Preparation, Characterization, and Properties of UV-Curable Coating Doped with Nano-SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
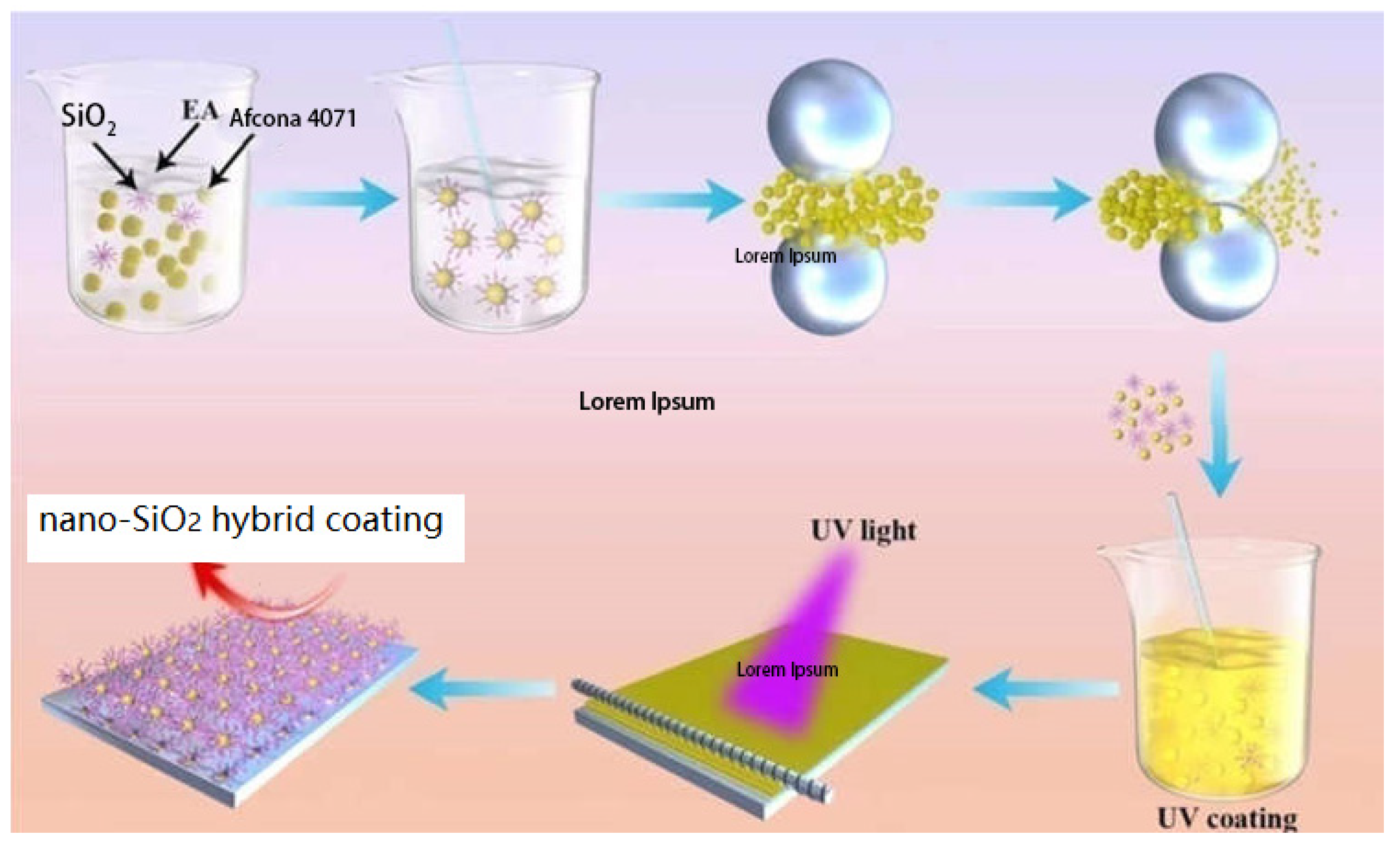
2.2. Modification of Nano-SiO2 via High-Energy Ball Milling
2.3. Preparation of Composite Coatings
2.4. Characterization
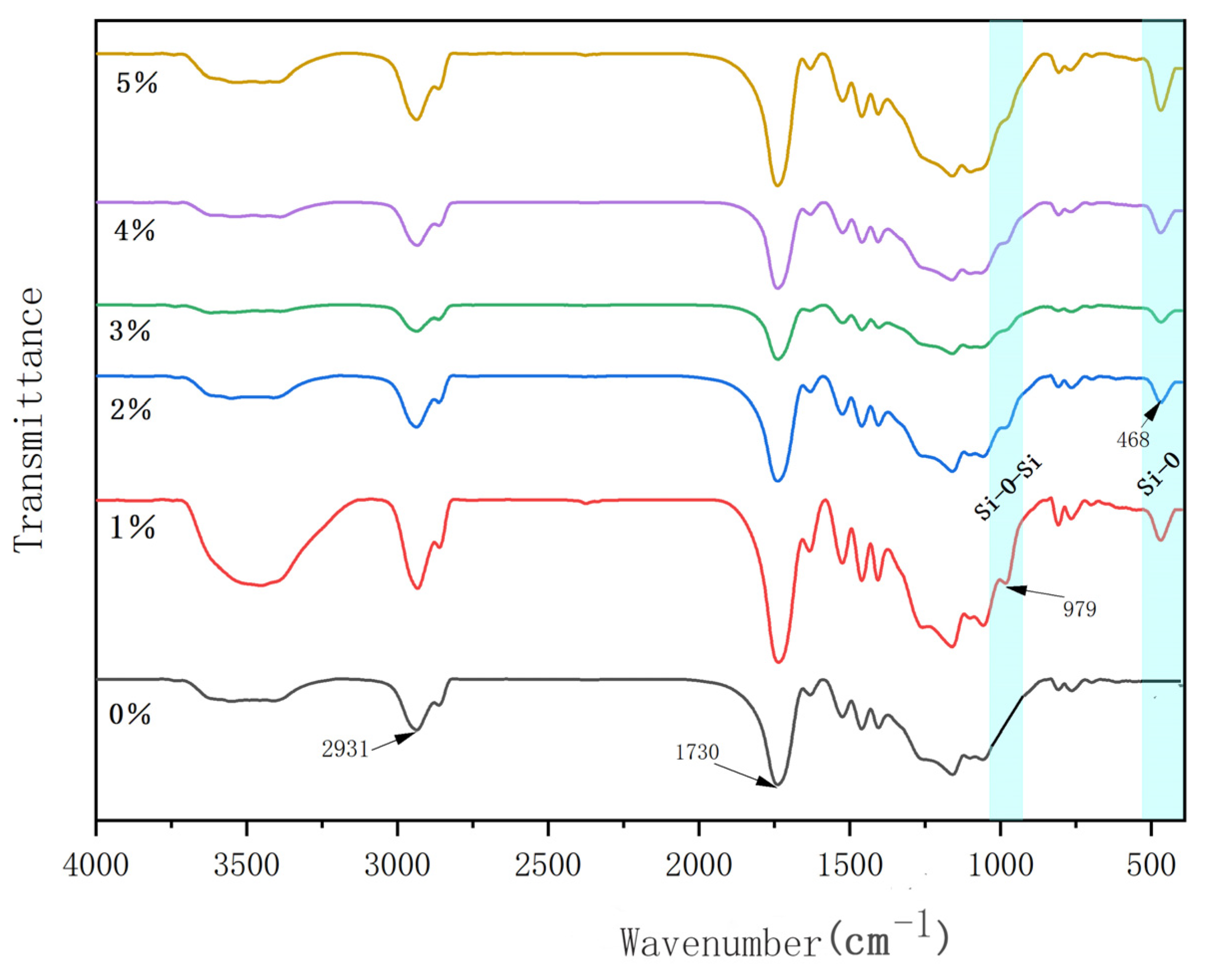
2.4.1. FTIR Spectra
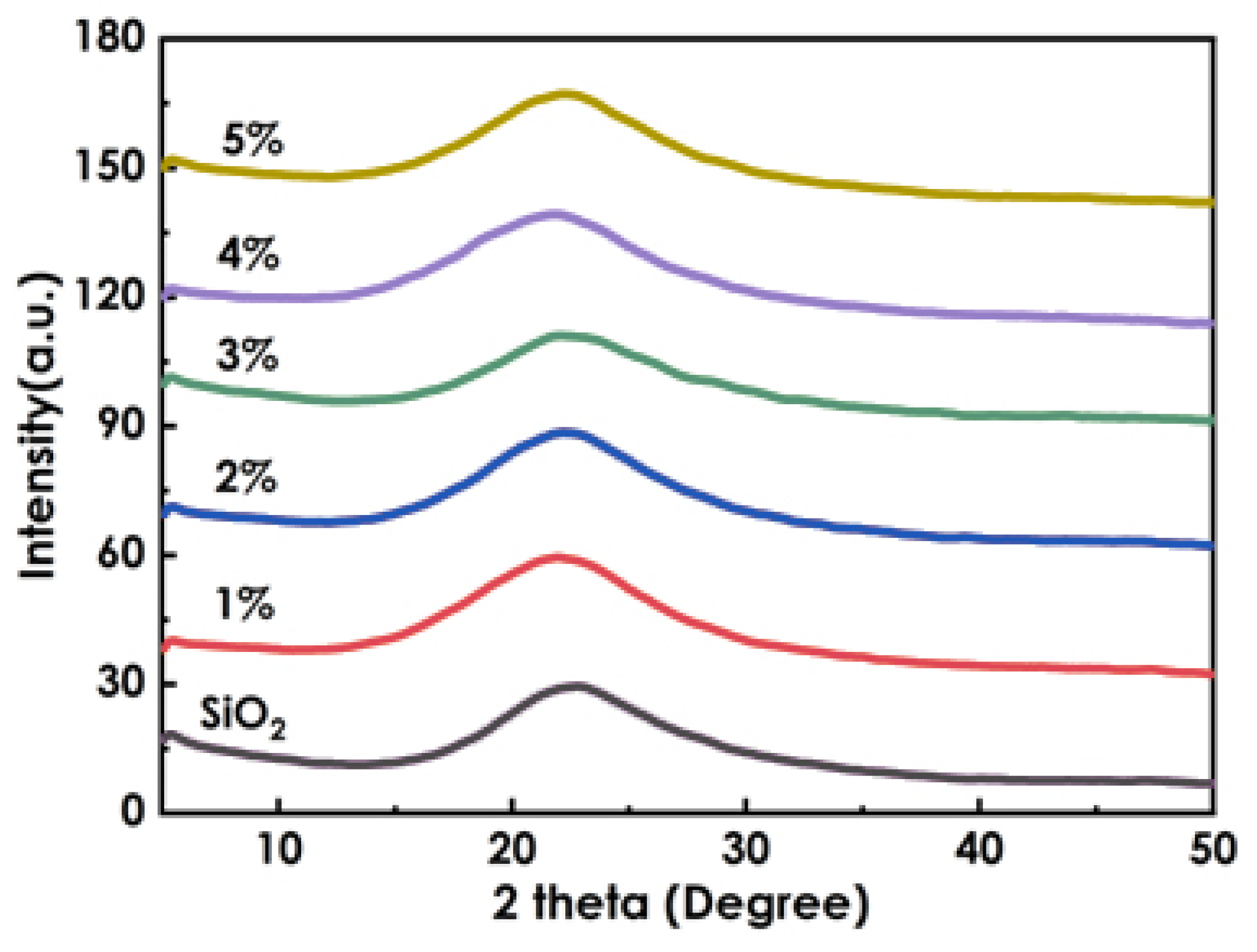
2.4.2. XRD Spectra
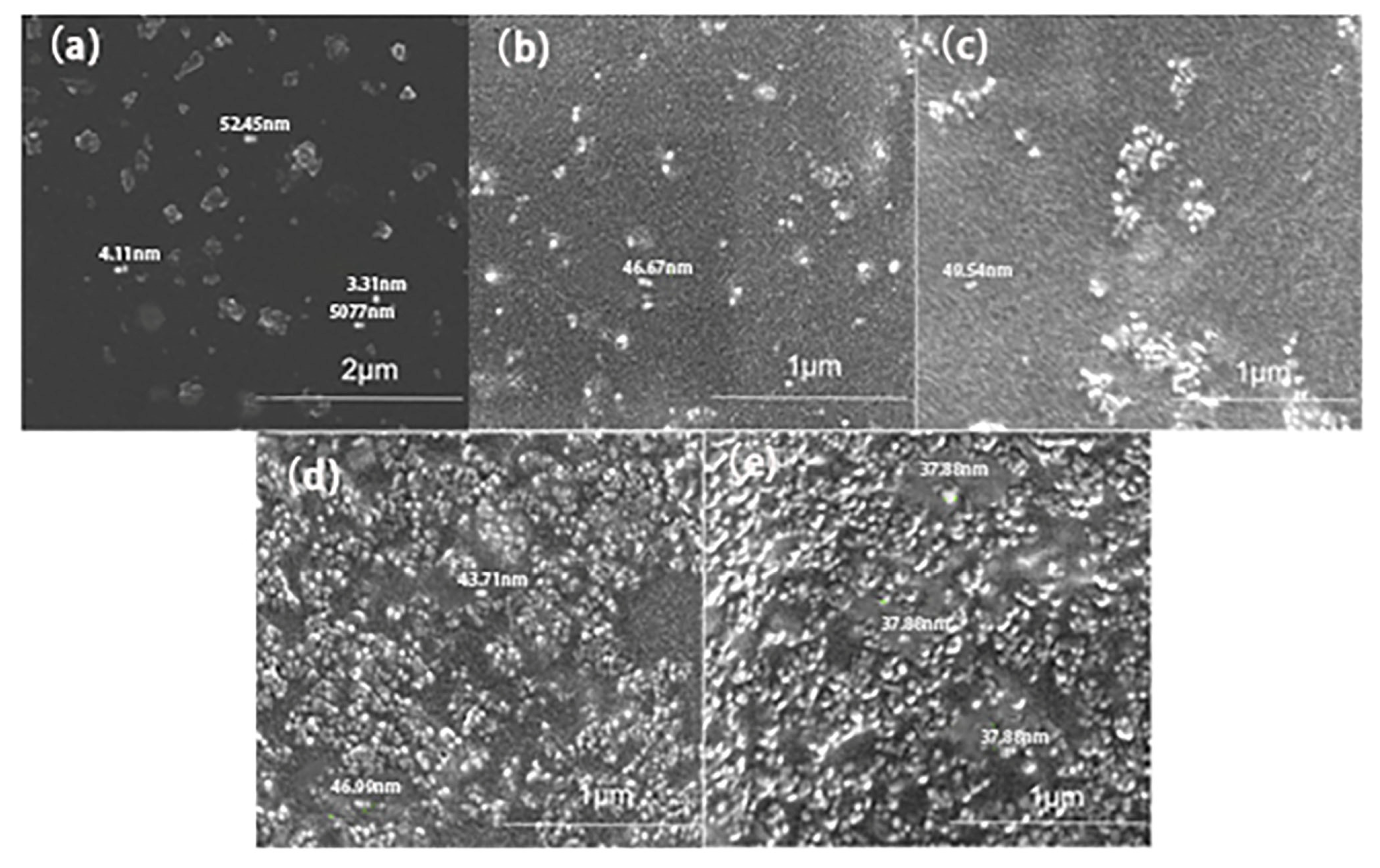
2.4.3. Scanning Electron Microscope (SEM) Analysis
2.4.4. Thermogravimetric Analysis
2.4.5. Mechanical Property Tests
2.4.6. Haze Tests
2.4.7. Wear Resistance Test
2.4.8. The Contact Angle Test
2.4.9. Particle Size Analysis
3. Results
3.1. Effect of Different Afcona 4071 Additions on the Particle Size of Nano-SiO2
3.2. Coating Formulation Optimization and Coating Performance Test
- (1)
- Effect of monomer type and dosage on coating properties
- (2)
- Effect of different resin ratios on coating properties
3.3. IR Testing of Composite Coatings
3.4. SEM Analysis of Composite Coatings
3.5. XRD Analysis of Composite Coatings
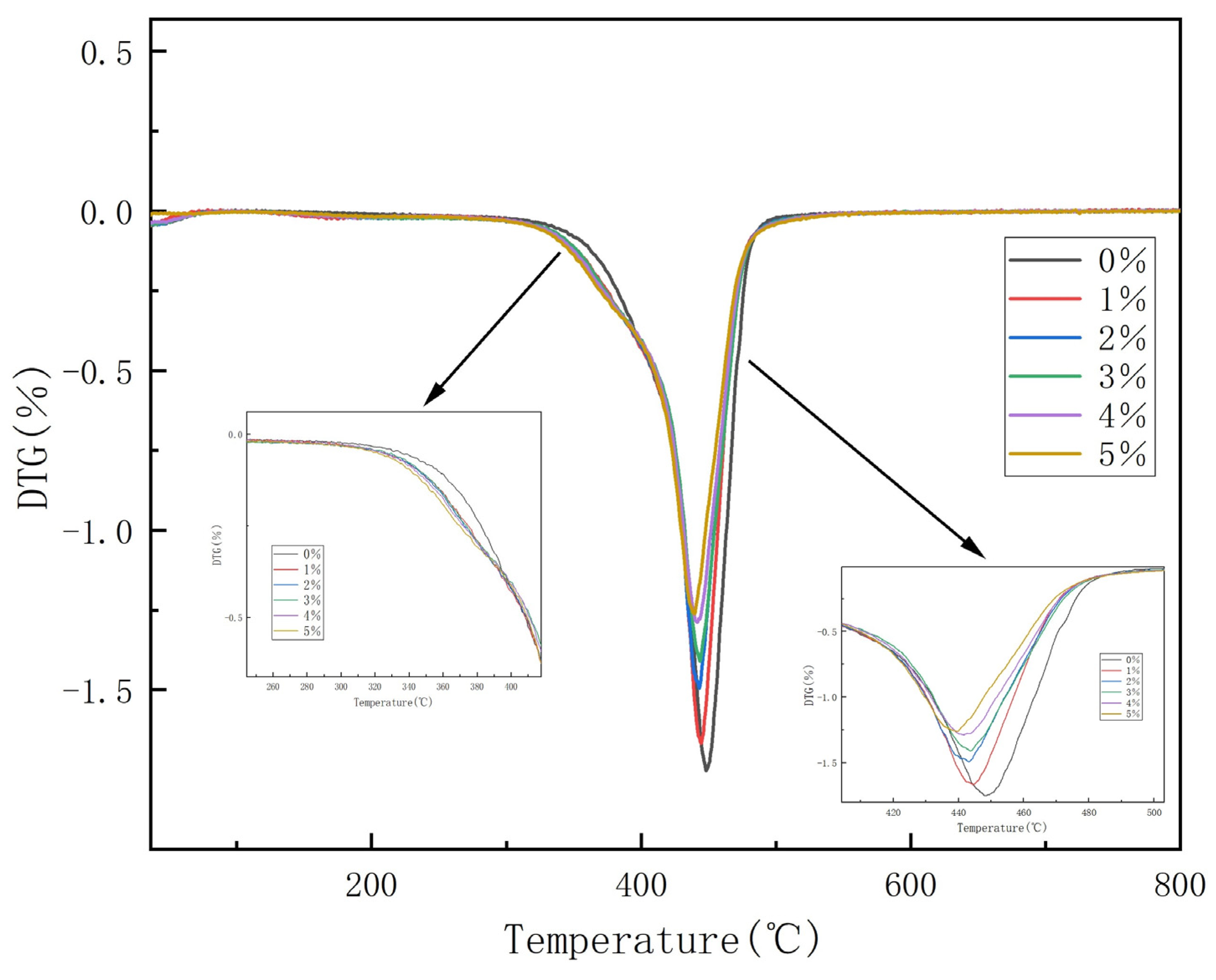
3.6. Thermogravimetric Analysis of Composite Coatings
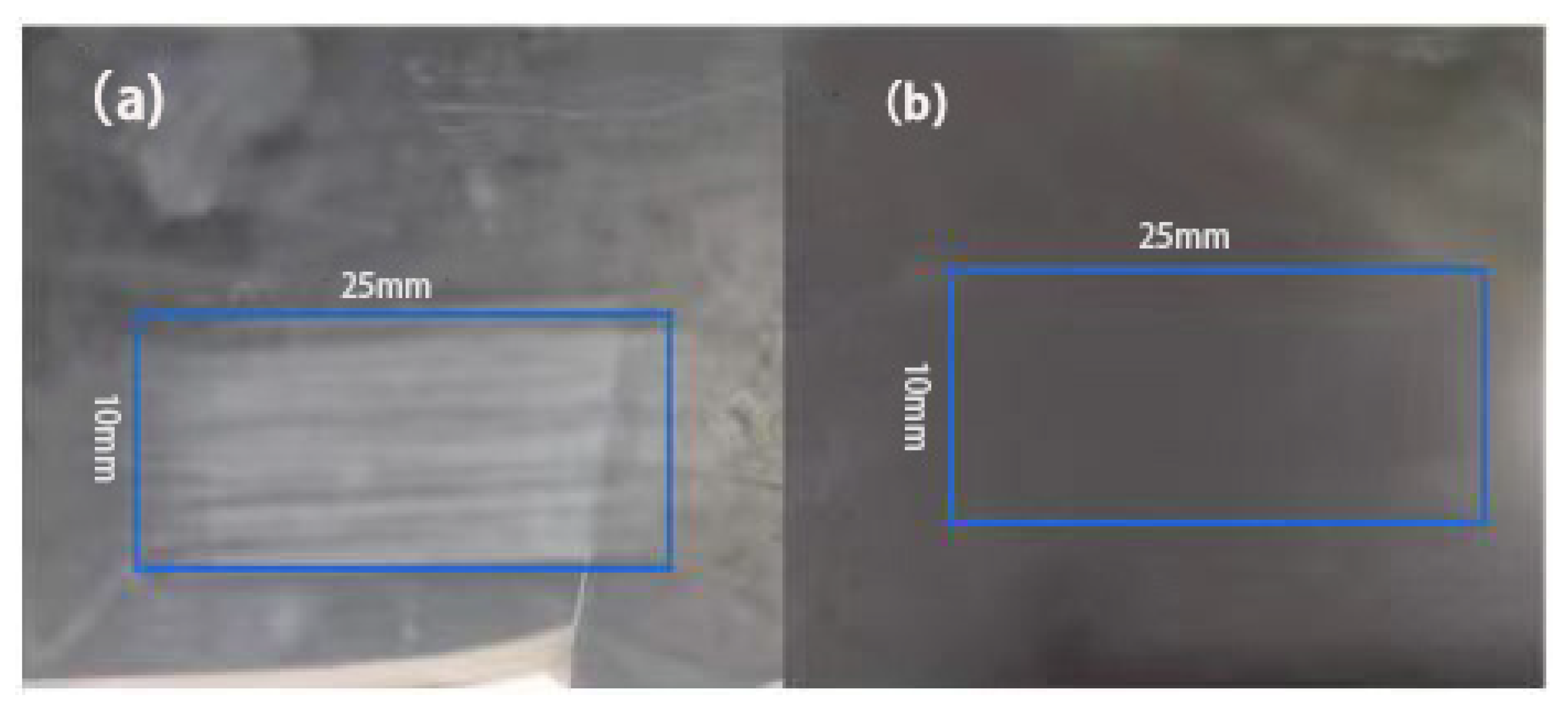
3.7. Wear Resistance Test of Composite Coatings
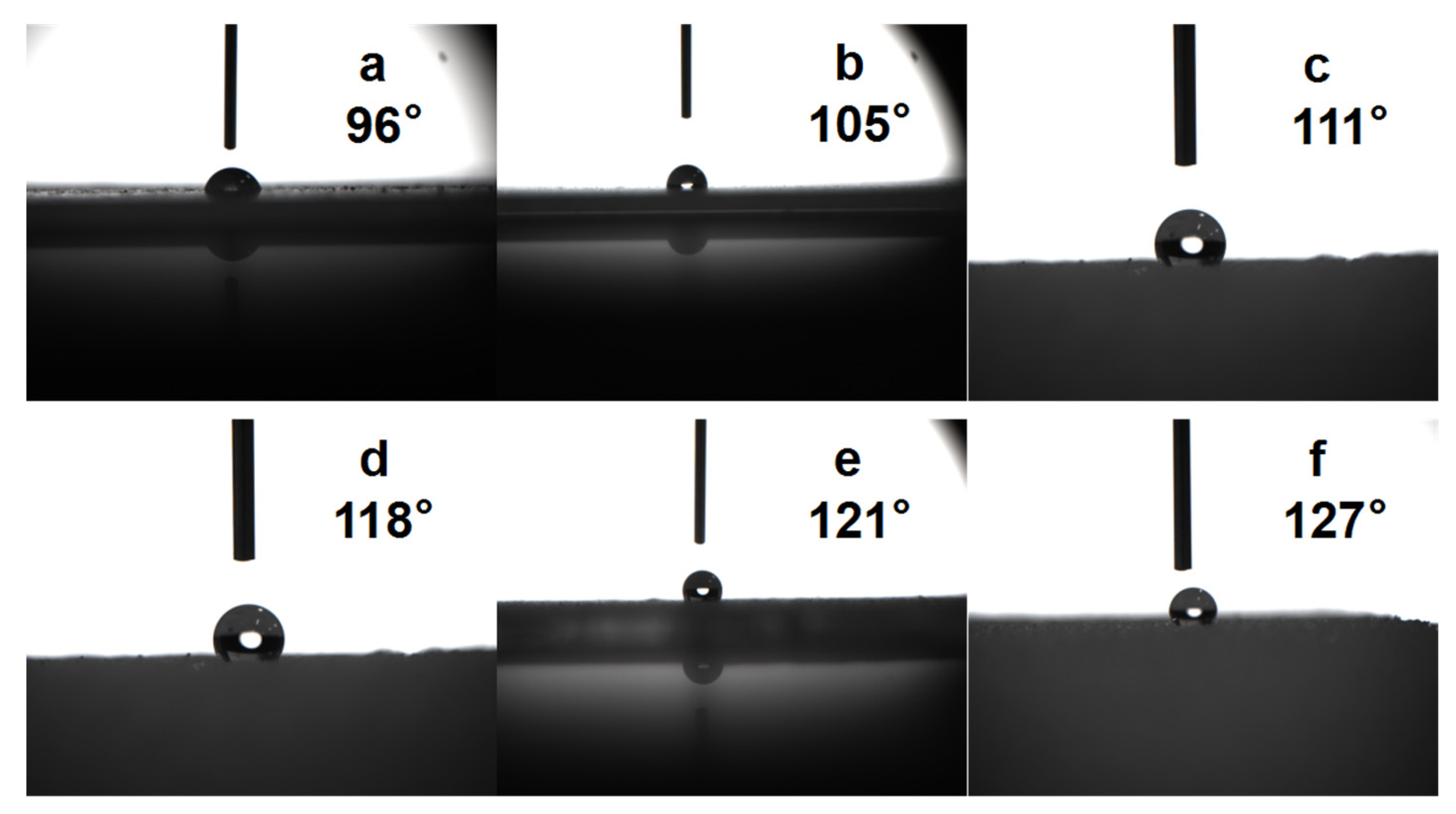
3.8. Contact Angle Test of Composite Coatings
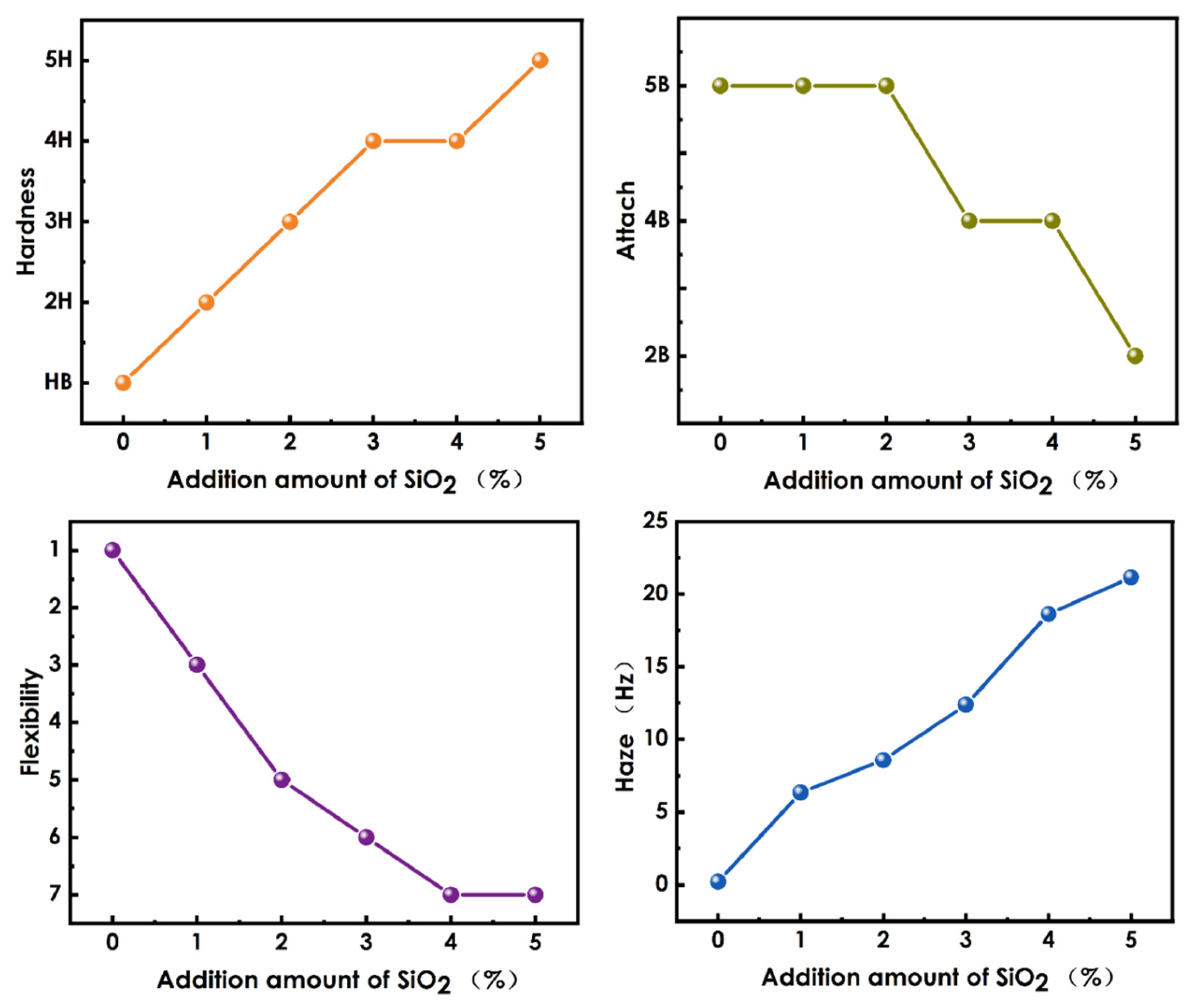
