Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants
Abstract
:1. Introduction
| S/No | Sorbents | Adsorbent Base | Adsorbent State | Adsorption Capacities (mg/g) | References |
|---|---|---|---|---|---|
| 1 | Banana peel | Cellulose | Modified | 32.40 68.92 99.09 | [19] |
| 2 | Sugar beet pulp | Cellulose | Modified | 73.53 | [20] |
| 3 | Rice husk | Cellulose | Modified | 6.0–9.0 | [21] |
| 4 | Vegetable fibres | Cellulose | Raw | 85.0 | [7] |
| 5 | Oil Palm empty fruit bunch | Cellulose | Modified | 7.0 | [22] |
| 6 | Posidonia oceanica (L.) | Cellulose | Raw + Modified | 4.74 12.80 | [23] |
| 7 | Sugarcane leaves straw Sugarcane bagasse | Cellulose | Raw | 8.0 6.6 | [24] |
| 8 | Green macroalgae | Cellulose | Modified | 19.38–23.08 | [25] |
| 9 | Coconut shell | Cellulose | Modified | 2.48 | [26] |
| 10 | Coconut fibre | Cellulose | Modified | 13.2–14.0 | [27] |
| 11 | Corn cob | Cellulose | Modified | 4.21–7.80 | [28] |
| 12 | Wheat straw | Cellulose | Modified | 41.84 | [29] |
| 13 | Pineapple leaf waste | Cellulose | Modified | 37.9 | [30] |
| 14 | Wheat bran | Cellulose | Modified | 62 | [31] |
| 15 | Hazelnut shells | Cellulose | Modified | 41.3 | [32] |
| 16 | Papaya seed | Cellulose | Modified | 55.6 37.43 | [33] |
| 17 | Sunflower stalk | Cellulose | Modified | 39 | [34] |
| 18 | Chicken feathers | Keratin | Modified | 6.1 | [1] |
| 19 | Human hair | Keratin | Raw + Modified | 8.1 5.5 | [3] |
| 20 | Mango peel | Cellulose | Modified | 46.09 39.75 28.21 | [35] |
| 21 | Palm ash | Cellulose | Modified | 61 | [36] |
| 22 | Palm shell | Cellulose | Modified | 83.33 | [37] |
| 23 | Sunflower stalk | Cellulose | Modified | 182.90 69.80 | [38] |
| 24 | Orange peel | Cellulose | Modified | 200 | [39] |
| 25 | Rice husk | Cellulose | Modified | 15.0 | [40] |
| 26 | Cashew nutshell | Cellulose | Modified | 22.11 | [41] |
| 27 | Cotton fibre | Cellulose | Modified | 25–75 | [42] |
| 28 | Spider cuticles | Keratin | Raw | 12.6–16.6 | [43] |
| 29 | Sugarcane bagasse | Cellulose | Modified | 38.03 | [44] |
| 30 | Bamboo leaf powder | Cellulose | Modified | 28.1 | [45] |
| 31 | Wastepaper | Cellulose | Modified | 24.4 | [46] |
| 32 | Silk fibre | Cellulose | Modified | 46.83 | [47] |
| 33 | Paper waste | Cellulose | Modified | 29.67 | [48] |
| 34 | Hazel nutshell | Cellulose | Modified | 28.18 | [49] |
| 35 | Wool fibre | Keratin | Raw + Modified | 12.0 | [50] |
| 36 | Wool fibre | Keratin | Raw | 11.06 | [51] |
| 37 | Grapefruits | Cellulose | Modified | 37.43 39.06 | [52] |
| 38 | Chicken feathers | Keratin | Modified | 50.0 | [53] |
| 39 | Garlic peel and Onion peel | Cellulose | Modified | 3.85 4.55 | [54] |
| 40 | Pine leaf powder | Cellulose | Modified | 3.27 | [55] |
| 41 | Banana stalk | Cellulose | Modified | 138 | [56] |
| 42 | Wheat straw | Cellulose | Modified | 6.91 | [57] |
| 43 | Banana | Cellulose | Modified | 5 | [58] |
| 44 | Rice husk | Cellulose | Modified | 6.22 | [59] |
| 45 | Rice husk | Cellulose | Modified | 19.66 | [60] |
| 46 | Kapok fibre | Cellulose | Modified | 46.9–58.8 | [61] |
| 47 | Corn stalk | Cellulose | Modified | 21.37 | [62] |
| 48 | Pigeon feathers | Keratin | Modified | 30.0 | [63] |
| 49 | Sugarcane bagasse | Cellulose | Modified | 13.72 | [64] |
2. Adsorbent Materials
2.1. Characteristics of Good Adsorbent Material
2.2. Chemical Composition and Physical Properties of Orange Peel
3. Testing Techniques and Results
3.1. Adsorption Properties of Orange Peel
3.2. Physical Modification
3.3. Chemical Modification
3.4. Thermal Modification
3.5. Thermochemical and Physical Modifications
4. Discussion
5. Conclusions
Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ifelebuegu, A.; Chinonyere, P. Oil Spill Clean-up from Sea Water using Waste Chicken Feathers. In Proceedings of the Fourth International Conference on Advances in Applied Science and Environmental Technology, Bangkok, Thailand, 21–22 February 2015; pp. 61–64. [Google Scholar]
