Mo3Ni2N Nanoparticle Generation by Spark Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Powder X-ray Diffraction (PXRD)
2.3. Transmission Electron Microscopy (TEM)
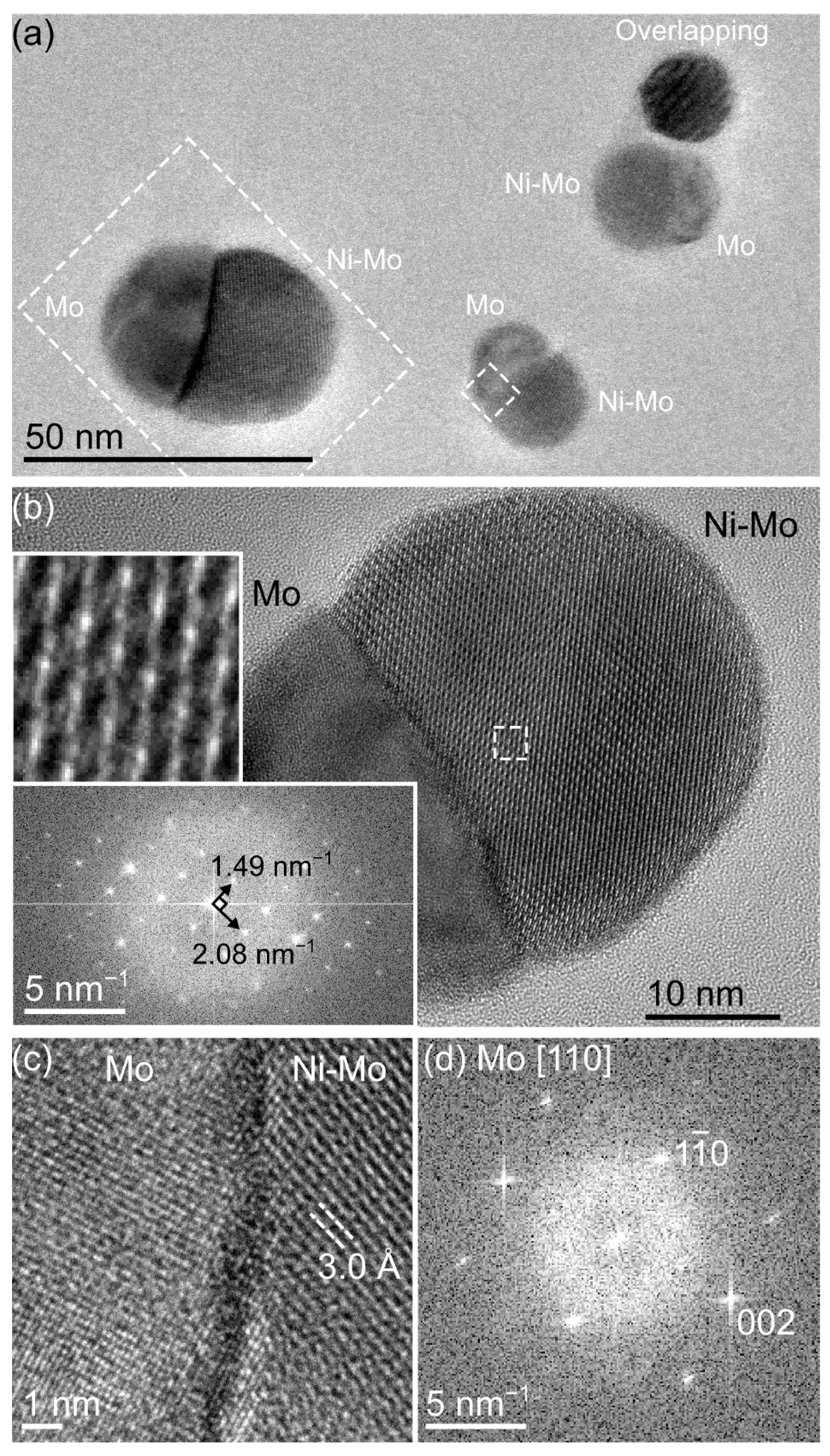
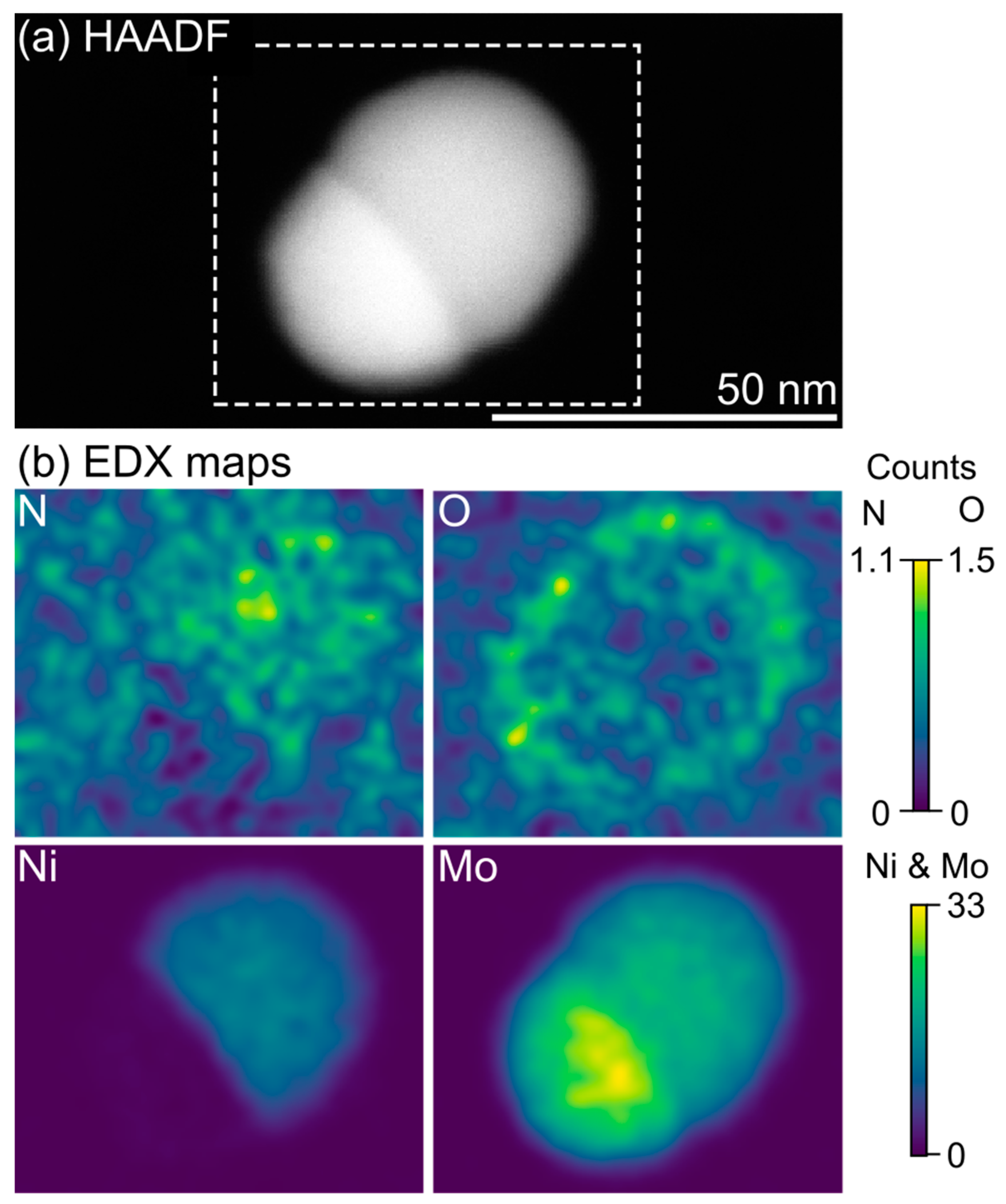
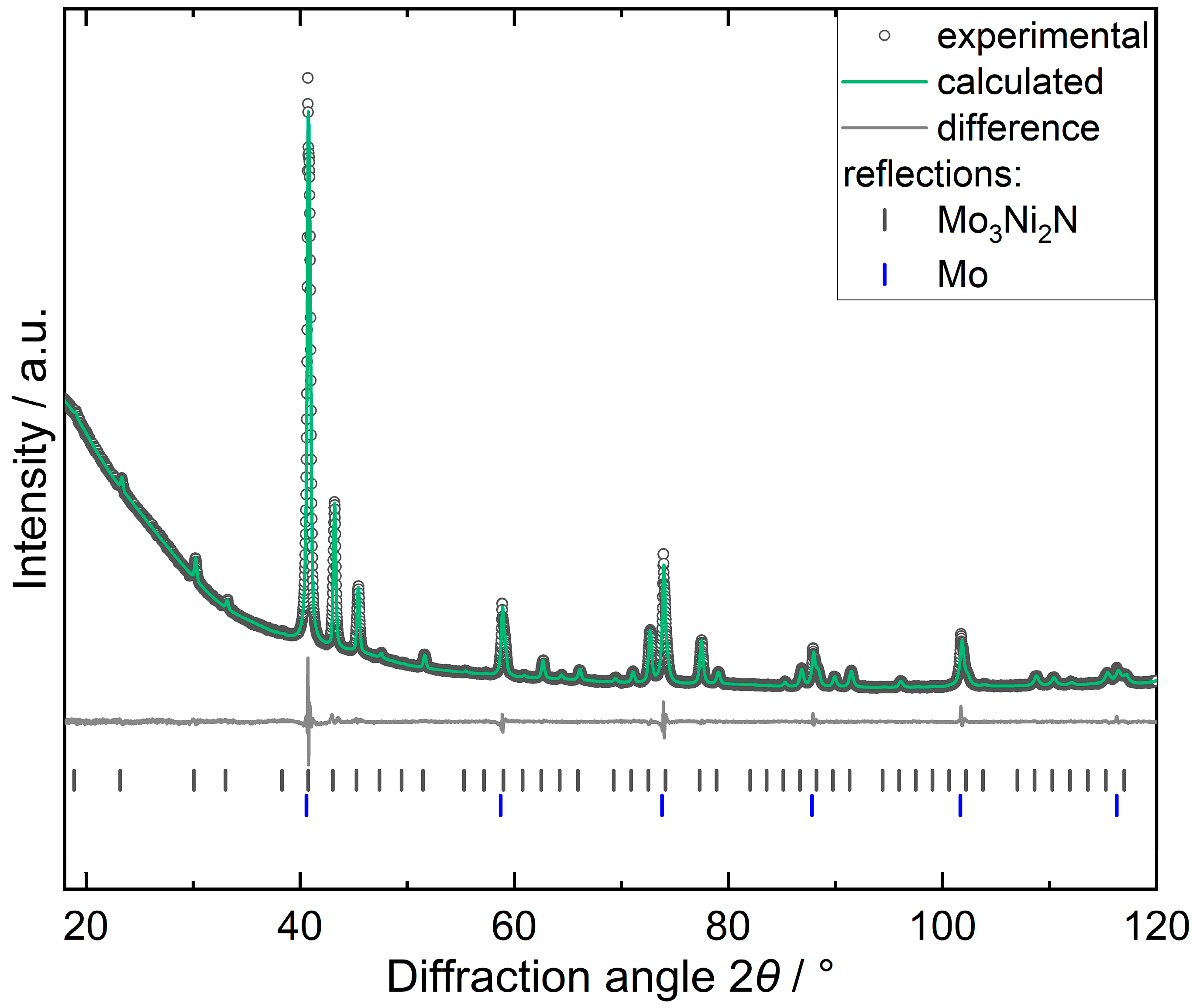
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyama, S.T. The Chemistry of Transition Metal Carbides and Nitrides; Blackie Academic & Professional: Glasgow, UK, 1996. [Google Scholar]
- Toth, L.E. Transition Metal Carbides and Nitrides; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.; Kim, J.; Kinsey, N.; Boltasseva, A. Alternative Plasmonic Materials. In Handbook of Surface Science; Richardson, N.V., Holloway, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 6; Volume 4, pp. 189–221. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Ghosh, A.; Gomathi, A. Functionalization and Solubilization of Carbon and Inorganic Nanostructures. In Comprehensive Nanoscience and Technology; Andrews, D.L., Scholes, G.D., Wiederrecht, G.P., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 445–490. [Google Scholar] [CrossRef]
