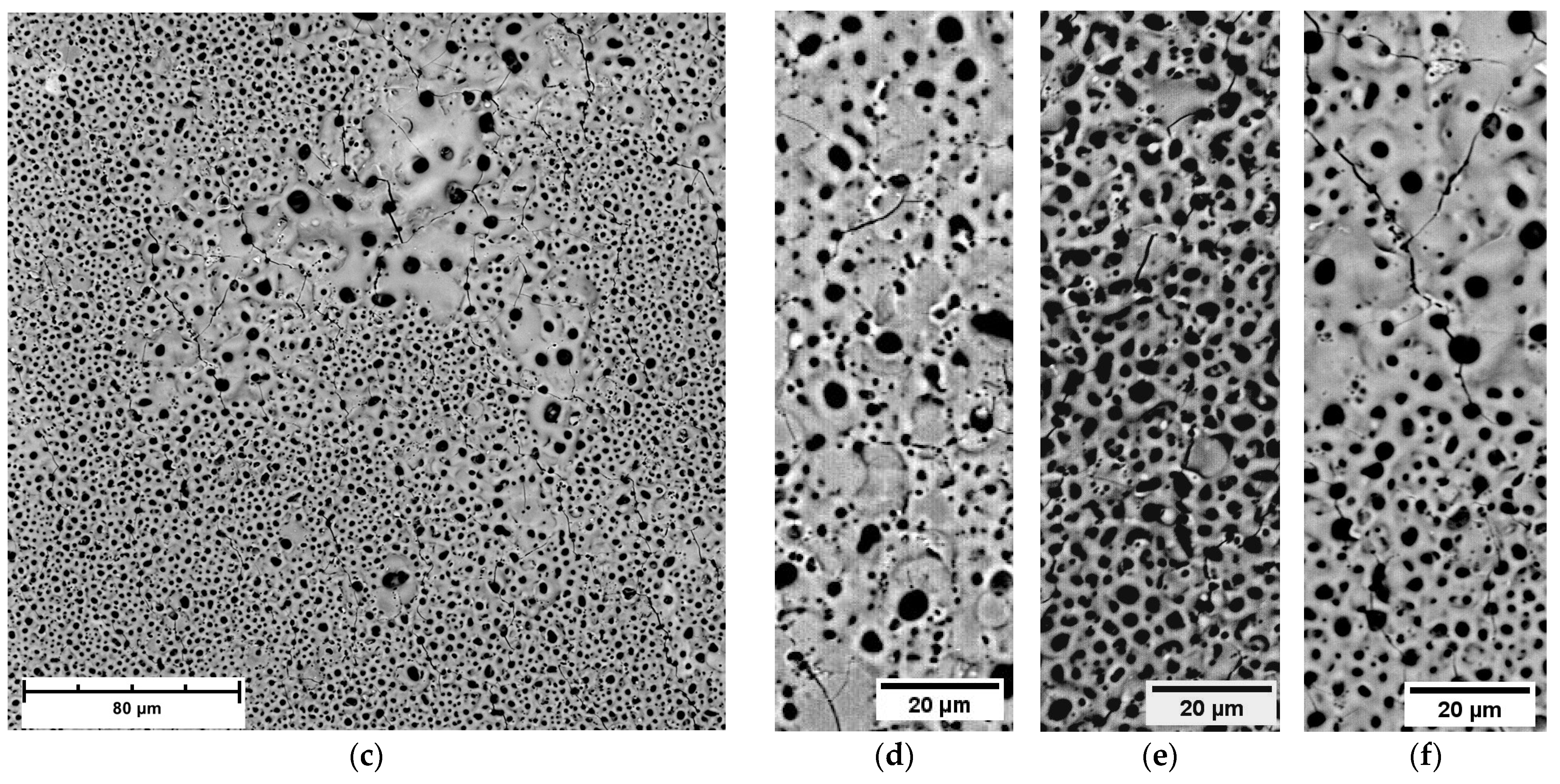
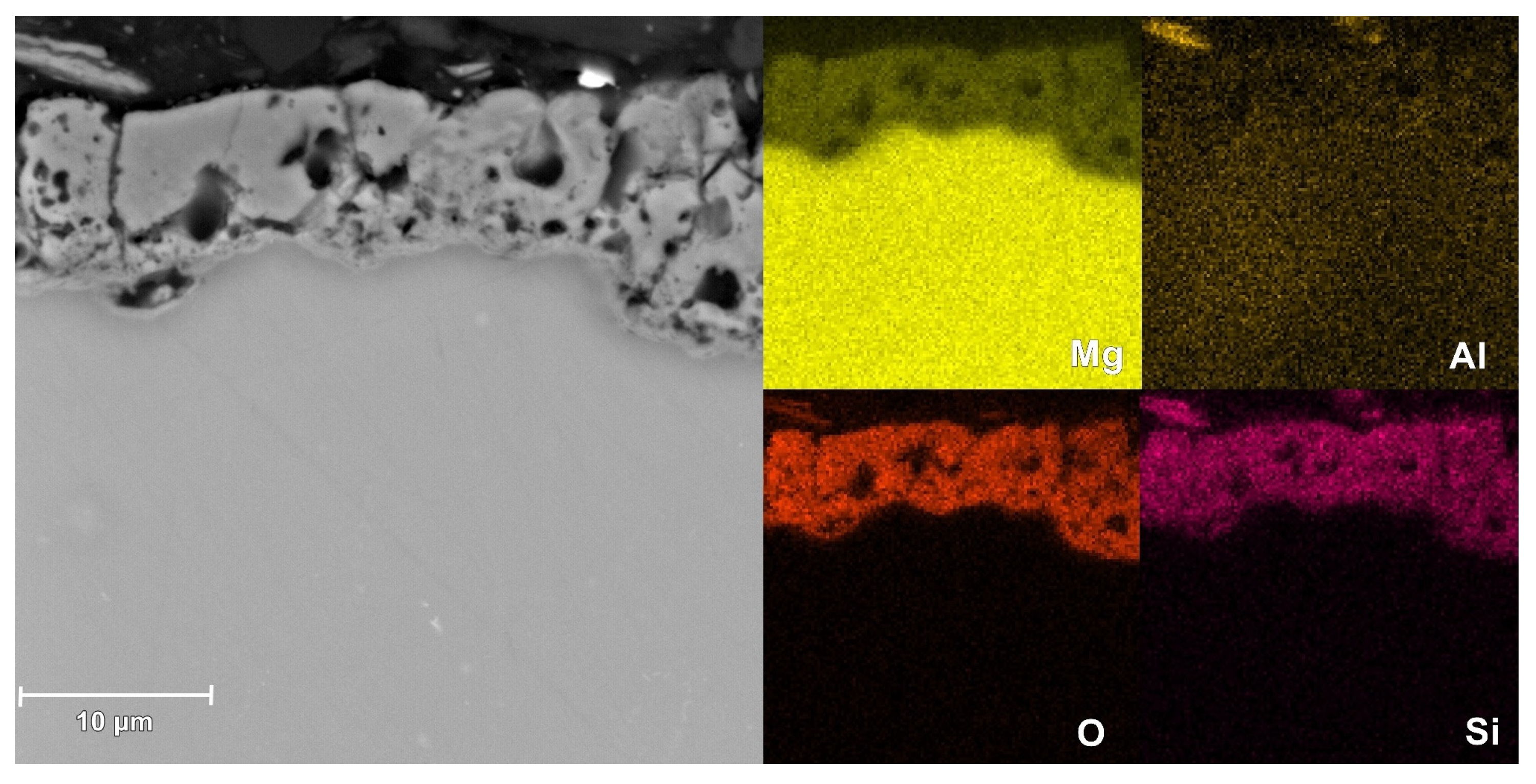
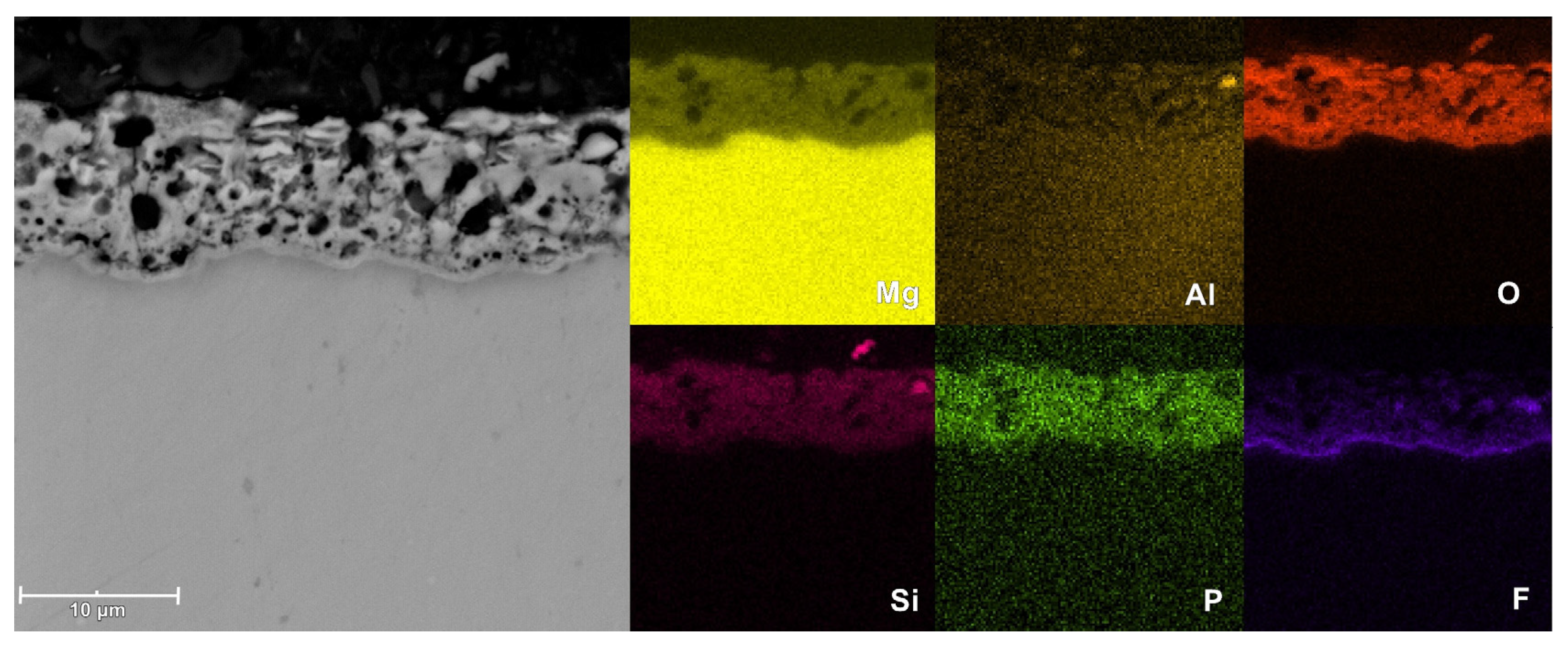
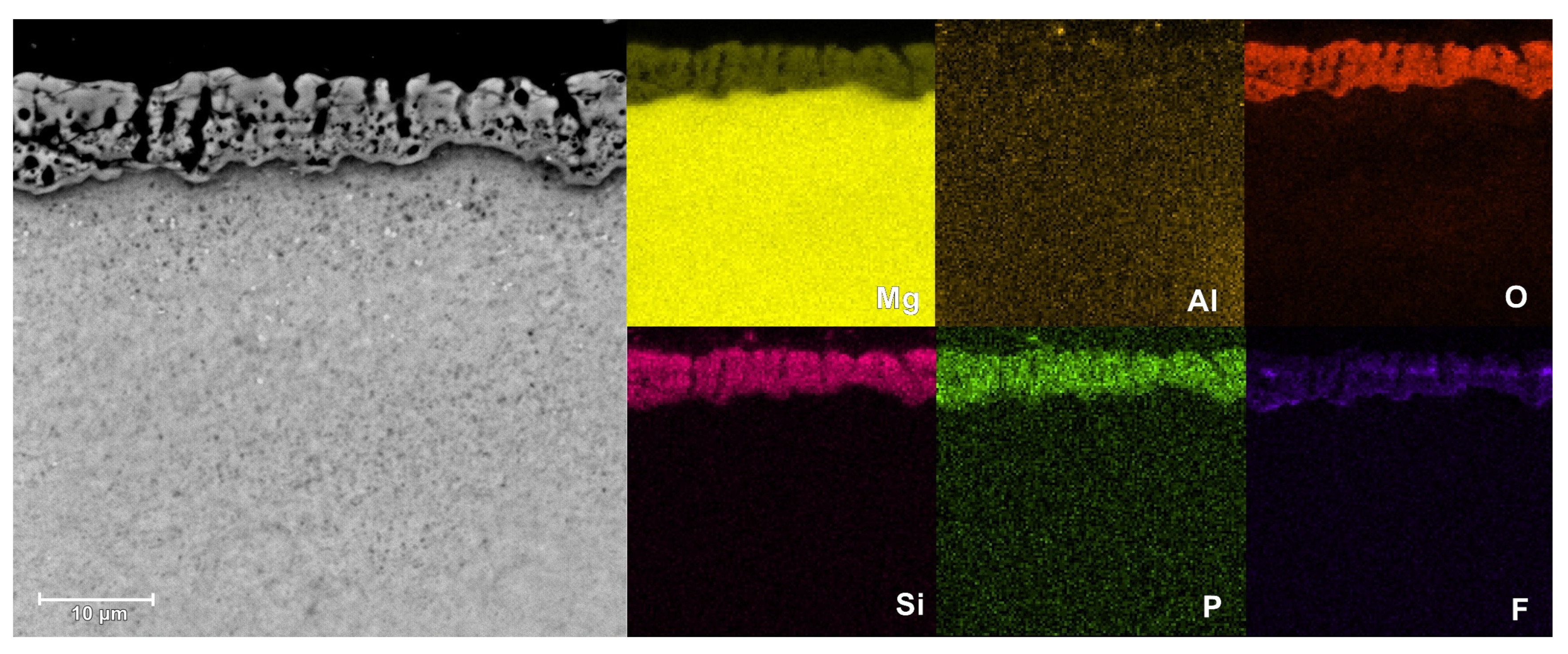
3.4. Morphological and Composition Characteristics of PEO Coatings
4. Conclusions
- The anticorrosive properties of the obtained coatings increase when the KPF6 concentration is increased to 2.5 g/L. The addition of larger amounts of KPF6 causes damage to the coating (a large increase in its roughness), probably due to the local formation of HF during the PEO process.
- The addition of KPF6 allows for better anticorrosive properties of the synthesized coating to be obtained, compared to a mixture of NaF and Na3PO4 with an equimolar content of fluorine and phosphorus.
- XPS measurements have shown that in coatings obtained in the presence of KPF6, as well as a mixture of NaF and Na3PO4 in the baths, the coating components derived from these additives are the same [MgF2 and Mg3(PO4)2]. Mg(PF6)2 was not present in the formed coatings, which is in contrast to the formation of Mg(BF4)2, when the silicate bath contained NaBF4 [46].
- The surface morphology of the PEO coatings produced in the KPF6-containing baths was more uniform and showed a sponge-like structure, in contrast to commonly reported crater-like structures. The sponge-like structure is similar to bone structure, and in combination with the presence of phosphates, it can increase the biocompatibility and the possibility of self-healing of this coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loukil, N. Alloying Elements of Magnesium Alloys: A Literature Review. In Magnesium Alloys Structure and Properties; Tański, T., Jarka, P., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 9. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy parts for automotive OEMs: A review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Luo, A.A. Applications: Aerospace, automotive and other structural applications of magnesium. In Fundamentals of Magnesium Alloy Metallurgy; Pekguleryuz, M.O., Kainer, K.U., Arslan Kaya, A., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 266–316. [Google Scholar]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Bălțatu, M.-S.; Cimpoeșu, R.; Ioanid, N. Microstructural and Electrochemical Influence of Zn in MgCaZn Biodegradable Alloys. Materials 2023, 16, 2487. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Chalisgaonkar, R. Insight in applications, manufacturing and corrosion behaviour of magnesium and its alloys—A review. Mater. Today Proc. 2020, 26, 1060–1071. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Z.; Hu, P.; Shao, J.; Li, X.; Du, C.; Jiang, B. The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution. Materials 2018, 11, 970. [Google Scholar] [CrossRef]
- Zeng, R.-c.; Zhang, J.; Huang, W.-j.; Dietzel, W.; Kainer, K.U.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferrous Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Y.-L.; Tian, Y.-Q.; Chen, L.-S. Research progress on surface protective coatings of biomedical degradable magnesium alloys. J. Alloys Compd. 2021, 885, 161001. [Google Scholar] [CrossRef]
- Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II—PEO and Anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef] [PubMed]
- Sampatirao, H.; Radhakrishnapillai, S.; Dondapati, S.; Parfenov, E.; Nagumothu, R. Developments in plasma electrolytic oxidation (PEO) coatings for biodegradable magnesium alloys. Mater. Today Proc. 2021, 46, 1407–1415. [Google Scholar] [CrossRef]
