Application of Petrographic and Stereological Analyses to Describe the Pore Space of Rocks as a Standard for the Characterization of Pores in Slags and Ashes Generated after the Combustion of Municipal Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Microscopic Analysis
2.3. Adsorption Measurements
2.4. Densimetric Research
3. Results
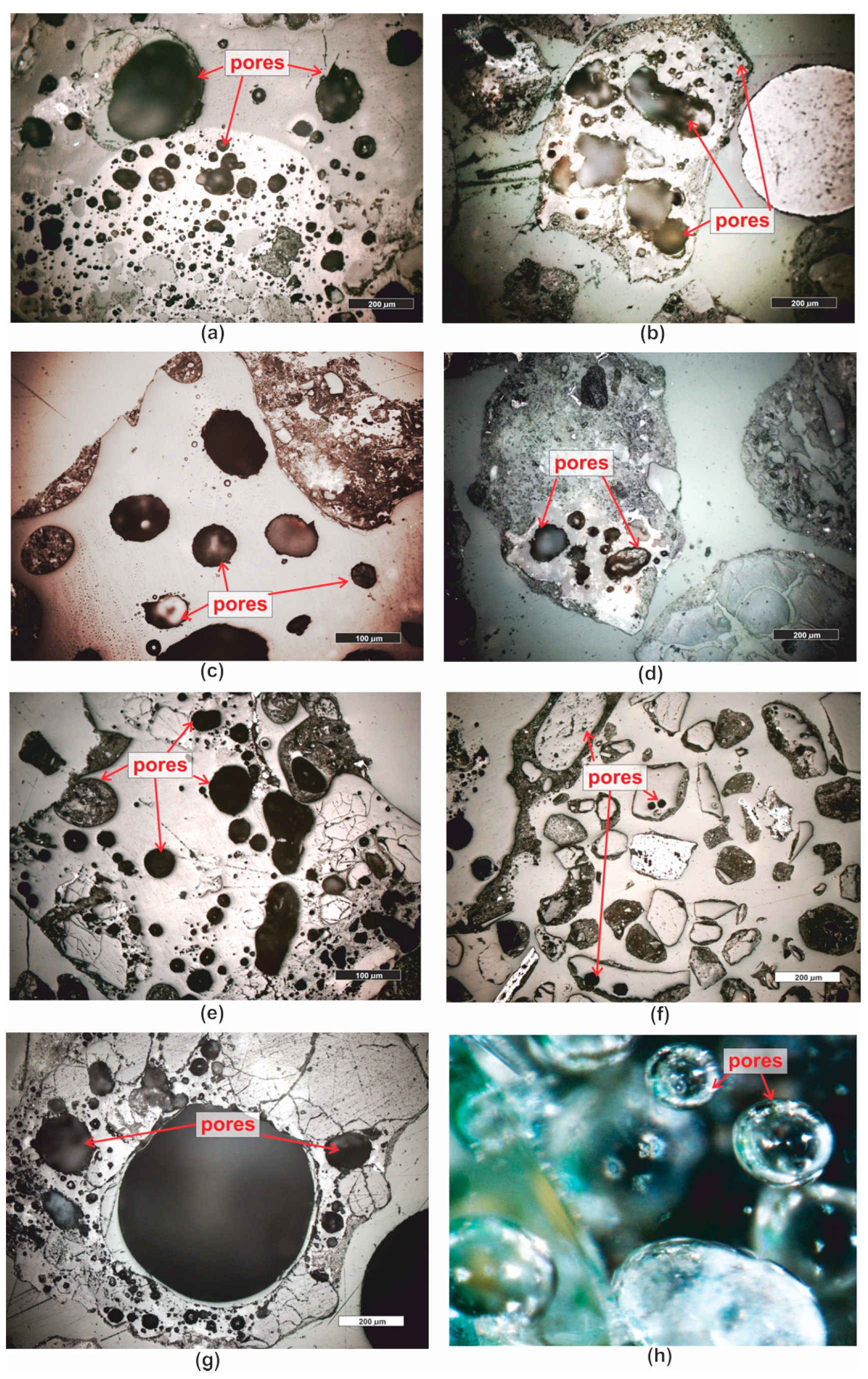
3.1. Microscopic Analyses
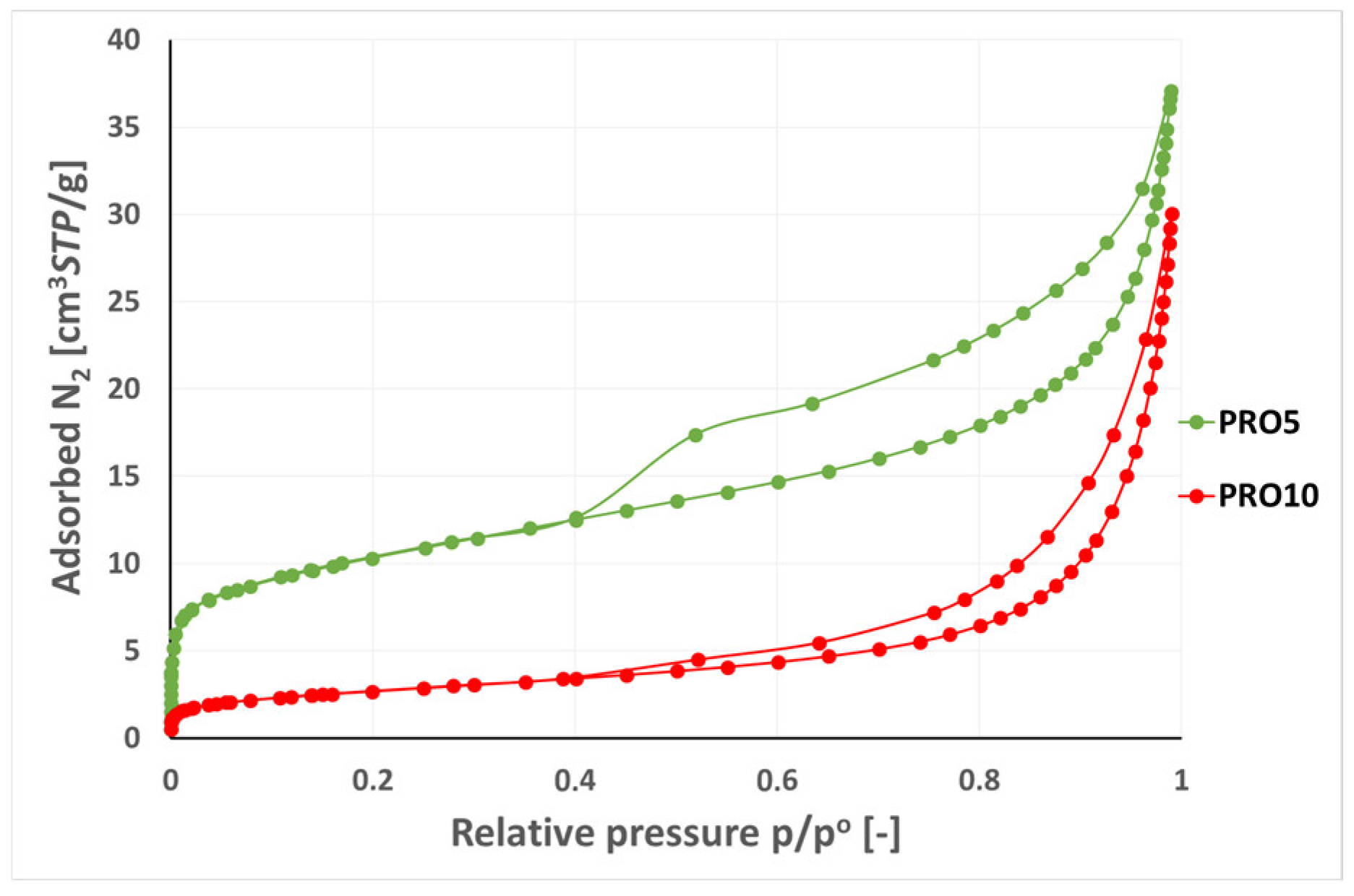
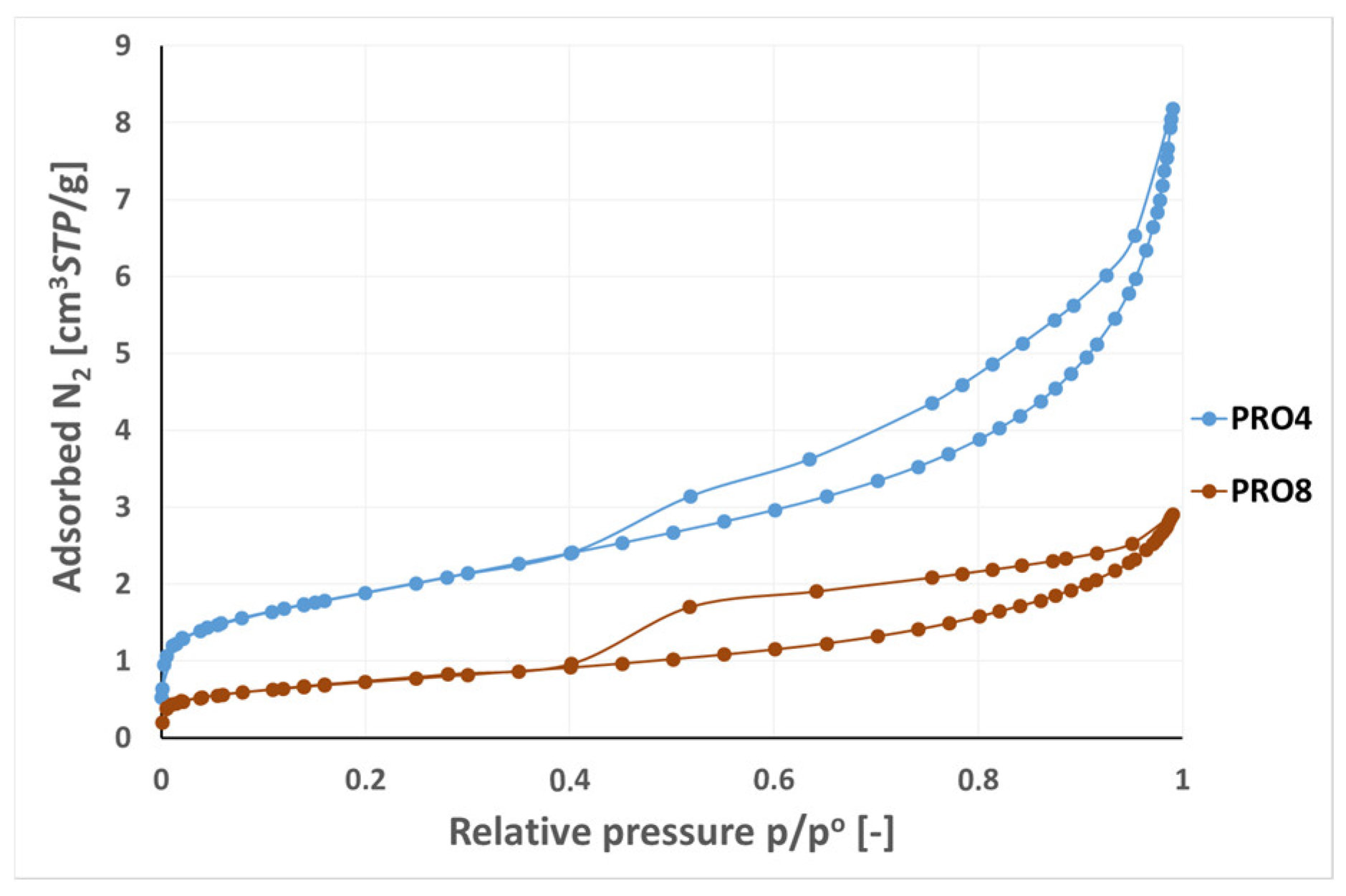
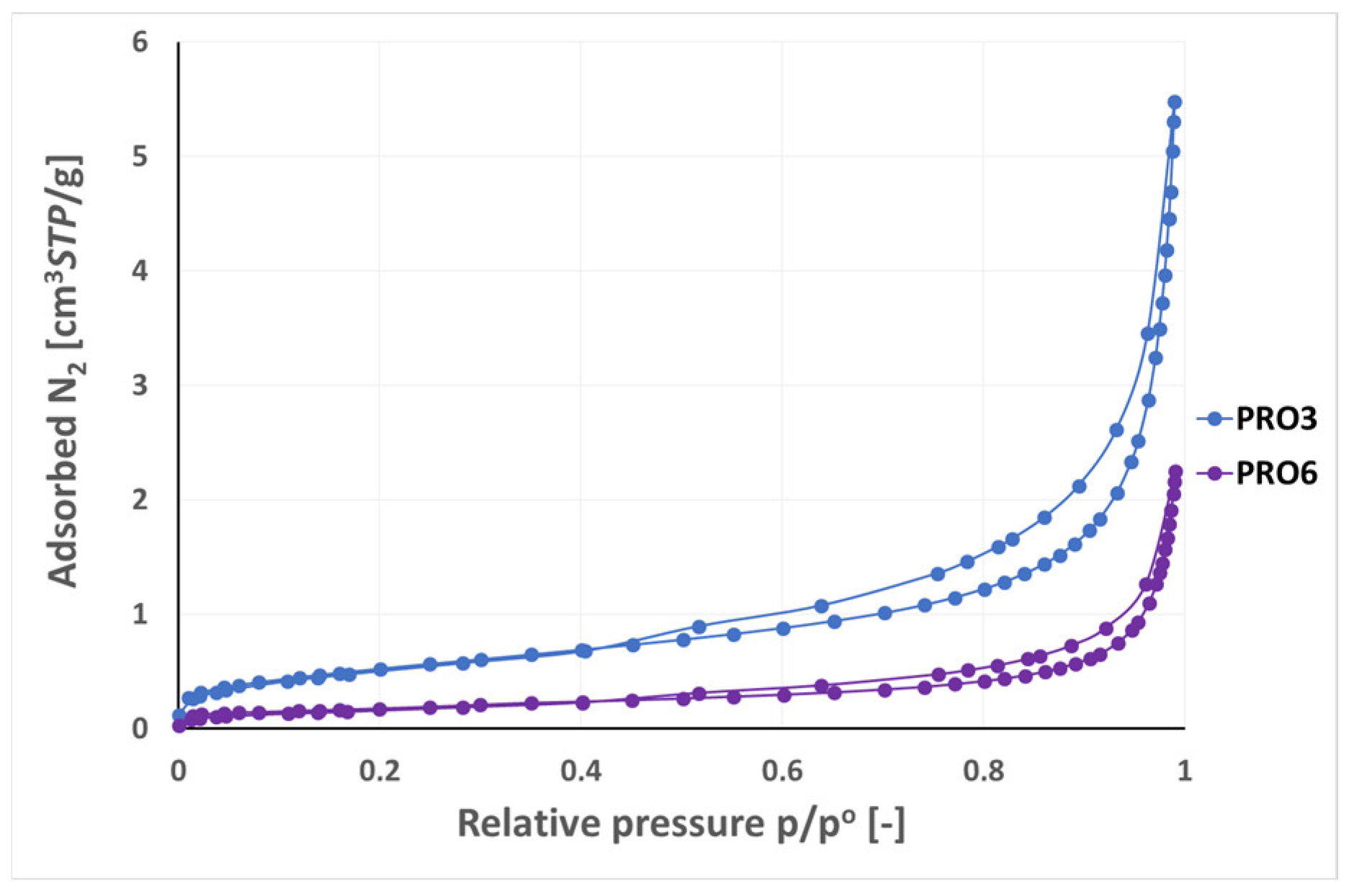
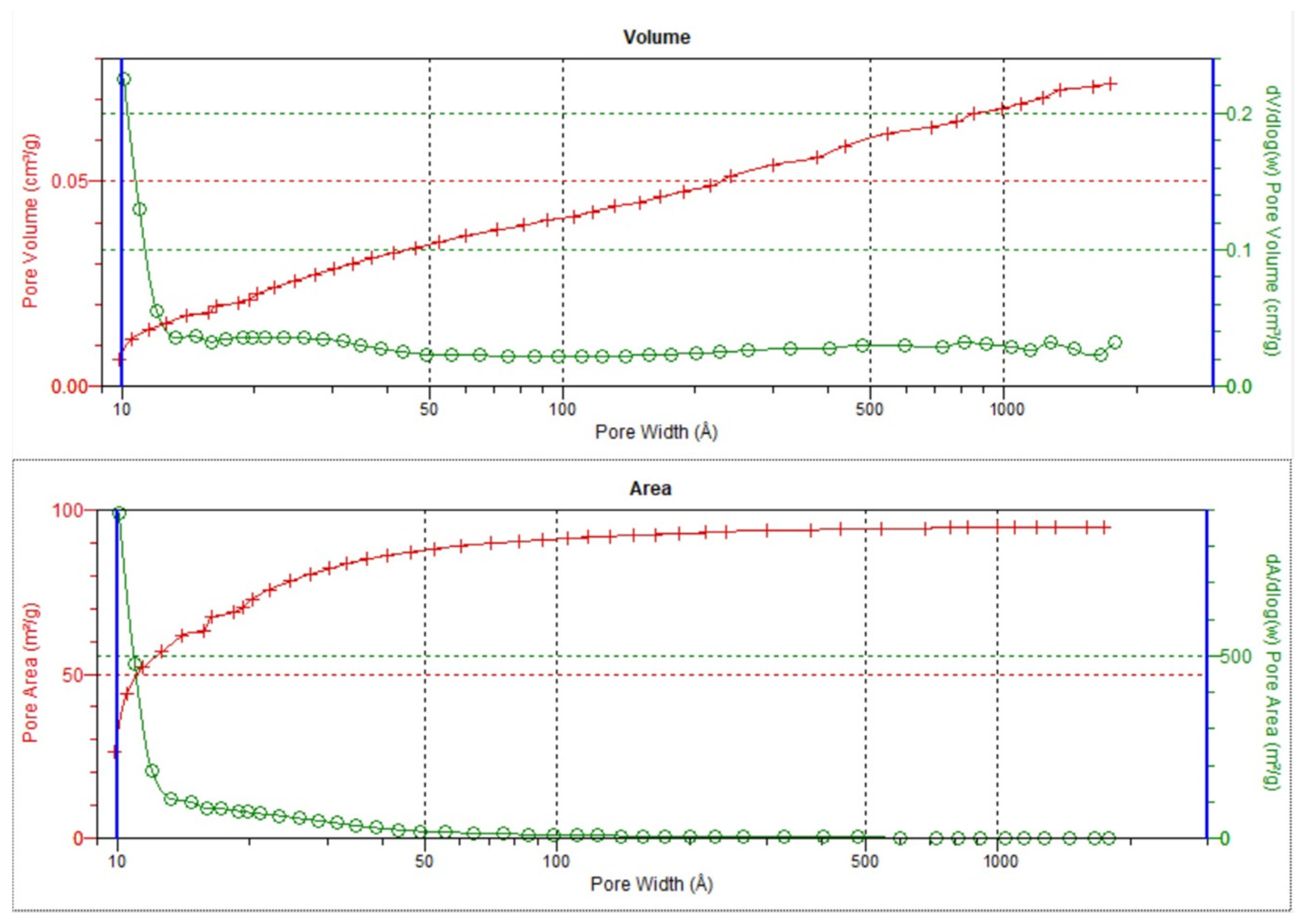
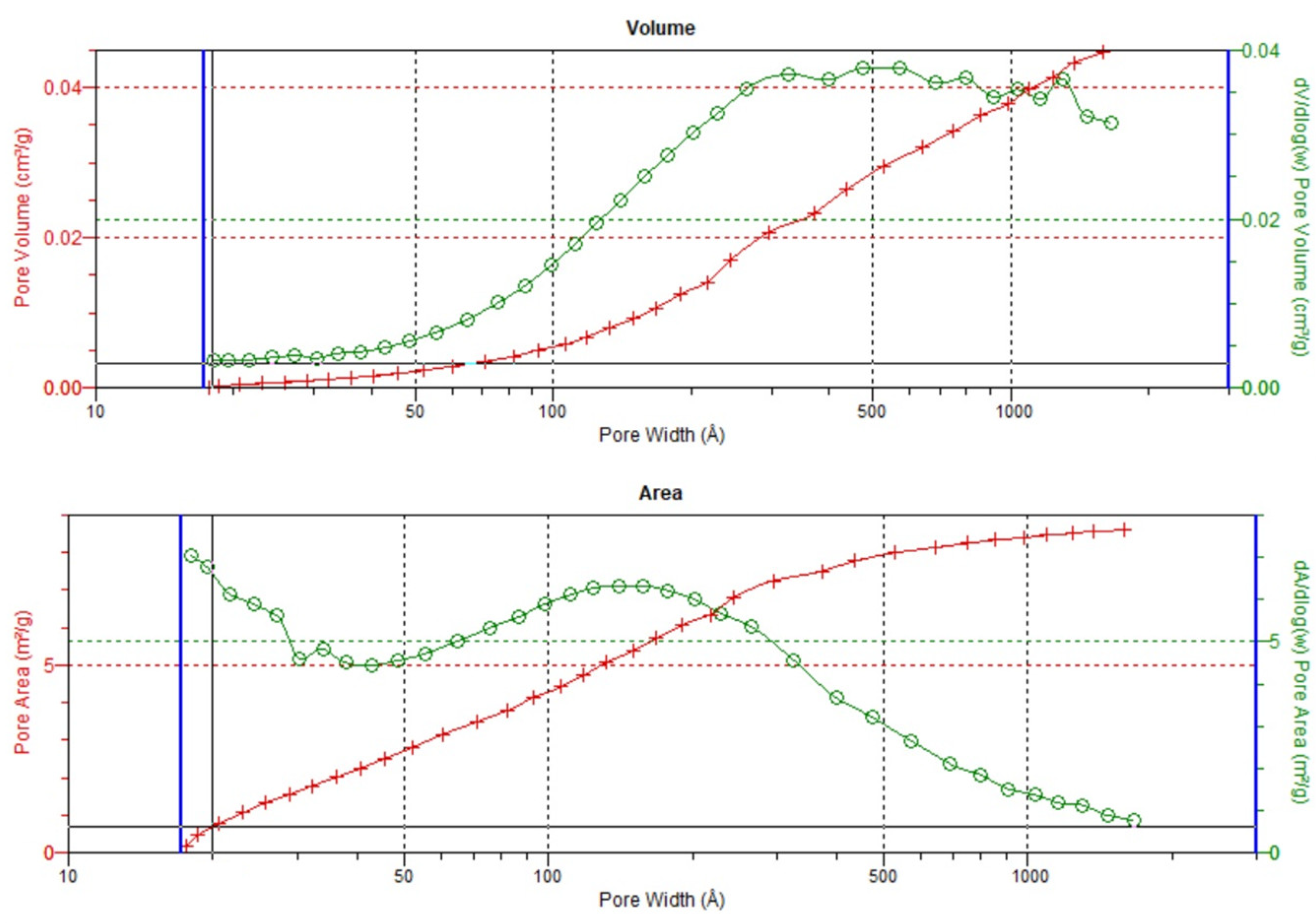
3.2. Analysis of Low-Pressure Isotherms
3.3. Textural Parameters
3.4. Densiometric Studies Results
4. Discussion and Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiab, D.; Donaldson, E.C. Petrophysics: Theory and Practice of Measuring Reservoir Rock and Fluid Transport Properties, 3rd ed.; Elsevier/Gulf Professional Publishing: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Chen, Y. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Zou, C.; Qiu, Z.; Zhang, J.; Li, Z.; Wei, H.; Liu, B.; Zhao, J.; Yang, T.; Zhu, S.; Tao, H.; et al. Unconventional petroleum sedimentology: A key to understanding unconventional hydrocarbon accumulation. Engineering 2022, 18, 62–78. [Google Scholar] [CrossRef]
- Hatherly, P. Overview on the application of geophysics in coal mining. Int. J. Coal Geol. 2013, 114, 74–84. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Misch, D.; Sachsenhofer, R.F. A review of influencing factors and study methods of carbon capture and storage (CCS) potential in coals. Int. J. Coal Geol. 2023, 277, 104351. [Google Scholar] [CrossRef]
- Dutka, B.; Godyń, K. Predicting variability of methane pressure with depth of coal seam. Przemysł Chem. 2018, 97, 1344–1348. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B.; Chuchro, M.; Młynarczuk, M. Synergy of Parameters Determining the Optimal Properties of Coal as a Natural Sorbent. Energies 2020, 13, 1967. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B. Sorption and Micro-Scale Strength Properties of Coals Susceptible to Outburst Caused by Changes in Degree of Coalification. Materials 2021, 14, 5807. [Google Scholar] [CrossRef] [PubMed]
- Dutka, B.; Godyń, K. Coalification as a process determining the methane adsorption ability of coal seams. Arch. Min. Sci. 2021, 66, 181–195. [Google Scholar] [CrossRef]
- Lerner, D.N.; Burston, M.W.; Bishop, P.K. Hydrogeology of the Coventry region (UK): An urbanized, multilayer, dual porosity aquifer system. J. Hydrol. 1993, 149, 111–135. [Google Scholar] [CrossRef]
