Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation of Ion-Selective Electrode
3. Results and Discussion
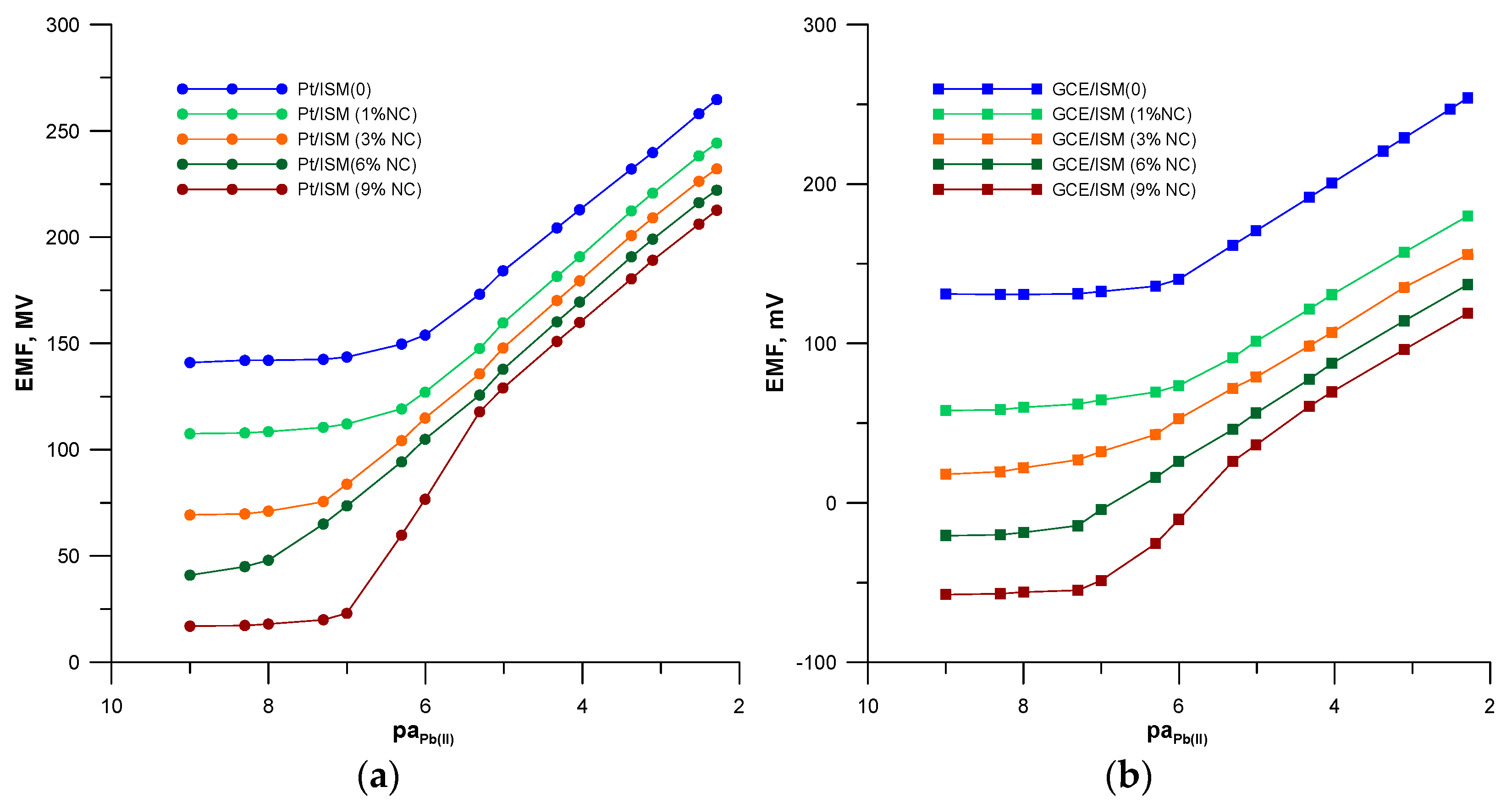
3.1. Potentiometric Response
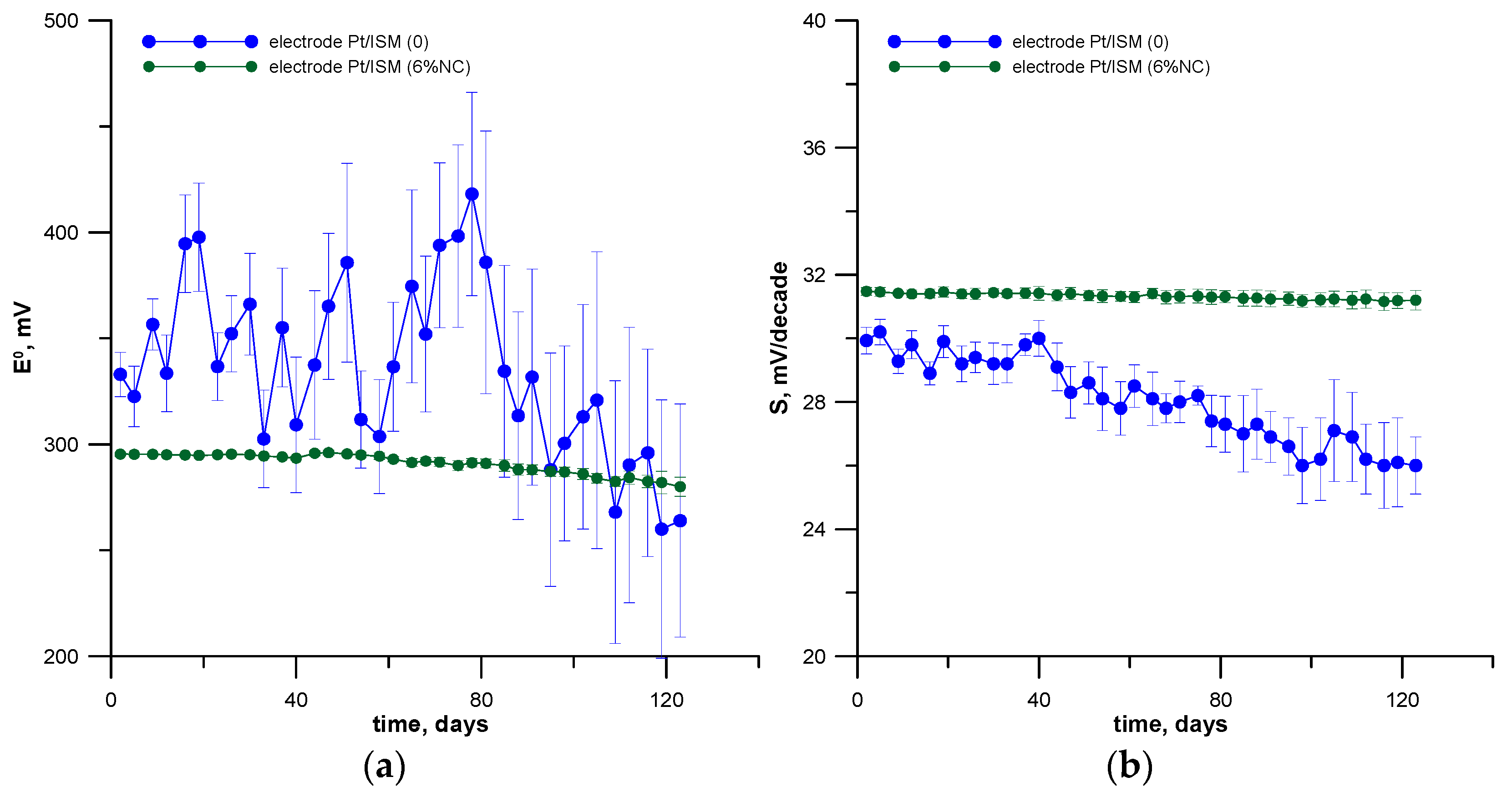
3.2. Potential Stability, Reversibility and Reproducibility
3.3. Selectivity
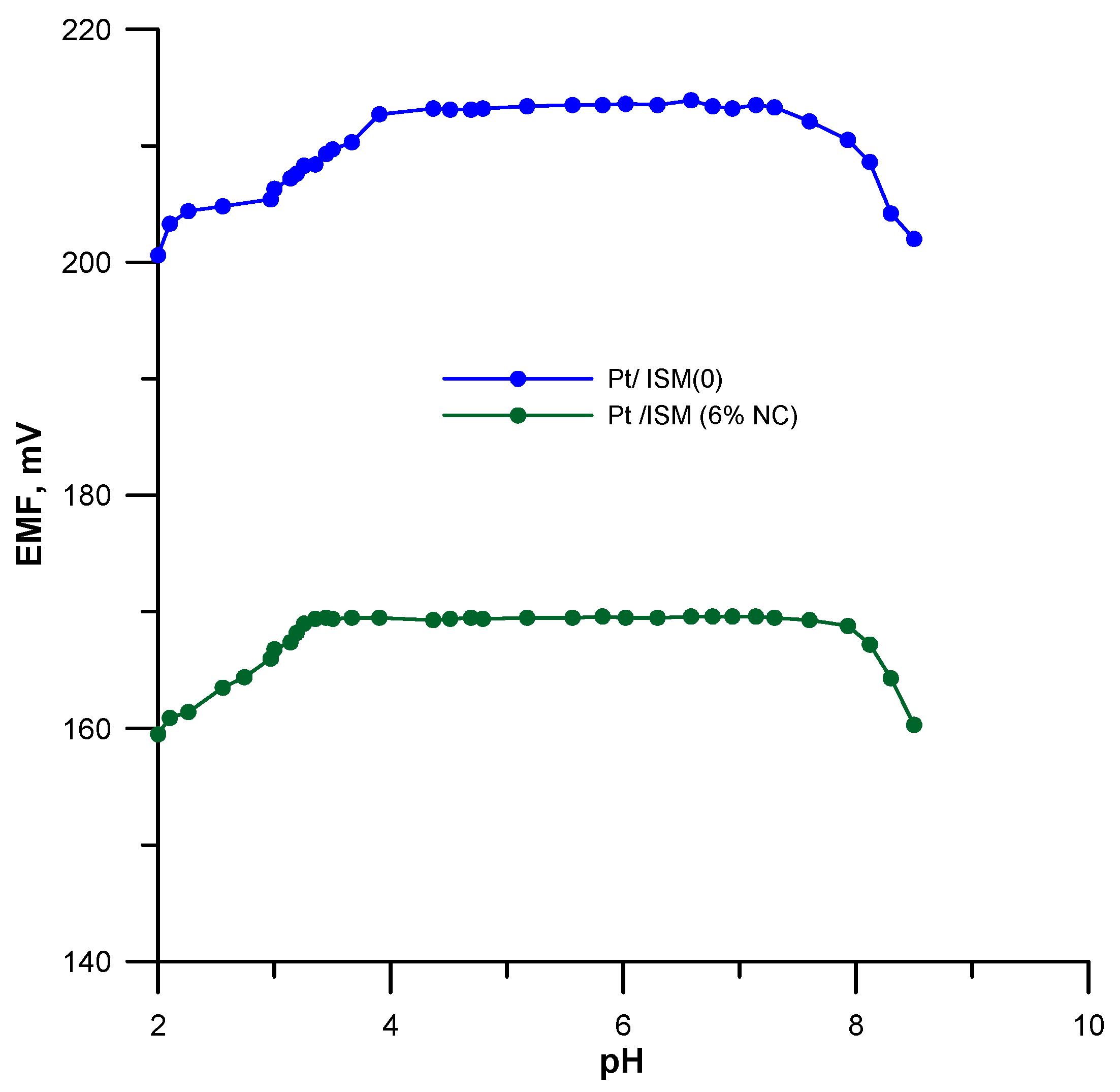
3.4. Optimal pH Range
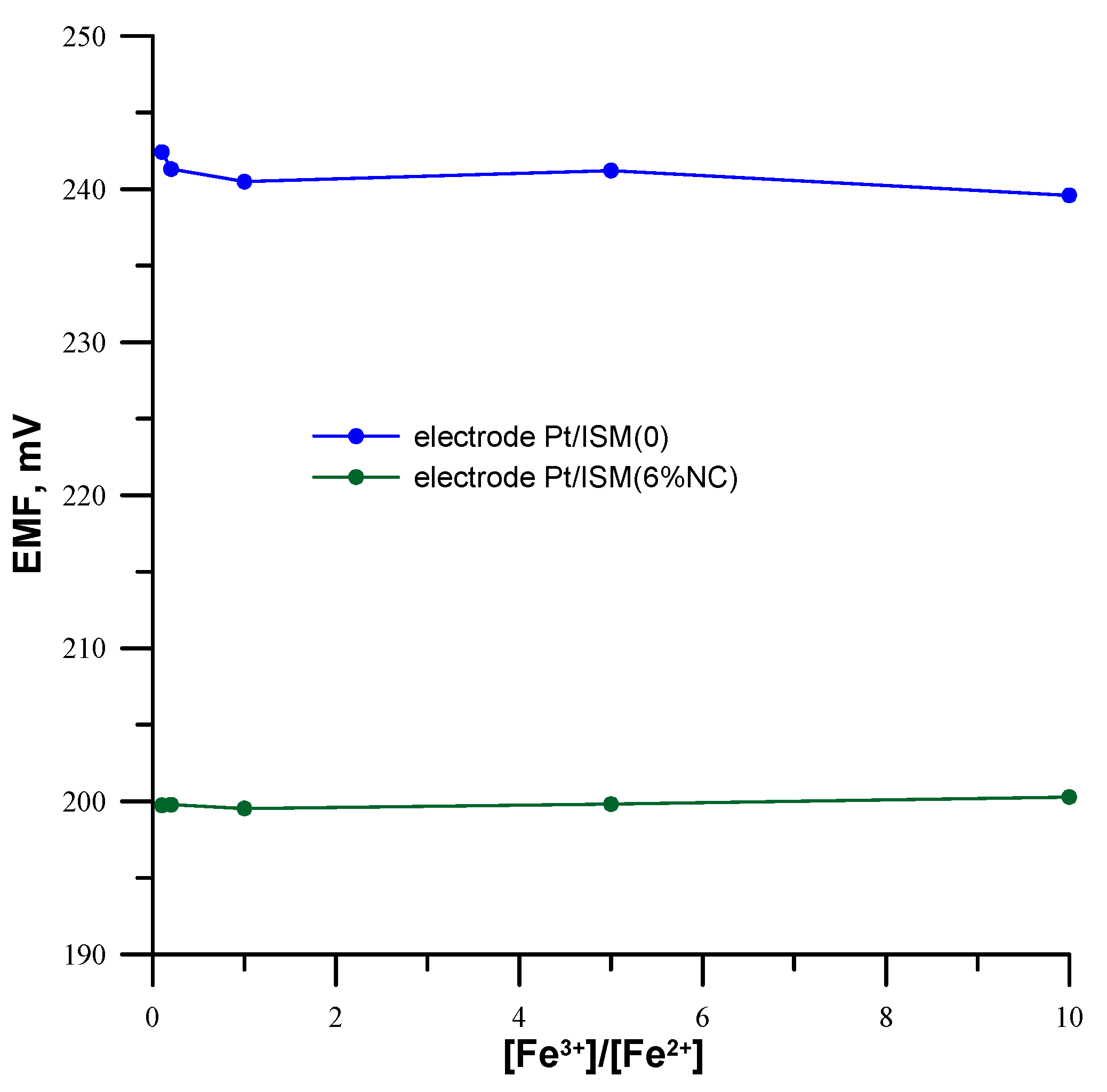
3.5. Redox Sensitivity
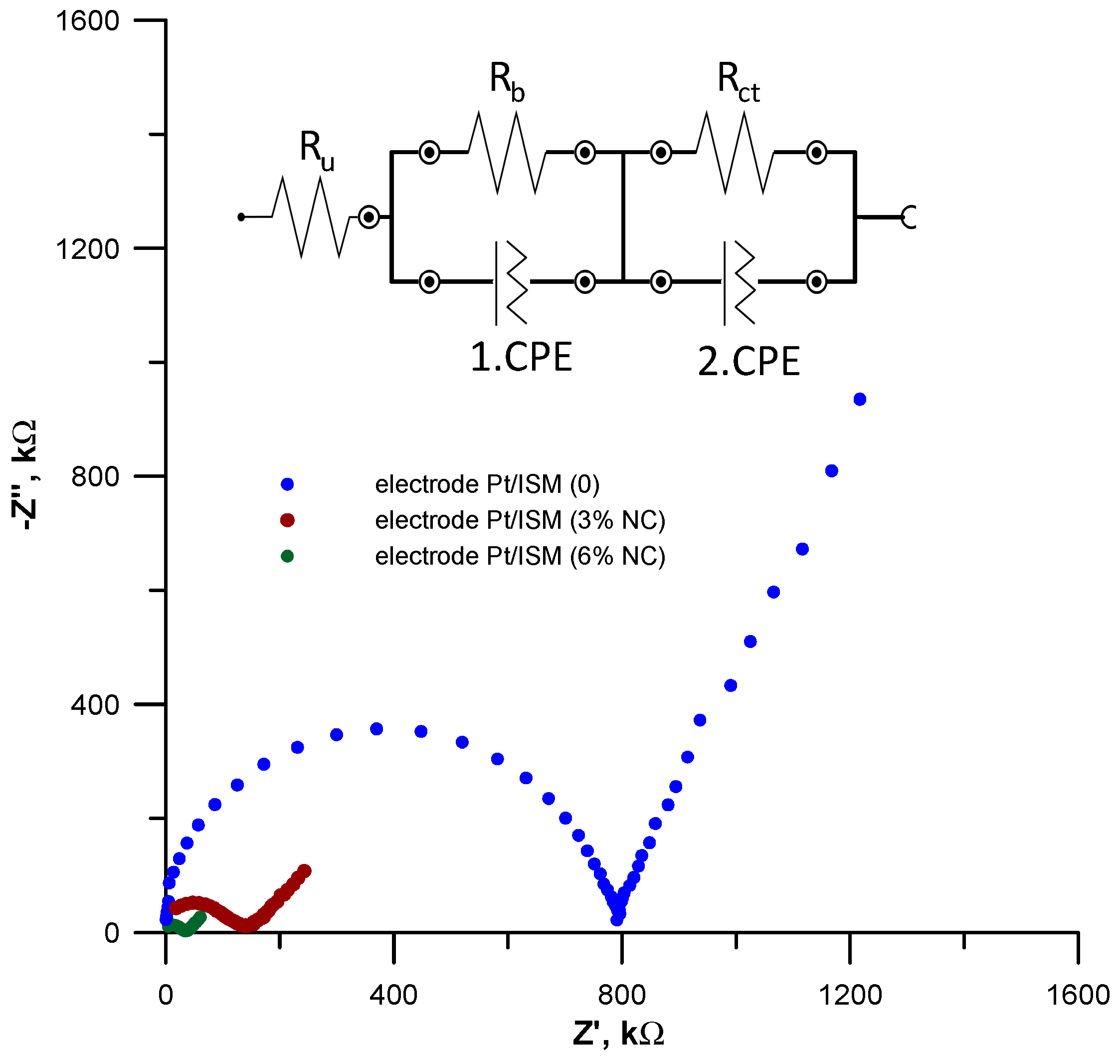
3.6. Electrochemical Impedance Spectroscopy Measurements
3.7. Analytical Application of Proposed Electrode for Lead Determination in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal-Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Mourya, A.; Mazumdar, B.; Sinha, S.K. Determination and quantification of heavy metal ion by electrochemical method. J. Environ. Chem. Eng. 2019, 7, 103459. [Google Scholar] [CrossRef]
- Tesfaye, E.; Chandravanshi, B.S.; Negash, N.; Tessema, M. A new modified carbon paste electrode using N1-hydroxy-N1,N2-diphenylbenzamidine for the square wave anodic stripping voltammetric determination of Pb(II) in environmental samples. Sens. Bio-Sens. Res. 2022, 38, 100520. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-contact ion-selective electrodes: Response mechanisms, transducer materials and wearable sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Wardak, C. A highly selective lead-sensitive electrode with solid contact based on ionic liquid. J. Hazard. Mater. 