Investigation of Microstructure and Wear Properties of Precipitates-Strengthened Cu-Ni-Si-Fe Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
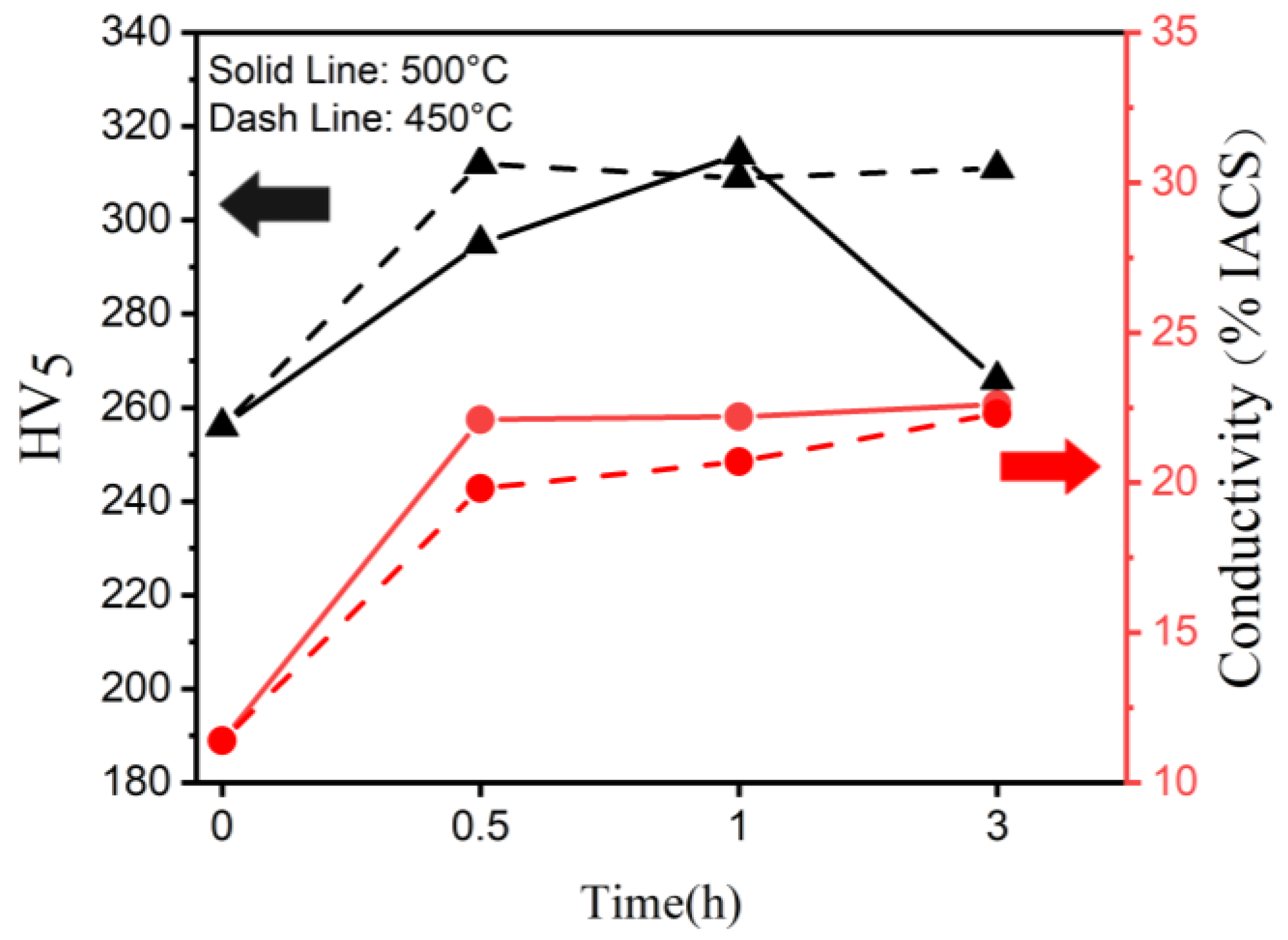
3.1. Mechanical and Electricaal Properties
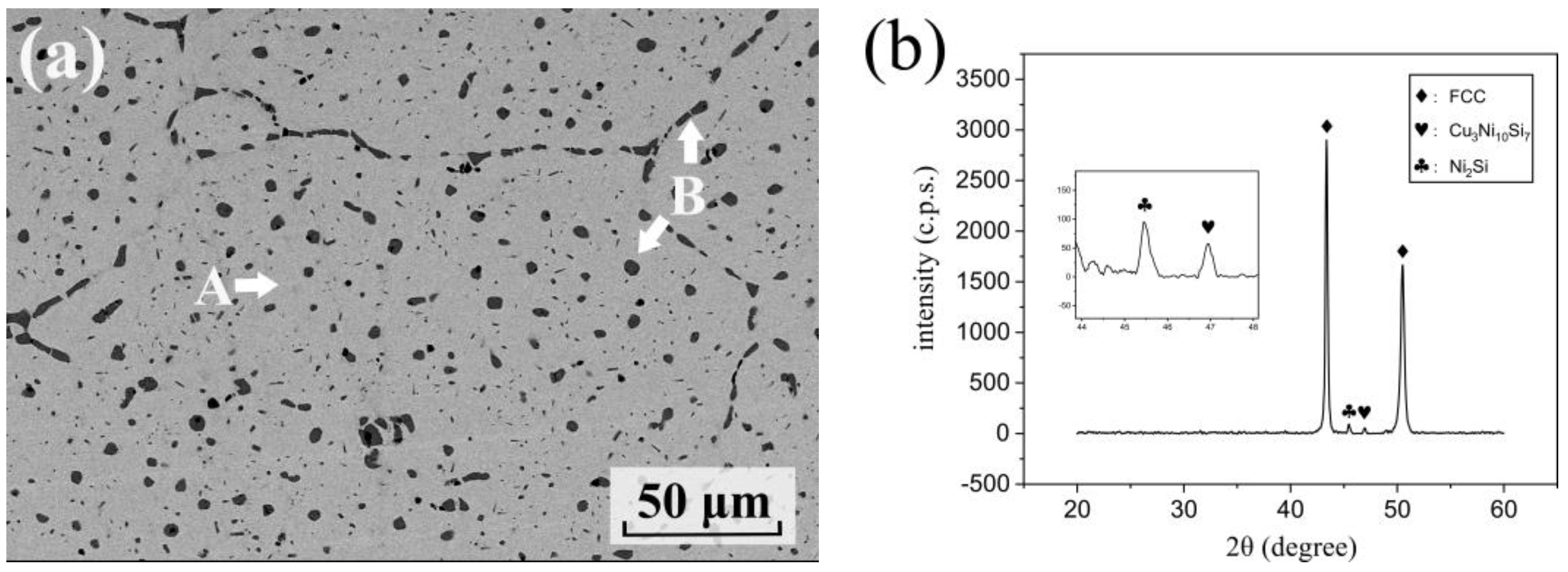
3.2. Microstructures of Cu86.5Ni7.4Si3.8Fe1.1
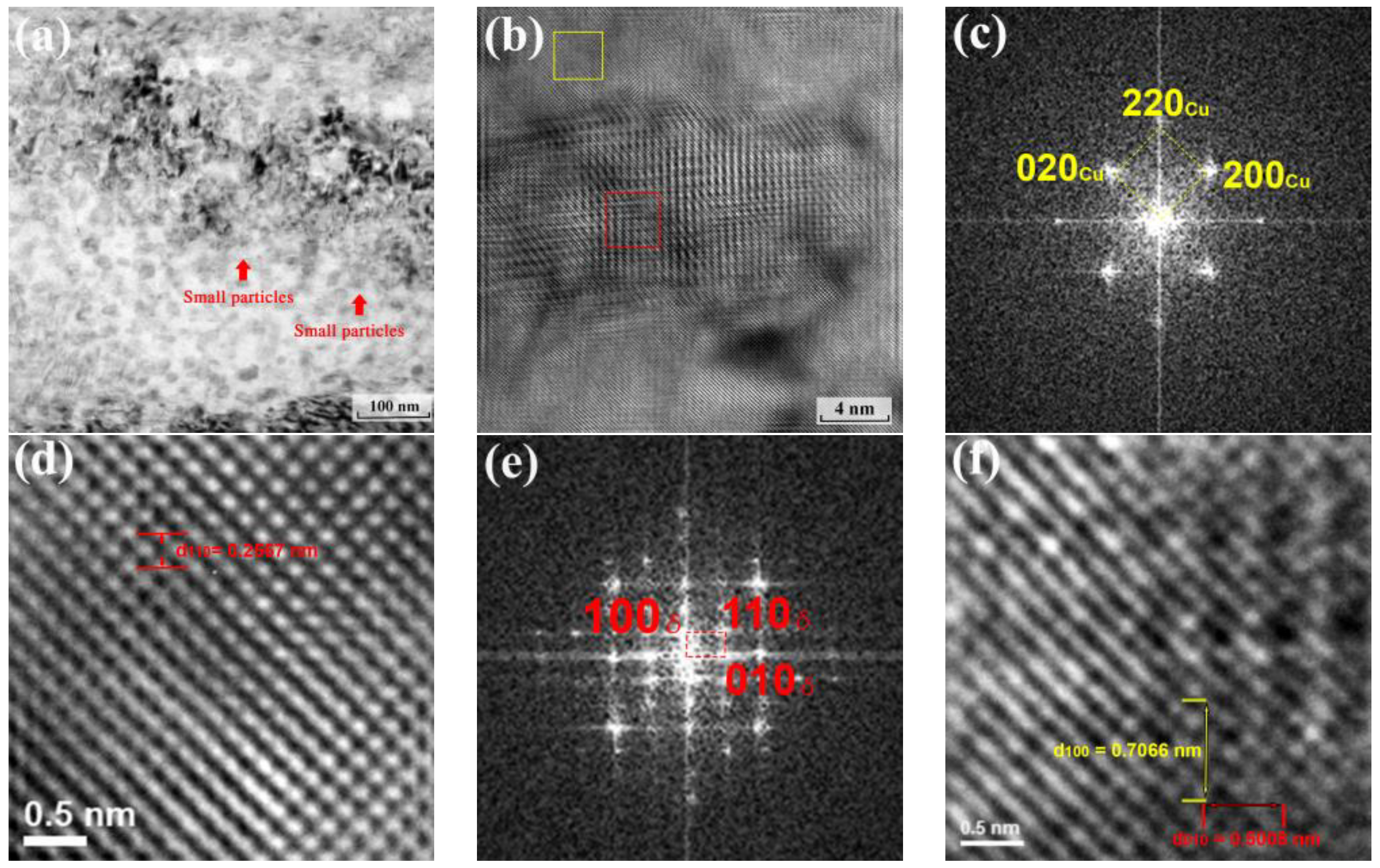
3.3. Nano Precipitates of Ni2Si
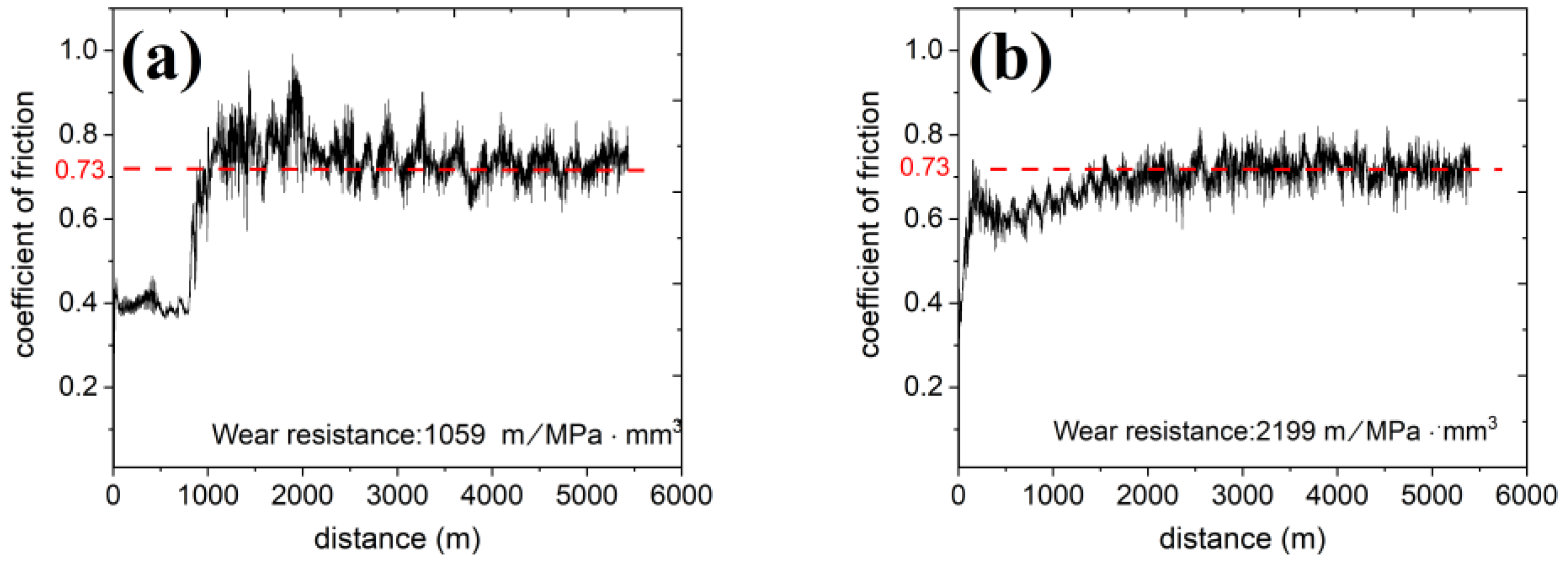
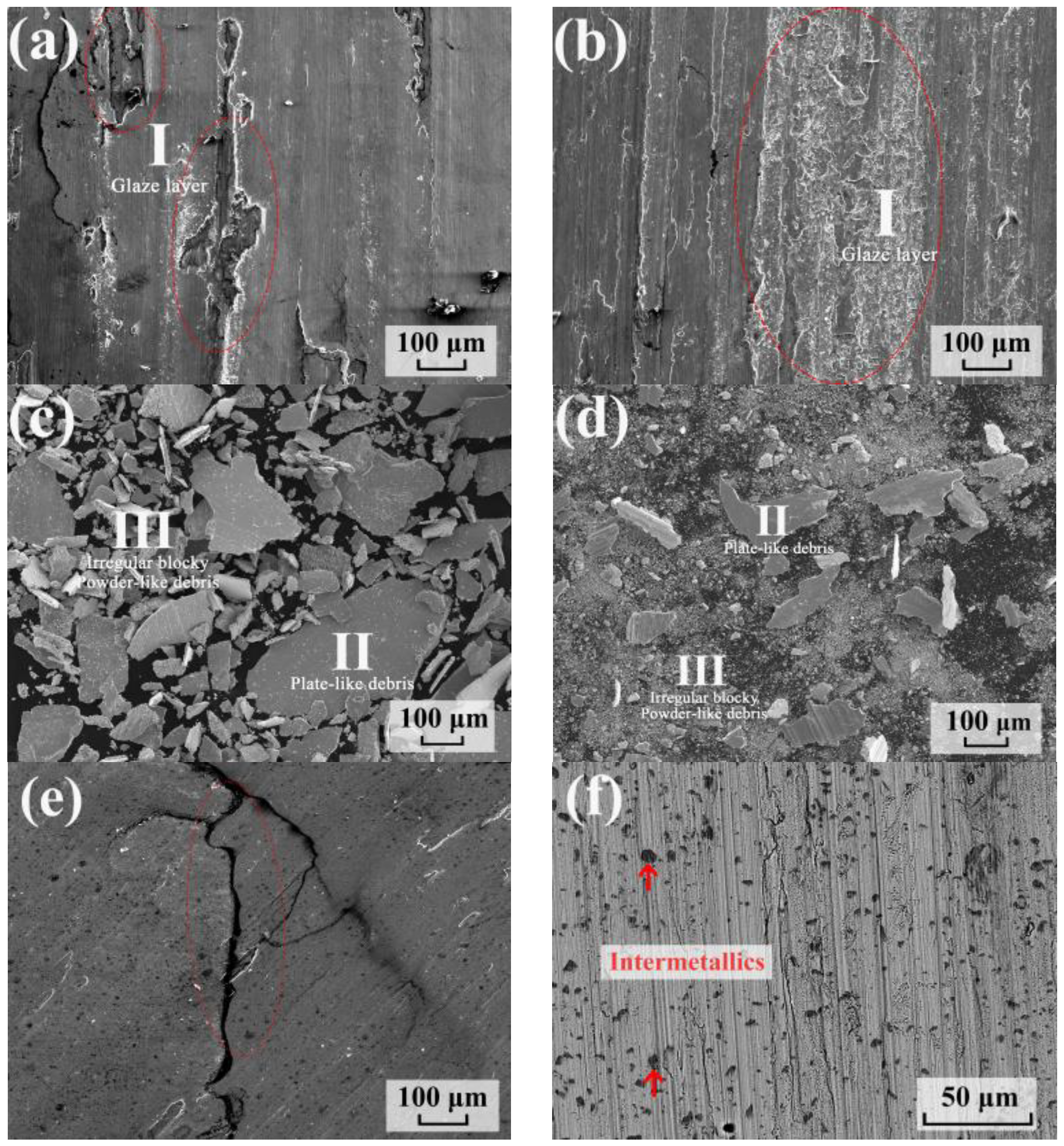
3.4. Wear Property of C17200 and the as-Aged Cu86.5 Alloy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Copper and Copper Alloys; ASM International: Almere, The Netherlands, 2001. [Google Scholar]
- Xie, G.; Wang, Q.; Mi, X.; Xiong, B.; Peng, L. The precipitation behavior and strengthening of a Cu–2.0wt% Be alloy. Mater. Sci. Eng. A 2012, 558, 326–330. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Tian, B.; Song, K.; Liu, P.; Jia, Y.; Chen, X.; An, J.; Zhao, Z.; Liu, Y.; et al. Review of nano-phase effects in high strength and conductivity copper alloys. Nanotechnol. Rev. 2019, 8, 383–395. [Google Scholar] [CrossRef]
- Courtney, T.H. Mechanical Behavior of Materials; Waveland Press: Long Grove, IL, USA, 2005. [Google Scholar]
- Nembach, E. Particle strengthening of metals and alloys. Mater. Sci. Technol. 1997, 13, 705. [Google Scholar]
- Basinski, S.; Basinski, Z. Plastic deformation and work hardening. Dislocat. Solids 1980, 4, 261–362. [Google Scholar]
- Gleiter, H.; Hornbogen, E. Precipitation hardening by coherent particles. Mater. Sci. Eng. 1968, 2, 285–302. [Google Scholar] [CrossRef]
