Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration
Abstract
:1. Introduction
2. Bioactive Glasses
3. Bioactive Glasses Containing Strontium
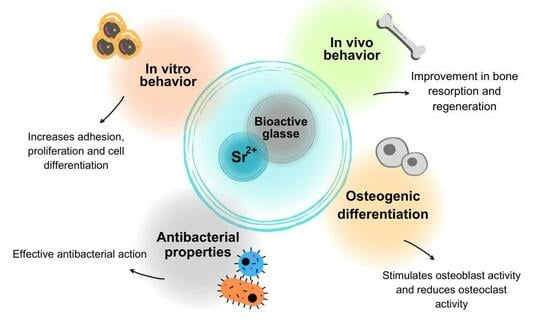
3.1. In Vitro Behavior
3.2. Osteogenic Differentiation
3.3. In Vivo Behavior
3.4. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Choy, K.L.; Schnabelrauch, M.; Wyrwa, R. Bioactive coatings. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Springer: Berlin/Heidelberg, Germany, 2018; pp. 361–406. [Google Scholar]
- Chacko, N.L.; Abraham, S.; Rao, H.S.; Sridhar, N.; Moon, N.; Barde, D.H. A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas®: A synthetic, resorbable material in treating periodontal infrabony defects. J. Int. Oral Health JIOH 2014, 6, 20. [Google Scholar] [PubMed]
- Blayney, A.; Bebear, J.-P.; Williams, K.; Portmann, M. Ceravital© in ossiculoplasty: Experimental studies* and early clinical results. J. Laryngol. Otol. 1986, 100, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.; Vallet-Regi, M.; Heikkilä, J. Use of bioactive glasses as bone substitutes in orthopedics and traumatology. In Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2018; pp. 337–364. [Google Scholar]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Chitra, S.; Bargavi, P.; Durgalakshmi, D.; Rajashree, P.; Balakumar, S. Role of sintering temperature dependent crystallization of bioactive glasses on erythrocyte and cytocompatibility. Process. Appl. Ceram. 2019, 13, 12–23. [Google Scholar] [CrossRef]
- Barbeck, M.; Alkildani, S.; Mandlule, A.; Radenković, M.; Najman, S.; Stojanović, S.; Jung, O.; Ren, Y.; Cai, B.; Görke, O.; et al. In Vivo Analysis of the Immune Response to Strontium-and Copper-doped Bioglasses. In Vivo 2022, 36, 2149–2165. [Google Scholar] [CrossRef]
- Stähli, C.; James-Bhasin, M.; Hoppe, A.; Boccaccini, A.R.; Nazhat, S.N. Effect of ion release from Cu-doped 45S5 Bioglass® on 3D endothelial cell morphogenesis. Acta Biomater. 2015, 19, 15–22. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Giannoulatou, V.; Theodorou, G.; Zorba, T.; Kontonasaki, E.; Papadopoulou, L.; Kantiranis, N.; Chrissafis, K.; Zachariadis, G.; Paraskevopoulos, K. Magnesium calcium silicate bioactive glass doped with copper ions; synthesis and in-vitro bioactivity characterization. J. Non-Cryst. Solids 2018, 500, 98–109. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Nardone, V.; Zonefrati, R.; Mavilia, C.; Romagnoli, C.; Ciuffi, S.; Fabbri, S.; Palmini, G.; Galli, G.; Tanini, A.; Brandi, M. In vitro effects of strontium on proliferation and osteoinduction of human preadipocytes. Stem Cells Int. 2015, 2015, 871863. [Google Scholar] [CrossRef] [PubMed]
- Ranga, N.; Poonia, E.; Jakhar, S.; Sharma, A.K.; Kumar, A.; Devi, S.; Duhan, S. Enhanced antimicrobial properties of bioactive glass using strontium and silver oxide nanocomposites. J. Asian Ceram. Soc. 2019, 7, 75–81. [Google Scholar] [CrossRef]
- Li, Y.; Stone, W.; Schemitsch, E.H.; Zalzal, P.; Papini, M.; Waldman, S.D.; Towler, M.R. Antibacterial and osteo-stimulatory effects of a borate-based glass series doped with strontium ions. J. Biomater. Appl. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.M.; Copes, R.M.; Dal Osto, L.C.; Flores, C.; Comim, F.V.; Premaor, M.O. Factors related with osteoporosis treatment in postmenopausal women. Medicine 2018, 97, e11524. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri, H.; Ghorbani, M.; Moosazadeh Moghaddam, M.; Nourani, M.R.; Makvandi, P.; Sharifi, E. Strontium doped bioglass incorporated hydrogel-based scaffold for amplified bone tissue regeneration. Sci. Rep. 2022, 12, 10160. [Google Scholar] [CrossRef]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced applications of strontium-containing biomaterials in bone tissue engineering. Mater. Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef]