- Keshawy, M.; Fathy, M.; Gaffer, A.; Hosny, R.; Abdel Moghny, T. Low-cost Bio-Adsorbent based on Amorphous Carbon Thin Film/chitosan composite for removal of Methylene Blue Dye: Kinetic and Isotherm. Egypt. J. Chem. 2019, 62, 2313–2329. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Momoh, Z. An Evaluation of the Adsorptive Properties of Coconut Husk for Oil Spill Clean-up. In Proceedings of the International Conference on Advances in Applied Science and Environmental Technology, Bangkok, Thailand, 3–4 January 2015. [Google Scholar]
- Huang, Y.; Li, H.; Bai, M.; Huang, X. Efficient extraction of perfluorocarboxylic acids in complex samples with a monolithic adsorbent combining fluorophilic and anion-exchange interactions. Anal. Chim. Acta 2018, 1011, 50–58. [Google Scholar] [CrossRef]
- Knaebel, S.P.; Ko, D.; Biegler, L.T. Simulation and Optimization of a Pressure Swing Adsorption System: Recovering Hydrogen from Methane. Adsorption 2005, 11 (Suppl. S1), 615–620. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Gulistan, A.S.; Khamis, M.I.; Ahmed, H.; Aidan, A. Produced water treatment using naturally abundant pomegranate peel. Desalin. Water Treat. 2016, 57, 6693–6701. [Google Scholar] [CrossRef]
- Spreen, T. Proyecciones de la producción y consumo mundial de los cítricos. In FAO Simposio Sobre Cítricos; FAO: Rome, Italy, 2010. [Google Scholar]
- Abdullah, M.; Muhamad, S.H.A.; Sanusi, S.N.A.; Iziuna, S. Preliminary Study of Oil Removal using Hybrid Peel Waste: Musa Balbisiana and Citrus Sinensis. J. Appl. Environ. Biol. Sci. 2016, 6, 59–63. [Google Scholar]
- El-Nemr, M.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; Tedone, L.; De Mastro, G.; Pantaleo, A. Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water. Molecules 2022, 27, 4840. [Google Scholar] [CrossRef]
- Sivaraj, R.; Namasivayam, C.; Kadirvelu, K. Orange peel as an adsorbent in the removal of Acid violet 17 (acid dye) from aqueous solutions. Waste Manag. 2001, 21, 105–110. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; Ahmad El Saqa, F.; Abd El Razek Amer, A.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Singh, A.K.; Ketan, K.; Singh, J.K. Simple and green fabrication of recyclable magnetic highly hydrophobic sorbents derived from waste orange peels for removal of oil and organic solvents from water surface. J. Environ. Chem. Eng. 2017, 5, 5250–5259. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M.; Tabrizi, N.S. Removal of dyes from coloured textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J. Colloid Interface Sci. 2005, 288, 371–376. [Google Scholar] [CrossRef]
- Khaled, A.; El Nemr, A.; El-Sikaily, A.; Abdelwahab, O. Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon. Desalination 2009, 238, 210–232. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Meniai, A.H. Application of chemically modified orange peels for removal of copper (II) from aqueous solutions. Theor. Found. Chem. Eng. 2012, 46, 732–739. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic (V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremediat. 2016, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Altundogan, H.S.; Arslan, N.E.; Tumen, F. Copper removal from aqueous solutions by sugar beet pulp treated by NaOH and citric acid. J. Hazard. Mater. 2007, 149, 432–439. [Google Scholar] [CrossRef]
- Angelova, D.; Uzunov, I.; Uzunova, S.; Gigova, A.; Minchev, L. Kinetics of oil and oil products adsorption by carbonized rice husks. Chem. Eng. J. 2011, 172, 306–311. [Google Scholar] [CrossRef]
- Asadpour, R.; Sapari, N.B.; Isa, M.H.; Kakooei, S.; Orji, K.U. Acetylation of corn silk and its application for oil sorption. Fibers Polym. 2015, 16, 1830–1835. [Google Scholar] [CrossRef]
- Ben Jmaa, S.; Kallel, A. Assessment of performance of Posidona oceanica (L.) as biosorbent for crude oil-spill clean-up in seawater. BioMed Res. Int. 2019, 2019, 6029654. [Google Scholar] [CrossRef] [Green Version]
- Behnood, R.; Anvaripour, B.; Jaafarzade Haghighi Fard, N.; Farasati, M. Application of natural sorbents in crude oil adsorption. Iran. J. Oil Gas Sci. Technol. 2013, 2, 1–11. [Google Scholar]
