Experimental Studies of Thermophysical Properties and Microstructure of X37CrMoV5-1 Hot-Work Tool Steel and Maraging 350 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
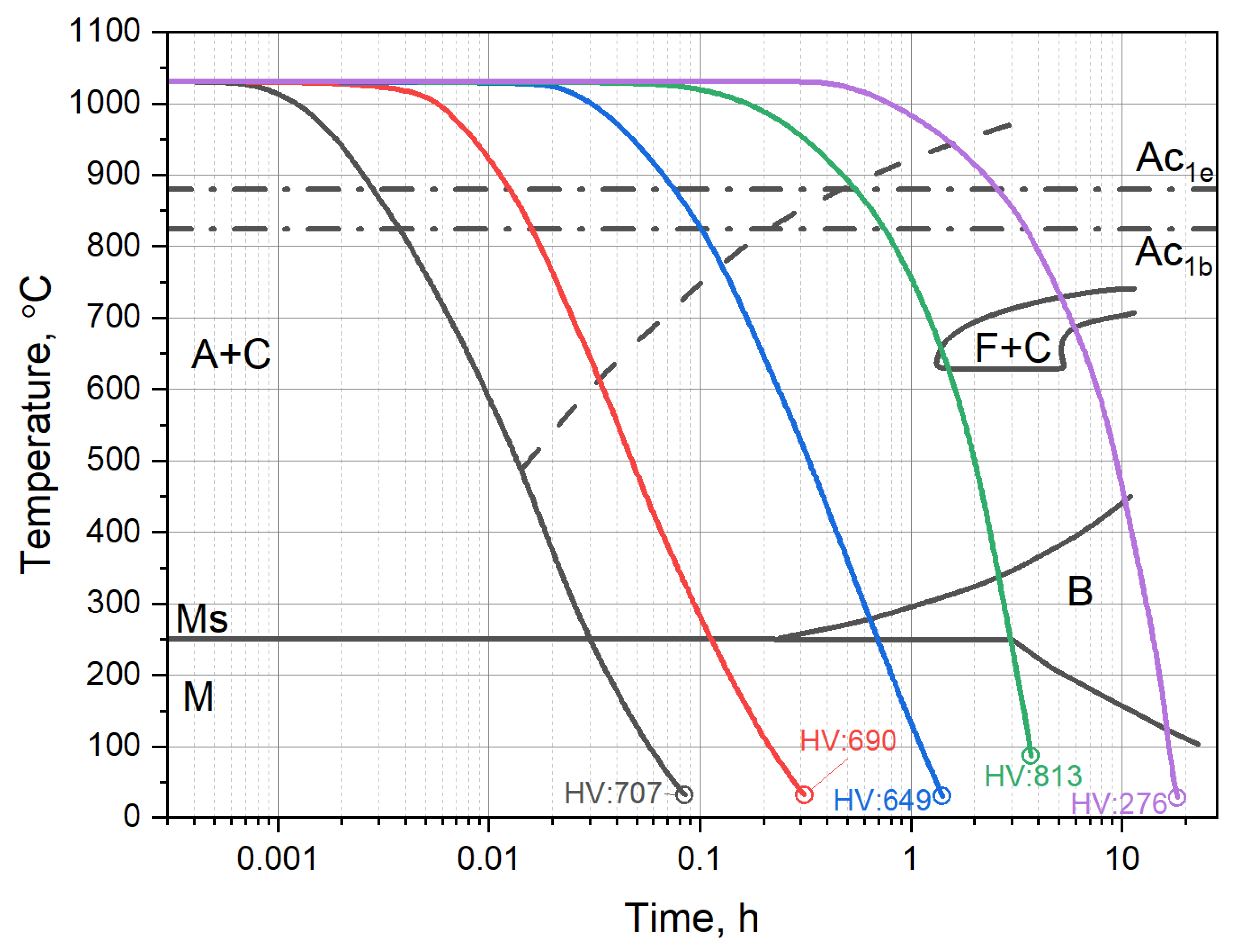
2.2. The X37CrMoV5-1 Time–Temperature-Transformation Diagram
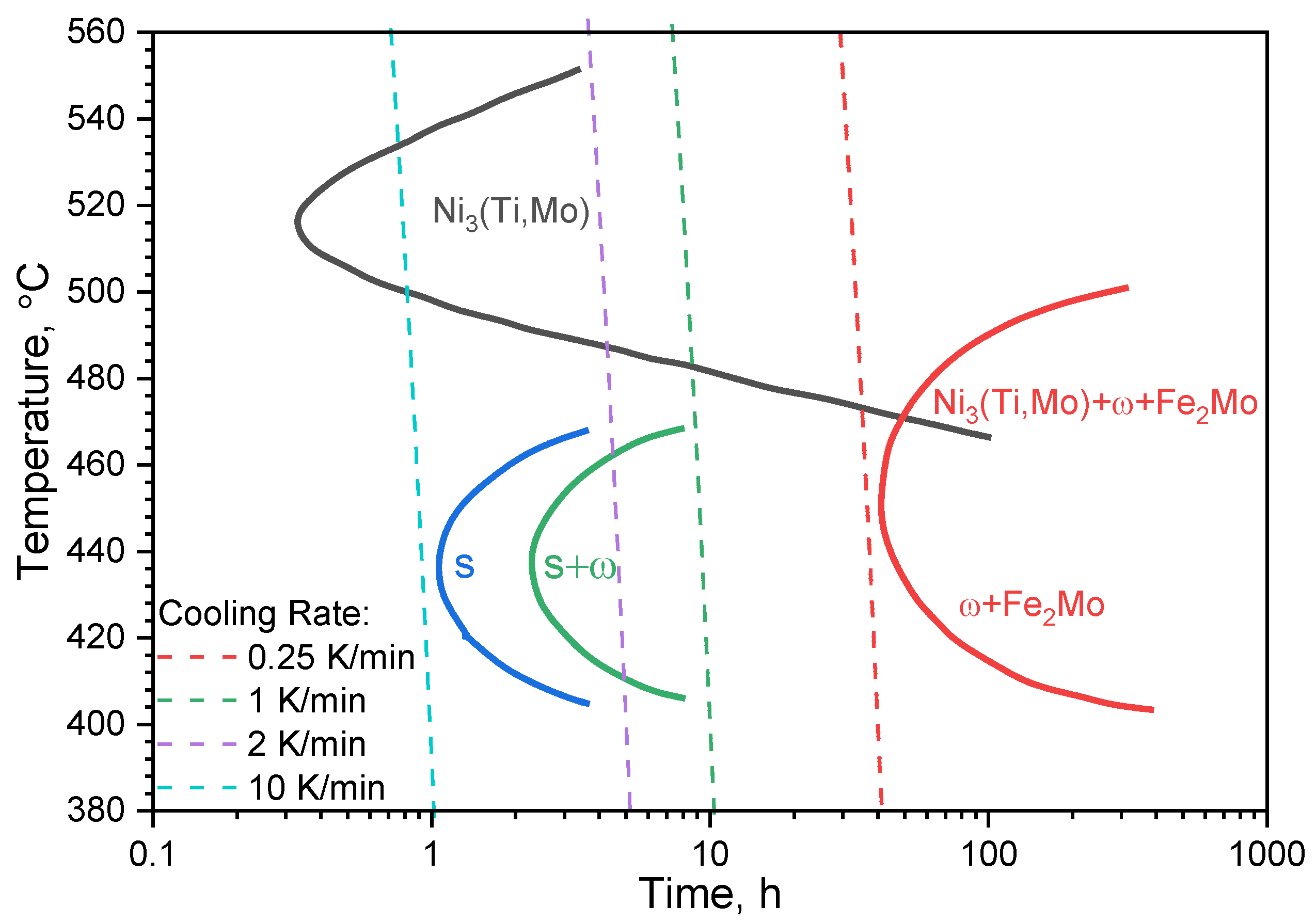
2.3. The Maraging 350 Time–Temperature-Transformation Diagram
2.4. Sample Preparation
2.5. Microstructure Analysis and Vickers Micro-Hardness Measurements
2.6. Thermal Analysis
2.6.1. LFA
2.6.2. DIL
2.6.3. DSC
3. Results and Discussion
3.1. Microstructural Analysis
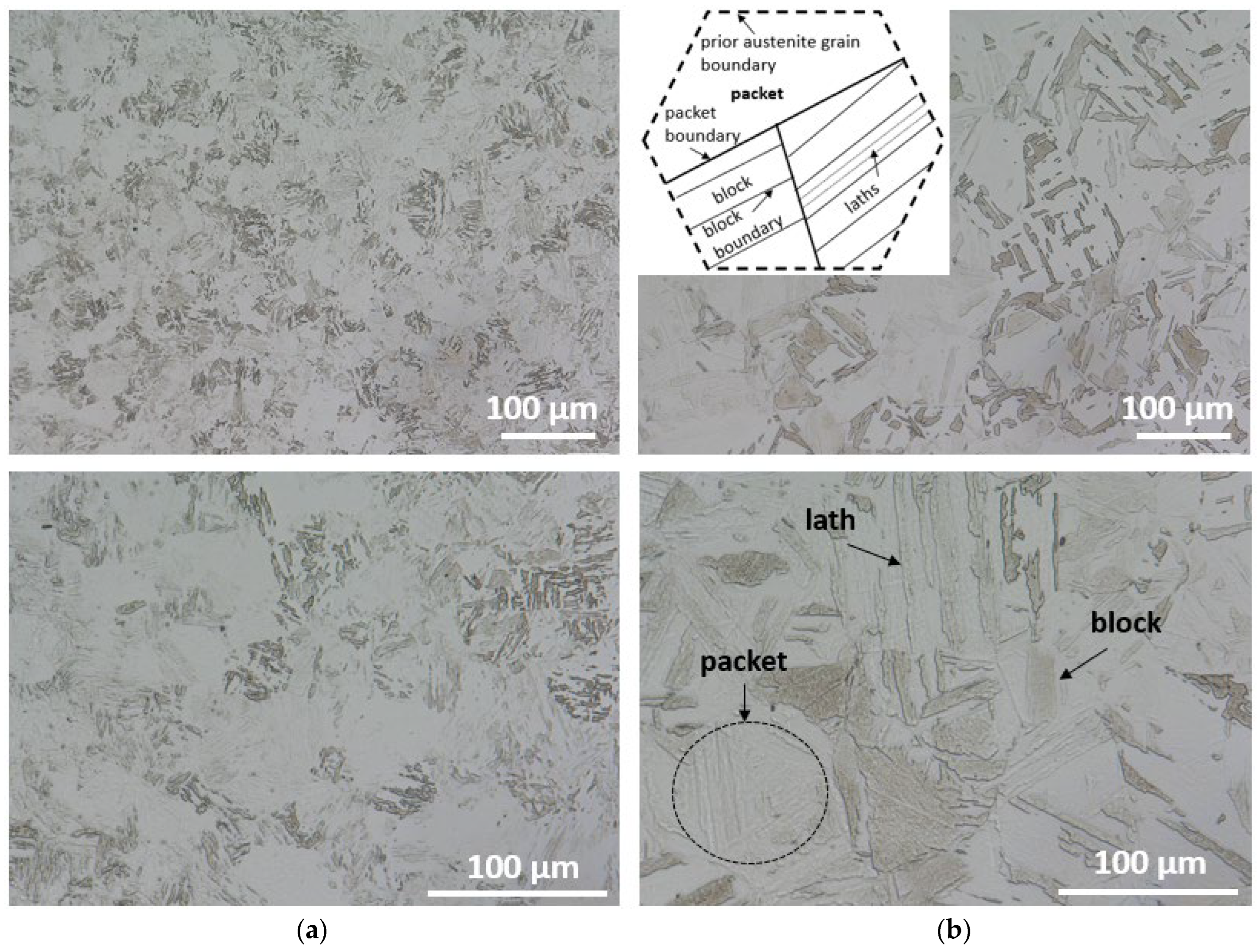
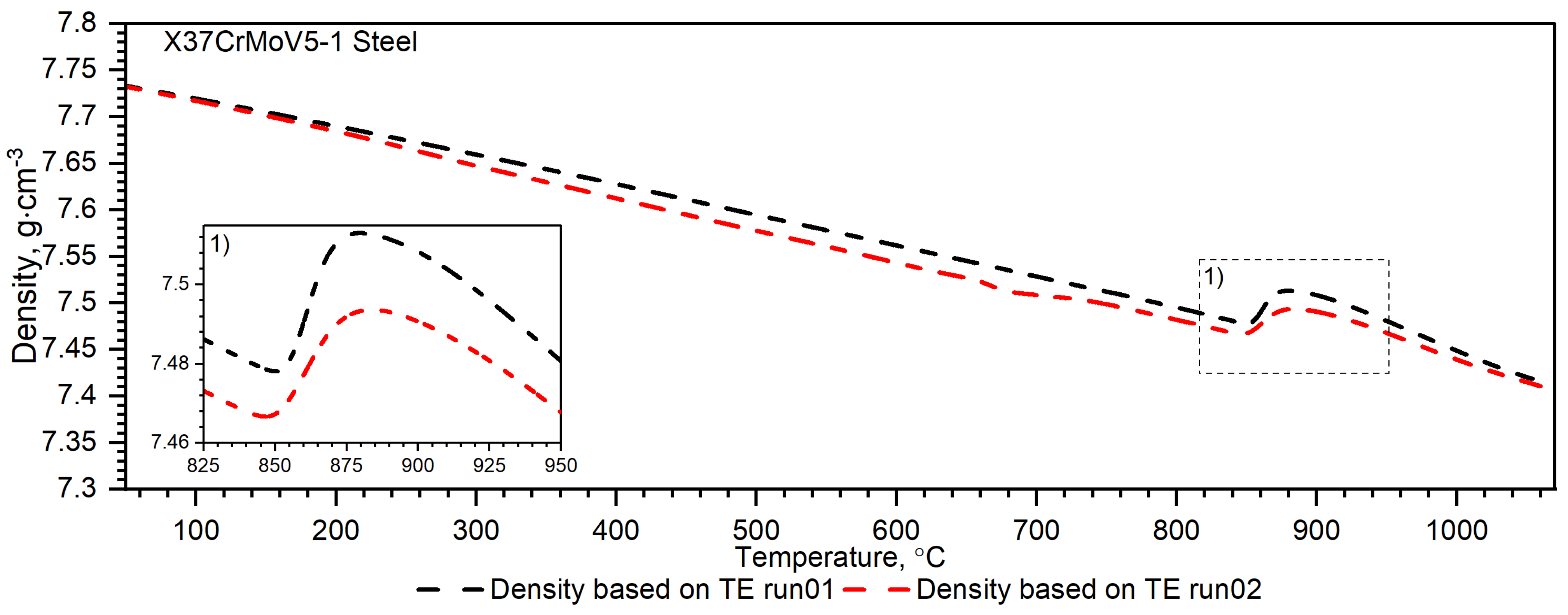
3.1.1. X37CrMoV5-1 Hot-Work Tool Steel
3.1.2. Maraging M350 Steel
3.2. Thermal Properties Investigations
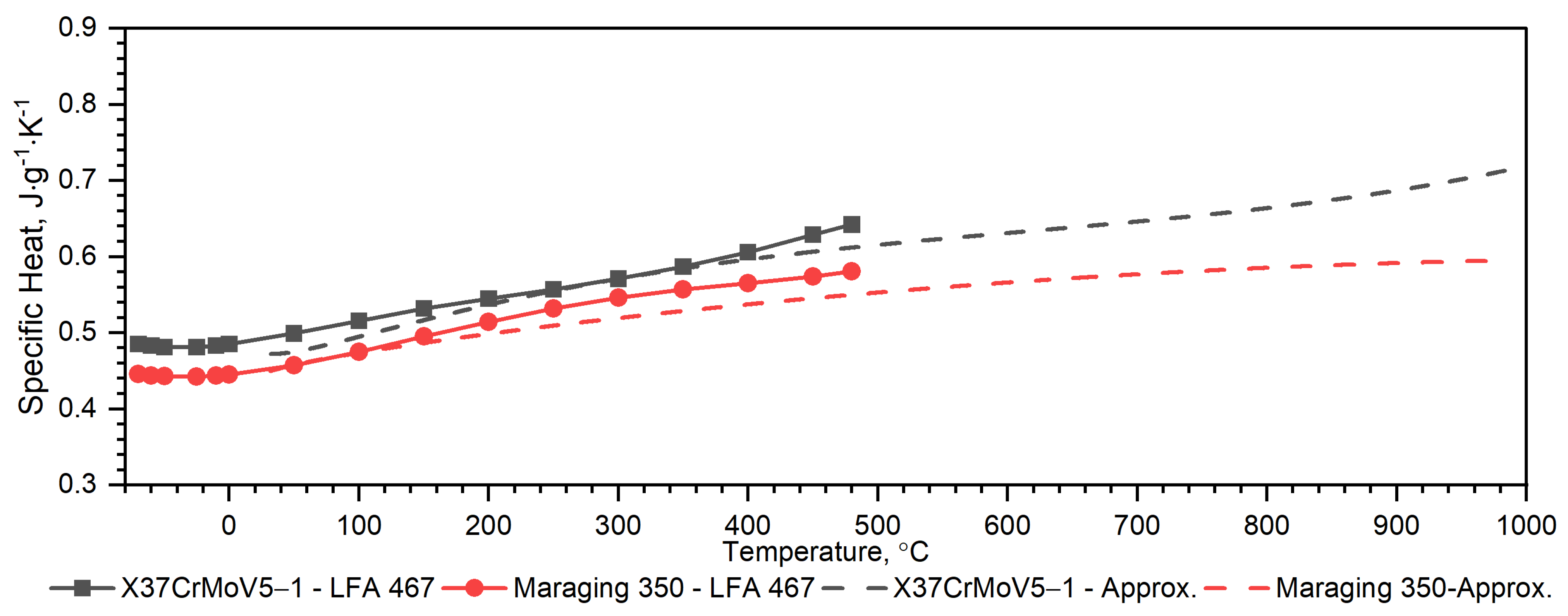
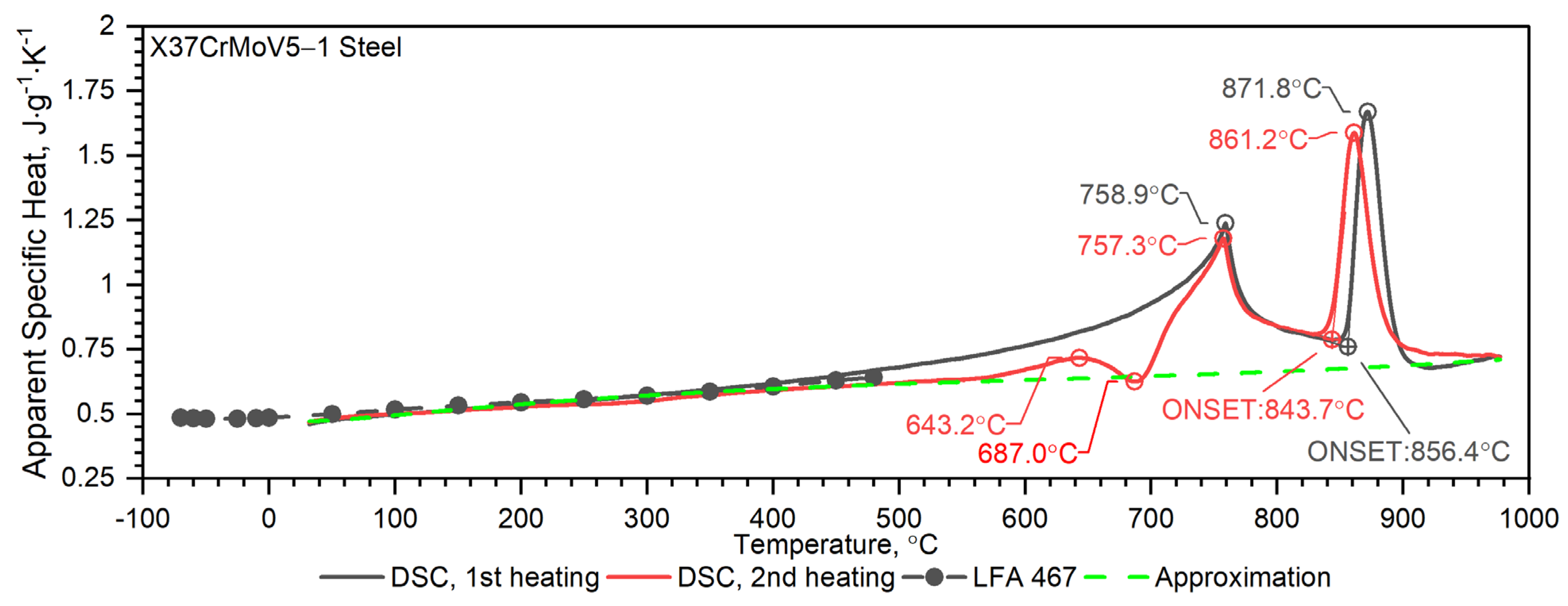
3.2.1. X37CrMoV5-1 Hot-Work Tool Steel
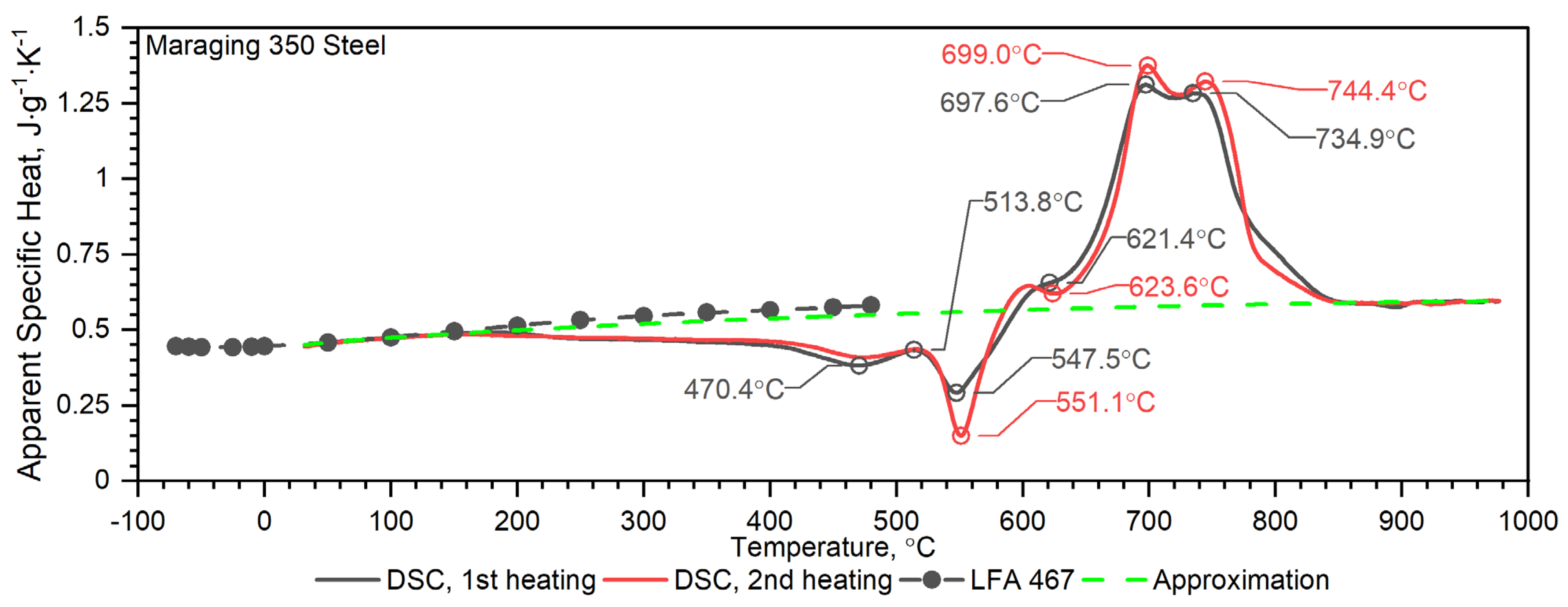
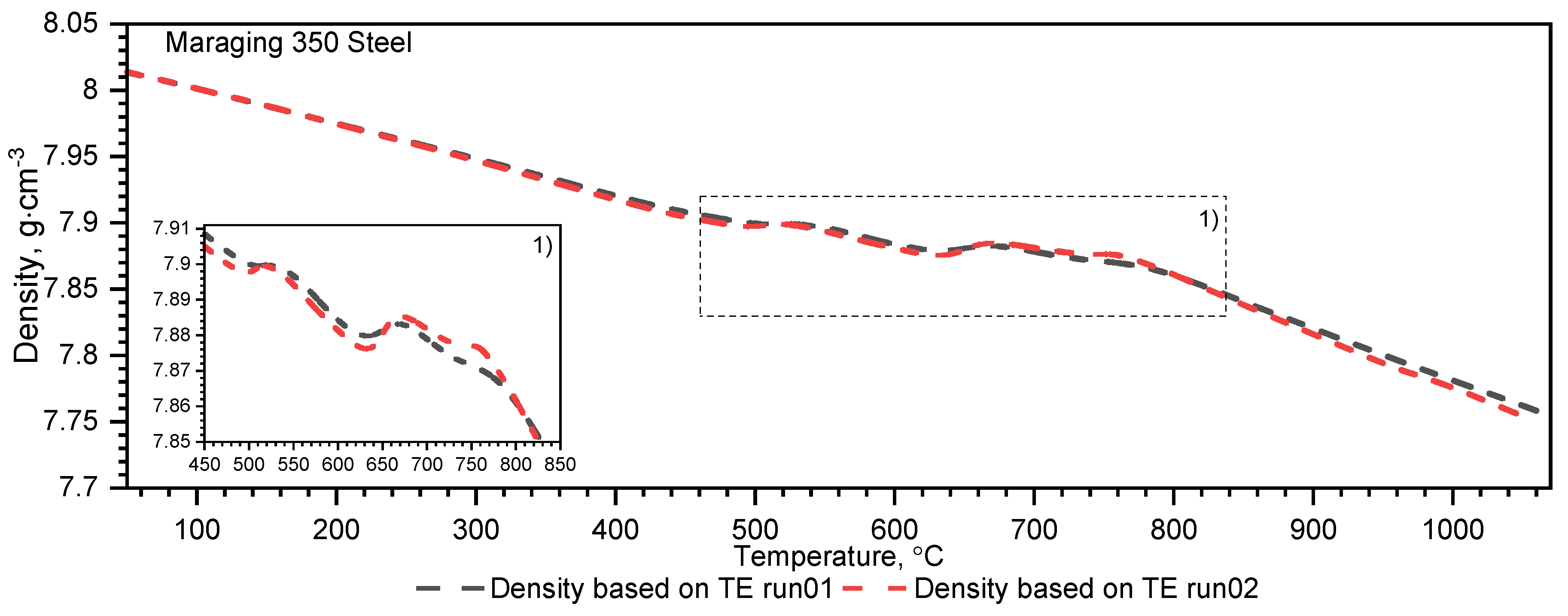
3.2.2. Maraging M350 Steel
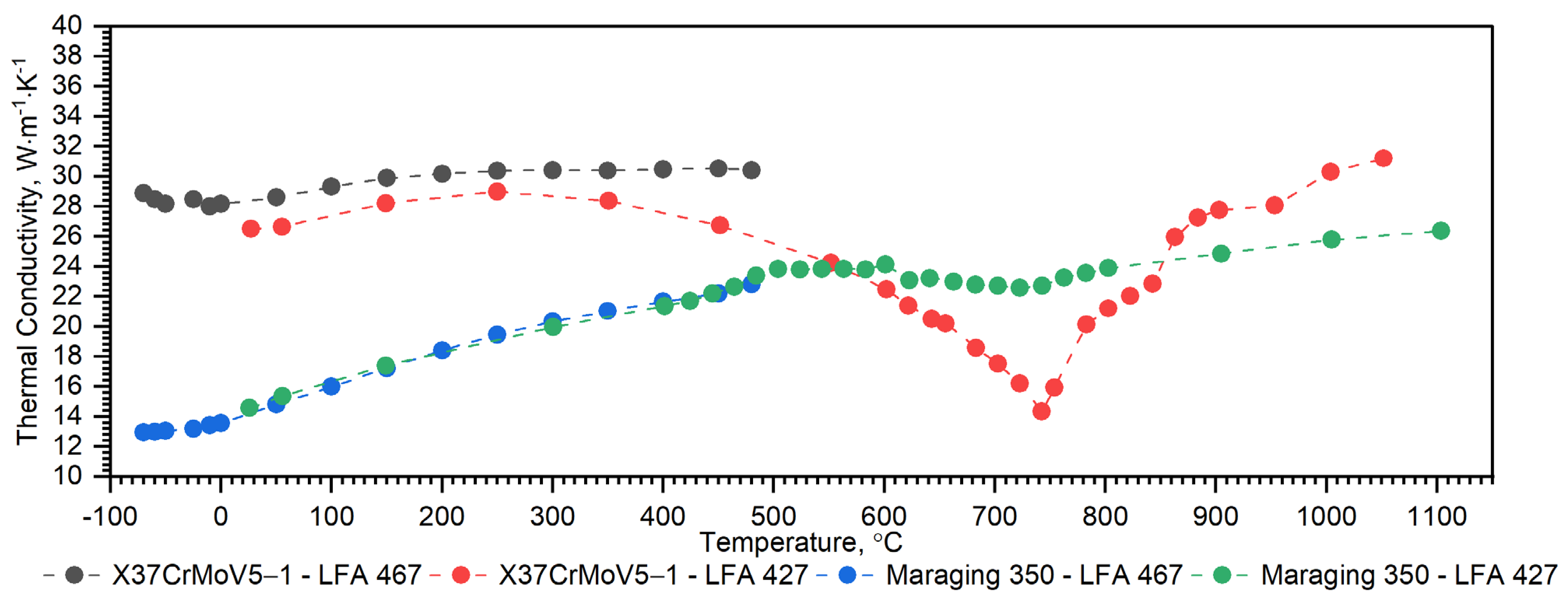
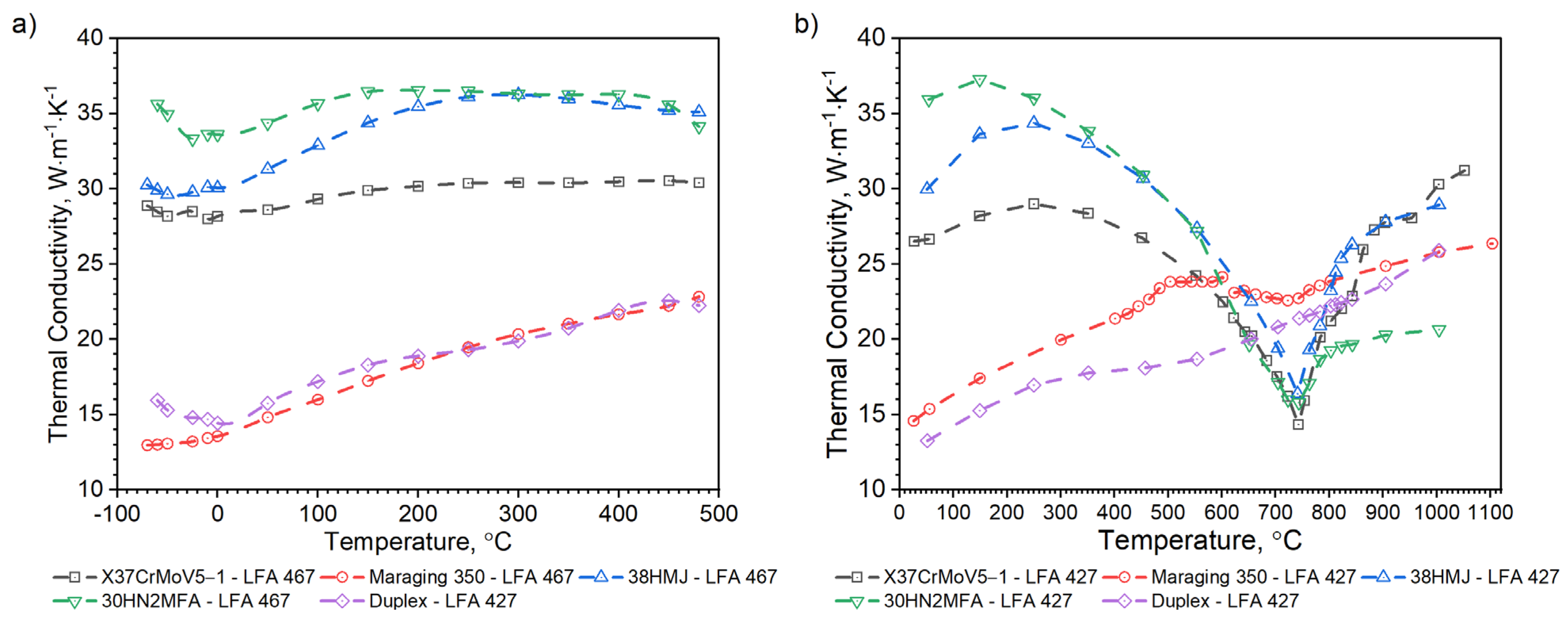