3.9. Evaluation of Haze and Mechanical Properties of Composite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Decker, C. Photoinitiated crosslinking polymerization. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Bajpai, M.; Vipin, S.; Arun, K. Film performance and UV curing of epoxy acrylate resins. Prog. Org. Coat. 2002, 44, 271–278. [Google Scholar] [CrossRef]
- Hang, Z.; Yu, H.; Luo, L.; Huai, X. Nanoporous g-C3N4/MOF: High-performance photoinitiator for UV-curable coating. J. Mater. Sci. 2019, 54, 13959–13972. [Google Scholar] [CrossRef]
- Ong, G.; Ramesh, K.; Ramesh, S. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- He, J.; Zhou, L.; Soucek, M.D.; Wollyung, K.M.; Wesdemiotis, C. UV-curable hybrid coatings based on vinylfunctionlized siloxane oligomer and acrylated polyester. J. Appl. Polym. Sci. 2007, 105, 2376–2386. [Google Scholar] [CrossRef]
- Nakayama, N.; Toyoharu, H. Synthesis of novel UV-curable difunctional thiourethane methacrylate and studies on organic–inorganic nanocomposite hard coatings for high refractive index plastic lenses. Prog. Org. Coat. 2008, 62, 274–284. [Google Scholar] [CrossRef]
- Lauppi, U.V. Radiation curing—An overview. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1990, 35, 30–35. [Google Scholar] [CrossRef]
- Fu, J.; Yu, H.; Wang, L.; Liang, R.; Zhang, C.; Jin, M. Preparation and properties of UV-curable polyurethane acrylate/SiO2 composite hard coatings. Prog. Org. Coat. 2021, 153, 106121. [Google Scholar] [CrossRef]
- Minh, H.; Chinh, N.; Van, T.; T Hoang, T. Ternary nanocomposites based on epoxy, modified silica, and tetrabutyl titanate: Morphology, characteristics, and kinetics of the curing process. J. Appl. Polym. Sci. 2019, 136, 47412. [Google Scholar] [CrossRef]