- Boleydei, H.; Mirghaffari, N.; Farhadian, O. Comparative study on adsorption of crude oil and spent engine oil from seawater and freshwater using algal biomass. Environ. Sci. Pollut. Res. 2018, 25, 21024–21035. [Google Scholar] [CrossRef]
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Adsorption of ammonium ion by coconut shell-activated carbon from aqueous solution: Kinetic, isotherm, and thermodynamic studies. Environ. Sci. Pollut. Res. 2013, 20, 533–542. [Google Scholar] [CrossRef]
- Cardoso, C.K.M.; Mattedi, S.; Lobato, A.K.D.C.L.; Moreira, Í.T.A. Remediation of petroleum contaminated saline water using value-added adsorbents derived from waste coconut fibres. Chemosphere 2021, 279, 130562. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yu, S.W. Biosorption of methylene blue from aqueous solution by agricultural bioadsorbent corncob. Environ. Eng. Res. 2019, 24, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Dhir, B.; Kumar, R. Adsorption of heavy metals by Salvinia biomass and agricultural residues. Int. J. Environ. Res. 2010, 4, 427–432. [Google Scholar]
- Do, N.H.; Tran, V.T.; Tran, Q.; Le, K.A.; Thai, Q.B.; Nguyen, P.T.; Duong, H.M.; Le, P.K. Recycling of pineapple leaf and cotton waste fibers into heat-insulating and flexible cellulose aerogel composites. J. Polym. Environ. 2021, 29, 1112–1121. [Google Scholar] [CrossRef]
- Farajzadeh, M.; Monji, A. Adsorption characteristics of wheat bran towards heavy metal cations. Sep. Purif. Technol. 2004, 38, 197–207. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low-cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef]
- Hameed, B.H. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J. Hazard. Mater. 2009, 162, 939–944. [Google Scholar] [CrossRef]
- Hashem, A.; Abou-Okeil, A.; El-Shafie, A.; El-Sakhawy, M. Grafting of high α-cellulose pulp extracted from sunflower stalks for removal of Hg (II) from aqueous solution. Polym.-Plast. Technol. Eng. 2006, 45, 135–141. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.H.; Lang, L.S.; Asaari, F.A.; Aziz, H.A.; Ramli, N.A.; Dhas, J.P.A. Low-cost removal of disperse dyes from aqueous solution using palm ash. Dyes Pigments 2007, 74, 446–453. [Google Scholar]
- Ismail, K.; Ishak, M.A.M.; Ab Ghani, Z.; Abdullah, M.F.; Safian, M.T.U.; Idris, S.S.; Tahiruddin, S.; Yunus, M.F.M.; Hakimi, N.I.N.M. Microwave-assisted pyrolysis of palm kernel shell: Optimization using response surface methodology (RSM). Renew. Energy 2013, 55, 357–365. [Google Scholar]
- Jalali, M.; Aboulghazi, F. Sunflower stalk, an agricultural waste, as an adsorbent for the removal of lead and cadmium from aqueous solutions. J. Mater. Cycles Waste Manag. 2013, 15, 548–555. [Google Scholar] [CrossRef]
- Kamsonlian, S.; Suresh, S.; Majumder, C.B.; Chand, S. Characterization of banana and orange peels: Biosorption mechanism. Int. J. Sci. Technol. Manag. 2011, 2, 1–7. [Google Scholar]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Murugesan, A.; Sivanesan, S. Removal of cadmium (II) from aqueous solution by agricultural waste cashew nutshell. Korean J. Chem. Eng. 2012, 29, 756–768. [Google Scholar] [CrossRef]
- Lv, N.; Wang, X.; Peng, S.; Luo, L.; Zhou, R. Superhydrophobic/superoleophilic cotton-oil absorbent: Preparation and its application in oil/water separation. RSC Adv. 2018, 8, 30257–30264. [Google Scholar] [CrossRef] [Green Version]
- Machałowski, T.; Wysokowski, M.; Petrenko, I.; Fursov, A.; Rahimi-Nasrabadi, M.; Moh’d, M.A.; Meissner, H.; Joseph, Y.; Fazilov, B.; Ehrlich, H.; et al. Naturally pre-designed biomaterials: Spider molting cuticle as a functional crude oil sorbent. J. Environ. Manag. 2020, 261, 110218. [Google Scholar] [CrossRef]
- Mohan, R.; Chui, E.A.; Biasi, L.A.; Soccol, C.R. Alternative invitro propagation: Use of sugarcane bagasse as a low-cost support material during rooting stage of strawberry cv. Dover. Braz. Arch. Biol. Technol. 2005, 48, 37–42. [Google Scholar] [CrossRef]