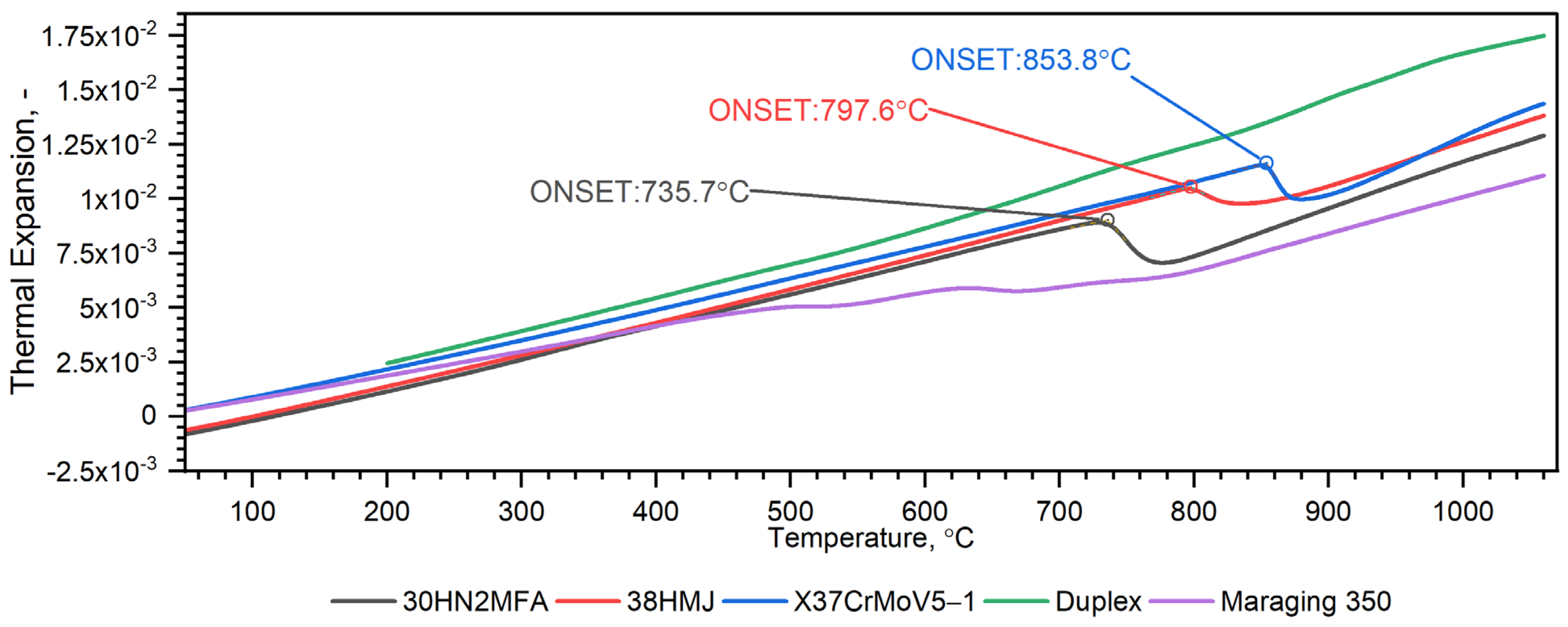
3.2.3. Comparison of Thermophysical Properties of X37CrMoV5-1, Maraging 350, 38HMJ, 30HN2MFA and Duplex Steels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koniorczyk, P.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Preiskorn, M.; Sienkiewicz, J. Investigation of Thermophysical Properties of Three Barrel Steels. Metals 2020, 10, 573. [Google Scholar] [CrossRef]
- Dębski, A.; Surma, Z.; Koperski, W. Material and Technological Optimization Research in Terms of Increasing the Durability of Small Arms; Military University of Technology: Warsaw, Poland, 2009. [Google Scholar]
- Li, H.; Chen, G.; Zhang, K.; Luo, G.; Ye, Z. Degradation failure features of chromium-plated gun barrels with a laser-discrete-quenched substrate. Surf. Coat. Technol. 2007, 201, 9558–9564. [Google Scholar] [CrossRef] [Green Version]
- Koniorczyk, P.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Cegła, M. Investigations of thermal diffusivity and thermal expansion for three types of the barrel steel. In Proceedings of