- Florczak, Ł.; Nawrat, G.; Kwolek, P.; Sieniawski, J.; Sobkowiak, A. Plasma electrolytic oxidation as a method for protection against corrosion of magnesium and its alloys. Przemysł Chem. 2018, 97, 2145–2153. (In Polish) [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.; Nevyantseva, R.R.; Gorbatkov, M.V.; Liang, C.J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. IMF 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Gawel, L.; Nieuzyla, L.; Nawrat, G.; Darowicki, K.; Slepski, P. Impedance monitoring of corrosion degradation of plasma electrolytic oxidation coatings (PEO) on magnesium alloy. J. Alloys Compd. 2017, 722, 406–413. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Anawati, A.; Hidayati, E.; Labibah, H. Characteristics of magnesium phosphate coatings formed on AZ31 Mg alloy by plasma electrolytic oxidation with improved current efficiency. Mater. Sci. Eng. B 2021, 272, 115354. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Moga, S.; Malinovschi, V.; Marin, A.; Coaca, E.; Negrea, D.; Craciun, V.; Lungu, M. Mechanical and corrosion-resistant coatings prepared on AZ63 Mg alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2023, 462, 129464. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Characterization of microarc oxidation coatings formed on AM60B magnesium alloy in silicate and phosphate electrolytes. Appl. Surf. Sci. 2007, 253, 4490–4496. [Google Scholar] [CrossRef]
- Mori, Y.; Koshi, A.; Liao, J.; Asoh, H.; Ono, S. Characteristics and corrosion resistance of plasma electrolytic oxidation coatings on AZ31B Mg alloy formed in phosphate—Silicate mixture electrolytes. Corros. Sci. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Yu, L.; Cao, J.; Cheng, Y. An improvement of the wear and corrosion resistances of AZ31 magnesium alloy by plasma electrolytic oxidation in a silicate–hexametaphosphate electrolyte with the suspension of SiC nanoparticles. Surf. Coat. Technol. 2015, 276, 266–278. [Google Scholar] [CrossRef]