2011, 186, 1131–1135. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly reproducible solid contact ion selective electrodes: Emerging opportunities for potentiometry—A review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, W.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Stable Pb(II) ion-selective electrodes with a low detection limit using silver nanoparticles/polyaniline as the solid contact. Microchim. Acta 2021, 188, 393. [Google Scholar] [CrossRef]
- Michalska, A. All-Solid-State Ion Selective and All-Solid-State Reference Electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2021, 93, 72–102. [Google Scholar] [CrossRef]
- Mousavi, z.; Teter, A.; Lewenstam, A.; Maj-Zurawska, M.; Ivaska, A.; Bobacka, J. Comparison of multi-walled carbon nanotubes and poly(3-octylthiophene) as ion-to-electron transducers in all solid-state potassium ion-selective electrodes. Electroanalysis 2011, 23, 1352. [Google Scholar] [CrossRef]
- Pietrzak, K.; Wardak, C.; Malinowski, S. Application of polyaniline nanofibers for the construction of nitrate all-solid-state ion-selective electrodes. Appl. Nanosci. 2021, 11, 2823–2835. [Google Scholar] [CrossRef]
- Parra, E.J.; Crespo, G.A.; Riu, J.; Ruiz, A.; Rius, F.X. Ion-selective electrodes using multi-walled carbon nanotubes as ion-to-electron transducers for the detection of perchlorate. Analyst 2009, 134, 1905–1910. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Radu, A.; Anastasova-Ivanova, S.; Paczosa-Bator, B.; Danielewski, M.; Bobacka, J.; Lewenstam, A.; Diamond, D. Diagnostic of functionality of polymer membrane—Based ion selective electrodes by impedance spectroscopy. Anal. Methods 2010, 2, 1490–1498. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Wardak, C. Solid contact cadmium ion-selective electrode based on ionic liquid and carbon nanotubes. Sens. Actuators B Chem. 2015, 209, 131–137. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Kałuza, D.; Jaworska, E.; Mazur, M.; Maksymiuk, K.; Michalska, A. Multiwalled Carbon Nanotubes-Poly(3-octylthiophene-2,5-diyl) Nanocomposite Transducer for Ion-Selective Electrodes: Raman Spectroscopy Insight into the Transducer/Membrane Interface. Anal. Chem. 2019, 91, 9010–9017. [Google Scholar] [CrossRef]
- Pietrzak, K.; Wardak, C. Comparative study of nitrate all solid state ion-selective electrode based on multiwalled carbon nanotubes-ionic liquid nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720. [Google Scholar] [CrossRef]
- Gadhari, N.S.; Gholave, J.V.; Patil, S.S.; Patil, V.R.; Upadhyay, S.S. Enantioselective high performance new solid contact ion-selective electrode potentiometric sensor based on sulphated γ-cyclodextrin-carbon nanofiber composite for determination of multichiral drug moxifloxacin. J. Electroanal. Chem. 2021, 882, 114981. [Google Scholar] [CrossRef]