- Wen, H.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J. Strengthening mechanisms in a high-strength bulk nanostructured Cu–Zn–Al alloy processed via cryomilling and spark plasma sintering. Acta Mater. 2013, 61, 2769–2782. [Google Scholar] [CrossRef]
- Fan, Z. A new approach to the electrical resistivity of two-phase composites. Acta Metall. Mater. 1995, 43, 43–49. [Google Scholar] [CrossRef]
- Botcharova, E.; Freudenberger, J.; Schultz, L. Mechanical and electrical properties of mechanically alloyed nanocrystalline Cu–Nb alloys. Acta Mater. 2006, 54, 3333–3341. [Google Scholar] [CrossRef]
- Qu, L.; Wang, E.; Han, K.; Zuo, X.; Zhang, L.; Jia, P.; He, J. Studies of electrical resistivity of an annealed Cu-Fe composite. J. Appl. Phys. 2013, 113, 173708. [Google Scholar] [CrossRef]
- Tian, L.; Anderson, I.; Riedemann, T.; Russell, A. Modeling the electrical resistivity of deformation processed metal–metal composites. Acta Mater. 2014, 77, 151–161. [Google Scholar] [CrossRef]
- Raeisinia, B.; Poole, W.J.; Lloyd, D.J. Examination of precipitation in the aluminum alloy AA6111 using electrical resistivity measurements. Mater. Sci. Eng. A 2006, 420, 245–249. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Tsai, C.-W.; Yang, N.-H.; Chang, S.-Y.; Yeh, J.-W.; Chen, S.-K.; Lin, S.-J. Intrinsic surface hardening and precipitation kinetics of Al0.3CrFe1.5MnNi0.5 multi-component alloy. J. Alloys Compd. 2013, 551, 12–18. [Google Scholar] [CrossRef]
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill Inc.: New York, NY, USA, 1993; p. 32835. [Google Scholar]