- Jarzyna, J.A.; Puskarczyk, E.; Motyka, J. Estimating porosity and hydraulic conductivity for hydrogeology on the basis of reservoir and elastic petrophysical parameters. J. Appl. Geophys. 2019, 167, 11–18. [Google Scholar] [CrossRef]
- Németh, G.; Mlinárik, L.; Török, Á. Adsorption and chemical precipitation of lead and zinc from contaminated solutions in porous rocks: Possible application in environmental protection. J. Afr. Earth Sci. 2016, 122, 98–106. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Shen, W.; Shao, J.; Vu, M.-N. Enhancing the elastoplastic damage constitutive model for clayey rocks: Incorporating anisotropy, saturation, time-dependent, and debonding effects. J. Rock Mech. Geotech. Eng. 2023, 15, 2291–2312. [Google Scholar] [CrossRef]
- Farquhar, R.A.; Somerville, J.M.; Smart, B.G.D. Porosity as a Geomechanical Indicator: An Application of Core and Log Data and Rock Mechanics. In Proceedings of the European Petroleum Conference, London, UK, 25–27 October 1994. [Google Scholar] [CrossRef]
- Choren, J.A.; Heinrich, S.M.; Silver-Thorn, M.B. Young’s modulus and volume porosity relationships for additive manufacturing applications. J. Mater. Sci. 2013, 48, 5103–5112. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, J. Engineering Geology for Underground Rocks; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Stalin, V.K.; Muttharam, M. Geotechnical Characterisation and Geoenvironmental Engineering; IGC 2016; Springer Nature Singapore Pte Ltd.: Singapore, 2019; Volume 1. [Google Scholar] [CrossRef]
- Bhawani, S.; Goel, R.K. Engineering Rock Mass Classification Tunneling, Foundations, and Landslides; Butterworth-Heinemann: Waltham, MA, USA; Oxford, UK, 2011. [Google Scholar]
- Zinszner, B.; Pellerin, F.-M. A Geoscientist’s Guide to Petrophysics 2007; Editions TECHNIP: Paris, France, 2007; 384p. [Google Scholar]
- Gradziński, R. Sedymentologia; Wydawnictwo Geologiczne: Warszawa, Poland, 1976. [Google Scholar]
- Manecki, A.; Muszyński, M. Przewodnik do Petrografii; Akademia Górniczo-Hutnicza, Uczelniane Wydawnictwa Naukowo—Dydaktyczne: Kraków, Poland, 2008. [Google Scholar]
- Milliken, K.L.; Curtis, M.E. Imaging pores in sedimentary rocks: Foundation of porosity prediction. Mar. Pet. Geol. 2016, 73, 590–608. [Google Scholar] [CrossRef]
- Zubrzycki, A. Podstawy Geologii Naftowej; Zakład Poligraficzny S.C.M.R. Wioska: Kraków, Poland, 2011. [Google Scholar]
- Lucia, F.J. Carbonate Reservoir Characterization, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Pajdak, A.; Godyń, K.; Kudasik, M. The use of selected research methods to describe the pore space of dolomite from copper ore mine, Poland. Environ. Earth Sci. 2017, 76, 389. [Google Scholar] [CrossRef]