- Niemiec, B.; Zambrzycki, M.; Piech, R.; Wardak, C.; Paczosa-Bator, B. Hierarchical Nanocomposites Electrospun Carbon NanoFibers/Carbon Nanotubes as a Structural Element of Potentiometric Sensors. Materials 2022, 15, 4803. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-bator, B. High Capacity Nanocomposite Layers Based on Nanoparticles of Carbon Materials and Ruthenium Dioxide for Potassium Sensitive Electrode. Materials 2021, 14, 1308. [Google Scholar] [CrossRef]
- Pietrzak, K.; Morawska, K.; Malinowski, S.; Wardak, C. Chloride ion-selective electrode with solid-contact based on polyaniline nanofibers and multiwalled carbon nanotubes nanocomposite. Membranes 2022, 12, 1150. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; El-rahman, M.K.A.; Tan, E.K.W.; Sigurslid, H.H.; Arkan, N.; Lane, J.S.; Whitesides, G.M.; Bühlmann, P. Ionic liquid-based reference electrodes for miniaturized ion sensors: What can go wrong ? Sens. Actuators B. Chem. 2019, 301, 127112. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Deyab, M.A. Ionic liquid as an electrolyte additive for high performance lead-acid batteries. J. Power Sources 2018, 390, 176–180. [Google Scholar] [CrossRef]