- Rui, Z.; Xiao, Y.; Xiangdong, Z.; Xingdong, Z. Application of Trace Element Strontium-Doped Biomaterials in the Field of Bone Regeneration. Prog. Chem. 2021, 33, 533. [Google Scholar]
- Wei, L.; Ke, J.; Prasadam, I.; Miron, R.J.; Lin, S.; Xiao, Y.; Chang, J.; Wu, C.; Zhang, Y. A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos. Int. 2014, 25, 2089–2096. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M.; Oudadesse, H.; Akbarzadeh, R.; Wers, E.; Lucas-Girot, A. Physical and biological characteristics of nanohydroxyapatite and bioactive glasses used for bone tissue engineering. Nanotechnol. Rev. 2014, 3, 527–552. [Google Scholar] [CrossRef]
- Hench, L.L. Sol-gel materials for bioceramic applications. Curr. Opin. Solid State Mater. Sci. 1997, 2, 604–610. [Google Scholar] [CrossRef]
- Lee, K.; Park, M.; Kim, H.; Lim, Y.; Chun, H.J.; Kim, H.; Moon, S. Ceramic bioactivity: Progresses, challenges and perspectives. Biomed. Mater. 2006, 1, R31. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Glass and glass-ceramic technologies to transform the world. Int. J. Appl. Glass Sci. 2011, 2, 162–176. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Lobel, K.; Hench, L. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 39, 575–579. [Google Scholar] [CrossRef]
- Ohgushi, H.; Dohi, Y.; Yoshikawa, T.; Tamai, S.; Tabata, S.; Okunaga, K.; Shibuya, T. Osteogenic differentiation of cultured marrow stromal stem cells on the surface of bioactive glass ceramics. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 32, 341–348. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Roether, J.; Gough, J.; Boccaccini, A.R.; Hench, L.; Maquet, V.; Jérôme, R. Novel bioresorbable and bioactive composites based on bioactive glass and polylactide foams for bone tissue engineering. J. Mater. Sci. Mater. Med. 2002, 13, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Production and potential of bioactive glass nanofibers as a next-generation biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Romeis, S.; Hoppe, A.; Eisermann, C.; Schneider, N.; Boccaccini, A.R.; Schmidt, J.; Peukert, W. Enhancing in vitro bioactivity of melt-derived 45S5 Bioglass® by comminution in a stirred media mill. J. Am. Ceram. Soc. 2014, 97, 150–156. [Google Scholar] [CrossRef]
- Wang, E.; Li, X.; Zhang, Y.; Ma, L.; Xu, Q.; Yue, Y.; Wang, W.; Li, Q.; Yu, J.; Chang, J. Multi-functional black bioactive glasses prepared via containerless melting process for tumor therapy and tissue regeneration. Adv. Funct. Mater. 2021, 31, 2101505. [Google Scholar] [CrossRef]
- Spirandeli, B.; Ribas, R.; Amaral, S.; Martins, E.; Esposito, E.; Vasconcellos, L.; Campos, T.; Thim, G.; Trichês, E. Incorporation of 45S5 bioglass via sol-gel in β-TCP scaffolds: Bioactivity and antimicrobial activity evaluation. Mater. Sci. Eng. C 2021, 131, 112453. [Google Scholar] [CrossRef]
- Ghiţulică, C.-D.; Damian-Buda, A.-I.; Cucuruz, A.; Voicu, G. Synthesis and characterization of ZnO (MgO)-CaO-SiO2-P2O5 bioglass obtained by sol-gel method in presence of surfactant agent. Gels 2021, 7, 187. [Google Scholar] [CrossRef]
- Canaveral, S.; Morales, D.; Vargas, A.F. Synthesis and characterization of a 58S bioglass modified with manganese by a sol-gel route. Mater. Lett. 2019, 255, 126575. [Google Scholar] [CrossRef]
- El Baakili, S.; El Mabrouk, K.; Bricha, M. Acellular bioactivity and drug delivery of new strontium doped bioactive glasses prepared through a hydrothermal process. RSC Adv. 2022, 12, 15361–15372. [Google Scholar] [CrossRef] [PubMed]
- Hoa, B.T.; Hoa, H.T.T.; Tien, N.A.; Khang, N.H.D.; Guseva, E.V.; Tuan, T.A.; Vuong, B.X. Green synthesis of bioactive glass 70SiO2-30CaO by hydrothermal method. Mater. Lett. 2020, 274, 128032. [Google Scholar] [CrossRef]