- Amyx, J.W.; Bass, D.M.; Whiting, R.L. Petroleum Reservoir Engineering; McGraw Hill Book Company: New York, NY, USA, 1960. [Google Scholar]
- Pappalardo, G.; Punturo, R.; Mineo, S.; Contrafatto, L. The role of porosity on the engineering geological properties of 1669 lavas from Mount Etna. Eng. Geol. 2017, 221, 16–28. [Google Scholar] [CrossRef]
- Tanyıldızı, M.; Gökalp, İ. Utilization of pumice as aggregate in the concrete: A state of art. Constr. Build. Mater. 2023, 377, 131102. [Google Scholar] [CrossRef]
- Plewa, M.; Plewa, S. Petrofizyka; Wydawnictwa Geologiczne: Warszawa, Poland, 1992. [Google Scholar]
- Ryncarz, T. Zarys Fizyki Górotworu; Śląskie Wydawnictwo Techniczne: Katowice, Poland, 1993. [Google Scholar]
- Twardowski, K.; Traple, J. Charakterystyka ilościowa porowatości ośrodków gruntowo-skalnych a zjawiska molekularno-powierzchniowe. Wiert. Naft. Gaz 2006, 23, 487–495. [Google Scholar]
- IUPAC. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- IUPAC. Reporting Physisorption Data. Pure Appl. Chem. 1985, 57, 603J. [Google Scholar]
- Skoczylas, N.; Godyń, K. Evaluating selected lithological features using photographs taken with an introscopic camera in boreholes. Int. J. Rock Mech. Min. Sci. 2014, 72, 319–324. [Google Scholar] [CrossRef]
- Karbownik, M.; Krawczyk, J.; Godyń, K.; Schlieter, T.; Ščučka, J. Analysis of the Influence of Coal Petrography on the Proper Application of the Unipore and Bidisperse Models of Methane Diffusion. Energies 2021, 14, 8495. [Google Scholar] [CrossRef]
- Dandekar, A. Petroleum Reservoir Rock and Fluid Properties; CRC Press: Boca Raton, FL, USA; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Peters, E.J. Advanced Petrophysics—Volumes 1 and 2; Greenleaf Book Group: Austin, TX, USA, 2012. [Google Scholar]
- Semyrka, R.; Semyrka, G.; Zych, I. Zmienność parametrów petrofizycznych subfacji dolomitu głównego zachodniej strefy półwyspu Grotowa w świetle badań porozymetrycznych. Geologia 2008, 34, 445–468. [Google Scholar]
- Such, P.; Leśniak, G. Porowatość, przepuszczalność i parametry przestrzeni porowej, w: Rzeczpospolita łupkowa: Studium wiedzy o gazie z formacji łupkowych. Pr. Inst. Naft. Gazu 2012, 183, 159–170. [Google Scholar]
- Zalewska, J. Rentgenowska mikrotomografia komputerowa w badaniu skał węglanowych: Praca zbiorowa. Pr. Nauk. Inst. Naft. Gazu 2010, 171, 263. [Google Scholar]
- Zalewska, J.; Dohnalik, M. Rentgenowska mikrotomografia komputerowa. Zobaczyć niewidzialne. Rynek Pol. Naft. Gazu 2012, 90–103. [Google Scholar]
