The Effect of Oxalic Acid and Citric Acid on the Modification of Wollastonite Surface
Abstract
:1. Introduction
2. Materials and Methods
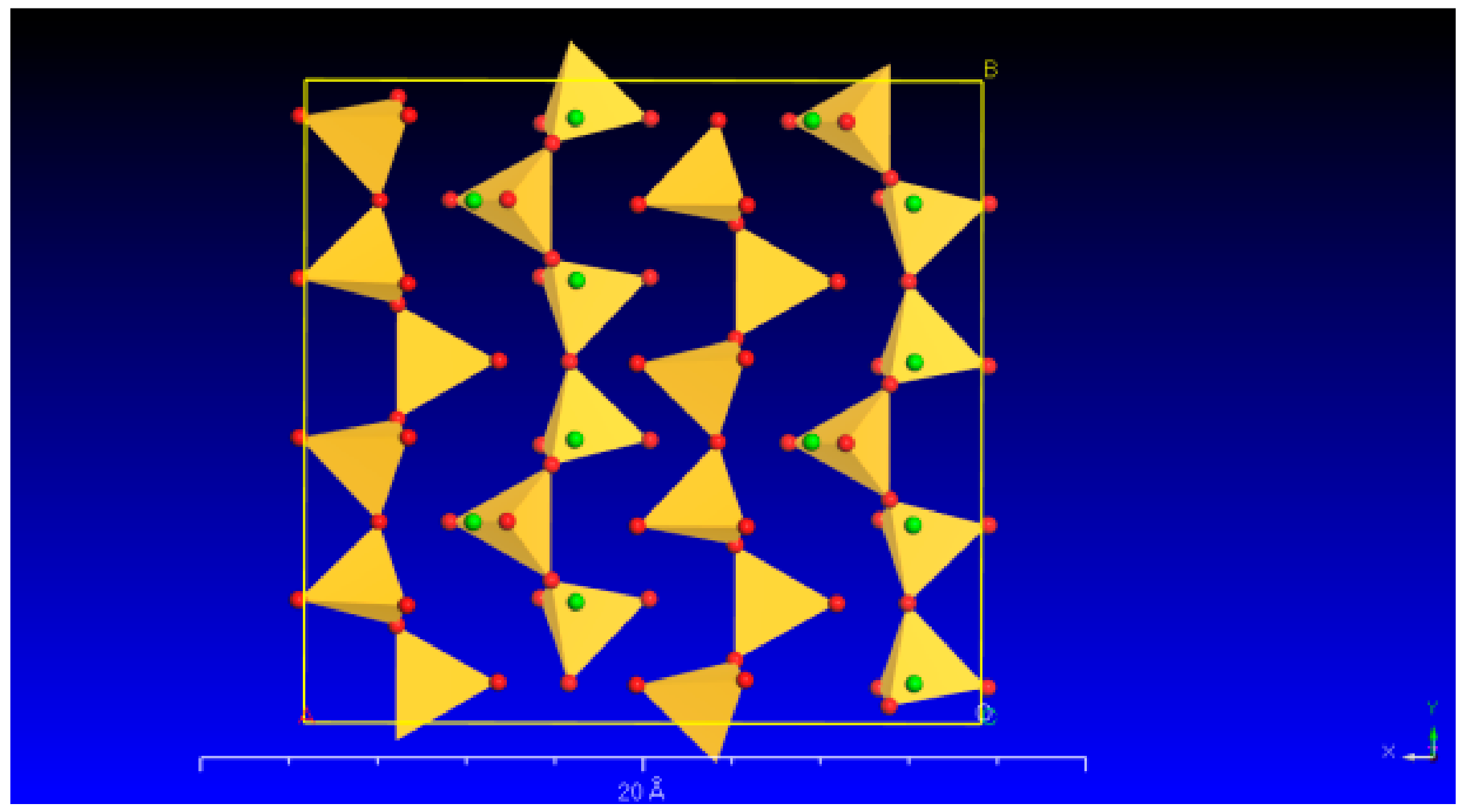
2.1. Materials
2.2. Experimental Procedure
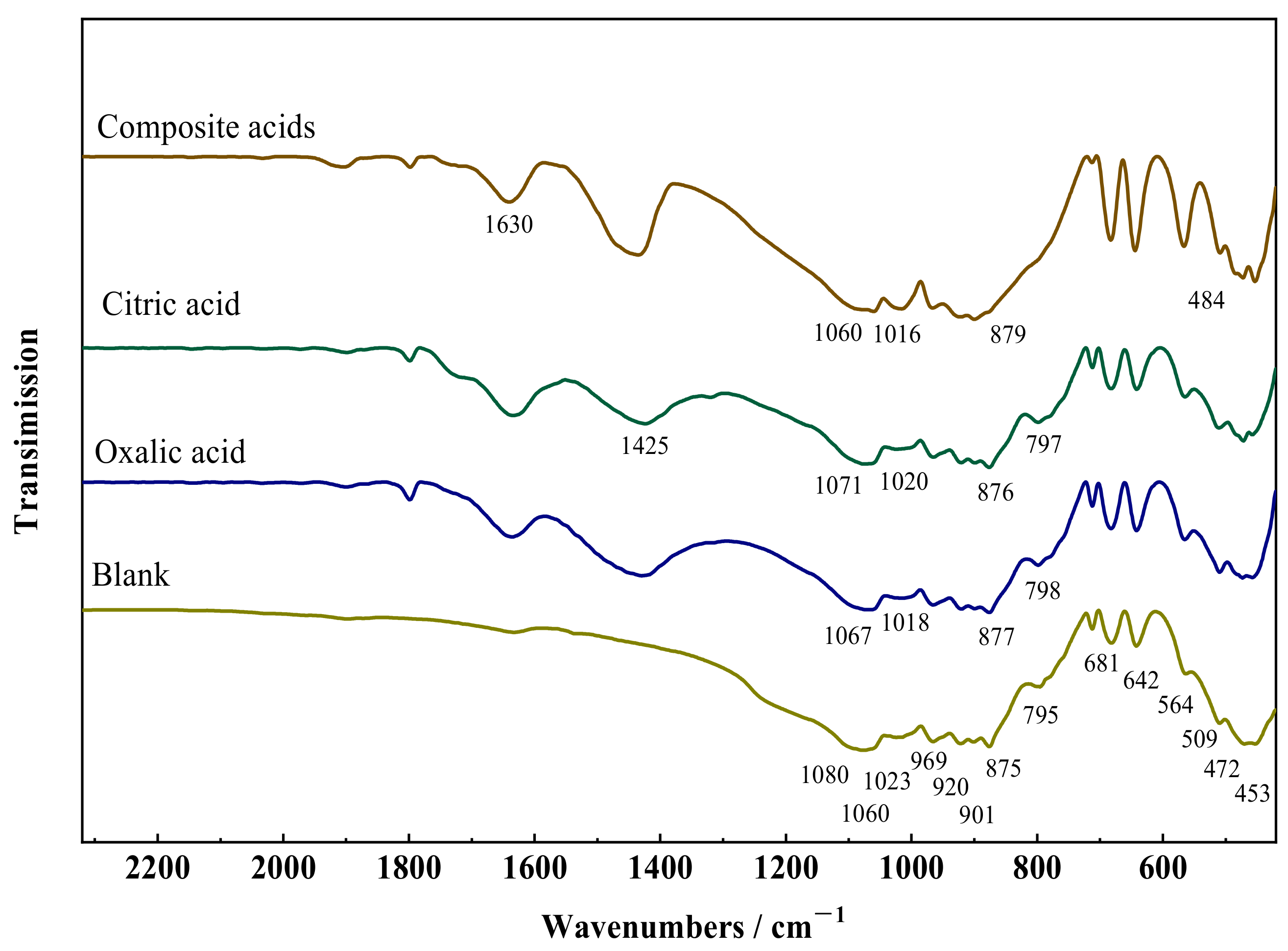
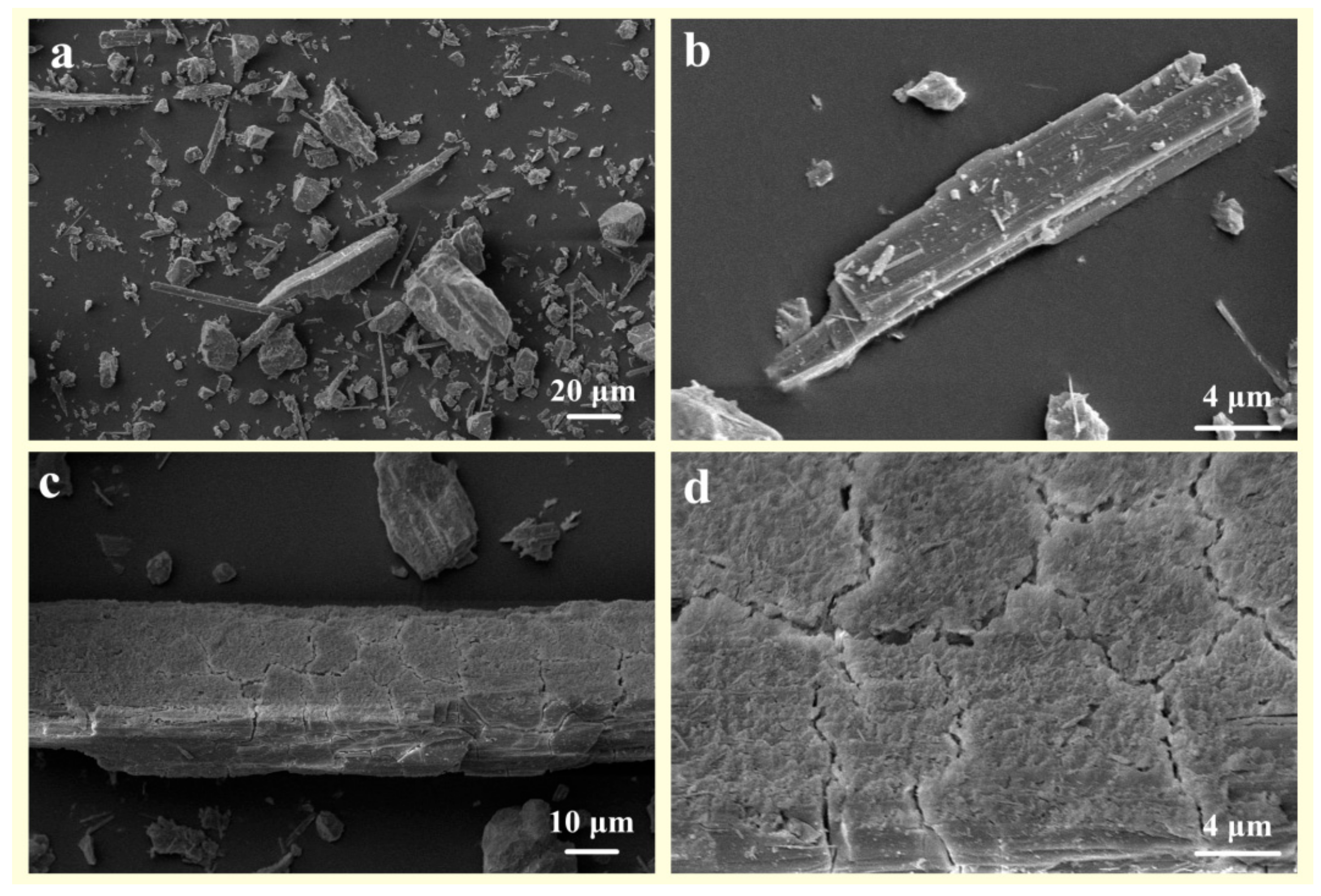
2.3. Characterization of the Solid
2.4. Characterization of the Dissolution Solutions
3. Results and Discussion
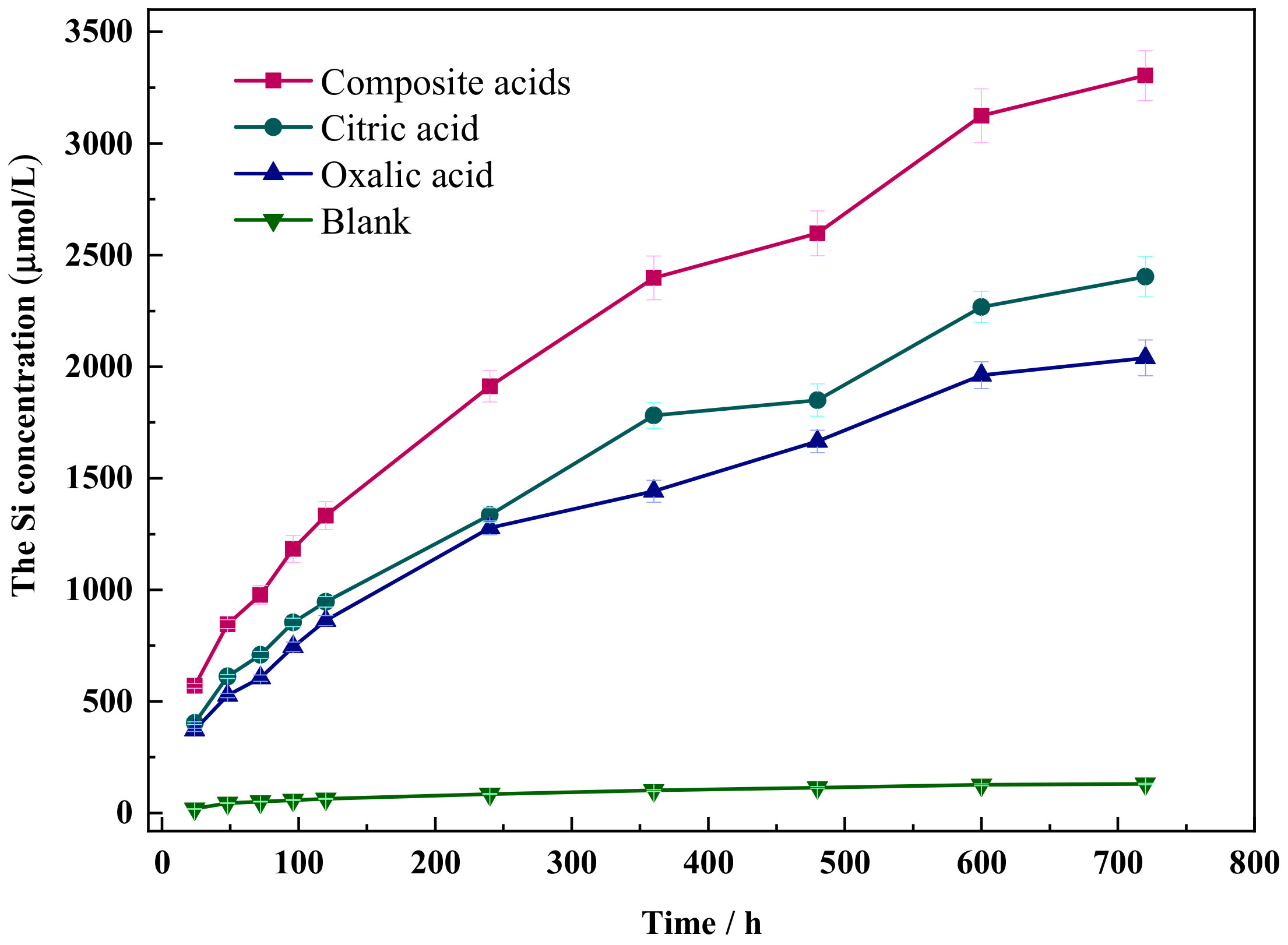
3.1. Dissolution Kinetics of Si in Different Organic Acids
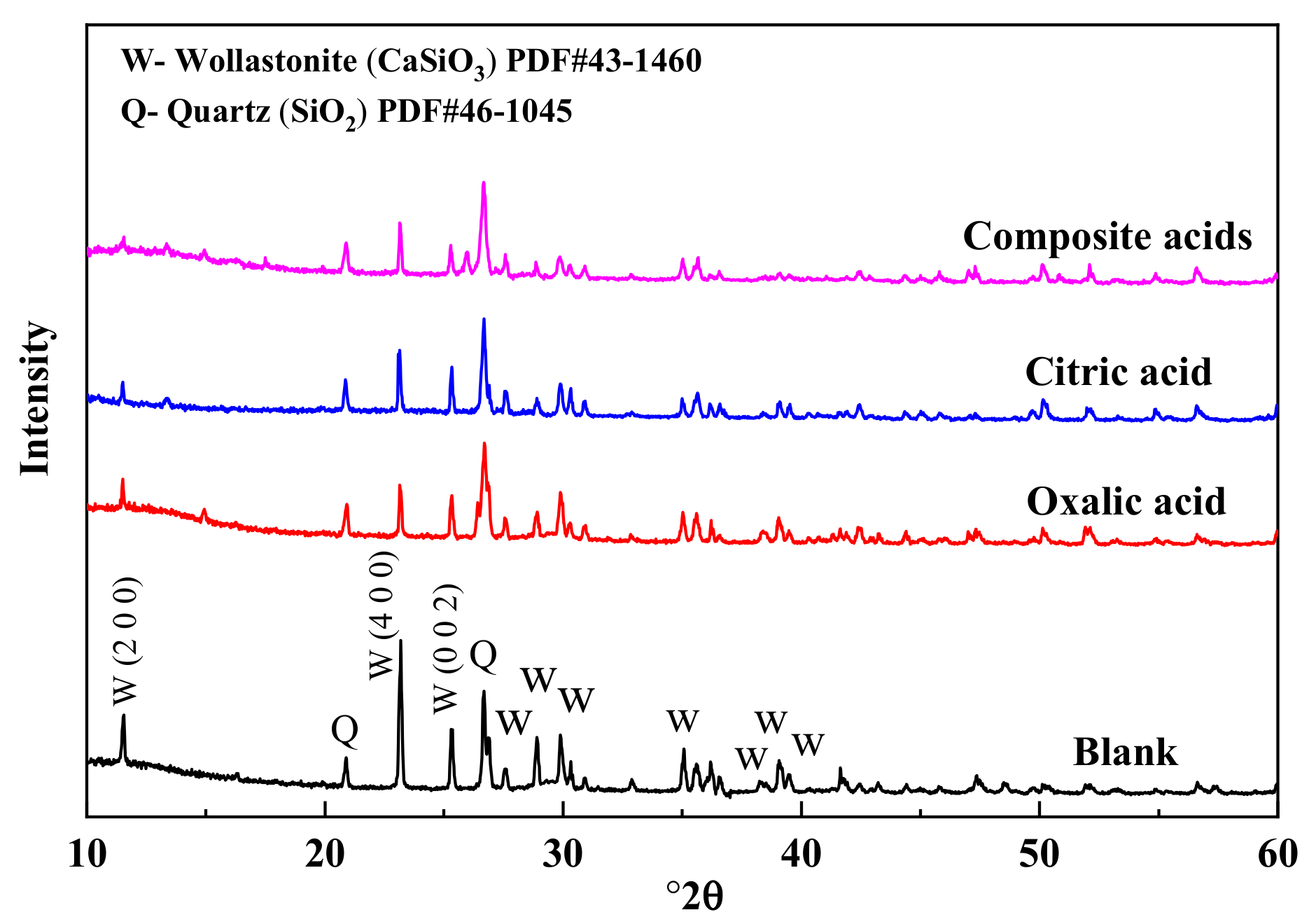
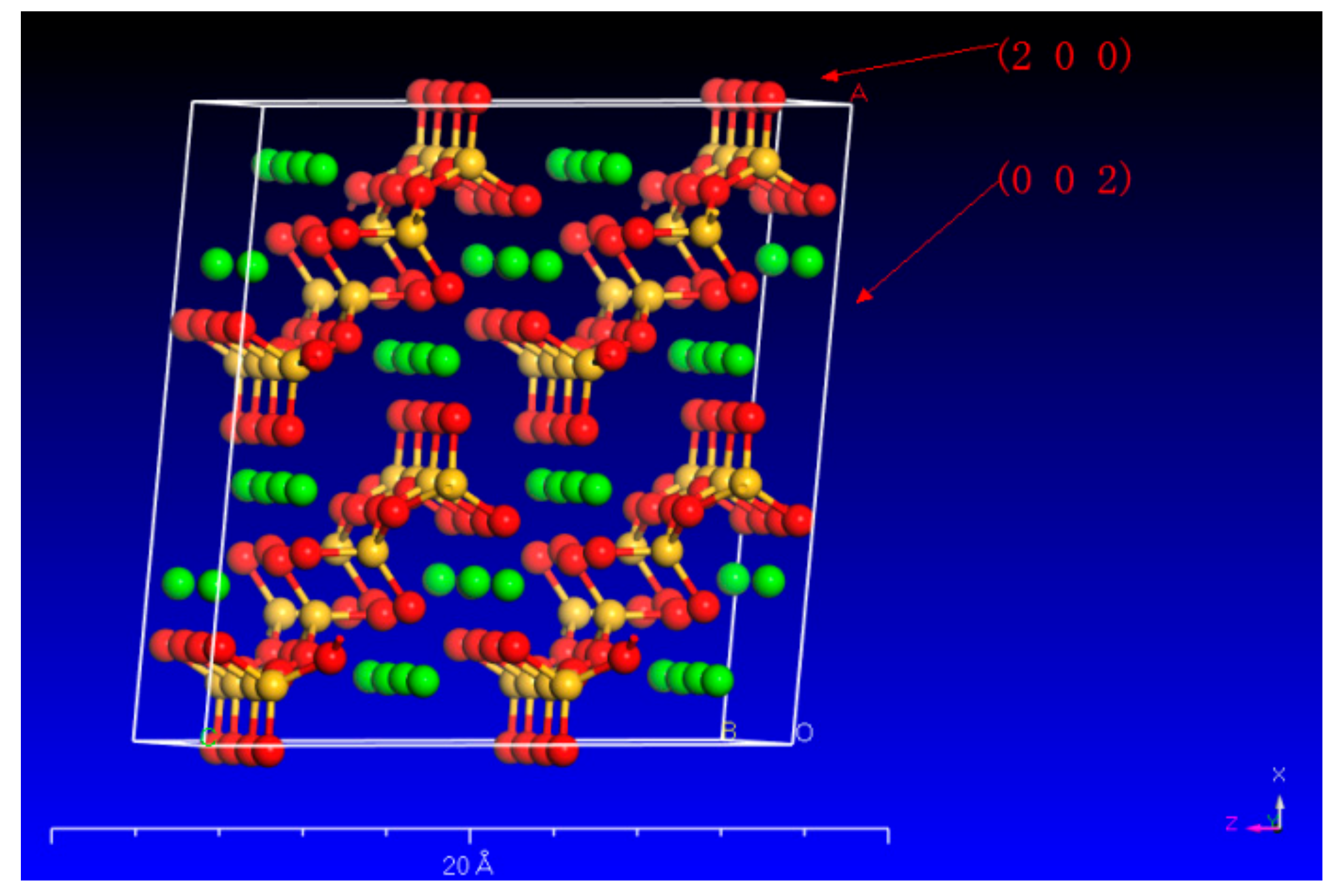
3.2. Changes in the Crystal Structure of Wollastonite after Modification
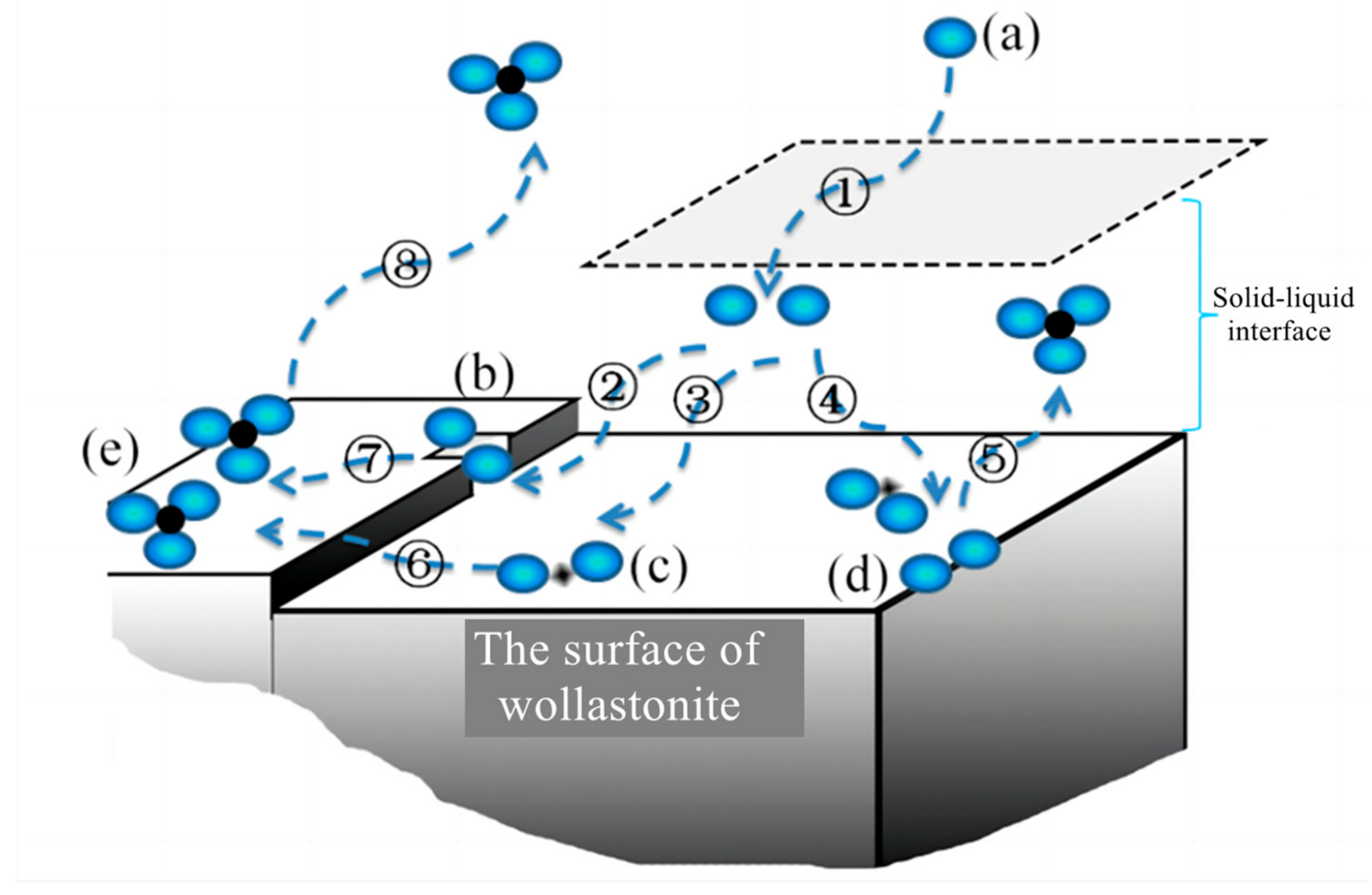
3.3. Modification Mechanism of Wollastonite in Aqueous Citric Acid, Oxalic Acid or Mixed Acids
- (1)
- From the solution phase, organic acids move toward the solid–liquid interface. To be more precise, oxalic acid exhibits a higher affinity for adsorption on the Ca reaction site, while citric acid has a stronger tendency to be adsorbed on the Si reaction site (Figure 7a,b);
- (2)
- Oxalic acid predominantly damages the (002) surface, while citric acid primarily attacks {100}. The synergistic effect of these two acids enhances the dissolution rate of wollastonite and leads to the destruction of more crystal faces (Figure 7c);