- Sovík, J.; Kajánek, D.; Pastorek, F.; Štrbák, M.; Florková, Z.; Jambor, M.; Hadzima, B. The Effect of Mechanical Pretreatment on the Electrochemical Characteristics of PEO Coatings Prepared on Magnesium Alloy AZ80. Materials 2023, 16, 5650. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Liu, W.; Duan, X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corros. Sci. 2013, 72, 118–124. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Monakhova, E.P.; Khabibullina, Z.V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Gladkova, A.A. Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys: Comparison of coatings formation mechanism. J. Magnes. Alloys 2020, 8, 587–600. [Google Scholar] [CrossRef]
- Yu, W.; Sun, R.; Guo, Z.; Wang, Z.; He, Y.; Lu, G.; Chen, P.; Chen, K. Novel fluoridated hydroxyapatite/MAO composite coating on AZ31B magnesium alloy for biomedical application. Appl. Surf. Sci. 2019, 464, 708–715. [Google Scholar] [CrossRef]
- Lujun, Z.; Hongzhan, L.; Qingmei, M.; Jiangbo, L.; Zhengxian, L. The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition. J. Magnes. Alloys 2023, 11, 2823–2832. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. Effect of potassium fluoride on structure and corrosion resistance of plasma electrolytic oxidation films formed on AZ31 magnesium alloy. J. Alloys Compd. 2009, 480, 469–474. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shin, S.H.; Hussain, I.; Koo, B.H. Structure and corrosion properties of the two-step PEO coatings formed on AZ91D Mg alloy in K2ZrF6-based electrolyte solution. Surf. Coat. Technol. 2016, 307, 484–490. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containing PEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Cui, X.-J.; Liu, C.-H.; Yang, R.-S.; Li, M.-T.; Lin, X.-Z. Self-sealing micro-arc oxidation coating on AZ91D Mg alloy and its formation mechanism. Surf. Coat. Technol. 2015, 269, 228–237. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Xu, D.; Li, J.; Chen, T. Characterization and formation mechanism of grey micro-arc oxidation coatings on magnesium alloy. Surf. Coat. Technol. 2015, 283, 281–285. [Google Scholar] [CrossRef]