- Salsamendi, M.; Morozova, S.M.; Lozinskaya, E.I.; Devaraj, S.; Vygodskii, Y.S.; Shaplov, A.S.; Mecerreyes, D. Polyimides as Cathodic Materials in Lithium Batteries: Effect of the Chemical Structure of the Diamine Monomer. J. Polym. Sci. 2018, 56, 714–723. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Wang, R.; Hu, G.; Miao, J.; Hai, K.; Lin, C. Determination of copper(II) ion in food using an ionic liquids-carbon nanotubes-based ion-selective electrode. J. Food Compos. Anal. 2017, 62, 63–68. [Google Scholar] [CrossRef]
- Zhan, T.; Tan, Z.; Tian, X.; Hou, W. Ionic liquid functionalized graphene oxide-Au nanoparticles assembly for fabrication of electrochemical 2,4-dichlorophenol sensor. Sens. Actuators B Chem. 2017, 246, 638–646. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Han, J.; Wang, L.; Chen, T.; Ni, L. Selective extraction and preconcentration of trace lead(II) in medicinal plant-based ionic liquid hollow fiber liquid phase microextraction system using dicyclohexyl-18-crown-6 as membrane carrier. Anal. Methods 2015, 7, 2339–2346. [Google Scholar] [CrossRef]
- Akhtar, A.; Kazi, T.G.; Afridi, H.I.; Baig, J.A.; Arain, M.B. A tandem ionic liquid-based dispersive microextraction method using in-syringe air-assisted vesicle system for rapid determination of lead and cadmium in artificial sweat extract of facial cosmetic products. Appl. Organomet. Chem. 2020, 34, e5784. [Google Scholar] [CrossRef]