- (3)
- As the reaction time increases, a considerable amount of Ca is leached from the wollastonite crystal. Both oxalic acid and citric acid have the potential to form substantial quantities of insoluble products. These materials create an isolation layer on the mineral surface (Figure 7d). As a result, the diffusion of reaction products into the liquid phase is impeded, resulting in a considerable decrease in the migration rate of organic acids to the reaction sites. Finally, the reaction rate gradually diminishes;
- (4)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saibaba, K.; Kondraivendhan, B. Investigation on enhancing the mechanical properties of Alkali-Activated concrete based on fly ash with wollastonite. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Elsayed, H.; Stabile, F.M.; Savio, G.; Bernardo, E. Masked stereolithography of wollastonite-diopside glass-ceramics from novel silicone-based liquid feedstock. Open Ceram. 2023, 16, 100474. [Google Scholar] [CrossRef]
- Wu, H.; Jian, S.; Xue, S.; Ma, Z.; Li, C.; Deng, S.; Feng, W.; Cao, Y.; Dou, Q.; Yang, L.; et al. Synergistic ion transport facilitated by one-dimension wollastonite in solid polymer electrolytes for stable lithium metal batteries. J. Power Sources 2023, 584, 233613. [Google Scholar] [CrossRef]
- Dong, H.; Akatsu, T.; Kamochi, N.; Inada, M.; Shiraishi, A. Near-zero sintering shrinkage in pottery with wollastonite addition. J. Eur. Ceram. Soc. 2023, 43, 700–707. [Google Scholar]
- He, M.; Zhang, H.; Wan, J.; Su, H. Calculation on the permittivity of the wollastonite ceramic matrix. Solid State Sci. 2012, 14, 1467–1470. [Google Scholar] [CrossRef]
- Dehghan, M.; Alizadeh, P.; Soltani, M. Investigation of sintering, crystallization and mechanical properties of silver nanoparticles-reinforced wollastonite glass-ceramic. J. Non-Cryst. Solids 2021, 571, 121028. [Google Scholar] [CrossRef]
- Mahdy, E.A.; Ahmed, H.Y.; Farag, M.M. Combination of Na-Ca-phosphate and fluorapatite in wollastonite-diopside glass-ceramic: Degradation and biocompatibility. J. Non-Cryst. Solids 2021, 566, 120888. [Google Scholar] [CrossRef]
- Lapčík, L.; Maňas, D.; Lapčíková, B.; Vašina, M.; Staněk, M.; Čépe, K.; Vlček, J.; Waters, K.E.; Greenwood, R.W.; Rowson, N.A. Effect of filler particle shape on plastic-elastic mechanical behavior of high density poly(ethylene)/mica and poly(ethylene)/wollastonite composites. Compos. Part B-Eng. 2018, 141, 92–99. [Google Scholar] [CrossRef]
- Yuhaida, I.; Salmah, H.; Hanafi, I.; Firuz, Z. The Effect of Acrylic Acid on Tensile and Morphology Properties of Wollastonite Filled High Density Polyethylene/Natural Rubber Composites. Procedia Chem. 2016, 19, 401–405. [Google Scholar] [CrossRef]