- Klaus, B.; Holzmann, C.; Rubatscher, E.; Schwarzinger, C.; Roessler, A.; Paulik, C. UV-curable coatings of highly crosslinked trimethylmelamine based acrylates and methacrylates. Eur. Polym. J. 2013, 49, 4141–4148. [Google Scholar]
- Schmitz, C.; Poplata, T.; Feilen, A.; Strehmel, B. Radiation crosslinking of pigmented coating material by UV LEDs enabling depth curing and preventing oxygen inhibition. Prog. Org. Coat. 2020, 144, 105663. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, J.; Luo, J. Phytic acid-based adhesion promoter for UV-curable coating: High performance, low cost, and eco-friendliness. Prog. Org. Coat. 2022, 167, 106834. [Google Scholar] [CrossRef]
- Yao, C.; Xing, W.; Ma, C.; Song, L.; Hu, Y.; Zhuang, Z. Synthesis of phytic acid-based monomer for UV-Cured coating to improve fire safety of PMMA. Prog. Org. Coat. 2020, 140, 105497. [Google Scholar] [CrossRef]
- Mejiritski, A.; Sarker, A.M.; Wheaton, B.; Neckers, D.C. Novel method of thermal epoxy curing based photogeneration ofpolymeric amines and negative-tone image formation. Chem. Mater. 1997, 9, 1488–1494. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Lai, G. Fabrication of UV-curable solvent-free epoxy modified silicone resin coating with high transparency and low volume shrinkage. Prog. Org. Coat. 2019, 129, 96–100. [Google Scholar] [CrossRef]
- Wu, H.; Han, X.; Zhao, W.; Zhang, Q.; Zhao, A.; Xia, J. Mechanical and electrochemical properties of UV-curable nanocellulose/urushiol epoxy acrylate anti-corrosive composite coatings. Ind. Crops Prod. 2022, 181, 114805. [Google Scholar] [CrossRef]