the THERMOPHYSICS 2019: 24th International Meeting of Thermophysics and 20th Conference REFRA, Smolenice, Slovakia, 22–24 October 2019; p. 20006. [Google Scholar]
- Sopok, S.; Rickard, C.; Dunn, S. Thermal–chemical–mechanical gun bore erosion of an advanced artillery system part one: Theories and mechanisms. Wear 2005, 258, 659–670. [Google Scholar] [CrossRef]
- Sopok, S.; Rickard, C.; Dunn, S. Thermal–chemical–mechanical gun bore erosion of an advanced artillery system part two: Modeling and predictions. Wear 2005, 258, 671–683. [Google Scholar] [CrossRef]
- Zieliński, M.; Koniorczyk, P.; Surma, Z.; Zmywaczyk, J.; Preiskorn, M. Numerical Study of Heat Transfer in a Gun Barrel Made of Selected Steels. Energies 2022, 15, 1868. [Google Scholar] [CrossRef]
- Dębski, A.; Koniorczyk, P.; Leciejewski, Z.; Preiskorn, M.; Surma, Z.; Zmywaczyk, J. Analysis of Heat Transfer in a 35 mm Barrel of an Anti-Aircraft Cannon. Probl. Mechatron. Armament Aviat. Saf. Eng. 2016, 7, 71–86. [Google Scholar] [CrossRef]