- Singh, U.P.; Biswas, B.K.; Ray, B.C. Evaluation of mechanical properties of polypropylene filled with wollastonite and silicon rubber. Mater. Sci. Eng. A 2009, 501, 94–98. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, J.; Wang, X.; Zhang, H.; Sun, S.; Li, Y.; Ding, H.; Chen, D.; Li, W.; Zhang, J.; et al. Superhydrophilic wollastonite-nanoTiO2 composite photocatalyst prepared by a wet grinding method: The effects of carriers and their application in the self-cleaning coatings. Ceram. Int. 2022, 48, 13770–13779. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N.; Koroleva, E.Y.; Goronchko, V.A. Optical properties degradation of wollastonite powders under the electron irradiation in vacuum. Opt. Mater. 2021, 119, 111342. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N.; Goronchko, V.A. Radiation stability of optical properties of Wollastonite powder with SiO2 nanoparticle addition. Opt. Mater. 2022, 129, 112478. [Google Scholar] [CrossRef]
- Balotiya, G.; Gaur, A.; Somani, P.; Sain, A. Investigating mechanical and durability aspects of concrete incorporating Wollastonite and bottom ash. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Zhu, D.; Wen, A.; Tang, A. Mechanical properties, durability and environmental assessment of low-carbon cementitious composite with natural fibrous wollastonite. Environ. Res. 2023, 234, 116552. [Google Scholar] [CrossRef]