- Mondal, D.K.; Nandi, B.K.; Purkait, M.K. Removal of mercury (II) from aqueous solution using bamboo leaf powder: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2013, 1, 891–898. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Patowary, M.; Pathak, K.; Ananthakrishnan, R. Robust superhydrophobic and oleophilic silk fibers for selective removal of oil from water surfaces. RSC Adv. 2016, 6, 73660–73667. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Uebe, J.; Karasu, A.U.; Anne, O. Investigation of cellulose-based aerogels for oil spill removal. Water Air Soil Pollut. 2020, 231, 424. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Radetić, M.M.; Jocić, D.M.; Jovančić, P.M.; Petrović, Z.L.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef]
- Radetic, M.; Ilic, V.; Radojevic, D.; Miladinovic, R.; Jocic, D.; Jovancic, P. Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525–530. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Tavares, T.; Pazos, M.; Sanromán, M.A. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Process Saf. Environ. Prot. 2016, 101, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, S.; Dadashian, F.; Eslahi, N. Recycling chicken feathers to produce adsorbent porous keratin-based sponge. Int. J. Environ. Sci. Technol. 2019, 16, 1119–1128. [Google Scholar] [CrossRef]
- Sayed, S.A.; Zayed, A.M. Investigation of the effectiveness of some adsorbent materials in oil spill clean-ups. Desalination 2006, 194, 90–100. [Google Scholar] [CrossRef]
- Shafique, U.; Ijaz, A.; Salman, M.; uz Zaman, W.; Jamil, N.; Rehman, R.; Javaid, A. Removal of arsenic from water using pine leaves. J. Taiwan Inst. Chem. Eng. 2012, 43, 256–263. [Google Scholar] [CrossRef]
- Shibi, I.G.; Anirudhan, T.S. Synthesis, characterization, and application as a mercury (II) sorbent of banana stalk (musa paradisiaca)—Polyacrylamide grafted copolymer bearing carboxyl groups. Ind. Eng. Chem. Res. 2002, 41, 5341–5352. [Google Scholar] [CrossRef]
- Sidiras, D.; Batzias, F.; Konstantinou, I.; Tsapatsis, M. Simulation of autohydrolysis effect on adsorptivity of wheat straw in the case of oil spill cleaning. Chem. Eng. Res. Des. 2014, 92, 1781–1791. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Vlaev, L.; Petkov, P.; Dimitrov, A.; Genieva, S. Cleanup of water polluted with crude oil or diesel fuel using rice husks ash. J. Taiwan Inst. Chem. Eng. 2011, 42, 957–964. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A. Acetylated modification of kapok fiber and application for oil absorption. Fibers Polym. 2013, 14, 1834–1840. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Superhydrophobic kapok fiber oil-absorbent: Preparation and high oil absorbency. Chem. Eng. J. 2012, 213, 1–7. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, C.; Dang, Z.; Zhang, H.; Yi, X.; Liu, C. Preparation of cellulose derived from corn stalk and its application for cadmium ion adsorption from aqueous solution. Carbohydr. Polym. 2012, 90, 1008–1015. [Google Scholar] [CrossRef]
- Zhou, L.T.; Yang, G.; Yang, X.X.; Cao, Z.J.; Zhou, M.H. Preparation of regenerated keratin sponge from waste feathers by a simple method and its potential use for oil adsorption. Environ. Sci. Pollut. Res. 2014, 21, 5730–5736. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zeng, H.; Liang, M.; Lu, R. Adsorption of chromium (VI) from aqueous solution by the iron (III)-impregnated sorbent prepared from sugarcane bagasse. Int. J. Environ. Sci. Technol. 2012, 9, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Fadeeva, E.; Schlie-Wolter, S.; Chichkov, B.N.; Paasche, G.; Lenarz, T. 5—Structuring of biomaterial surfaces with ultrashort pulsed laser radiation. In Laser Surface Modification of Biomaterials Techniques and Applications; Woodhead Publishing: Sawston, UK, 2016; pp. 145–172. [Google Scholar]
- Dong, T.; Xu, G.; Wang, F. Adsorption, and adhesiveness of kapok fiber to different oils. J. Hazard. Mater. 2015, 296, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Przyklenk, M. Studies on Kapok. 2. Chem. Investig. 1986, 40, 325–330. [Google Scholar]