- Tang, M.; Feng, Z.; Li, G.; Zhang, Z.; Zhang, R. High-corrosion resistance of the microarc oxidation coatings on magnesium alloy obtained in potassium fluotitanate electrolytes. Surf. Coat. Technol. 2015, 264, 105–113. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Ahn, B.H.; Song, J.I.; Koo, B.H. Effect of Na2SiF6 concentration on the plasma electrolytic oxidation of AZ31B magnesium alloy. Int. J. Surf. Sci. Eng. 2016, 10, 456–467. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Heun Koo, B.; Choi, D. Influence of Complex SiF62− Ions on the PEO Coatings Formed on Mg–Al6–Zn1 Alloy for Enhanced Wear and Corrosion Protection. Coatings 2020, 10, 94. [Google Scholar] [CrossRef]
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a nearly defect-free coating via high-energy plasma sparks. Sci. Rep. 2017, 7, 2378. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Wei, B.; Liu, Q. Electrochemical performance of microarc oxidation films formed on AZ91D magnesium alloy in silicate and phosphate electrolytes. Surf. Coat. Technol. 2006, 200, 3727–3733. [Google Scholar] [CrossRef]
- Florczak, Ł.; Nawrat, G.; Darowicki, K.; Ryl, J.; Sieniawski, J.; Wierzbińska, M.; Raga, K.; Sobkowiak, A. The Effect of Sodium Tetrafluoroborate on the Properties of Conversion Coatings Formed on the AZ91D Magnesium Alloy by Plasma Electrolytic Oxidation. Processes 2022, 10, 2089. [Google Scholar] [CrossRef]
- Ponikvar, M.; Žemva, B.; Liebman, J.F. The analytical and descriptive inorganic chemistry of the hydrolysis of hexafluoropnictate ions, PnF6− (Pn = P, As, Sb, Bi). J. Fluor. Chem. 2003, 123, 217–220. [Google Scholar] [CrossRef]