- Nair, N.A.; Sairam, V. Research initiatives on the influence of wollastonite in cement-based construction material—A review. J. Clean. Prod. 2021, 283, 124665. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Kalantari, A.; Avramidis, S. Effect of wollastonite nanofibers and exposure to Aspergillus niger fungus on air flow rate in paper. Measurement 2019, 136, 307–313. [Google Scholar] [CrossRef]
- Xue, H.; Wang, G.; Hu, M.; Chen, B. Modification of wollastonite by acid treatment and alkali-induced redeposition for use as papermaking filler. Powder Technol. 2015, 276, 193–199. [Google Scholar] [CrossRef]
- Baino, F.; Vitale-Brovarone, C. Wollastonite-containing bioceramic coatings on alumina substrates: Design considerations and mechanical modelling. Ceram. Int. 2015, 41, 11464–11470. [Google Scholar] [CrossRef]
- Garcia, E.; Miranzo, P.; Sainz, M.A. Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceram. Int. 2018, 44, 12896–12904. [Google Scholar] [CrossRef]
- Sivamohan, R.; Vachot, P. A comparative study of stirred and vibratory mills for the fine grinding of muscovite, wollastonite and kaolinite. Powder Technol. 1990, 61, 119–129. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Zheng, S. Characterization, organic modification of wollastonite coated with nano-Mg(OH)2 and its application in filling PA6. Mater. Res. Bull. 2014, 50, 273–278. [Google Scholar] [CrossRef]
- Khan, R.I.; Haque, M.I.; Siddique, S.; Landis, E.N.; Ashraf, W. Effects of amino acids on the multiscale properties of carbonated wollastonite composites. Constr. Build. Mater. 2023, 374, 130816. [Google Scholar] [CrossRef]
- Hu, H.; Cheng, P.; Wang, X. Dissolution of Kaolinite Induced by Citric, Oxalic and Malic Acids. Chin. J. Soil. Sci. 2013, 44, 6. [Google Scholar]
- Cappelli, C.; Van Driessche, A.E.S.; Cama, J.; Huertas, F.J. Alteration of trioctahedral micas in the presence of inorganic and organic acids. Appl. Clay Sci. 2023, 238, 106923. [Google Scholar] [CrossRef]
- Xiao, Y.; Lasaga, A.C. Ab initio quantum mechanical studies of the kinetics and mechanisms of quartz dissolution: OH− catalysis. Geochim. Cosmochim. Acta 1996, 60, 2283–2295. [Google Scholar] [CrossRef]
- Schott, J.; Pokrovsky, O.S.; Spalla, O.; Devreux, F.O.; Gloter, A.; Mielczarski, J.A. Formation, growth and transformation of leached layers during silicate minerals dissolution: The example of wollastonite. Geochimica et Cosmochimica Acta 2012, 98, 259–281. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Chu, G.; Zhang, G.; Luo, D.; Yue, H.; Liang, B.; Li, C. Energy-efficient mineral carbonation of CaSO4 derived from wollastonite via a roasting-leaching route. Hydrometallurgy 2019, 184, 151–161. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Sun, D.; Zhang, M. Structural effects of silicate minerals on the growth, metabolism and desilicification of a strain of silicate bacterium. J. Chongqing Univ. 2014, 37, 98–103. [Google Scholar]
- Lin, S.-M.; Yu, Y.-L.; Zhong, M.-F.; Yang, H.; Zhang, C.-Y.; Zhang, Z.-J.; Wu, Y.-Y. The Dissolution Behavior of Feldspar Minerals in Various Low-Molecular-Weight Organic Acids. Materials 2023, 16, 6704. [Google Scholar] [CrossRef]
- Galhardo, C.X.; Masini, J.C. Spectrophotometric determination of phosphate and silicate by sequential injection using molybdenum blue chemistry. Anal. Chim. Acta 2000, 417, 191–200. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef]

| Sample | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | K2O | TiO2 | P2O5 | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wollastonite | 53.82 | 0.87 | 41.2 | 1.53 | 0.21 | 0.12 | 0.21 | 0.05 | 0.03 | 0.03 | 1.93 |
| The Solution | ||||||
|---|---|---|---|---|---|---|
| b | R2 | b | R2 | k | R2 | |
| Blank | 5.22 | 0.981 | 32.54 | 0.979 | 3.91 × 10−3 | 0.953 |