- Chung, B.-Y.; Cho, J.Y.; Lee, M.H.; Wi, S.G.; Kim, J.H.; Kim, J.S.; Kang, P.H.; Nho, Y.C. Adsorption of Heavy Metal Ions onto Chemically Oxidized Ceiba pentandra (L.) Gaertn. (Kapok) Fibers. J. Appl. Biol. Chem. 2008, 51, 28–35. [Google Scholar] [CrossRef]
- Hori, K.; Flavier, M.E.; Kuga, S.; Lam, T.B.T.; Iiyama, K. Excellent oil absorbent kapok [Ceiba pentandra (L.) Gaertn.] fiber: Fiber structure, chemical characteristics, and application. J. Wood Sci. 2000, 46, 401–404. [Google Scholar] [CrossRef]
- Deschamps, G.; Caruel, H.; Borredon, M.-E.; Albasi, C.; Riba, J.-P.; Bonnin, C.; Vignoles, C. Oil Removal from Water by Sorption on Hydrophobic Cotton Fibers. 2. Study of Sorption Properties in Dynamic Mode. Environ. Sci. Technol. 2003, 37, 5034–5039. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Han, G.; Mao, Z.; Zhang, Y.; Duan, H.; Huang, J.; Qu, L. Structural characteristics and physical properties of lotus fibers obtained from Nelumbo nucifera petioles. Carbohydr. Polym. 2011, 85, 188–195. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z. Hybrid aerogels derived from banana peel and wastepaper for efficient oil absorption and emulsion separation. J. Clean. Prod. 2018, 199, 411–419. [Google Scholar] [CrossRef]
- Kale, P.N.; Adsule, P.G. Citrus. In Handbook of Fruit Science and Technology: Production, Composition, Storage, and Processing; Salunkhe, D.K., Kadam, S.S., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 39–65. [Google Scholar]
- Mafra, M.R.; Igarashi-Mafra, L.; Zuim, D.R.; Vasques, É.C.; Ferreira, M.A. Adsorption of remazol brilliant blue on an orange peel adsorbent. Braz. J. Chem. Eng. 2013, 30, 657–665. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Zapata, B.; Balmaseda, J.; Fregoso-Israel, E.; Torres-García, E. Thermo-kinetics study of orange peel in air. J. Therm. Anal. Calorim. 2009, 98, 309–315. [Google Scholar] [CrossRef]
- Santos, C.M.; Dweck, J.; Viotto, R.S.; Rosa, A.H.; de Morais, L.C. Application of orange peel waste in the production of solid biofuels and biosorbents. Bioresour. Technol. 2015, 196, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Richards, H.; Baker, P.; Iwuoha, E. Metal Nanoparticle Modified Polysulfone Membranes for Use in Wastewater Treatment: A Critical Review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 183. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Ku, X.; Lin, J.; Jin, H. Modelling the combustion of thermally thick biomass particles. Powder Technol. 2019, 353, 110–124. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Hydrolysis of Biopolymers in Near-Critical and Subcritical Water. In Water Extraction of Bioactive Compounds from Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent developments in pre-treatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- El-Said, W.A.; Fouad, D.M.; Ali, M.H.; El-Gahami, M.A. Green synthesis of magnetic mesoporous silica nanocomposite and its adsorptive performance against organochlorine pesticides. Int. J. Environ. Sci. Technol. 2017, 15, 1731–1744. [Google Scholar] [CrossRef]
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Ngaini, Z.; Nourouzi, M.M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Kumar, K.; Patavardhan, S.S.; Lobo, S.; Gonsalves, R. Equilibrium study of dried orange peel for its efficiency in removal of cupric ions from water. Int. J. Phytoremediat. 2018, 20, 593–598. [Google Scholar] [CrossRef]
- FENG, N.; GUO, X.; LIANG, S. Enhanced Cu (II) adsorption by orange peel modified with sodium hydroxide. Trans. Nonferrous Met. Soc. China 2010, 20, s146–s152. [Google Scholar] [CrossRef]
- Sha, L.; Xueyi, G.; Ningchuan, F.; Qinghua, T. Adsorption of Cu2+ and Cd2+ from aqueous solution by mercapto-acetic acid modified orange peel. Colloids Surf. B Biointerfaces 2009, 73, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Guo, X.; Tian, Q. Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulphured orange peel. Desalination 2011, 275, 212–216. [Google Scholar] [CrossRef]
- Marín, A.B.P.; Ortuño, J.F.; Aguilar, M.I.; Meseguer, V.F.; Sáez, J.; Lloréns, M. Use of chemical modification to determine the binding of Cd (II), Zn (II) and Cr (III) ions by orange waste. Biochem. Eng. J. 2010, 53, 2–6. [Google Scholar] [CrossRef]