- Magnoni, F.; Rannée, A.; Marasinghe, L.; El-Fouhaili, B.; Allonas, X.; Croutxé-Barghorn, C. Correlation between the scratch resistance of UV-cured PUA-based coatings and the structure and functionality of reactive diluents. Prog. Org. Coat. 2018, 124, 193–199. [Google Scholar] [CrossRef]
- Teijido, R.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog. Org. Coat. 2022, 163, 106684. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Zhang, Z.; Ma, W. In situ assembly of halloysite nanotubes@cerium oxide nanohybrid for highly UV-shielding and superhydrophobic coating. J. Alloys Compd. 2019, 811, 151986. [Google Scholar] [CrossRef]
- Liu, M.J.; Zhang, M.; Zhang, X.F.; Li, G.R.; Zhang, Q.; Li, C.X.; Li, C.J.; Yang, G.J. Transport and deposition behaviors of vapor coating materials in plasma spray-physical vapor deposition. Appl. Surf. Sci. 2019, 486, 80–92. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, P.; Zhao, H.; Li, Z.; Liu, X.; Zhang, K.; Liu, B. SiC coating on HTR graphite spheres prepared by fluidized-bed chemical vapor deposition. Ann. Nuclear Energy 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Babur, M.Z.; Iqbal, Z.; Shafiq, M.; Naz, M.Y.; Makhlouf, M.M. Hybrid TiN-CCPN coating of AISI-201 stainless steel by physical vapor deposition combined with cathodic cage plasma nitriding for improved tribological properties. J. Build. Eng. 2022, 45, 103512. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liao, R.M.; Lai, L.W.; Jeng, M.S.; Liu, D.S. Organosilicon/silicon oxide gas barrier structure encapsulated flexible plastic substrate by using plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2012, 206, 4685–4691. [Google Scholar] [CrossRef]