| Oxalic acid | 77.44 | 0.995 | 515.91 | 0.951 | 4.23 × 10−3 | 0.962 |
| Citric acid | 91.82 | 0.993 | 603.57 | 0.939 | 4.49 × 10−3 | 0.956 |
| Complex acids | 126.06 | 0.996 | 829.86 | 0.946 | 6.07 × 10−3 | 0.961 |
| Organic Acids | Composite Acids | Citric Acid | Oxalic Acid |
|---|---|---|---|
| [I(200) + I(400)]/I(002) | 2.05 | 1.86 | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Wang, W.; Wu, L.; Zhong, M.; Zhang, C.; Yu, Y.; Zhang, Z.; Wu, Y. The Effect of Oxalic Acid and Citric Acid on the Modification of Wollastonite Surface. Materials 2023, 16, 7704. https://doi.org/10.3390/ma16247704
Lin S, Wang W, Wu L, Zhong M, Zhang C, Yu Y, Zhang Z, Wu Y. The Effect of Oxalic Acid and Citric Acid on the Modification of Wollastonite Surface. Materials. 2023; 16(24):7704. https://doi.org/10.3390/ma16247704
Chicago/Turabian StyleLin, Shaomin, Weijie Wang, Linguang Wu, Mingfeng Zhong, Chenyang Zhang, Yaling Yu, Zhijie Zhang, and Yunying Wu. 2023. "The Effect of Oxalic Acid and Citric Acid on the Modification of Wollastonite Surface" Materials 16, no. 24: 7704. https://doi.org/10.3390/ma16247704
APA StyleLin, S., Wang, W., Wu, L., Zhong, M., Zhang, C., Yu, Y., Zhang, Z., & Wu, Y. (2023). The Effect of Oxalic Acid and Citric Acid on the Modification of Wollastonite Surface. Materials, 16(24), 7704. https://doi.org/10.3390/ma16247704