- Gomell, L.; Haeger, T.; Roscher, M.; Bishara, H.; Heiderhoff, R.; Riedl, T.; Scheu, C.; Gault, B. Microstructure manipulation by laser-surface remelting of a full-Heusler compound to enhance thermoelectric properties. Acta Mater. 2022, 223, 117501. [Google Scholar] [CrossRef]
- Gomell, L.; Tsai, S.P.; Roscher, M.; Bueno Villoro, R.; Konijnenberg, P.; Zaefferer, S.; Scheu, C.; Gault, B. In situ nitriding of Fe 2 VAl during laser surface remelting to manipulate microstructure and crystalline defects. Phys. Rev. Mater. 2022, 6, 085405. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Heterogeneous catalysis with metal nitrides. Coord. Chem. Rev. 2013, 257, 2015–2031. [Google Scholar] [CrossRef]
- Nagai, M. Transition-metal nitrides for hydrotreating catalyst—Synthesis, surface properties, and reactivities. Appl. Catal. A Gen. 2007, 322, 178–190. [Google Scholar] [CrossRef]
- Furimsky, E. Metal carbides and nitrides as potential catalysts for hydroprocessing. Appl. Catal. A Gen. 2003, 240, 1–28. [Google Scholar] [CrossRef]
- Bernard, P.; Stelmachowski, P.; Broś, P.; Makowski, W.; Kotarba, A. Demonstration of the Influence of Specific Surface Area on Reaction Rate in Heterogeneous Catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef]