- European Standard EN 1753:2019; Magnesium and Magnesium Alloys. Magnesium Alloy Ingots and Castings. European Committee for Standardization: Brussels, Belgium, 2019.
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Jangde, A.; Kumar, S.; Blawert, C. Evolution of PEO coatings on AM50 magnesium alloy using phosphate-based electrolyte with and without glycerol and its electrochemical characterization. J. Magnes. Alloys 2020, 8, 692–715. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, protective, inhibitory and semiconducting properties of PEO coatings containing CeO2 nanoparticles formed on AZ31 Mg alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Parfenov, E.; Matthews, A. Application of Voltage Pulse Transient Analysis during Plasma Electrolytic Oxidation for Assessment of Characteristics and Corrosion Behaviour of Ca- and P-containing Coatings on Magnesium. Electrochim. Acta 2014, 149, 218–230. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.-H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; John, D.S.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Barnes, P.; Smith, K.; Parrish, R.; Jones, C.; Skinner, P.; Storch, E.; White, Q.; Deng, C.; Karsann, D.; Lau, M.L.; et al. A non-aqueous sodium hexafluorophosphate-based electrolyte degradation study: Formation and mitigation of hydrofluoric acid. J. Power Sources 2020, 447, 227363. [Google Scholar] [CrossRef]
- Terborg, L.; Nowak, S.; Passerini, S.; Winter, M.; Karst, U.; Haddad, P.R.; Nesterenko, P.N. Ion chromatographic determination of hydrolysis products of hexafluorophosphate salts in aqueous solution. Anal. Chim. Acta 2012, 714, 121–126. [Google Scholar] [CrossRef]
- Biesinger, M.C. X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com (accessed on 31 October 2023).