- Vieira, L.D.; Moreira, A.C.; Mantovani, I.F.; Honorato, A.R.; Prado, O.F.; Becker, M.; Fernandes, C.S.; Waichel, B.L. The influence of secondary processes on the porosity of volcanic rocks: A multiscale analysis using 3D X-ray microtomography. Appl. Radiat. Isot. 2021, 172, 109657. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Jaroniec, M. An improved methodology for adsorption characterization of unmodified and modified silica gels. J. Colloid Interface Sci. 2003, 266, 168–174. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B. Preliminary Studies of Slag and Ash from Incinerated Municipal Waste for Prospective Applications. Energies 2023, 16, 117. [Google Scholar] [CrossRef]
- Available online: https://stat.gov.pl/ (accessed on 15 November 2022).
- Jendrus, R.; Pach, G.; Strózik, G. Assessment of the determined ground compaction of anthropogenic soil containing hard coal mine waste using the DPSH dynamic probe. Arch. Min. Sci. 2023, 68, 227–249. [Google Scholar]
- Wielgosiński, G.; Wasiak, D.; Zawadzka, A. The Use of Sequential Extraction for Assessing Environmental Risks of Waste Incineration Bottom Ash. Ecol. Chem. Eng. S 2014, 21, 3. [Google Scholar] [CrossRef]
- Wielgosinski, G. Termiczne przekształcanie odpadów. Wydaw. Nowa Energ. 2020. [Google Scholar]
- Rosik-Dulewska, C. Podstawy Gospodarki Odpadami. Środowisko; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2023. [Google Scholar]
- Mądrawski, J.; Kostrzewski, W.; Smoczkiewicz-Wojciechowska, A. Problematyka wykorzystania odpadów typu żużle ze spalarni śmieci komunalnych do produkcji betonów. Przegląd Bud. 2017, 88, 105–107. [Google Scholar]
- de Lorena Diniz Chaves, G.; Ribeiro Siman, R.; Mattos Ribeiro, G.; Chang, N.-B. Synergizing environmental, social, and economic sustainability factors for refuse derived fuel use in cement industry: A case study in Espirito Santo, Brazil. J. Environ. Manag. 2021, 288, 112401. [Google Scholar] [CrossRef] [PubMed]
- Rozporządzenie Ministra Klimatu z Dnia 2 Stycznia 2020 r. w Sprawie Katalogu. Available online: https://isap.sejm.gov.pl (accessed on 15 November 2022).
- Szewski, A.; Budziszewski, A. Ewidencja odpadów 2023 Poradnik BDO—Ewidencja Odpadów oraz Sprawozdawczość Odpadowa. Available online: www.kartaewidencji.pl (accessed on 10 January 2022).
- Ambaye, T.G.; Djellabi, R.; Vaccari, M.; Prasad, S.; Aminabhavi, T.M.; Rtimi, S. Emerging technologies and sustainable strategies for municipal solid waste valorization: Challenges of circular economy implementation. J. Clean. Prod. 2023, 423, 138708. [Google Scholar] [CrossRef]
- Available online: https://portalprzemyslowy.pl/ (accessed on 15 November 2022).