- Yuan, Y.; Adimi, S.; Guo, X.; Thomas, T.; Zhu, Y.; Guo, H.; Priyanga, G.S.; Yoo, P.; Wang, J.; Chen, J.; et al. A Surface-Oxide-Rich Activation Layer (SOAL) on Ni2Mo3N for a Rapid and Durable Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 18036–18041. [Google Scholar] [CrossRef]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Alcohol Oxidation and Hydrogen Evolution. In Interface Science and Technology; Kakaei, K., Esrafili, M.D., Ehsani, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 27, pp. 253–301. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, T.H.; Lee, M.H.; Kawashima, K.; Mullins, C.B.; Lim, H.K.; Youn, D.H. Highly active and stable nickel–molybdenum nitride (Ni2Mo3N) electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2021, 9, 4945–4951. [Google Scholar] [CrossRef]
- Iffat, A.; Syed, R.; Mudassir, I. A Comprehensive Review on the Synthesis and Energy Applications of Nano-structured Metal Nitrides. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D Porous Hierarchical Nickel-Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221. [Google Scholar] [CrossRef]
- Wise, R.S.; Markel, E.J. Catalytic NH3 Decomposition by Topotactic Molybdenum Oxides and Nitrides: Effect on Temperature Programmed γ-Mo2N Synthesis. J. Catal. 1994, 145, 335–343. [Google Scholar] [CrossRef]
- Meuller, B.O.; Messing, M.E.; Engberg, D.L.J.; Jansson, A.M.; Johansson, L.I.M.; Norlén, S.M.; Tureson, N.; Deppert, K. Review of Spark Discharge Generators for Production of Nanoparticle Aerosols. Aerosol Sci. Technol. 2012, 46, 1256–1270. [Google Scholar] [CrossRef]
- Hallberg, R.T.; Ludvigsson, L.; Preger, C.; Meuller, B.O.; Dick, K.A.; Messing, M.E. Hydrogen-assisted spark discharge generated metal nanoparticles to prevent oxide formation. Aerosol Sci. Technol. 2018, 52, 347–358. [Google Scholar] [CrossRef] [Green Version]
- De la Peña, F.; Prestat, E.; Tonaas Fauske, V.; Burdet, P.; Jokubauskas, P.; Nord, M.; Furnival, T.; Ostasevicius, T.; MacArthur, K.E.; Johnstone, D.N.; et al. hyperspy/hyperspy: HyperSpy 1.6.0, version 1.6.0; Zenodo. 2020. Available online: https://zenodo.org/record/3973513#.Y9O-OHbMKUk (accessed on 6 December 2022).