- Surovka, D.; Pertile, E. Sorption of Iron, Manganese, and Copper from Aqueous Solution Using Orange Peel: Optimization, Isothermic, Kinetic, and Thermodynamic Studies. Pol. J. Environ. Stud. 2017, 26, 795–800. [Google Scholar] [CrossRef]
- Khaled, A.; Nemr, A.E.; El-Sikaily, A.; Abdelwahab, O. Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: Adsorption isotherm and kinetic studies. J. Hazard. Mater. 2009, 165, 100–110. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions. J. Hazard. Mater. 2009, 170, 425–429. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S. Adsorption study of copper (II) by chemically modified orange peel. J. Hazard. Mater. 2009, 164, 1286–1292. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption Using Modified Orange Peel as Adsorbent. Adv. Mater. Res. 2011, 236, 237–240. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Fernando, P.S.A.; Ahmed, R.T.; Srinath, R.; Priyadharshini, M.; Vignesh, A.M.; Thanjiappan, A. Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid-treated orange peel. Chem. Eng. Commun. 2014, 201, 1526–1547. [Google Scholar] [CrossRef]
- Baltrenas, P.; Vaišis, V. Experimental investigation of thermal modification influence on sorption qualities of biosorbents. J. Environ. Eng. Landsc. Manag. 2005, 13, 3–8. [Google Scholar] [CrossRef]
- Hussein, M.; Amer, A.A.; Sawsan, I.I. Oil spill sorption using carbonized pith bagasse. J. Anal. Appl. Pyrolysis 2008, 82, 205–211. [Google Scholar] [CrossRef]
- Kalderis, D.; Koutoulakis, D.; Paraskeva, P.; Diamadopoulos, E.; Otal, E.; del Valle, J.O.; Fernández-Pereira, C. Adsorption of polluting substances on activated carbons prepared from rice husk and sugarcane bagasse. Chem. Eng. J. 2008, 144, 42–50. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Bendjamaa, I.; Bensid, T.; Meniai, A.H.; Derbal, K. Effect of Calcination on Orange Peels Characteristics: Application of an Industrial Dye Adsorption. Chem. Eng. Trans. 2014, 38, 361–366. [Google Scholar]
- Malook, K. Orange Peel Powder: A Potential Adsorbent for Pb (II) Ions Removal from Water. Theor. Found. Chem. Eng. 2021, 55, 518–526. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Feng, N.C.; Guo, X.Y.; Liang, S. Kinetic and thermodynamic studies on biosorption of Cu (II) by chemically modified orange peel. Trans. Nonferrous Met. Soc. China 2009, 19, 1365–1370. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Feng, N.C.; Guo, X.Y. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Khormaei, M.; Nasernejad, B.; Edrisi, M.; Eslamzadeh, T. Copper biosorption from aqueous solutions by sour orange residue. J. Hazard. Mater. 2007, 149, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd (II), Cu (II) and Pb (II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Irem, S.; Mahmood Khan, Q.; Islam, E.; Jamal Hashmat, A.; Anwar ul Haq, M.; Afzal, M.; Mustafa, T. Enhanced removal of reactive navy-blue dye using powdered orange waste. Ecol. Eng. 2013, 58, 399–405. [Google Scholar] [CrossRef]
- Dey, S.; Basha, S.R.; Babu, G.V.; Nagendra, T. Characteristic and biosorption capacities of orange peels biosorbents for removal of ammonia and nitrate from contaminated water. Clean. Mater. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Dhorabe, P.T.; Lataye, D.H.; Ingole, R.S. Adsorptive Removal of 4-Nitrophenol from Aqueous Solution by Activated Carbon Prepared from Waste Orange Peels. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016015. [Google Scholar] [CrossRef]
- Eddy, N.O.; Garg, R.; Garg, R.; Aikoye, A.O.; Ita, B.I. Waste to resource recovery: Mesoporous adsorbent from orange peel for the removal of trypan blue dye from aqueous solution. Biomass Convers. Biorefin. 2022, in press. [Google Scholar] [CrossRef]