- Giron, R.G.P.; Ferguson, G.S. Tetrafluoroborate and Hexafluorophosphate Ions are not Interchangeable: A Density Functional Theory Comparison of Hydrogen Bonding. ChemistrySelect 2017, 2, 10895–10901. [Google Scholar] [CrossRef]



| Al | Mn | Zn | Si | Fe | Cu | Ni | Mg |
|---|---|---|---|---|---|---|---|
| 4.40–5.50 | min. 0.1 | max. 0.02 | max. 0.1 | max. 0.005 | max. 0.01 | 0.002 | balance |
| Concentration of KPF6, g/L | Time to Reach Critical Voltage, min | Final Voltage, V |
|---|---|---|
| 0.0 | 8.8 | 455 |
| 0.5 | 8.3 | 461 |
| 1.0 | 8.0 | 465 |
| 1.5 | 7.0 | 466 |
| 2.0 | 5.9 | 467 |
| 2.5 | 5.0 | 470 |
| 3.0 | 9.5 | 453 |
| 4.0 | - | 346 |
| Concentration of KPF6, g/L | ECORR, V | jCORR, µA/cm2 | RP,kΩ∙cm2 |
|---|---|---|---|
| 0.0 | −1.630 | 33.01 | 1.04 |
| 0.5 | −1.606 | 23.11 | 1.46 |
| 1.0 | −1.596 | 9.35 | 3.81 |
| 1.5 | −1.595 | 4.55 | 7.88 |
| 2.0 | −1.593 | 3.91 | 9.41 |
| 2.5 | −1.587 | 1.76 | 21.09 |
| 3.0 | −1.555 | 2.94 | 13.09 |
| 4.0 | −1.453 | 5.94 | 7.25 |
| Concen. of KPF6, g/L | RS, Ω·cm2 | QOL, µFn/cm2 | nOL | ROL, Ω·cm2 | QIL, µFn/cm2 | nIL | RIL, kΩ·cm2 | Cdl, mF/cm2 | Rct, kΩ·cm2 | L kH·cm2 | RL kΩ·cm2 | Rtotal kΩ·cm2 | Χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 155.5 | - | - | - | 5.713 | 0.877 | 3.296 | 0.469 | 1.533 | 25.5 | 0.75 | 3.80 | 5.12 × 10−4 |
| 0.5 | 146.8 | 1.032 | 0.905 | 37.9 | 4.189 | 0.917 | 3.476 | 0.516 | 1.927 | 53.8 | 3.87 | 4.80 | 2.88 × 10−4 |
| 1.0 | 147.9 | 0.784 | 0.913 | 42.5 | 3.347 | 0.926 | 4.971 | 0.348 | 2.928 | 24.2 | 18.64 | 7.54 | 4.25 × 10−4 |
| 1.5 | 135.9 | 0.814 | 0.858 | 79.1 | 2.051 | 0.922 | 5.728 | 0.178 | 4.361 | 200.7 | 21.60 | 9.44 | 3.31 × 10−4 |
| 2.0 | 153.9 | 0.803 | 0.873 | 74.2 | 1.974 | 0.922 | 6.222 | 0.180 | 4.550 | 233.1 | 15.92 | 9.84 | 2.74 × 10−4 |
| 2.5 | 141.1 | 0.392 | 0.847 | 218.2 | 0.819 | 0.892 | 13.400 | 0.093 | 9.271 | 231.5 | 28.51 | 20.61 | 5.13 × 10−4 |
| 3.0 | 151.1 | 0.425 | 0.867 | 129.4 | 1.431 | 0.894 | 7.921 | 0.189 | 7.069 | 293.1 | 16.92 | 13.04 | 3.30 × 10−4 |
| 4.0 | 144.9 | 1.115 | 0.896 | 64.9 | 2.818 | 0.903 | 5.975 | 0.345 | 5.035 | - | - | 11.07 | 6.37 × 10−4 |
| Sample | Composition of Electrolyte, g/L | Molar Contents of Fluorine, mM/L | Molar Contents of Phosphorus, mM/L |
|---|---|---|---|
| Base | Na2SiO3·5H2O: 10 | - | - |
| NaOH: 4 | |||
| 2.5PF6 | Na2SiO3·5H2O: 10 | 81.5 | 13.6 |
| NaOH: 4 | |||
| KPF6: 2.5 | |||
| FPO4 | Na2SiO3·5H2O: 10 | 81.5 | 13.6 |
| NaOH: 4 | |||