- Parker, T.; Daniel, B.; Demaree, J. Polymeric barrier coatings via initiated chemical vapor deposition. Surf. Coat. Technol. 2011, 206, 1680–1683. [Google Scholar] [CrossRef]
- Kong, Q.; Li, Z.; Zhang, Z.; Ren, X. Functionlization of PET fabric via silicone based organic-inorganic hyhrid coarings. J. Ind. Eng. Chem. 2020, 83, 430–437. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, K.; Lee, Y.; Seo, J.; Seo, K.; Han, H.; Khan, S. Preparation and characterization of UV-cured polyurethane acrylate/ZnO nanocomposite films based on surface modified ZnO. Prog. Org. Coat. 2012, 74, 435–442. [Google Scholar] [CrossRef]
- Rêgo, G.C.; Bandeira, R.M.; van Drunen, J.; Tremiliosi-Filho, G.; Casteletti, L.C. Multi-layer organic-inorganic hybrid anticorrosive coatings for protectionof medium carbon steel. Mater. Chem. Phys. 2023, 303, 127841. [Google Scholar] [CrossRef]
- Karataş, S.; Hoşgör, Z.; Menceloğlu, Y.; Kayaman-Apohan, N.; Güngör, A. Synthesis and characterization of flame retarding UV-curable organic-inorganic hybrid coatings. J. Appl. Polym. Sci. 2006, 102, 1906–1914. [Google Scholar] [CrossRef]
- Soumya, S.; Sheemol, V.N.; Amba, P.; Mohamed, A.P.; Ananthakumar, S. Sn and Ag doped ZnO quantum dots with PMMA by in situ polymerization for UV/IR protective, photochromic multifunctional hybrid coatings. Sol. Energy Mater. Sol. Cells 2018, 174, 554–565. [Google Scholar] [CrossRef]