- Wardak, C. 1-Hexyl-3-methylimidazolium hexafluorophosphate as new component of polymeric membrane of lead ion-selective electrode. Desalin. Water Treat. 2013, 51, 658–664. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Ozcelikay, G.; Budak, F.; Ozkan, S.A. Carbon Nanomaterials-Based Novel Hybrid Platforms for Electrochemical Sensor Applications in Drug Analysis. Crit. Rev. Anal. Chem. 2022; in press. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wang, D.; Zhuang, H.L.; Li, S.; Fan, L.; Wang, X.; He, Y.; Lu, Y. Ionic liquid-reinforced carbon nano fi ber matrix enabled lean-electrolyte Li-S batteries via electrostatic attraction. Energy Storage Mater. 2020, 26, 378–384. [Google Scholar] [CrossRef]
- Tang, X.; Wan, P.-Y.; Buchter, G. Ion-Selective Electrodes for Detection of Lead (II) in Drinking Water: A Mini-Review. Environments 2018, 5, 95. [Google Scholar] [CrossRef]
- Horvai, G.; GráF, E.; TóTh, K.; Pungor, E.; Buck, R.P. Plasticized Poly(vinyl chloride) Properties and Characteristics of Valinomycin Electrodes. 1. High-Frequency Resistances and Dielectric Properties. Anal. Chem. 1986, 58, 2735–2740. [Google Scholar] [CrossRef]
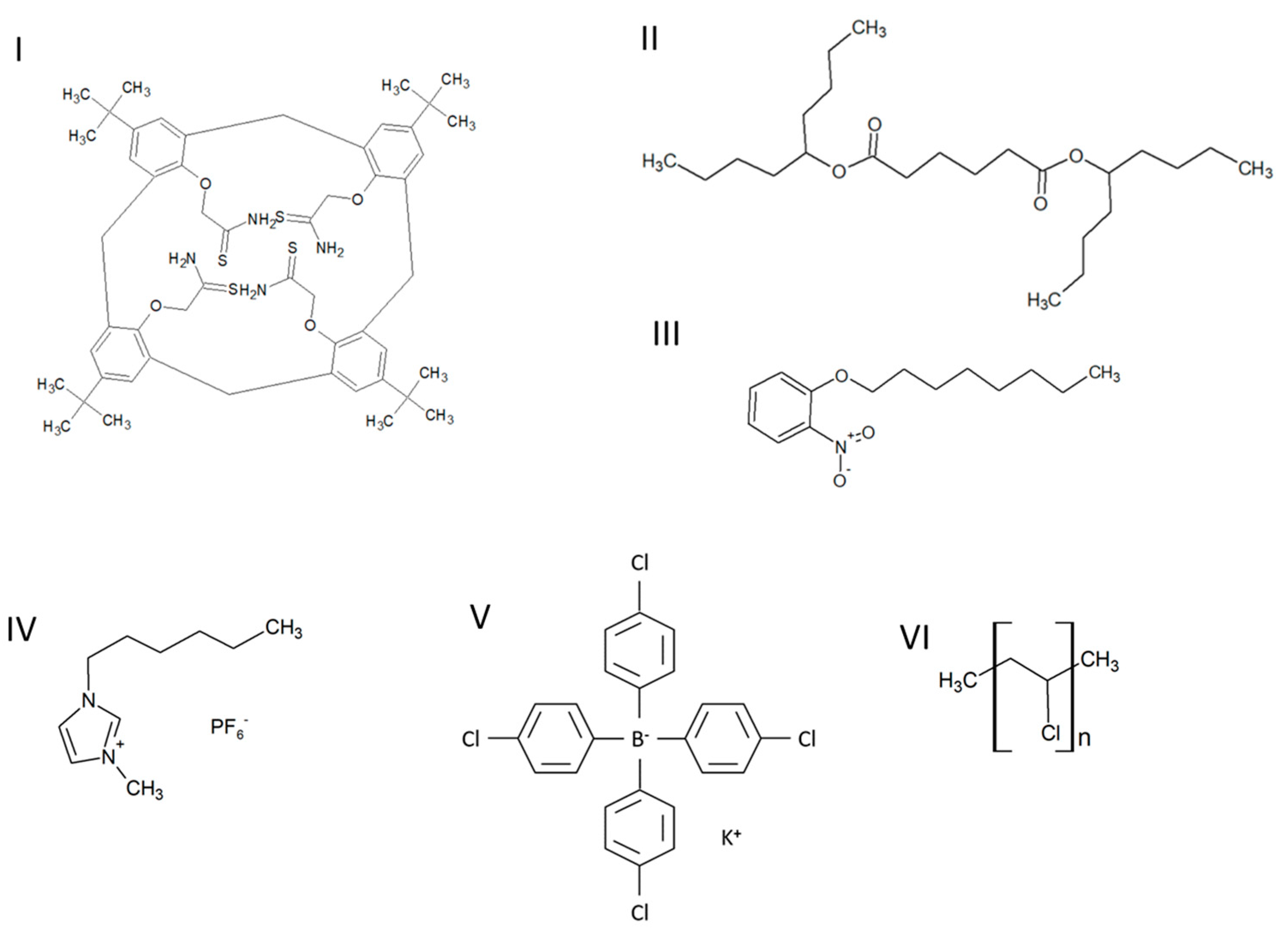
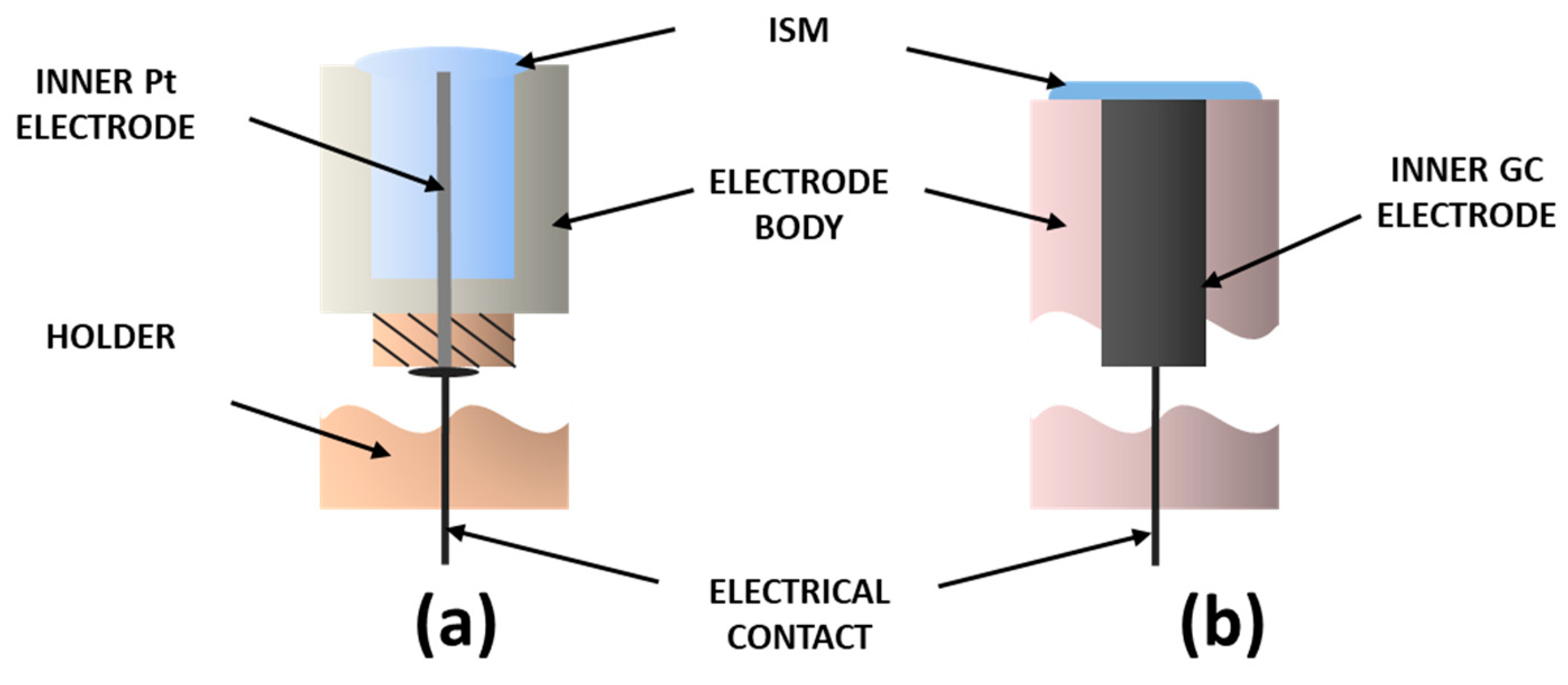

| ISE | Inner Electrode | Membrane Composition, % wt. | |||||
|---|---|---|---|---|---|---|---|
| Ionophore | KTpClPB | NC | PVC | BBPA | NPOE | ||