- Fikus, B.; Dorochowicz, A.; Surma, Z.; Kijewski, J.; Leciejewski, Z.; Michalski, J.; Trębiński, R. Investigations of Middle-Caliber Anti-Aircraft Cannon Interior Ballistics including Heat Transfer Problem in Estimation of Critical Burst Length. Processes 2022, 10, 607. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels. Part II: Carbides and Nitrides. Metallogr. Microstruct. Anal. 2013, 2, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Charles, J. Composition and properties of duplex stainless steels. Weld. World/Soudage Dans Monde 1995, 36, 43–54. [Google Scholar]
- Koniorczyk, P.; Sienkiewicz, J.; Zmywaczyk, J.; Dębski, A.; Zieliński, M.; Preiskorn, M. Effect of Microstructure on Thermophysical Properties of Heat-Treated Duplex Steel. Materials 2021, 14, 6043. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Mazumder, S.; Batra, I.S.; Dey, G.K.; Banerjee, S. Precipitation in 18 wt% Ni maraging steel of grade 350. Acta Mater. 2000, 48, 1187–1200. [Google Scholar] [CrossRef]
- Kaschnitz, E.; Hofer-Hauser, P.; Funk, W. Electrical resistivity measured by millisecond pulse-heating in comparison to thermal conductivity of the hot work tool steel AISI H11 (1.2343) at elevated temperature. HTHP 2020, 49, 75–87. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Li, D. Quantification of phase transformation kinetics of 18 wt.% Ni C250 maraging steel. Mater. Sci. Eng. A 2004, 373, 10–20. [Google Scholar] [CrossRef]
- Jarfors, A.E.W.; Matsushita, T.; Siafakas, D.; Stolt, R. On the nature of the anisotropy of Maraging steel (1.2709) in additive manufacturing through powder bed laser-based fusion processing. Mater. Des. 2021, 204, 109608. [Google Scholar] [CrossRef]
- Kapoor, R.; Kumar, L.; Batra, I.S. A dilatometric study of the continuous heating transformations in 18 wt.% Ni maraging steel of grade 350. Mater. Sci. Eng. A 2003, 352, 318–324. [Google Scholar] [CrossRef]
- Skołek, E.; Marciniak, S.; Świątnicki, W.A. Thermal Stability of Nanocrystalline Structure in X37CrMoV5-l Steel. Arch. Metall. Mater. 2015, 60, 511–516. [Google Scholar] [CrossRef]