- Scarber, R.E.; Salaam, A.D.; Thomas, V.; Janowski, G.M.; Dean, D. Direct sol–gel electrospinning of fibrous bioglass scaffolds for bone tissue engineering. J. Biomater. Tissue Eng. 2013, 3, 440–447. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Balakumar, S. Phase separation induced shell thickness variations in electrospun hollow Bioglass 45S5 fiber mats for drug delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 15316–15323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, Y.; Liu, D.; Liu, Y.; Ke, Q.; Xu, H. A bioglass sustained-release scaffold with ECM-like structure for enhanced diabetic wound healing. Nanomedicine 2020, 15, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.L.; Gomes, D.S.; Santos, A.M.; Vieira, R.H.; de Lima, I.L.; Rocha, F.S.; Castro-Filice, L.d.S.; Medeiros, E.S.; Neves, G.A.; Menezes, R.R. 3D nanofibrous bioactive glass scaffolds produced by one-step spinning process. Ceram. Int. 2021, 47, 102–110. [Google Scholar] [CrossRef]
- Quintero, F.; Pou, J.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; Mann, A.B.; Hill, R.G.; Wu, Z.Y.; Jones, J.R. Laser spinning of bioactive glass nanofibers. Adv. Funct. Mater. 2009, 19, 3084–3090. [Google Scholar] [CrossRef]
- Riveiro, A.; Amorim, S.; Solanki, A.; Costa, D.S.; Pires, R.A.; Quintero, F.; Del Val, J.; Comesaña, R.; Badaoui, A.; Lusquiños, F. Hyaluronic acid hydrogels reinforced with laser spun bioactive glass micro-and nanofibres doped with lithium. Mater. Sci. Eng. C 2021, 126, 112124. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, I.; Grant, D.; Nommeots-Nomm, A.; Hussain, T. Effect of processing on microstructure, mechanical properties and dissolution behaviour in SBF of Bioglass (45S5) coatings deposited by Suspension High Velocity Oxy Fuel (SHVOF) thermal spray. Surf. Coat. Technol. 2019, 372, 229–238. [Google Scholar] [CrossRef]
- Bano, S.; Romero, A.R.; Grant, D.; Nommeots-Nomm, A.; Scotchford, C.; Ahmed, I.; Hussain, T. In-vitro cell interaction and apatite forming ability in simulated body fluid of ICIE16 and 13–93 bioactive glass coatings deposited by an emerging suspension high velocity oxy fuel (SHVOF) thermal spray. Surf. Coat. Technol. 2021, 407, 126764. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Wang, Y.; Cheng, D. Preparation and characterization of the system Si2-CaO-P2O5 bioactive glasses by microemulsion approach. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 1053–1057. [Google Scholar] [CrossRef]
- Boccardi, E.; Philippart, A.; Juhasz-Bortuzzo, J.; Novajra, G.; Vitale-Brovarone, C.; Boccaccini, A.R. Characterisation of Bioglass based foams developed via replication of natural marine sponges. Adv. Appl. Ceram. 2015, 114, S56–S62. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P (3HB) microsphere/45S5 Bioglass®-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gong, J.; Zhang, H.; Ni, Y.; Cheng, L.; Song, Q.; Tang, L.; Xing, F.; Liu, M.; Zhou, C. Preparation and Characterization of 3D Printed Porous 45S5 Bioglass Bioceramic for Bone Tissue Engineering Application. Int. J. Bioprinting 2022, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, M.N.; Peña, J.; Ivanovski, S.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. Multiscale porosity in mesoporous bioglass 3D-printed scaffolds for bone regeneration. Mater. Sci. Eng. C 2021, 120, 111706. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, M.N.; Lozano, D.; Arcos, D.; Vallet-Regi, M.; Vaquette, C. The effect of biomimetic mineralization of 3D-printed mesoporous bioglass scaffolds on physical properties and in vitro osteogenicity. Mater. Sci. Eng. C 2020, 109, 110572. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Wu, X.; Meng, G.; Wang, S.; Wu, F.; Huang, W.; Gu, Z. Zn and Sr incorporated 64S bioglasses: Material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater. Sci. Eng. C 2015, 52, 242–250. [Google Scholar] [CrossRef]
- Hope, T.C.I. Account of a mineral from strontian, and of a peculiar species of earth which it contains. Earth Environ. Sci. Trans. R. Soc. Edinb. 1798, 4, 3–39. [Google Scholar] [CrossRef]
- Watts, P.; Howe, P. Strontium and Strontium Compounds; World Health Organization: Geneva, Switzerland, 2010.