- Masamichi, M.; Yoshikiyo, O. Effects of Co, Ni and Ti additions on the cellular precipitation in Cu-2% Be alloy. Mater. Trans. JIM 1994, 35, 163–167. [Google Scholar]
- Buggy, M.; Conlon, C. Material selection in the design of electrical connectors. J. Mater. Process. Technol. 2004, 153–154, 213–218. [Google Scholar] [CrossRef]
- Schuler, C.R.; Kent, M.S.; Deubner, D.C.; Berakis, M.T.; McCawley, M.; Henneberger, P.K.; Rossman, M.D.; Kreiss, K. Process-related risk of beryllium sensitization and disease in a copper–beryllium alloy facility. Am. J. Ind. Med. 2005, 47, 195–205. [Google Scholar] [CrossRef]
- Kuhn, H.; Altenberger, I.; Käufler, A.; Hölzl, H.; Fünfer, M. Properties of high performance alloys for electromechanical connectors. In Copper Alloys: Early Applications and Current Performance—Enhancing Processes; IntechOpen: London, UK, 2012; pp. 52–68. [Google Scholar]
- Dong, Q.-y.; Shen, L.-n.; Wang, M.-p.; Jia, Y.-l.; Zhou, L.; Feng, C.; Chang, C. Microstructure and properties of Cu–2.3 Fe–0.03 P alloy during thermomechanical treatments. Trans. Nonferrous Met. Soc. China 2015, 25, 1551–1558. [Google Scholar] [CrossRef]
- Wang, W.; Zou, C.L.; Li, R.G.; Wen, W.; Kang, H.J.; Wang, T.M. In Situ Synchrotron X-ray Diffraction Study of a Deformed Cu-Fe-P Alloy during Heating. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2016; pp. 191–196. [Google Scholar]