- Ryś, J. Stereology of Materials; Fotobit Design: Kraków, Poland, 1995. [Google Scholar]
- Faass, G.S. Correlation of Gas Adsorption, Mercury Intrusion, and Electron Microscopy Pore Property Data for Porous Glasses. Ga. Inst. Technol. 1981, 260. [Google Scholar]
- Pettijohn, F.J.; Potter, P.E. Sand and Sandstone; Springer: Berlin, Germany, 1972. [Google Scholar]
- Dunham, R.J. Classification of Carbonate Rocks According to Depositional Texture; Ham, W.E., Ed.; Classification of Carbonate Rocks; AAPG: Tulsa, OK, USA, 1962; pp. 108–121. [Google Scholar]
- Ryka, W.; Maliszewska, A. Słownik Petrograficzny; Wydawnictwo Geologiczne: Warszawa, Poland, 1991. [Google Scholar]
- Olszewska, K.; Magnes, C.; Ziółkowski, J.; Kuhl, J. Atlas Petrograficzny Górnośląskich Kamiennych węgli Humusowych; Główny Instytut Górnictwa: Katowice, Poland; Wydawnictwo Śląsk: Katowice, Poland, 1965. [Google Scholar]
- Gao, H.; Lan, Y.; Guo, N. Pore Structural Features of Granite under Different Temperatures. Materials 2021, 14, 6470. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B. Slags and ashes generated in the process of incineration of municipal waste as one of the cogs of the circular economy. In Proceedings of the SEEP 2022, Brunel University London, Uxbridge, UK, 12–15 September 2022; pp. 12–15. [Google Scholar]
- Nowak-Winiarska, K.; Wrobel, S.; Sienkiewicz-Cholewa, U. Application of sequential analysis with the BCR method in the estimation of effects of chemical remediation of soil polluted with copper. Chem. Speciat. Bioavailab. 2012, 24, 53–59. [Google Scholar] [CrossRef]