- Rosenthal, H.L.; Eves, M.M.; Cochran, O.A. Common strontium concentrations of mineralized tissues from marine and sweet water animals. Comp. Biochem. Physiol. 1970, 32, 445–450. [Google Scholar] [CrossRef]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef] [PubMed]
- Papillon, M. Recherches experimentales sur les modifications de la composition immediate des os. CR Acad. Sci 1870, 71, 372–374. [Google Scholar]
- Altman, G.B.; Lee, C.A. Strontium-89 for treatment of painful bone metastasis from prostate cancer. Oncol. Nurs. Forum 1996, 23, 523–527. [Google Scholar] [PubMed]
- Zhao, B.; Li, X.; Xu, H.; Jiang, Y.; Wang, D.; Liu, R. Influence of simvastatin-strontium-hydroxyapatite coated implant formed by micro-arc oxidation and immersion method on osteointegration in osteoporotic rabbits. Int. J. Nanomed. 2020, 15, 1797–1807. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef]
- Amudha, S.; Ramya, J.R.; Arul, K.T.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.-L.; Kalkura, S.N. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Liu, X.; Huang, H.; Zhang, J.; Sun, T.; Zhang, W.; Li, Z. Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering 2023, 10, 414. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Yazdanian, M.; Tebyanian, H.; Tahmasebi, E.; Yazdanian, A.; Seifalian, A.; Tavakolizadeh, M. Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J. Mater. Res. Technol. 2020, 9, 14799–14817. [Google Scholar] [CrossRef]
- Morohashi, T.; Izumisawa, T.; Matsumoto, A.; Yamada, S. The effects of stable strontium on calcium metabolism: I. Kinetic analysis of calcium metabolism in strontium-fed rats. J. Bone Miner. Metab. 1993, 11, 31–38. [Google Scholar] [CrossRef]
- Marie, P.; Garba, M.-T.; Hott, M.; Miravet, L. Effect of stable strontium on bone metabolism in rats. In Proceedings of the Metals in Bone: Proceedings of the a EULEP Symposium on the Deposition, Retention and Effects of Radioactive and Stable Metals in Bone and Bone Marrow Tissues, Angers, France, 11–13 October 1984; Springer: Berlin/Heidelberg, Germany, 1985; pp. 117–125. [Google Scholar]
- Marie, P.J.; Hott, M. Short-term effects of fluoride and strontium on bone formation and resorption in the mouse. Metabolism 1986, 35, 547–551. [Google Scholar] [CrossRef]
- Mourino, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Della Bella, E.; Fini, M.; Bigi, A. Combined effect of strontium and zoledronate on hydroxyapatite structure and bone cell responses. Biomaterials 2014, 35, 5619–5626. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bhatia, A.; Varma, H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent. Mater. 2016, 32, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.-W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hill, R.G.; Rawlinson, S.C. Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater. 2016, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Y.; Zhou, Y. Strontium functionalized in biomaterials for bone tissue engineering: A prominent role in osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.-I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K. PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, J.W. Multifunctional effects of a modification of SLA titanium implant surface with strontium-containing nanostructures on immunoinflammatory and osteogenic cell function. J. Biomed. Mater. Res. Part A 2018, 106, 3009–3020. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, D.; Zhao, F.; Gao, W.; Sun, L.; Li, X.; Chen, X. Synergistic effect of strontium and silicon in strontium-substituted sub-micron bioactive glass for enhanced osteogenesis. Mater. Sci. Eng. C 2018, 89, 245–255. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Wang, W.; Zhang, X.; Hu, T.; He, Y.; Lin, K.; Liu, X. Strontium released bi-lineage scaffolds with immunomodulatory properties induce a pro-regenerative environment for osteochondral regeneration. Mater. Sci. Eng. C 2019, 103, 109833. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Gu, Z.; He, Y.; Xu, J.; Xu, C.; Li, L.; Ye, Q. Microenvironment construction of strontium–calcium-based biomaterials for bone tissue regeneration: The equilibrium effect of calcium to strontium. J. Mater. Chem. B 2018, 6, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Aimaiti, A.; Wahafu, T.; Keremu, A.; Yicheng, L.; Li, C. Strontium ameliorates glucocorticoid inhibition of osteogenesis via the ERK signaling pathway. Biol. Trace Elem. Res. 2020, 197, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sriranganathan, D.; Kanwal, N.; Hing, K.A.; Hill, R.G. Strontium substituted bioactive glasses for tissue engineered scaffolds: The importance of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2016, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Blackwood, K.A.; Doustgani, A.; Poh, P.P.; Steck, R.; Stevens, M.M.; Woodruff, M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 3140–3153. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- O’donnell, M.; Hill, R. Influence of strontium and the importance of glass chemistry and structure when designing bioactive glasses for bone regeneration. Acta Biomater. 2010, 6, 2382–2385. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [CrossRef]
- Dai, L.L.; Nudelman, F.; Chu, C.H.; Lo, E.C.M.; Mei, M.L. The effects of strontium-doped bioactive glass and fluoride on hydroxyapatite crystallization. J. Dent. 2021, 105, 103581. [Google Scholar] [CrossRef]
- Ningsih, H.S.; Liu, Y.-C.; Chen, J.-W.; Chou, Y.-J. Effects of strontium dopants on the in vitro bioactivity and cytotoxicity of strontium-doped spray-dried bioactive glass microspheres. J. Non-Cryst. Solids 2022, 576, 121284. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, M.; Peroglio, M.; Stanciuc, A.-M.; Machado, G.; Campbell, I.; Kržmanc, M.M.; Alini, M.; Zhang, X. The influence of strontium release rate from bioactive phosphate glasses on osteogenic differentiation of human mesenchymal stem cells. J. Eur. Ceram. Soc. 2018, 38, 887–897. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Santocildes-Romero, M.E.; Crawford, A.; Hatton, P.V.; Goodchild, R.L.; Reaney, I.M.; Miller, C.A. The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses. J. Tissue Eng. Regen. Med. 2015, 9, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Characterization, in vitro bioactivity and biological studies of sol-gel synthesized SrO substituted 58S bioactive glass. Ceram. Int. 2017, 43, 14880–14890. [Google Scholar] [CrossRef]
- Yin, H.; Yang, C.; Gao, Y.; Wang, C.; Li, M.; Guo, H.; Tong, Q. Fabrication and characterization of strontium-doped borate-based bioactive glass scaffolds for bone tissue engineering. J. Alloys Compd. 2018, 743, 564–569. [Google Scholar] [CrossRef]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The incorporation of strontium to improve bone-regeneration ability of mesoporous bioactive glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef]
- Swe, T.T.; Shariff, K.A.; Mohamad, H.; Ishikawa, K.; Hayashi, K.; Bakar, M.H.A. Behavioural response of cells and bacteria on single and multiple doped Sr and Ag S53P4 sol-gel bioglass. Ceram. Int. 2020, 46, 17881–17890. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef]
- Araujo, M.; Silva, A.; Cabal, B.; Bartolomé, J.; Mello-Castanho, S. In vitro bioactivity and antibacterial capacity of 45S5 Bioglass®-based compositions containing alumina and strontium. J. Mater. Res. Technol. 2021, 13, 154–161. [Google Scholar] [CrossRef]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Synthesis, characterization, and in vitro apatite formation of strontium-doped sol-gel-derived bioactive glass nanoparticles for bone regeneration applications. Ceram. Int. 2023, 49, 23020–23034. [Google Scholar] [CrossRef]
- Shaojing, W.C. Preparation of Strontium-Containing Bioglass Powder and Preparation Method of Strontium-Containing Porous Bioglass Bracket. CN 103848574A, 11 June 2014. [Google Scholar]
- Jarrow Edouard, R.J.; Nedelk, J.-M.; Sautier, J.-M.; Isaac, J. Bioactive Glass with Strontium. P5469598, 2014. [Google Scholar]
- Bao, Y.; Qing, Z.Y.; Xu, Z.; Peng, P. A Kind of 3D-Printing Bracket of Mesoporous Bioglass Containing Strontium of Biological Absorbable and Preparation Method Thereof. CN 106267374B, 20 September 2019. [Google Scholar]
- Haewon Kim, K.K.; Mandahbayar, N.E.; Park, J.; Ahmed, E.; Lee, J.; Haehyung, L.; Jonathan, N. Nanobioactive Glass Cement Comprising Strontium Doped Bioactive Glass Nanoparticle and Preparation Method Thereof. 10-2021-0069759, 2021. [Google Scholar]