- Xiao, X.; Xu, H.; Chen, J.; Liang, Q.; Wang, J.; Zhang, J. Aging properties and precipitates analysis of Cu–2.3 Fe–0.03 P alloy by thermomechanical treatments. Mater. Res. Express 2017, 4, 116511. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.M.; Yang, W.W.; Chen, D.L. Effect of aluminum on microstructure and property of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Gao, Y.; Peng, X.; Derby, B. Microstructure and mechanical properties of a high strength Cu-Ni-Si alloy treated by combined aging processes. J. Alloys Compd. 2017, 695, 2413–2423. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, X.; Liu, C.; Shen, Y.; Zhang, X.; Sakai, T. Enhancement of strength and ductility of Cu–Sn–Zn alloy by iron addition. Mater. Sci. Eng. A 2014, 612, 246–252. [Google Scholar] [CrossRef]
- Gao, W.; Cao, D.; Jin, Y.; Zhou, X.; Cheng, G.; Wang, Y. Microstructure and properties of Cu-Sn-Zn-TiO2 nano-composite coatings on mild steel. Surf. Coat. Technol. 2018, 350, 801–806. [Google Scholar] [CrossRef]
- Fuxiang, H.; Jusheng, M.; Honglong, N.; Zhiting, G.; Chao, L.; Shumei, G.; Xuetao, Y.; Tao, W.; Hong, L.; Huafen, L. Analysis of phases in a Cu–Cr–Zr alloy. Scr. Mater. 2003, 48, 97–102. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Ge, Y.; Wang, J.; Cui, J.-Z. Effect of processing and heat treatment on behavior of Cu-Cr-Zr alloys to railway contact wire. Metall. Mater. Trans. A 2006, 37, 3233–3238. [Google Scholar] [CrossRef]
- Su, J.; Liu, P.; Li, H.; Ren, F.; Dong, Q. Phase transformation in Cu–Cr–Zr–Mg alloy. Mater. Lett. 2007, 61, 4963–4966. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, Q.M.; Liu, P.; Kang, B.X.; Huang, J.L.; Jin, Z.H. Aging behavior of Cu–Ni–Si alloy. Mater. Sci. Eng. A 2003, 361, 93–99. [Google Scholar] [CrossRef]