| Pt/ISM (0) | Pt | 1 | 0.5 | - | 33 | 33 | 32.5 |
| Pt/ISM (1%NC) | Pt | 1 | - | 1 | 33 | 32.5 | 32.5 |
| Pt/ISM (3%NC) | Pt | 1 | - | 3 | 33 | 32.5 | 30.5 |
| Pt/ISM (6%NC) | Pt | 1 | - | 6 | 33 | 32.5 | 27.5 |
| Pt/ISM (9%NC) | Pt | 1 | - | 9 | 33 | 32.5 | 24.5 |
| GCE/ISM (0) | GCE | 1 | 0.5 | - | 33 | 33 | 32.5 |
| GCE/ISM (1%NC) | GCE | 1 | - | 1 | 33 | 32.5 | 32.5 |
| GCE/ISM (3%NC) | GCE | 1 | - | 3 | 33 | 32.5 | 30.5 |
| GCE/ISM (6%NC) | GCE | 1 | - | 6 | 33 | 32.5 | 27.5 |
| GCE/ISM (9%NC) | GCE | 1 | - | 9 | 33 | 32.5 | 24.5 |
| Electrode | Slope 1, mV/pa | Limit of Detection, mol L−1 | Linear Range, mol L−1 | Response Time, s |
|---|---|---|---|---|
| Pt/ISM (0) | 29.9 | 4.0 × 10−7 | 1 × 10−6–1 × 10−2 | 15 |
| Pt/ISM (1%NC) | 31.9 | 2.4 × 10−7 | 5 × 10−6–1 × 10−2 | 8 |
| Pt/ISM (3%NC) | 31.8 | 3.1 × 10−8 | 1 × 10−7–1 × 10−2 | 5 |
| Pt/ISM (6%NC) | 31.5 | 6.0 × 10−9 | 1 × 10−8–1 × 10−2 | 5 |
| Pt/ISM (9%NC) | 30.7 | 3.1 × 10−6 | 5 × 10−6–1 × 10−2 | 5 |
| 58.4 | - | 1 × 10−7–5 × 10−6 | - | |
| GCE/ISM (0) | 30.6 | 5.0 × 10−7 | 1 × 10−6–1 × 10−2 | 20 |
| GCE/ISM (1%NC) | 28.9 | 5.1 × 10−7 | 1 × 10−6–1 × 10−2 | 12 |
| GCE/ISM (3%NC) | 28.2 | 1.0 × 10−7 | 5 × 10−7–1 × 10−2 | 5 |
| GCE/ISM (6%NC) | 30.4 | 2.4 × 10−8 | 5 × 10−8–1 × 10−2 | 5 |
| GCE/ISM (9%NC) | 29.9 | 2.8 × 10−6 | 5 × 10−6–1 × 10−2 | 5 |
| 52.4 | - | 5 × 10−7–5 × 10−6 |
| Interfering Ion | −logKpotPb(II)/M | |