- Haas, A.; dos Santos, E.P. Characterization, and application of orange peel as an adsorbent for cationic dye removal from aqueous solution. Rev. Eletrôn. Gestão Educ. E Tecnol. Amb. 2021, 25, e16. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation, and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt, and nickel. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; Gonzalez-Gutierrez, L.V.; Baldenegro-Perez, L.A. Biosorbents prepared from orange peels using Instant Controlled Pressure Drop for Cu (II) and phenol removal. Ind. Crops Prod. 2016, 84, 344–349. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]







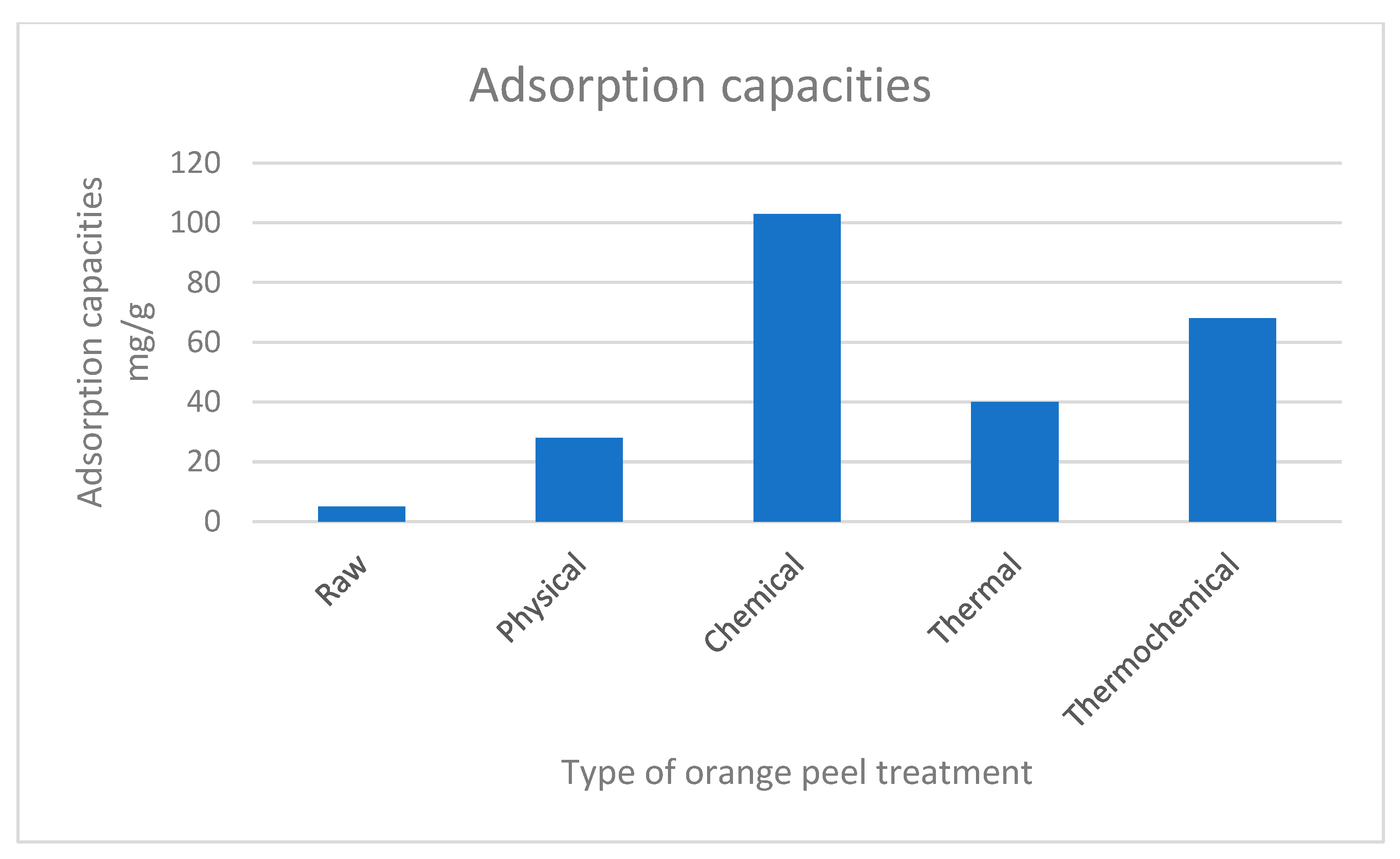
| S/No | Adsorbent | Water Contact Angle | Sorbate | Maximum Adsorption Capacity mg/g | References |
|---|---|---|---|---|---|
| 1 | Kapok fibre | 138.6–151.2° | Diesel Soybean | 38.1 49.1 | [61] |
| 2 | Cotton fibre | 100° | Vegetable oil | 30 | [70] |
| 3 | Wool fibre | 155° | Diesel Vegetable oil | 10.6 14.5 | [51] |
| 4 | Lotus fibre | 161° | Dye | 24.14 | [71] |
| 5 | Banana peel | 110° | Oil | 5 | [72] |
| 6 | Orange peel | 0° | Dye | 5 | [14] |
| Chemical Composition | Mass% [76] | Mass% [77] | Mass% [78] | Mass% This Study |
|---|---|---|---|---|
| Carbon | 49.59 | 44.5 | 47.0 | 48.67 |
| Hydrogen | 6.95 | 6.1 | 6.0 | - |
| Oxygen | 39.7 | 47.3 | 44.71 | 36.46 |
| Na | - | - | - | 4.44 |
| Nitrogen | 0.66 | 1.5 | 1.3 | - |
| Potassium | - | - | - | 0.95 |
| Calcium | - | - | - | 1.08 |
| Sulphur | 0.06 | 0.4 | 0.09 | - |
| Chloride | 0.001 | - | 0.001 | 8.39 |
| Ash | 3.05 | 4.0 | - | - |
| Water | 2.73 | - | - | - |
| References | Author | Adsorbent State | Adsorption Capacities (mg/g) | Adsorbate |
|---|---|---|---|---|
| Application of chemically modified orange peels for removal of copper (II) from aqueous solutions | [90] | Thermochemical | - | Heavy metal –copper (II) |
| Effect of calcination on orange peels characteristics: Application of an Industrial Dye Adsorption | [94] | Thermal -calcination | 9.74 | Dye –methylene blue (MB) |
| Oil spill sorption capacity of raw and thermally modified orange peel waste | [95] | Raw Thermal | 30–50 | Oil |
| Potential application of orange peel as an eco-friendly adsorbent for textile dyeing effluents | [88] | Dried (crushed) | - | Dye |
| Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid–treated orange peel | [97] | Thermochemical | Dye | |
| Equilibrium and thermodynamic studies of Cd (II) biosorption by chemically modified orange peel | [74] | Chemical | Heavy metal | |
| Equilibrium study of dried orange peel for its efficiency in removal of cupric ions from water | [102] | Dried (crushed) | 33.99 mg/g | Heavy metal –copper ion |
| Functionalised adsorbents prepared from fruit peels: Equilibrium, kinetics, and thermodynamic studies for copper adsorption in aqueous solution | [103] | Physical—Chemical | 163 mg/g | Heavy metal –Cu (II) |
| Preliminary study of oil removal using hybrid peel waste: Musa Balbisiana and Citrus Sinensis | [10] | Chemical (NAOH) | - | Oil (Light and Heavy) |
| Removal of Direct N Blue—106 from artificial textile dye effluent using activated carbon from orange peel: Adsorption Isotherm and Kinetic studies | [17] | Chemical | 107.53 mg/g | Dye |
| Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon | [93] | Chemical—Thermal | 75.76 mg/g | Dye |
| Sorption of Iron, Manganese, and Copper from Aqueous solution using orange peel: Optimization, Isothermic, Kinetic, and Thermodynamic studies | [92] | Chemical | 15 mg/g | Heavy metal |
| Adsorption of Remazol Brilliant Blue on an Orange Peel Adsorbent | [74] | Dried (crushed) | 9.7 mg/g | Dye |
| Simple and green fabrication of recyclable magnetic highly hydrophobic sorbents derived from waste orange peels for removal of oil and organic solvents from water surface | [14] | Chemical | 54.20 mg/g | Oil |
| Investigation of aqueous Cr (VI) adsorption characteristics of orange peels powder | [102] | Chemical | 4.69 mg/g | Heavy metal |
| Application of orange peel Xanthate for the adsorption of Pb2+ from aqueous solutions | [94] | Chemical | 204.50 mg/g | Heavy metal |
| Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulphured orange peel | [90] | Chemical | 160 mg/g 80 mg/g | Heavy metal Pb2+ Zn2+ |
| Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption using modified orange peel as adsorbent | [96] | Chemical | 14.1 mg/g–45.29 mg/g | Heavy metal |
| Adsorption of CU2+ and Cd2+ from aqueous solution by Mercapto—acetic acid modified orange peel | [89] | Chemical | 70.67 mg/g 136.05 mg/g | Heavy metal Cu2+ Cd2+ |
| Adsorption study of copper (II) by chemically modified orange peel | [95] | Chemical | 289.0 mg/g | Heavy metal Copper (II) |
| Kinetic and thermodynamic studies on biosorption of Cu (II) by chemically modified orange peel | [104] | Chemical | 72.73 mg/g | Copper (II) |
| Biosorption of heavy metals from aqueous solutions by chemically modified orange peel | [105] | Chemical | 162.6 | Heavy metals |
| Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead, and zinc by modified orange peel | [106] | Chemical | 70.73 209.8 56.18 | Heavy metal Cu2+ Pb2+ Zn2+ |
| Copper biosorption from aqueous solutions by sour orange residue | [107] | Chemical | 21.7 | Heavy metal |
| Adsorption of Remazol Brilliant Blue on an orange peel adsorbent | [74] | Dried (crushed) | 9.7 mg/g | Dye |
| Enhanced Cu (II) adsorption by orange peel modified with sodium hydroxide | [88] | Chemical | 50.25 mg/g | Heavy metal Cu (II) |
| Use of chemical modification to determine the binding of Cd (II), Zn (II), and Cr (II) ions by orange waste | [91] | Chemical | 41.59 mg/g 32.04 mg/g 40.35 mg/g | Heavy metal Cd2+ Zn2+ Cr2+ |
| Application of orange peel waste in the production of solid biofuels and biosorbents | [77] | Thermal (pyrolysis) | - | Heavy metal |
| Adsorption/desorption of Cd (II), Cu (II), and Pb (II) using chemically modified orange peel: Equilibrium and Kinetic studies | [108] | Chemical | 13.7 mg/g 15.27 mg/g 73.53 mg/g | Heavy metals Cd Cu Pb |
| Removal of dyes from coloured textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies | [16] | Physical (dried) | 10.72 mg/g and 21.05 mg/g | Dyes |
| Enhanced removal of reactive navy-blue dye using powdered orange waste | [109] | Physical (dried) | 30.28 mg/g | Dye |
| Arsenic(V) biosorption by charred orange peel in aqueous environments | [19] | Thermochemical | 60.9 mg/g | Dye |
| Characteristic and biosorption capacities of orange peels biosorbents for removal of ammonia and nitrate from contaminated water | [110] | Chemical | 100% | Ammonia Nitrate |
| Adsorptive Removal of 4-Nitrophenol from Aqueous Solution by Activated Carbon Prepared from Waste Orange Peels | [111] | Chemical | 73.35 | 4-Nitrophenol |
| Waste to resource recovery: mesoporous adsorbent from orange peel for the removal of trypan blue dye from aqueous solution | [112] | Chemical | 97.10% | Dye |
| Characterization of banana and orange peels: biosorption mechanism | [39] | Physical | - | - |
| Characterization and application of orange peel as an adsorbent for cationic dye removal from aqueous solution | [113] | Physical | 3.96 | Dye |
| Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt, and nickel | [114] | Chemical | 130% | Metals |
| Biosorbents prepared from orange peels using Instant Controlled Pressure Drop for Cu (II) and phenol removal | [115] | Thermal | 32.51 106.91 | Metals |
| Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater | [116] | Chemical | 33 38 | Dye |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O.; Eyo, E.U. Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials 2023, 16, 1092. https://doi.org/10.3390/ma16031092
Michael-Igolima U, Abbey SJ, Ifelebuegu AO, Eyo EU. Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials. 2023; 16(3):1092. https://doi.org/10.3390/ma16031092
Chicago/Turabian StyleMichael-Igolima, Uloaku, Samuel J. Abbey, Augustine O. Ifelebuegu, and Eyo U. Eyo. 2023. "Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants" Materials 16, no. 3: 1092. https://doi.org/10.3390/ma16031092
APA StyleMichael-Igolima, U., Abbey, S. J., Ifelebuegu, A. O., & Eyo, E. U. (2023). Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials, 16(3), 1092. https://doi.org/10.3390/ma16031092