- Yang, B.; Wu, M.; Li, X.; Zhang, J.; Wang, H. Effects of cold working and corrosion on fatigue properties and fracture behaviors of precipitate strengthened Cu-Ni-Si alloy. Int. J. Fatigue 2018, 116, 118–127. [Google Scholar] [CrossRef]
- Lockyer, S.A.; Noble, F.W. Precipitate structure in a Cu-Ni-Si alloy. J. Mater. Sci. 1994, 29, 218–226. [Google Scholar] [CrossRef]
- Hu, T.; Chen, J.H.; Liu, J.Z.; Liu, Z.R.; Wu, C.L. The crystallographic and morphological evolution of the strengthening precipitates in Cu–Ni–Si alloys. Acta Mater. 2013, 61, 1210–1219. [Google Scholar] [CrossRef]
- Wang, W.; Kang, H.; Chen, Z.; Chen, Z.; Zou, C.; Li, R.; Yin, G.; Wang, T. Effects of Cr and Zr additions on microstructure and properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2016, 673, 378–390. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, B.; Volinsky, A.A.; Sun, H.; Chai, Z.; Liu, P.; Chen, X.; Liu, Y. Microstructure and precipitate’s characterization of the Cu-Ni-Si-P alloy. J. Mater. Eng. Perform. 2016, 25, 1336–1341. [Google Scholar] [CrossRef]
- Lei, Q.; Xiao, Z.; Hu, W.; Derby, B.; Li, Z. Phase transformation behaviors and properties of a high strength Cu-Ni-Si alloy. Mater. Sci. Eng. A 2017, 697, 37–47. [Google Scholar] [CrossRef]
- Yi, J.; Jia, Y.; Zhao, Y.; Xiao, Z.; He, K.; Wang, Q.; Wang, M.; Li, Z. Precipitation behavior of Cu-3.0 Ni-0.72 Si alloy. Acta Mater. 2019, 166, 261–270. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Effect of Ni/Si Mass Ratio and Thermomechanical Treatment on the Microstructure and Properties of Cu-Ni-Si Alloys. Materials 2019, 12, 2076. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chen, H.-G.; Gu, J.-W.; Hsu, C.-E.; Wu, C.-Y. Improvement in strength and thermal conductivity of powder metallurgy produced Cu–Ni–Si–Cr alloy by adjusting Ni/Si weight ratio and hot forging. J. Alloys Compd. 2015, 633, 59–64. [Google Scholar] [CrossRef]
- Patchett, J.; Abbaschian, G. Grain refinement of copper by the addition of iron and by electromagnetic stirring. Metall. Trans. B 1985, 16, 505–511. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibutani, N.; Mimura, K.; Isshiki, M.; Waseda, Y. Improvement in strength and electrical conductivity of Cu–Ni–Si alloys by aging and cold rolling. J. Alloys Compd. 2006, 417, 116–120. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu–Ni–Si alloy. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Z.; Li, Z.; Zhou, Z.; Yang, Z.; Lei, Q. Microstructure and properties of a novel Cu-Mg-Ca alloy with high strength and high electrical conductivity. J. Alloys Compd. 2017, 723, 1162–1170. [Google Scholar] [CrossRef]
- Lee, E.; Han, S.; Euh, K.; Lim, S.; Kim, S. Effect of Ti addition on tensile properties of Cu-Ni-Si alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Tang, B.B.; Yu, F.X.; Shen, B. Evaluation of nanoscaled precipitates in a Cu–Ni–Si–Cr alloy during aging. J. Alloys Compd. 2014, 614, 189–195. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.; Zhao, Y.; Jia, L.; Xie, H.; Tao, S. Disintegration of the net-shaped grain-boundary phase by multi-directional forging and its influence on the microstructure and properties of Cu–Ni–Si alloy. Mater. Res. Express 2017, 4, 096511. [Google Scholar] [CrossRef]