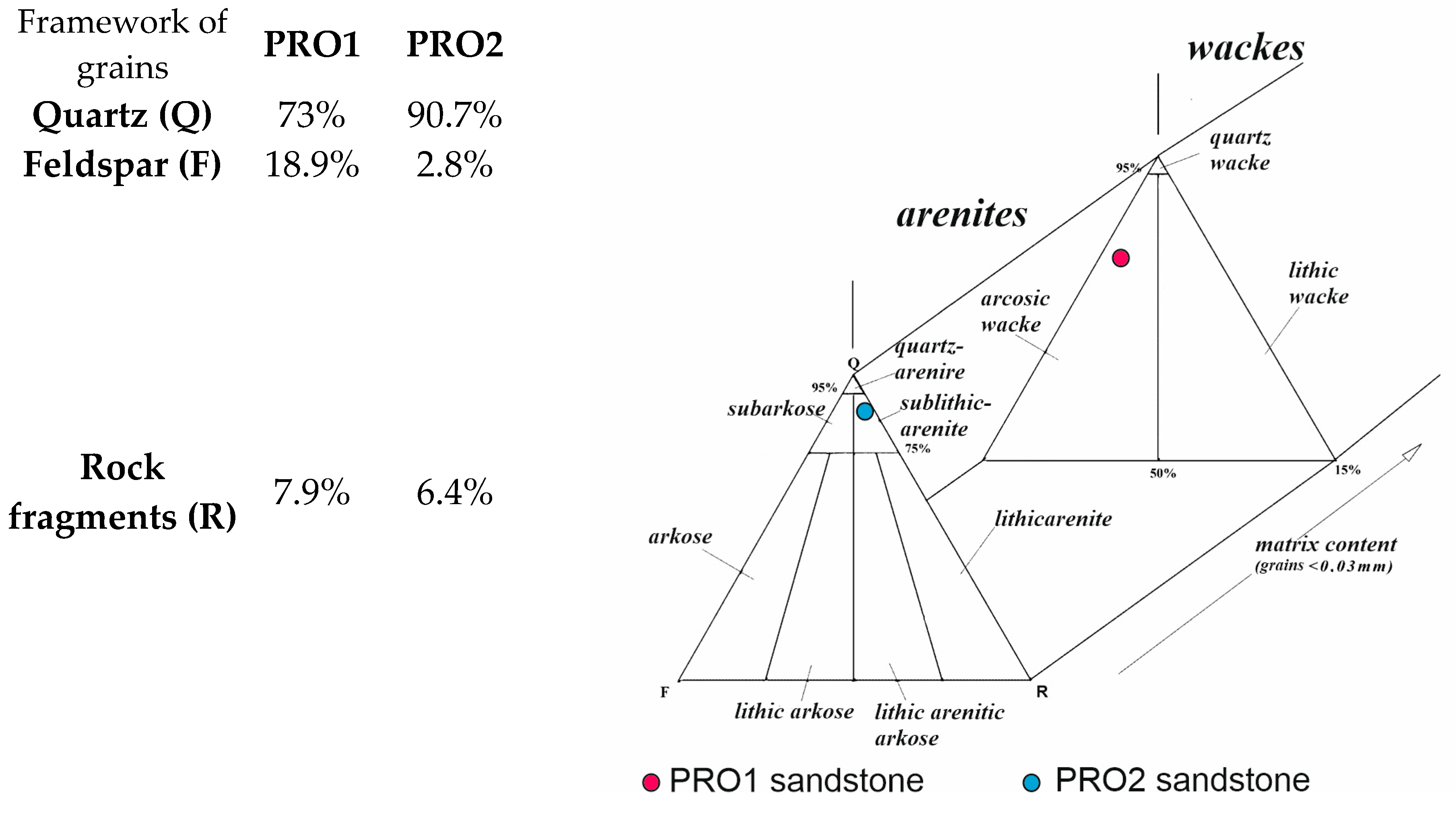
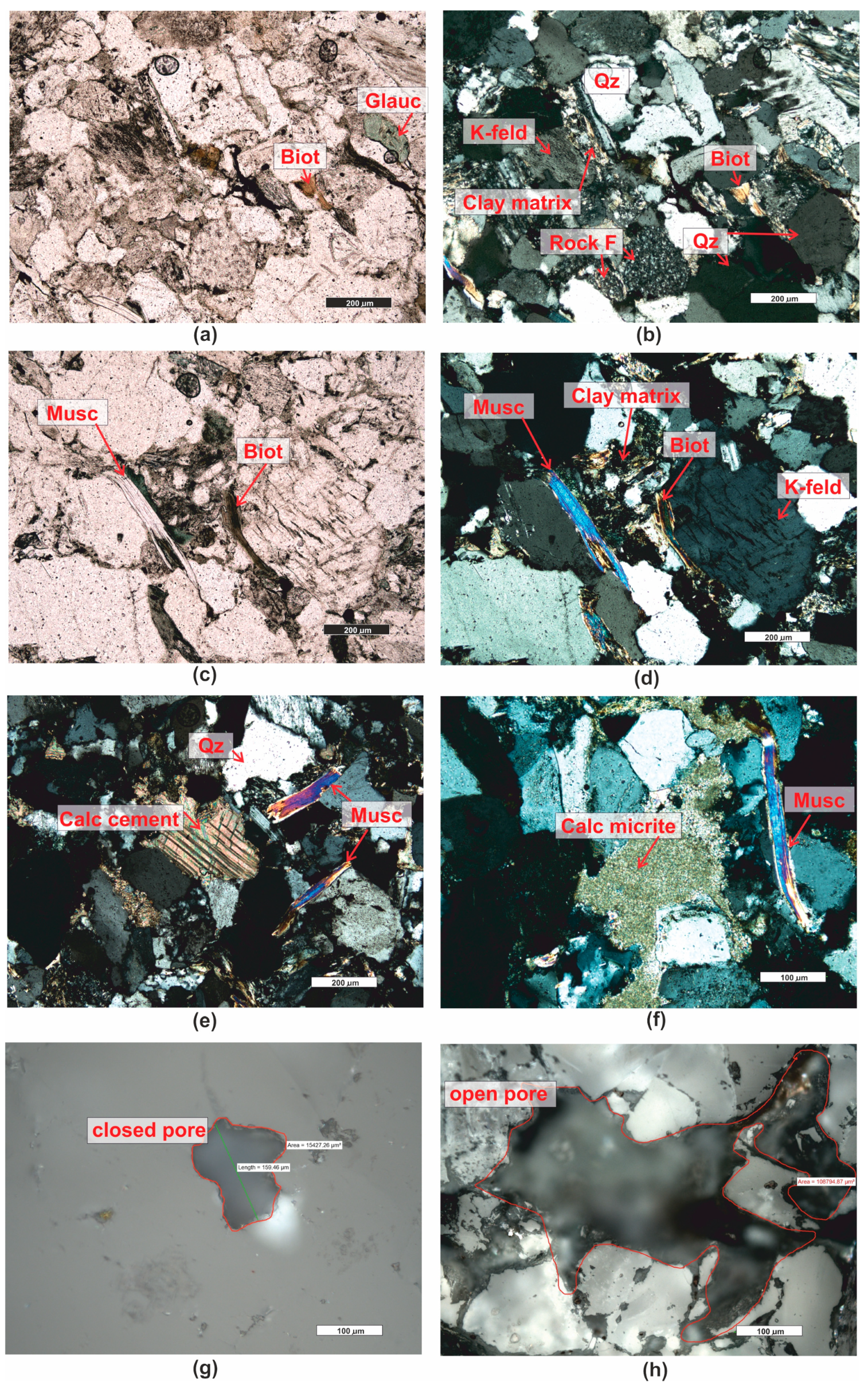
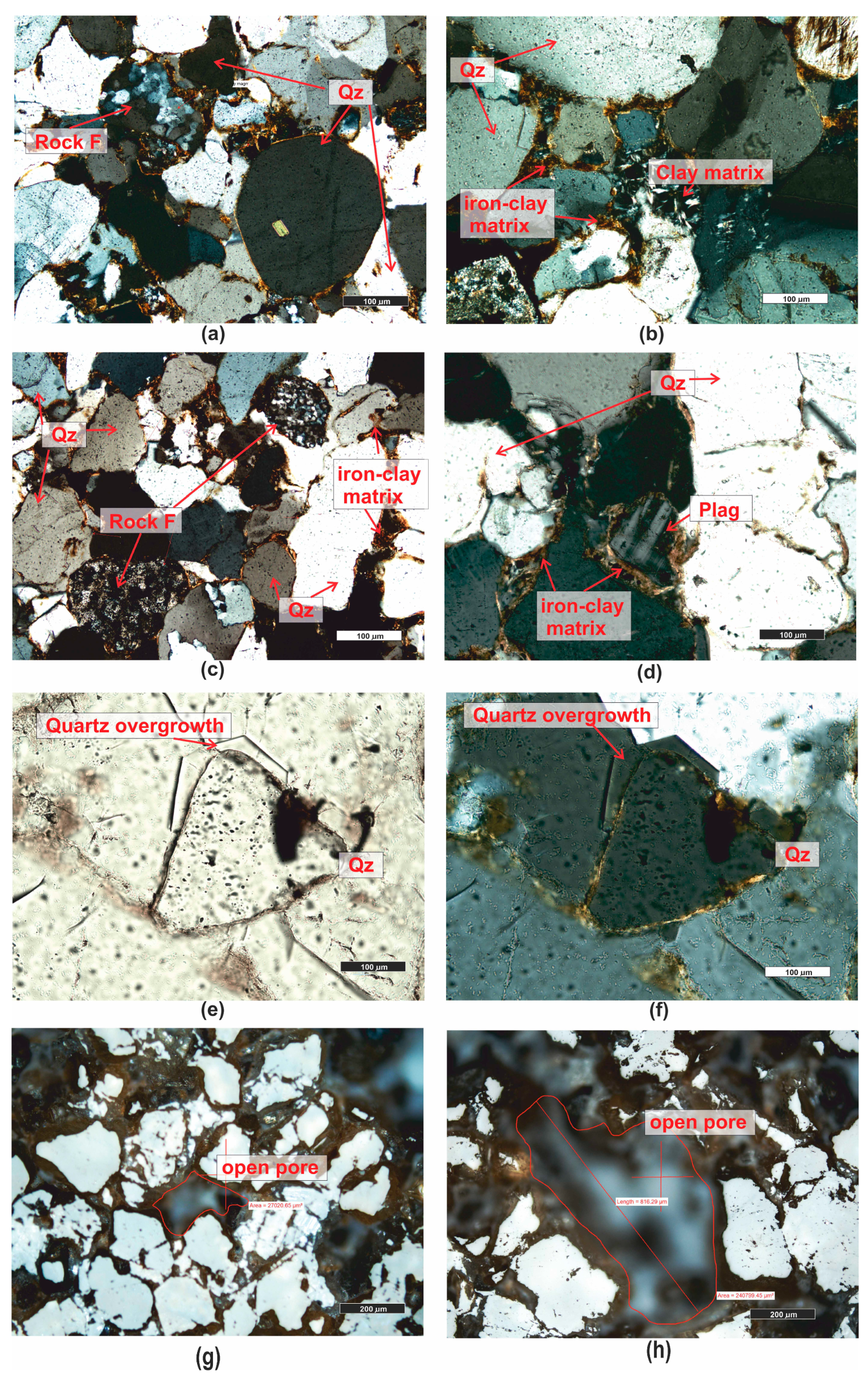
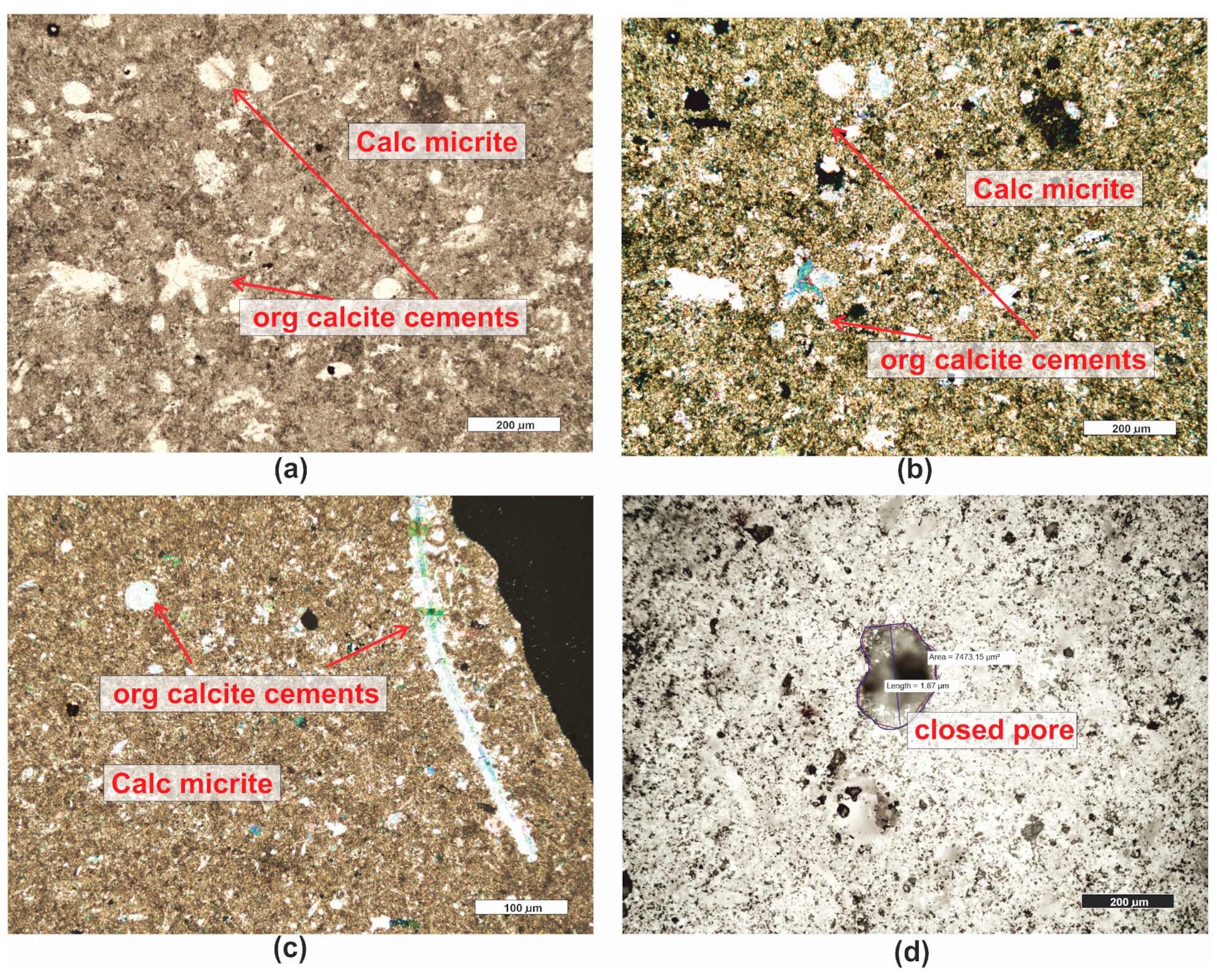
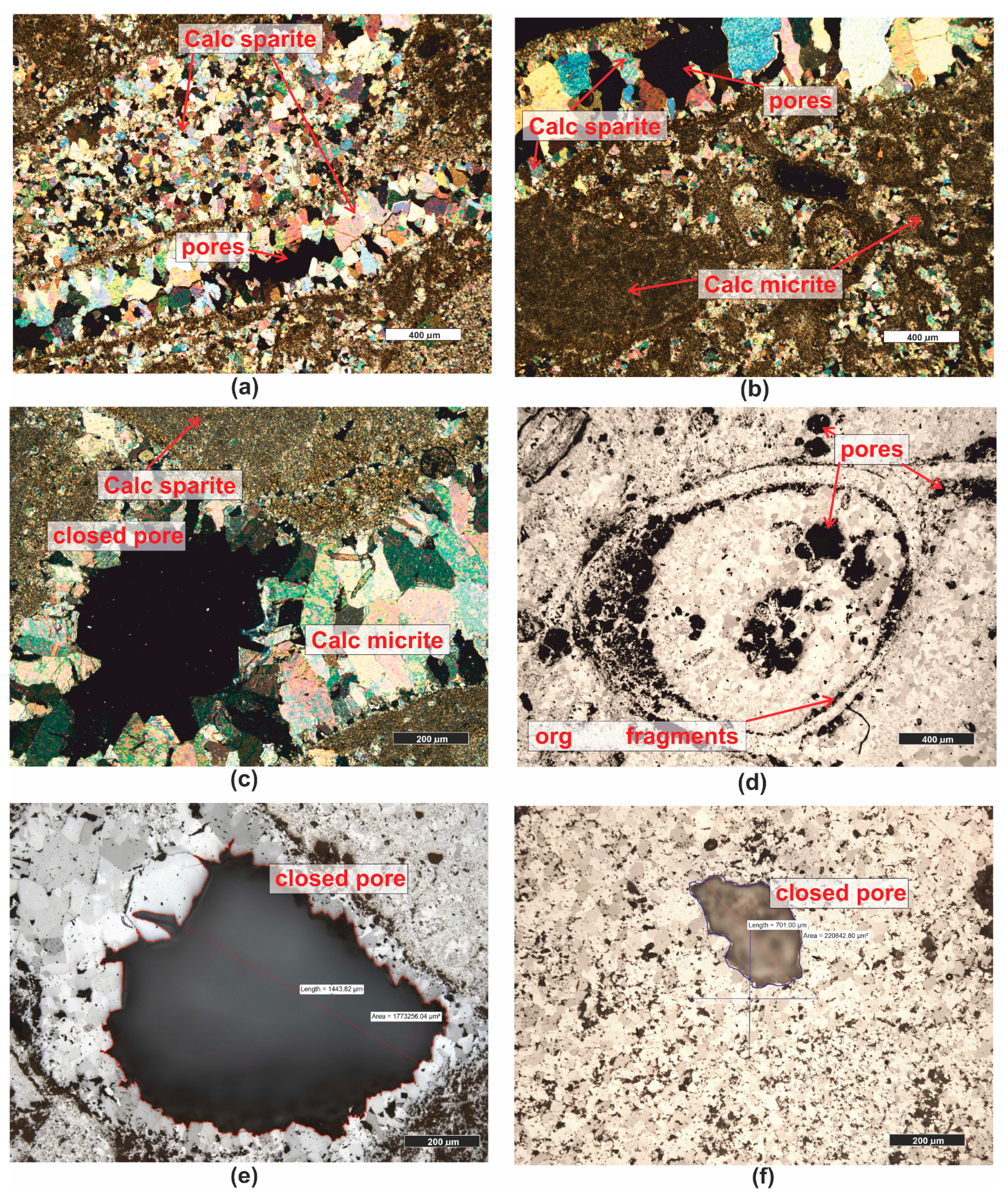

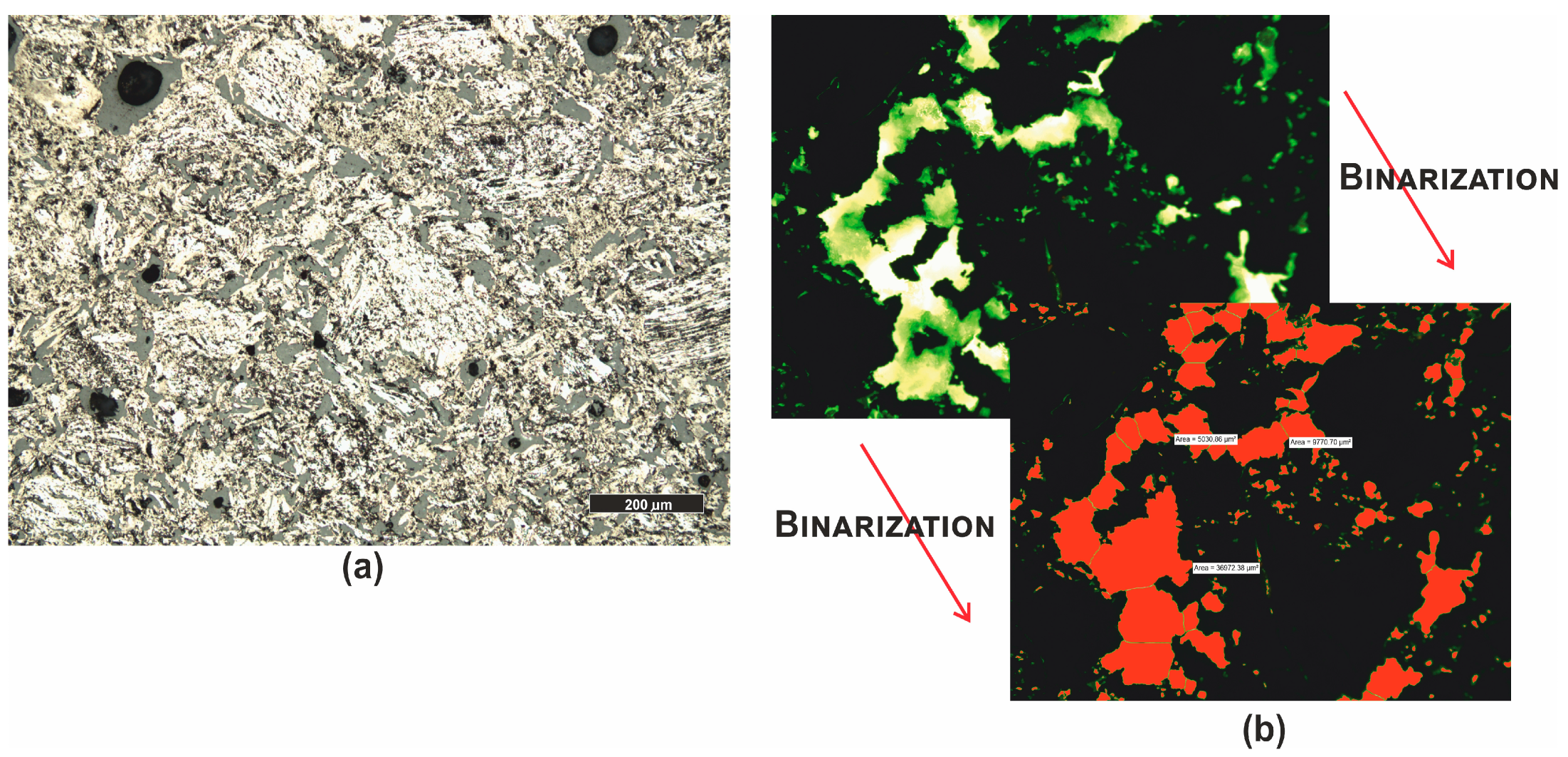

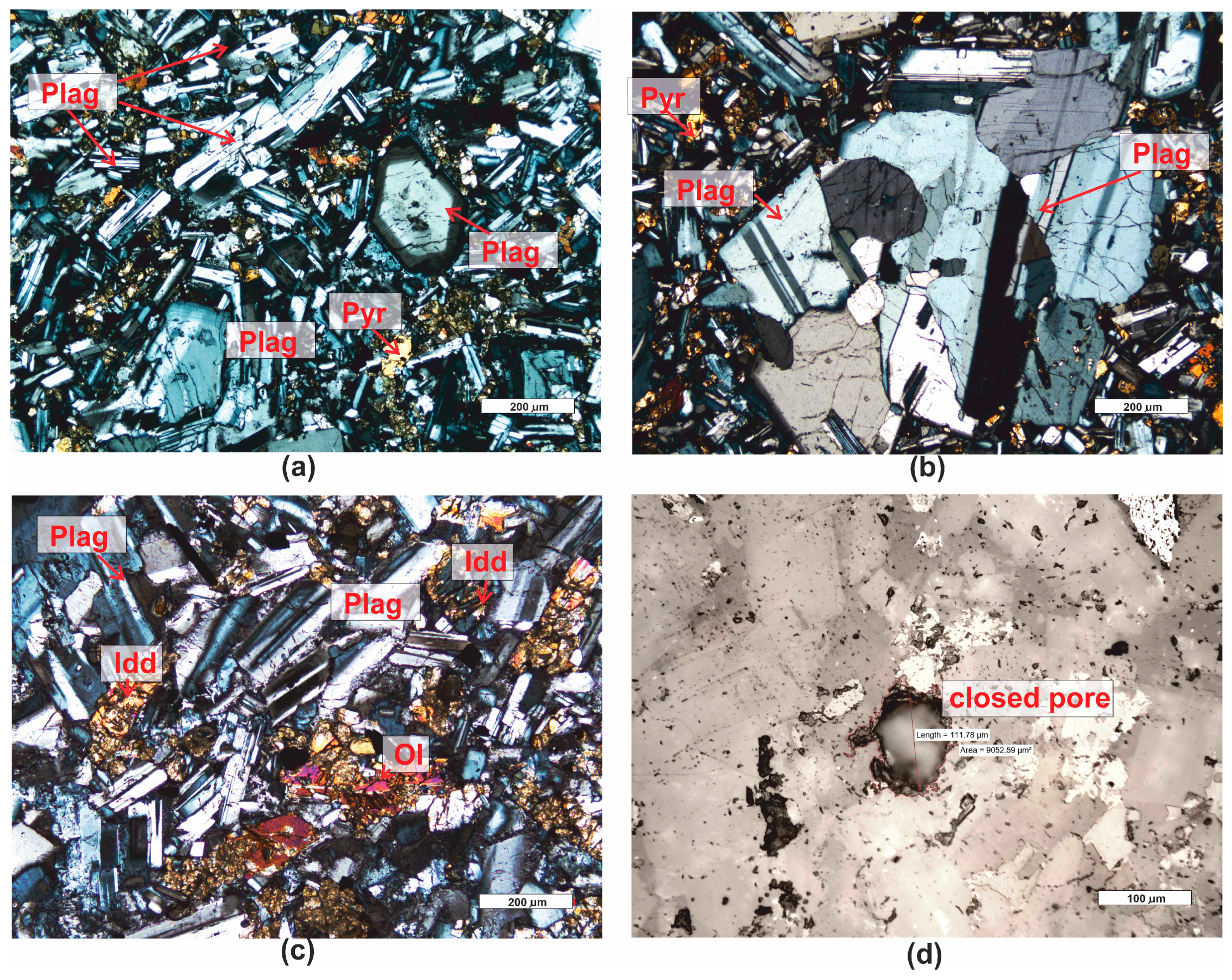
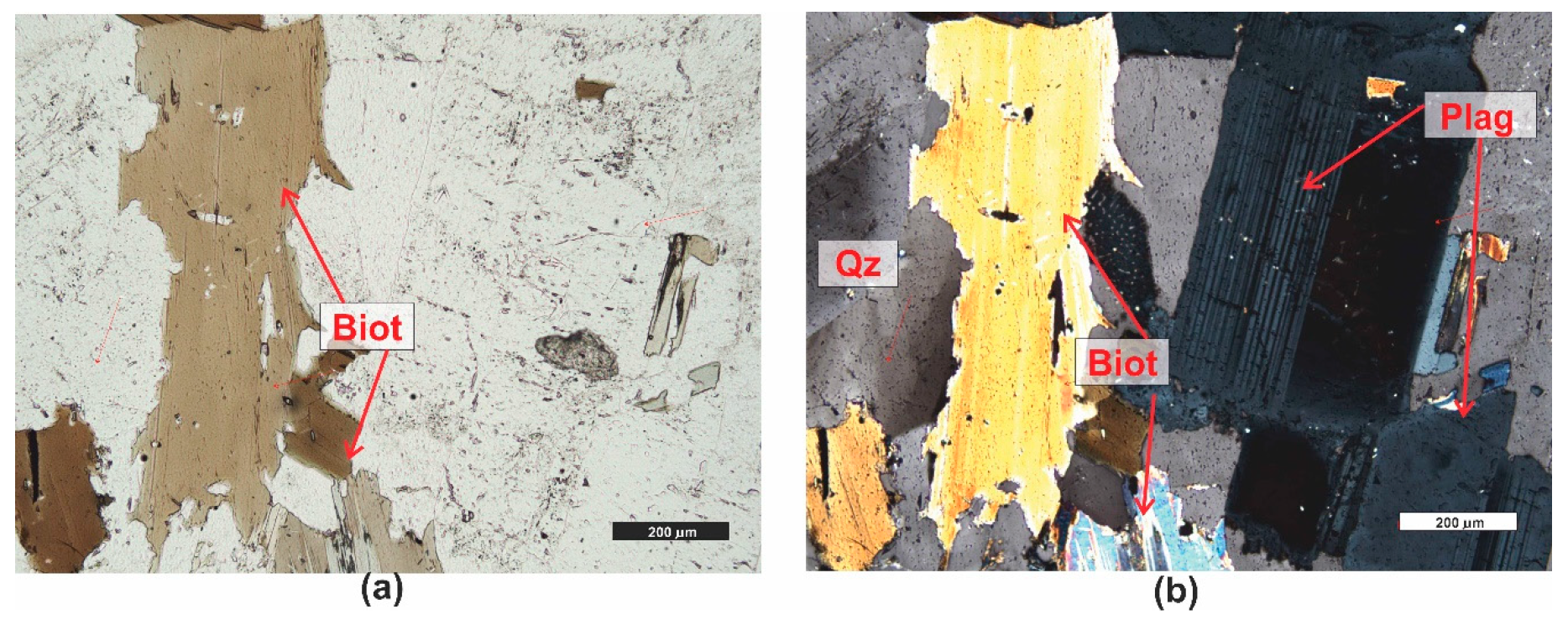


| Grain fraction | 4–2.5 | 2.5–1.6 | 1.6–1 | 1–0.63 | 0.63–0.4 | 0.4–0.25 |
| Porosity [%] | 19.81 | 9.97 | 11.55 | 11.32 | 5.87 | 4.15 |
| N2, −196 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRO1 | PRO2 | PRO3 | PRO4 | PRO5 | PRO6 | PRO6 | PRO7 | PRO8 | PRO9 | PRO10 | ||
| Adsorption capacity (aN2) | cm3/g | 4.44 | 2.95 | 5.48 | 8.18 | 37.06 | 2.96 | 2.245 | 3.21 | 2.908 | 1.6498 | 30.04 |
| BET surface area 0.05 < p/p° ≤ 0.35 | m2/g | 2.080 | 1.320 | 1.920 | 6.650 | 35.800 | 1.670 | 0.642 | 1.161 | 2.560 | 0.650 | 9.490 |
| t-plot micropore area | 0.000 | 0.000 | 0.v000 | 0.515 | 12.070 | 0.000 | 0.249 | 0.000 | 0.101 | 0.000 | 0.694 | |
| t-plot external surface area | 2.080 | 1.290 | 1.922 | 6.133 | 23.014 | 1.654 | 0.476 | 1.170 | 2.449 | 0.619 | 8.791 | |
| BJH cumulative surface area of pores 1.7–300 nm | 2.080 | 1.320 | 1.996 | 5.900 | 35.800 | 1.670 | 0.630 | 1.237 | 2.550 | 0.670 | 8.700 | |
| BJH total pore volume (Vt) | cm3/g | 0.0069 | 0.0046 | 0.0085 | 0.0127 | 0.0750 | 0.0046 | 0.0035 | 0.0048 | 0.0045 | 0.0026 | 0.0465 |