- Zhihong, O. Oral Composition Added with Bioactive Glass and Strontium Chloride and Application Thereof. CN 109431834 B, 2021. [Google Scholar]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef]
- Solgi, S.; Khakbiz, M.; Shahrezaee, M.; Zamanian, A.; Tahriri, M.; Keshtkari, S.; Raz, M.; Khoshroo, K.; Moghadas, S.; Rajabnejad, A. Synthesis, characterization and in vitro biological evaluation of sol-gel derived Sr-containing nano bioactive glass. Silicon 2017, 9, 535–542. [Google Scholar] [CrossRef]
- Dubok, V.A. Bioceramics―Yesterday, today, tomorrow. Powder Metall. Met. Ceram. 2000, 39, 381–394. [Google Scholar] [CrossRef]
- Branda, F.; Arcobello-Varlese, F.; Costantini, A.; Luciani, G. Effect of the substitution of M2O3 (M = La, Y, In, Ga, Al) for CaO on the bioactivity of 2.5 CaO–2SiO2 glass. Biomaterials 2002, 23, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. Role of magnesium oxide and strontium oxide as modifiers in silicate-based bioactive glasses: Effects on thermal behaviour, mechanical properties and in-vitro bioactivity. Mater. Sci. Eng. C 2017, 72, 566–575. [Google Scholar] [CrossRef]
- Galliano, P.; López, J.P.; Varetti, E.; Sobrados, I.; Sanz, J. Analysis by nuclear magnetic resonance and raman spectroscopies of the structure of bioactive alkaline-earth silicophosphate glasses. Mater. Res. Bull. 1994, 29, 1297–1306. [Google Scholar] [CrossRef]
- Massera, J.; Hupa, L. Influence of SrO substitution for CaO on the properties of bioactive glass S53P4. J. Mater. Sci. Mater. Med. 2014, 25, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, L.; Gremillard, L.; Chevalier, J.; Zenati, R.; Bernache-Assolant, D. Sintering behaviour of 45S5 bioactive glass. Acta Biomater. 2008, 4, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Samadikuchaksaraie, A.; Hill, R.G.; Shah, P.A.; Milan, P.B.; Mozafari, M.; Fathi, M.; Joghataei, M.T. Synthesis, physico-chemical and biological characterization of strontium and cobalt substituted bioactive glasses for bone tissue engineering. J. Non-Cryst. Solids 2016, 449, 133–140. [Google Scholar] [CrossRef]
- Goel, A.; Rajagopal, R.R.; Ferreira, J.M. Influence of strontium on structure, sintering and biodegradation behaviour of CaO–MgO–SrO–SiO2–P2O5–CaF2 glasses. Acta Biomater. 2011, 7, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Jiang, W.; Chen, X.; Li, Y.; Liang, Q. The effects of Sr concentration on physicochemical properties, bioactivity and biocompatibility of sub-micron bioactive glasses spheres. Adv. Powder Technol. 2017, 28, 2713–2722. [Google Scholar] [CrossRef]
- Goudarzi, Z.; Ijadi, A.; Bakhtiari, A.; Eskandarinezhad, S.; Azizabadi, N.; Jazi, M.A. Sr-doped bioactive glasses for biological applications. J. Compos. Compd. 2020, 2, 105–109. [Google Scholar] [CrossRef]
- Oliveira, I.; Barbosa, A.; Santos, K.; Lança, M.; Lima, M.; Vieira, T.; Silva, J.; Borges, J. Properties of strontium-containing BG 58S produced by alkali-mediated sol-gel process. Ceram. Int. 2022, 48, 11456–11465. [Google Scholar] [CrossRef]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. BoneKEy Rep. 2014, 3, 481. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E608–E616. [Google Scholar] [CrossRef]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.; Nohra, J.; Lao, J.; Jallot, E.; Nedelec, J.-M.; Berdal, A.; Sautier, J.-M. Effects of strontium-doped bioactive glass on the differentiation of cultured osteogenic cells. Eur. Cell Mater. 2011, 21, e43. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass (MBG) powder. J. Sol Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Greasley, S.L.; Chen, S.; Porter, A.E.; Jones, J.R. Monodispersed strontium containing bioactive glass nanoparticles and MC3T3-E1 cellular response. Biomed. Glas. 2016, 2, 72–81. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Li, S.; Pinna, A.; Barrak, F.; Chen, S.; Redpath, A.N.; Rankin, S.M.; Porter, A.E.; Jones, J.R. Interaction of monodispersed strontium containing bioactive glass nanoparticles with macrophages. Biomater. Adv. 2022, 133, 112610. [Google Scholar] [CrossRef]