- Potapov, P.; Longo, P.; Okunishi, E. Enhancement of noisy EDX HRSTEM spectrum-images by combination of filtering and PCA. Micron 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, N.W.M.; Newbury, D.E.; Davis, J.M. EDS Measurements of X-Ray Intensity at WDS Precision and Accuracy Using a Silicon Drift Detector Microscopy and Microanalysis. Microsc. Microanal. 2012, 184, 892–904. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef] [Green Version]
- George, S.J.; Drury, O.B.; Fu, J.; Friedrich, S.; Doonan, C.J.; George, G.N.; White, J.M.; Young, C.G.; Cramer, S.P. Molybdenum X-ray absorption edges from 200 to 20,000eV: The benefits of soft X-ray spectroscopy for chemical speciation. J. Inorg. Biochem. 2009, 103, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Herle, P.S.; Hegde, M.S.; Sooryanarayana, K.; Guru Row, T.N.; Subbanna, G.N. Ni2Mo3N: A New Ternary Interstitial Nitride with a Filled β-Manganese Structure. Inorg. Chem. 1998, 37, 4128–4130. [Google Scholar] [CrossRef]
- Conway, J.O.; Prior, T.J. Interstitial nitrides revisited—A simple synthesis of MxMo3N (M = Fe, Co, Ni). J. Alloys Compd. 2019, 774, 69–74. [Google Scholar] [CrossRef]
- Cao, B.; Neuefeind, J.C.; Adzic, R.R.; Khalifah, P.G. Molybdenum nitrides as oxygen reduction reaction catalysts: Structural and electrochemical studies. Inorg. Chem. 2015, 54, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.L.; McMillan, P.F.; Soignard, E.; Leinenweber, K. Determination of the crystal structure of delta-(Mo N) by neutron diffraction. J. Solid State Chem. 2004, 177, 1488–1492. [Google Scholar] [CrossRef]
- Shoemaker, C.B.; Shoemaker, D.P. The crystal structure of the delta phase, Mo-Ni. Acta Crystallogr. 1963, 16, 997–1009. [Google Scholar] [CrossRef]
- Straumanis, M.E.; Shodhan, R.P. Lattice parameter and thermal expansion coefficient of molybdenum between 15 and 65 C. Trans. Metall. Soc. AIME 1968, 242, 1185–1186. [Google Scholar]
- Jette, E.; Foote, F. Precision determination of lattice constants. J. Chem. Phys. 1935, 3, 605–616. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Xu, Q.; van der Pers, N.M.; Lafont, U.; Schmidt-Ott, A. Synthesis of mixed metallic nanoparticles by spark discharge. J. Nanopart. Res. 2009, 11, 1209–1218. [Google Scholar] [CrossRef]
- Anastasopol, A.; Pfeiffer, T.V.; Middelkoop, J.; Lafont, U.; Canales-Perez, R.J.; Schmidt-Ott, A.; Mulder, F.M.; Eijt, S.W.H. Reduced enthalpy of metal hydride formation for MgTi nanocomposites produced by spark discharge generation. J. Am. Chem. Soc. 2013, 135, 7891–7900. [Google Scholar] [CrossRef]
- Vons, V.A.; Leegwater, H.; Legerstee, W.J.; Eijt, S.W.H.; Schmidt-Ott, A. Hydrogen storage properties of spark generated palladium nanoparticles. Int. J. Hydrog. Energy 2010, 35, 5479–5489. [Google Scholar] [CrossRef]
- Roth, C.; Ferron, G.A.; Karg, E.; Lentner, B.; Schumann, G.; Takenaka, S.; Heyder, J. Generation of ultrafine particles by spark discharging. Aerosol Sci. Technol. 2004, 38, 228–235. [Google Scholar] [CrossRef]
- Vons, V.A.; Anastasopol, A.; Legerstee, W.J.; Mulder, F.M.; Eijt, S.W.H.; Schmidt-Ott, A. Low-temperature hydrogen desorption and the structural properties of spark discharge generated Mg nanoparticles. Acta Mater. 2011, 59, 3070–3080. [Google Scholar] [CrossRef]