| t-plot micropore volume | 0 | 0 | 0 | 0.0003 | 0.0205 | 0 | 0.0001 | 0 | 0.0001 | 0 | 0.0032 | |
| BJH cumulative volume of pores 1.7–300 nm | 0.0068 | 0.0045 | 0.0082 | 0.0129 | 0.0545 | 0.0044 | 0.0034 | 0.0047 | 0.0046 | 0.0024 | 0.0446 | |
| BJH average pore diameter | nm | 12 | 12 | 16.3 | 7.39 | 7.05 | 9.2 | 19.2 | 15.1 | 6.7 | 13.6 | 18 |
| Sample | Real Density [g/cm3] | Aparent Density [g/cm3] | Porosity [%] | Pore Volume [cm3/g] |
|---|---|---|---|---|
| PRO1 | 2.6926 | 2.3592 | 12.4 | 0.0525 |
| PRO2 | 2.6707 | 2.3000 | 13.9 | 0.0603 |
| PRO3 | 2.7247 | 2.4384 | 10.5 | 0.0431 |
| PRO4 | 2.7108 | 2.4906 | 8.1 | 0.0326 |
| PRO5 | 2.5252 | 2.2260 | 11.8 | 0.0532 |
| PRO6 | 1.8681 | 1.4303 | 23.4 | 0.1639 |
| PRO7 | 2.7073 | 1.8554 | 31.5 | 0.1696 |
| PRO8 | 2.7550 | 2.6453 | 4.0 | 0.0151 |
| PRO9 | 2.7895 | 2.5907 | 7.1 | 0.0275 |
| PRO10 | 2.5850 | 1.9570 | 24.3 | 0.1241 |
| Sample | Microscopic [% Porosity] | Densimetric [% Porosity] | Adsorption [% Porostiy] |
|---|---|---|---|
| >1000 nm (Macroporosity) | <25,000 nm (Micro, Meso, Macro) | <300 nm (Micro and Meso) | |
| PRO1 | 17.5 | 12.4 | 0 |
| PRO2 | 11.9 | 13.9 | 0 |
| PRO3 | 11.6 | 10.5 | 0 |
| PRO4 | 10.4 | 8.1 | 2.7 |
| PRO5 | 0 | 11.8 | 8.5 |
| PRO6 | 25 | 23.4 | 2.6 |
| PRO7 | 26.4 | 31.5 | 0 |
| PRO8 | 1.5 | 4.0 | 2.0 |
| PRO9 | 0 | 7.1 | 0 |
| PRO10 | 4.2 | 24.3 | 6.9 |
| Comparison—Features/Analysis | Clastic Rock of the Sandstone Type | Slags and Process Ashes |