|---|---|---|
| Electrode Pt/ISM (0) | Electrode Pt/ISM (6%NC) | |
| Na+ | 4.1 | 8.3 |
| K+ | 4.6 | 8.5 |
| Li+ | 4.8 | 7.2 |
| Ca2+ | 4.9 | 7.2 |
| Mg2+ | 5.2 | 8.1 |
| Ba2+ | 4.8 | 6.4 |
| Cu2+ | 3.0 | 4.4 |
| Cd2+ | 3.2 | 5.5 |
| Co2+ | 6.0 | 6.4 |
| Ni2+ | 6.0 | 6.5 |
| Zn2+ | 5.8 | 6.1 |
| Electrode | Ru, kΩ | Rb, kΩ | CPE1 Y0(N), pF | Rct kΩ | CPE2 Y0 (N), µF |
|---|---|---|---|---|---|
| Pt/ISM (0) | 16.8 | 762 | 15.4 (0.982) | 9832 | 0.044 (0.991) |
| Pt/ISM (3%NC) | 12.4 | 178 | 387 (0.847) | 196 | 20.7 (0.879) |
| Pt/ISM (6%NC) | 11.6 | 46.5 | 1231 (0.789) | 28.6 | 82.4 (0.886) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardak, C.; Morawska, K.; Paczosa-Bator, B.; Grabarczyk, M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003. https://doi.org/10.3390/ma16031003
Wardak C, Morawska K, Paczosa-Bator B, Grabarczyk M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials. 2023; 16(3):1003. https://doi.org/10.3390/ma16031003
Chicago/Turabian StyleWardak, Cecylia, Klaudia Morawska, Beata Paczosa-Bator, and Malgorzata Grabarczyk. 2023. "Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite" Materials 16, no. 3: 1003. https://doi.org/10.3390/ma16031003
APA StyleWardak, C., Morawska, K., Paczosa-Bator, B., & Grabarczyk, M. (2023). Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials, 16(3), 1003. https://doi.org/10.3390/ma16031003