- Pfeiffer, T.V.; Feng, J.; Schmidt-Ott, A. New developments in spark production of nanoparticles. Adv. Powder Technol. 2014, 25, 56–70. [Google Scholar] [CrossRef]
- Johnston, G.P.; Muenchausen, R.E.; Smith, D.M.; Foltyn, S.R. Reactive Laser Ablation Synthesis of Nanosize Aluminum Nitride. J. Am. Ceram. Soc. 1992, 75, 3465–3468. [Google Scholar] [CrossRef]
- Luo, Q.; Congcong Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2022, in press. [CrossRef]
- Duan, Y.; Wu, Y.; Zhang, O.; Ding, R.; Chen, Y.; Liu, J.; Yang, M. Towards conversion of octanoic acid to liquid hydrocarbon via hydrodeoxygenation over Mo promoter nickel-based catalyst. J. Mol. Catal. A Chem. 2015, 398, 72–78. [Google Scholar] [CrossRef]
- Maluf, S.S.; Assaf, E.M. Ni catalysts with Mo promoter for methane steam reforming. Fuel 2009, 88, 1547–1553. [Google Scholar] [CrossRef]
- Eijsbouts, S.; van den Oetelaar, L.C.A.; van Puijenbroek, R.R. MoS2 morphology and promoter segregation in commercial Type 2 Ni–Mo/Al2O3 and Co–Mo/Al2O3 hydroprocessing catalysts. J. Catal. 2005, 229, 352–364. [Google Scholar] [CrossRef]

| Composition | Mo3Ni2N |
| Space group, Pearson symbol | P4132 (No. 213), cP24 |
| Unit cell parameters | |
| a [Å] | 6.6394(1) |
| V [Å3] | 292.67(1) |
| Formula units Z | 4 |
| Diffractometer | Stoe Stadi MP, Mythen 1k detector, Cu-Kα radiation, λ = 1.5406 Å, transmission mode |
| Measurement range, 2θ step | 16.535 ≤ 2θ ≤ 121.085, Δ2θ = 0.015 |
| Measured points/reflections | 7740/46 |
| Residuals and GOF | R = 0.0169, wR = 0.0198, GOF = 2.1451 |
| Atom | Site | a/x | b/y | c/z | Uiso/aniso |
|---|---|---|---|---|---|
| Mo | 12d | 1/8 | 0.2019(1) | 0.4519(1) | 0.0075(2) |
| Ni | 8c | 0.0675(1) | 0.0675(1) | 0.0675(1) | 0.0045(2) * |
| N | 4a | 3/8 | 3/8 | 3/8 | 0.010(2) |
| Atoms | Distance/Å | Atoms | Distance/Å | ||
|---|---|---|---|---|---|
| Mo | 2 N | 2.0825(2) | Ni | 3 Ni | 2.469(1) |
| 2 Ni | 2.7309(9) | 3 Mo | 2.7309(9) | ||
| 2 Ni | 2.7459(9) | 3 Mo | 2.7459(9) | ||
| 4 Mo | 2.7796(4) | 3 Mo | 2.8206(9) | ||
| 2 Mo | 2.8153(5) | ||||
| 2 Ni | 2.8206(9) | N | 8 Mo | 2.0825(2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmroth Nordlander, J.; Bermeo, M.; Ternero, P.; Wahlqvist, D.; Schmeida, T.; Blomberg, S.; Messing, M.E.; Ek, M.; Hübner, J.-M. Mo3Ni2N Nanoparticle Generation by Spark Discharge. Materials 2023, 16, 1113. https://doi.org/10.3390/ma16031113
Elmroth Nordlander J, Bermeo M, Ternero P, Wahlqvist D, Schmeida T, Blomberg S, Messing ME, Ek M, Hübner J-M. Mo3Ni2N Nanoparticle Generation by Spark Discharge. Materials. 2023; 16(3):1113. https://doi.org/10.3390/ma16031113
Chicago/Turabian StyleElmroth Nordlander, Jonas, Marie Bermeo, Pau Ternero, David Wahlqvist, Toni Schmeida, Sara Blomberg, Maria E. Messing, Martin Ek, and Julia-Maria Hübner. 2023. "Mo3Ni2N Nanoparticle Generation by Spark Discharge" Materials 16, no. 3: 1113. https://doi.org/10.3390/ma16031113
APA StyleElmroth Nordlander, J., Bermeo, M., Ternero, P., Wahlqvist, D., Schmeida, T., Blomberg, S., Messing, M. E., Ek, M., & Hübner, J.-M. (2023). Mo3Ni2N Nanoparticle Generation by Spark Discharge. Materials, 16(3), 1113. https://doi.org/10.3390/ma16031113