- Mayer, S.; Scheu, C.; Leitner, H.; Siller, I.; Clemens, H. Correlation between heat treatment, microstructure and mechanical properties of a hot-work tool steel. Int. J. Mater. Res. 2009, 100, 86–91. [Google Scholar] [CrossRef]
- Maraging Data Sheet. Available online: https://www.ssa-corp.com/documents/Data%20Sheet%20Maraging.pdf (accessed on 9 December 2022).
- Garrett, R.P.; Xu, S.; Lin, J.; Dean, T.A. A model for predicting austenite to bainite phase transformation in producing dual phase steels. Int. J. Mach. Tools Manuf. 2004, 44, 831–837. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. The bainite transformation: Unresolved issues. Mater. Sci. Eng. A 1999, 273–275, 58–66. [Google Scholar] [CrossRef]
- Eschmann Stahl Katalog. Available online: https://www.eschmannstahl.de/app/uploads/sites/30/2018/08/Technischer_katalog_EN_2603-18.pdf (accessed on 9 December 2022).
- Sha, W.; Cerezo, A.; Smith, G.D.W. Phase chemistry and precipitation reactions in maraging steels: Part IV. Discussion and conclusions. Metall. Mater. Trans. A 1993, 24, 1251–1256. [Google Scholar] [CrossRef]