| NaF: 3.42 | |||
| Na3PO4·12H2O: 5.17 |
| Sample | ECORR, V | jCORR, µA/cm2 | RP,kΩ∙cm2 |
|---|---|---|---|
| Uncoated AM50 | −1.607 | 140.10 | 0.22 |
| Base | −1.630 | 33.01 | 1.04 |
| 2.5PF6 | −1.587 | 1.76 | 21.09 |
| FPO4 | −1.501 | 2.75 | 13.41 |
| Sample | RS, Ω·cm2 | QOL, µFn/cm2 | nOL | ROL, Ω·cm2 | QIL, µFn/cm2 | nIL | RIL, kΩ·cm2 | Cdl, mF/cm2 | Rct, kΩ·cm2 | L kH·cm2 | RL kΩ·cm2 | Rtotal kΩ·cm2 | Χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | 155.5 | - | - | - | 5.713 | 0.877 | 3.296 | 0.469 | 1.533 | 25.5 | 0.75 | 3.80 | 5.12 × 10−4 |
| 2.5PF6 | 141.1 | 0.392 | 0.847 | 218.2 | 0.819 | 0.892 | 13.400 | 0.093 | 9.271 | 231.5 | 28.51 | 20.61 | 5.13 × 10−4 |
| FPO4 | 130.6 | 0.828 | 0.890 | 63.7 | 2.411 | 0.910 | 10.270 | 0.016 | 5.271 | - | - | 15.60 | 5.73 × 10−4 |
| Sample | RS,Ω·cm2 | QC,µFn/cm2 | nC | RC,kΩ·cm2 | Cdl,mF/cm2 | Rct, kΩ·cm2 | L kH·cm2 | RL kΩ·cm2 | Rtotal kΩ·cm2 | Χ2 | |||
| Uncoated AM50 | 122.9 | 0.302 | 0.873 | 0.073 | 7.627 | 0.029 | 1.53 | 0.153 | 0.061 | 2.50 × 10−4 |
| Sample | Layer | Elements Content, at.% | |||||
|---|---|---|---|---|---|---|---|
| Mg | O | Si | Al | F | P | ||
| Base | outer | 49.1 | 37.3 | 11.2 | 2.4 | - | - |
| inner | 53.8 | 37.4 | 6.4 | 2.5 | - | - | |
| 2.5PF6 | outer | 39.6 | 43.0 | 10.1 | 3.7 | 2.6 | 1.1 |
| inner | 39.6 | 40.5 | 9.1 | 3.3 | 6.3 | 1.1 | |
| FPO4 | outer | 45.0 | 41.3 | 7.6 | 3.1 | 1.7 | 1.4 |
| inner | 38.9 | 42.8 | 7.4 | 3.7 | 5.2 | 1.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florczak, Ł.; Kościelniak, B.; Kramek, A.; Sobkowiak, A. The Influence of Potassium Hexafluorophosphate on the Morphology and Anticorrosive Properties of Conversion Coatings Formed on the AM50 Magnesium Alloy by Plasma Electrolytic Oxidation. Materials 2023, 16, 7573. https://doi.org/10.3390/ma16247573
Florczak Ł, Kościelniak B, Kramek A, Sobkowiak A. The Influence of Potassium Hexafluorophosphate on the Morphology and Anticorrosive Properties of Conversion Coatings Formed on the AM50 Magnesium Alloy by Plasma Electrolytic Oxidation. Materials. 2023; 16(24):7573. https://doi.org/10.3390/ma16247573
Chicago/Turabian StyleFlorczak, Łukasz, Barbara Kościelniak, Agnieszka Kramek, and Andrzej Sobkowiak. 2023. "The Influence of Potassium Hexafluorophosphate on the Morphology and Anticorrosive Properties of Conversion Coatings Formed on the AM50 Magnesium Alloy by Plasma Electrolytic Oxidation" Materials 16, no. 24: 7573. https://doi.org/10.3390/ma16247573