- Qiao, Q.; Shen, Z.Q.; Wu, X.S.; Wang, X.Z.; Pei, W.B.; Liu, S.X.; Ren, X.M. Glowing kaolinite intercalated with N-Methyl imidazole and Eu3+/Tb3+ salts and potential application in UV-to-red light conversion. Appl. Clay Sci. 2020, 186, 105473. [Google Scholar] [CrossRef]
- Pan, C.; Shen, L.; Shang, S.; Xing, Y. Preparation of superhydrophobic and UV blocking cotton fabric via sol–gel method and self-assembly. Appl. Surf. Sci. 2012, 259, 110–117. [Google Scholar] [CrossRef]
- You, Z.; Zhang, M.; Wang, J.; Pei, W.; Li, G. Experimental study of optical and cooling performances of CuO and TiO2 near-infrared reflective blending coatings. Sol. Energy 2021, 225, 19–32. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Hong, P.; Chen, L.; Liu, X. Synthesis of organic and inorganic hybrid nanoparticles as multifunctional photoinitiator and its application in UV-curable epoxy acrylate-based coating systems. Prog. Org. Coat. 2020, 141, 105565. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyen, A.H.; Le, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, J.; Shi, J.; Zhao, Y.; He, M.; Ding, L. Bio-polyols based waterborne polyurethane coatings reinforced with chitosan-modified ZnO nanoparticles. Int. J. Biol. Macromol. 2022, 208, 97–104. [Google Scholar] [CrossRef]
- Dao, P.H.; Nguyen, T.D.; Nguyen, T.C.; Nguyen, A.H.; Tran, H.T.; Phung, T.L.; Vu, Q.T.; Vu, D.H.; Ngo, T.C.Q.; Vu, M.C.; et al. Assessment of some characteristics, properties of a novel waterborne acrylic coating incorporated TiO2 nanoparticles modified with silane coupling agent and Ag/Zn zeolite. Prog. Org. Coat. 2022, 163, 106641. [Google Scholar] [CrossRef]