- Cao, Y.; Han, S.Z.; Choi, E.-A.; Ahn, J.H.; Mi, X.; Lee, S.; Shin, H.; Kim, S.; Lee, J. Effect of inclusion on strength and conductivity of Cu-Ni-Si alloys with discontinuous precipitation. J. Alloys Compd. 2020, 843, 156006. [Google Scholar] [CrossRef]
- Xie, H.; Jia, L.; Lu, Z. Microstructure and solidification behavior of Cu–Ni–Si alloys. Mater. Charact. 2009, 60, 114–118. [Google Scholar] [CrossRef]
- Jia, Y.-l.; Wang, M.-p.; Chen, C.; Dong, Q.-y.; Wang, S.; Li, Z. Orientation and diffraction patterns of δ-Ni2Si precipitates in Cu–Ni–Si alloy. J. Alloys Compd. 2013, 557, 147–151. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Purcek, G.; Yanar, H.; Saray, O.; Karaman, I.; Maier, H.J. Effect of precipitation on mechanical and wear properties of ultrafine-grained Cu–Cr–Zr alloy. Wear 2014, 311, 149–158. [Google Scholar] [CrossRef]
- Stott, F. The role of oxidation in the wear of alloys. Tribol. Int. 1998, 31, 61–71. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Abouei, V.; Mortazavi, N.; Razavi, S.H.; Hooshyar, H.; Esmaily, M. Effects of cold working and heat treatment on microstructure and wear behaviour of Cu–Be alloy C17200. Tribol.-Mater. Surf. Interfaces 2015, 9, 118–127. [Google Scholar] [CrossRef]
| Cu | Ni | Si | Fe | |
|---|---|---|---|---|
| Overall | Balanced | 7.4 | 3.8 | 1.1 |
| Cu-rich matrix (A) | Balanced | 5.3 | 2.3 | 0.7 |
| Intermetallics (B) | Balanced | 47.7 | 27.0 | 7.6 |