| Color/pleochroism | Yes | Yes/maybe |
| Relief | Yes | Yes |
| Cracks and pores | Yes | Yes |
| Structure and texture | Yes | Yes or not |
| Cement, matrix | Yes | If present, yes |
| Shape and size of ingredients | Yes | Yes |
| Interference colors | Yes | Yes or not |
| Crystal twinning | Yes | Yes or not |
| Qualitative assessment | Yes | Yes or not |
| Stereological analyses—quantitative analysis | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godyń, K.; Dutka, B.; Tram, M. Application of Petrographic and Stereological Analyses to Describe the Pore Space of Rocks as a Standard for the Characterization of Pores in Slags and Ashes Generated after the Combustion of Municipal Waste. Materials 2023, 16, 7706. https://doi.org/10.3390/ma16247706
Godyń K, Dutka B, Tram M. Application of Petrographic and Stereological Analyses to Describe the Pore Space of Rocks as a Standard for the Characterization of Pores in Slags and Ashes Generated after the Combustion of Municipal Waste. Materials. 2023; 16(24):7706. https://doi.org/10.3390/ma16247706
Chicago/Turabian StyleGodyń, Katarzyna, Barbara Dutka, and Maciej Tram. 2023. "Application of Petrographic and Stereological Analyses to Describe the Pore Space of Rocks as a Standard for the Characterization of Pores in Slags and Ashes Generated after the Combustion of Municipal Waste" Materials 16, no. 24: 7706. https://doi.org/10.3390/ma16247706