- Xu, T.; Zvonkina, I.J.; Soucek, M.D. UV-curable polyurethane inorganic–organic hybrid coatings. J. Coat. Technol. Res. 2021, 18, 1461–1479. [Google Scholar] [CrossRef]
- Habib, S.; Hassanein, A.; Kahraman, R.; Ahmed, E.M.; Shakoor, R.A. Self-healing behavior of epoxy-based double-layer nanocomposite coatings modified with Zirconia nanoparticles. Mater. Des. 2021, 207, 109839. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, R.; Lai, T.; Chen, T. Preparation of UV-curable nano-WO3 coatingand its infrared shielding properties. Nanomaterials 2022, 12, 12213920. [Google Scholar]
- Lai, T.; Qin, W.; Cao, C.; Zhong, R.; Ling, Y.; Xie, Y. Preparation of a microwave absorbing UV coating using a BaFe12O19 polypyrrole nanocomposite filler. Polymers 2023, 15, 5081839. [Google Scholar] [CrossRef]
- ASTM D3363-05; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2008.
- GB/T 1731-1993; Determination of Flexibility of Films. National Standardization Technical Committee Pigment Coatings: Changzhou, China, 1993.
- Liu, J.; Wu, K.; Liu, R.; Sun, G.; Luo, J. Robust SiO2/polymer hybrid microcapsule synthesized via emulsion photo-polymerization and its application in self-lubricating coatings. Prog. Org. Coat. 2023, 184, 107856. [Google Scholar] [CrossRef]



| B-619W | HDDA | DPHA | 184 | |
|---|---|---|---|---|
| Formula 1 | 80 | 15 | 0 | 5 |
| Formula 2 | 80 | 10 | 5 | 5 |
| Formula 3 | 80 | 7.5 | 7.5 | 5 |