- Kumar, A.; Banrjee, S.; Roy, P.; Xu, H.; Mariappan, C. Osteogenic commitment of strontium nanoparticles doped mesoporous bioactive glass-ceramics. Mater. Sci. Eng. B 2022, 286, 116068. [Google Scholar] [CrossRef]
- Martin, T.J. Historically significant events in the discovery of RANK/RANKL/OPG. World J. Orthop. 2013, 4, 186. [Google Scholar] [CrossRef]
- Song, C.; Yang, X.; Lei, Y.; Zhang, Z.; Smith, W.; Yan, J.; Kong, L. Evaluation of efficacy on RANKL induced osteoclast from RAW264. 7 cells. J. Cell. Physiol. 2019, 234, 11969–11975. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Tsouderos, Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. 2002, 450, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, C.; Gao, C.; Wu, Z. Alendronate-decorated strontium-containing bioactive glass nanoparticles with dual osteogenic and anti-osteoclastic effects for osteoporosis. J. Non-Cryst. Solids 2023, 622, 122681. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, L.; Yan, R.; Shi, J.; Gu, H.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Strontium-incorporated bioceramic scaffolds for enhanced osteoporosis bone regeneration. Bone Res. 2022, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.; Crotti, T.; Zreiqat, H. Regulation of osteoclast activity in peri-implant tissues. Biomaterials 2004, 25, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Døskeland, A.; Avery, S.J.; Fuoco, T.; Mohamed-Ahmed, S.; Lygre, H.; Finne-Wistrand, A.; Sloan, A.J.; Waddington, R.J.; Mustafa, K. Efficacy of copolymer scaffolds delivering human demineralised dentine matrix for bone regeneration. J. Tissue Eng. 2019, 10, 2041731419852703. [Google Scholar] [CrossRef]
- Khan, P.K.; Mahato, A.; Kundu, B.; Nandi, S.K.; Mukherjee, P.; Datta, S.; Sarkar, S.; Mukherjee, J.; Nath, S.; Balla, V.K. Influence of single and binary doping of strontium and lithium on in vivo biological properties of bioactive glass scaffolds. Sci. Rep. 2016, 6, 32964. [Google Scholar] [CrossRef]
- Neves, N.; Linhares, D.; Costa, G.; Ribeiro, C.; Barbosa, M. In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: A systematic review. Bone Jt. Res. 2017, 6, 366–375. [Google Scholar] [CrossRef]
- Bari, A.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Novel multifunctional strontium-copper co-substituted mesoporous bioactive particles. Mater. Lett. 2018, 223, 37–40. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Steimetz, T.; Cabrini, R.L.; Porto López, J.M. Osteoconductivity of strontium-doped bioactive glass particles: A histomorphometric study in rats. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, L.; Wu, C.; Miron, R.J. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS ONE 2014, 9, e104527. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Montazeri, M.; Nourani, M.R.; Harandi, M. Use of bioactive glass doped with magnesium or strontium for bone regeneration: A rabbit critical-size calvarial defects study. Dent. Res. J. 2022, 19, 18. [Google Scholar]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol–gel method. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Echezarreta-López, M.; Landin, M. Using machine learning for improving knowledge on antibacterial effect of bioactive glass. Int. J. Pharm. 2013, 453, 641–647. [Google Scholar] [CrossRef]





| Composition of Bioactive Glass (mol%) | Concentration (mol%) | Precursor ion Sr | Process | Sr Function in Bioactive Glass | Reference |
|---|---|---|---|---|---|
| 45SiO2–24.5Na2O–24.5CaO–6P2O5 | 1 | SrO | Hydrothermal | Improves the osteogenic ability and stimulates new blood vessel formation in critical-sized rat calvarial defects. | [93] |
| 46SiO2–24.5Na2O–26.9(CaO + SrO)–2.6P2O5 | 50 and 100 | SrO | Melt quench | Stimulates genes associated with osteoblastic differentiation (including Alpl e Bglap) and promotes osteogenesis in a differentiating model of bone cells culture. | [99] |
| (59 − x)B2O3–3P2O5–13CaCO3–15Na2CO3–10TiO2–xSrCO3 | 5–25 | SrCO3 | Sol–gel | Promotes osteoblastic cells (MC3T3-E1) proliferation (in concentrations of 20 and 25 mol%) and exhibits antibacterial effects against S. aureus in the short term (1–7 days). | [17] |
| 60SiO2–36CaO–4P2O5 | 5 and 10 | Sr(NO3)2 | Sol–gel | Increases MC3T3-E1 differentiation, proliferation and ALP activity. Promotes the formation of hydroxycarbonate apatite on the BG surfaces and exhibits antibacterial effect against S. aureus. | [100] |