| Alloys | C17200 | Cu86.5Ni7.4Si3.8Fe1.1 | |||||
|---|---|---|---|---|---|---|---|
| Elements | Cu | O | Cu | Ni | Si | Fe | O |
| Glaze layer (I) | 67.5 | 32.5 | Bal. | 4.8 | 3.2 | 2.0 | 31.0 |
| Plate-like debris (II) | 94.8 | 5.2 | 0.7 | 0.6 | - | 6.1 | |
| Irregular blocky/Powder-like debris (III) | 76.9 | 23.1 | 4.2 | 2.7 | 0.9 | 29.1 | |
| Intermetallic | N/A | N/A | 42.8 | 29.1 | 7.4 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-H.; Hou, P.-Y.; Lin, W.-S.; Shen, P.-K.; Huang, H.-H.; Yeh, J.-W.; Yen, H.-W.; Huang, C.-Y.; Tsai, C.-W. Investigation of Microstructure and Wear Properties of Precipitates-Strengthened Cu-Ni-Si-Fe Alloy. Materials 2023, 16, 1193. https://doi.org/10.3390/ma16031193
Peng C-H, Hou P-Y, Lin W-S, Shen P-K, Huang H-H, Yeh J-W, Yen H-W, Huang C-Y, Tsai C-W. Investigation of Microstructure and Wear Properties of Precipitates-Strengthened Cu-Ni-Si-Fe Alloy. Materials. 2023; 16(3):1193. https://doi.org/10.3390/ma16031193
Chicago/Turabian StylePeng, Chun-Hao, Po-Yu Hou, Woei-Shyang Lin, Pai-Keng Shen, Hao-Hsuan Huang, Jien-Wei Yeh, Hung-Wei Yen, Cheng-Yao Huang, and Che-Wei Tsai. 2023. "Investigation of Microstructure and Wear Properties of Precipitates-Strengthened Cu-Ni-Si-Fe Alloy" Materials 16, no. 3: 1193. https://doi.org/10.3390/ma16031193
APA StylePeng, C.-H., Hou, P.-Y., Lin, W.-S., Shen, P.-K., Huang, H.-H., Yeh, J.-W., Yen, H.-W., Huang, C.-Y., & Tsai, C.-W. (2023). Investigation of Microstructure and Wear Properties of Precipitates-Strengthened Cu-Ni-Si-Fe Alloy. Materials, 16(3), 1193. https://doi.org/10.3390/ma16031193