| Formula 4 | 80 | 5 | 10 | 5 |
| Formula 5 | 80 | 0 | 15 | 5 |
| Hardness | Adhesion | Flexibility | |
|---|---|---|---|
| Formula 1 | H/500 g | 5B | 3 |
| Formula 2 | 2H/500 g | 4B | 4 |
| Formula 3 | 2H/750 g | 4B | 6 |
| Formula 4 | 2H/1000 g | HB | 7 |
| Formula 5 | 3H/500 g | 0B | 8 |
| B-619W | HDDA | DPHA | 184 | |
|---|---|---|---|---|
| Formula 6 | 70 | 12.5 | 12.5 | 5 |
| Formula 7 | 75 | 10 | 10 | 5 |
| Formula 8 | 80 | 7.5 | 7.5 | 5 |
| Formula 9 | 85 | 5 | 5 | 5 |
| Formula 10 | 90 | 2.5 | 2.5 | 5 |
| Hardness | Adhesion | Flexibility | |
|---|---|---|---|
| Formula 6 | 2H/500 g | 5B | 8 |
| Formula 7 | HB/750 g | 5B | 1 |
| Formula 8 | 2H/750 g | 4B | 6 |
| Formula 9 | H/1000 g | 3B | 6 |
| Formula 10 | H/750 g | 2B | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Zhong, R.; Wang, Z. Preparation, Characterization, and Properties of UV-Curable Coating Doped with Nano-SiO2. Materials 2023, 16, 7576. https://doi.org/10.3390/ma16247576
Chen T, Zhong R, Wang Z. Preparation, Characterization, and Properties of UV-Curable Coating Doped with Nano-SiO2. Materials. 2023; 16(24):7576. https://doi.org/10.3390/ma16247576
Chicago/Turabian StyleChen, Tianlei, Rong Zhong, and Zhengjie Wang. 2023. "Preparation, Characterization, and Properties of UV-Curable Coating Doped with Nano-SiO2" Materials 16, no. 24: 7576. https://doi.org/10.3390/ma16247576
APA StyleChen, T., Zhong, R., & Wang, Z. (2023). Preparation, Characterization, and Properties of UV-Curable Coating Doped with Nano-SiO2. Materials, 16(24), 7576. https://doi.org/10.3390/ma16247576