| 18SiO2–36B2O3–22CaO–6Na2O–8K2O–8MgO–2P2O5 | 3, 6 and 9 | SrO | Melt quench | Suppresses the fast release of boron and minimizes cytotoxicity. Also improves MG-63 cell proliferation. | [101] |
| SiO2–CaO | 2 and 4 | SrCl2 | Aerosol-Assisted Spray Drying and Sol–gel | Stimulates the expression of pro-osteogenic genes, like COLL1A1, SPARC and OPG. Promotes apatite layer formation after 1 day of SBF immersion and does not significantly affect the surface ion-exchange kinetics. | [102] |
| 55SiO2–40CaO–(5 − x)SrO–xAg2O | 1, 3 and 5 | _ | Sol–gel | Positive effect on the materials’ bioactivity, promoting hydroxyapatite layer formation after 7 days of immersion in SBF. | [16] |
| 53.85SiO2–1.72P2O5–21.77Na2O–(22.65 − x)CaO–1Al2O3–xSrO | 3 | Sr(NO3)2 | Sol–gel | Formation of high density of needlelike shape HAp crystals 7 days after immersion in SBF. Increases MC3T3-E1 osteoblast-like cell proliferation, ALP activity and bone nodule formation. | [103] |
| 45SiO2–6P2O5–24.5Na2O–(24.5 − x)CaO–xSrO | 5, 10 and 20 | SrO | Melt quench | Promotes the regeneration of osteoporotic bone defects via early improvement in autophagy and late activation of the Akt/mTOR signaling pathway. | [104] |
| 45.2SiO2–2.5P2O5–23.9Na2O–26.4CaO–2Al2O3–2SrO | 2 | Sr(NO3)2 | Melt quench | Inhibitory effects on E. coli and cell viability greater than 90% by using the NCTC clone 929. | [105] |
| 60SiO2–35CaO–5P2O5 | 1, 3 and 5 | Sr(NO3)2 | Spray drying | Positive effect on the bioactivity and significant increase in osteoblast cell viability. | [95] |
| 46.1SiO2–26.9CaO–2.6P2O5–24.35Na2O | 1 and 2 | Sr(NO3)2 | Melt quench | Antibacterial activity against E. coli and a positive effect on the viability of Saos-2 cells (ATCC ® HTB-85™ human osteosarcoma cell line). | [98] |
| 74SiO2–(26 − x) CaO–xSrO | 1, 3 and 5 | Sr(NO3)2 | Sol–gel | Affects the microstructure of nanoparticles, resulting in a decrease in specific surface area on the pore size of nanoparticles and reduced polymerization of the glass network. | [106] |
| Title | Patent Request | Process | Description | BG (%mol) | Request Year | Reference |
|---|---|---|---|---|---|---|
| Preparation of strontium-containing bioglass powder and preparation method of strontium-containing porous bioglass bracket | CN 103848574 A | High-temperature melting | Repair, treatment and promotion of bone growth | 40CaO–28SiO2–20Na2O–2K2O–2.5MgO–3P2O5–4B2O3–0.5SrO | 2014 | [107] |
| Bioactive glass with strontium added | P5469598 | Sol–gel | BG for bone repair or reconstruction | 45–75 SiO2–15–30 CaO– 2–8 SrO–0–10 P2O5 Other elements: 0–1 | 2014 | [108] |
| A kind of 3D-printing bracket of mesoporous bioglass containing strontium of biological absorbable and preparation method thereof | CN 106267374 B | 3D printing | Scaffolds using treatment for bone defect | 57.2SiO2–7.5P2O5–35.3(SrO + CaO) | 2016 | [109] |
| Nanobioactive glass cement comprising strontium-doped bioactive glass nanoparticle and preparation method thereof | 10-2439780 | High-temperature melting | Apatite deposition and tissue regeneration | 85SiO2–10CaO–5SrO | 2019 | [110] |
| Oral composition added with bioactive glass and strontium chloride and application thereof | CN 109431834 B | Oral composition | BG for oral hygiene with anti-allergy effect and tooth-defect-repair effect. | The bioactive glass and of the strontium chloride are both 0.5 to 15 wt% | 2021 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.V.; Gomes, D.d.S.; Victor, R.d.S.; Santana, L.N.d.L.; Neves, G.A.; Menezes, R.R. Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration. Materials 2023, 16, 7654. https://doi.org/10.3390/ma16247654
Silva AV, Gomes DdS, Victor RdS, Santana LNdL, Neves GA, Menezes RR. Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration. Materials. 2023; 16(24):7654. https://doi.org/10.3390/ma16247654
Chicago/Turabian StyleSilva, Amanda Vieira, Déborah dos Santos Gomes, Rayssa de Sousa Victor, Lisiane Navarro de Lima Santana, Gelmires Araújo Neves, and Romualdo Rodrigues Menezes. 2023. "Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration" Materials 16, no. 24: 7654. https://doi.org/10.3390/ma16247654