- Tohme, E. A Contribution to the Understanding of Hydrogen Diffusion and Embrittlement in Metallic Materials Based on SKPFM Measurements and Mechanical Testing; Université de Lyon: Lyon, France, 2019. [Google Scholar]
- Netzsch Proteus ver. 7.1 Software Manual. Available online: https://www.netzsch-thermal-analysis.com/en/products-solutions/software/proteus/ (accessed on 14 September 2022).
- Michaud, P.; Delagnes, D.; Lamesle, P.; Mathon, M.H.; Levaillant, C. The effect of the addition of alloying elements on carbide precipitation and mechanical properties in 5% chromium martensitic steels. Acta Mater. 2007, 55, 4877–4889. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wu, X.; Shi, N.; Li, J.; Min, N. Microstructure evolution and kinetic analysis of DM hot-work die steels during tempering. Mater. Sci. Eng. A 2011, 528, 5696–5700. [Google Scholar] [CrossRef]
- Raju, S.; Ganesh, B.J.; Banerjee, A.; Mohandas, E. Characterisation of thermal stability and phase transformation energetics in tempered 9Cr–1Mo steel using drop and differential scanning calorimetry. Mater. Sci. Eng. A 2007, 465, 29–37. [Google Scholar] [CrossRef]
- Casati, R.; Coduri, M.; Lecis, N.; Andrianopoli, C.; Vedani, M. Microstructure and mechanical behavior of hot-work tool steels processed by Selective Laser Melting. Mater. Charact. 2018, 137, 50–57. [Google Scholar] [CrossRef]
- Carasi, G.; Yu, B.; Hutten, E.; Zurob, H.; Casati, R.; Vedani, M. Effect of Heat Treatment on Microstructure Evolution of X38CrMoV5-1 Hot-Work Tool Steel Produced by L-PBF. Metall. Mater. Trans. A 2021, 52, 2564–2575. [Google Scholar] [CrossRef]
- Chen, H.; Yue, Z.; Ren, D.; Zeng, H.; Wei, T.; Zhao, K.; Yang, R.; Qiu, P.; Chen, L.; Shi, X. Thermal Conductivity during Phase Transitions. Adv. Mater. 2019, 31, e1806518. [Google Scholar] [CrossRef]












| Component | C | Mn | Cr | Ni | Mo | V | P | Si | S |
|---|---|---|---|---|---|---|---|---|---|
| Concentration [wt.%] | 0.39 | 0.36 | 5.52 | 0.40 | 1.30 | 0.45 | 0.012 | 0.84 | <0.002 |
| Fe | |||||||||
| Balance | |||||||||
| Component | C | Si | Mn | Ni | Co | Mo | Ti | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Concentration [wt.%] | 0.03 max | 0.10 max | 0.10 max | 18.50 | 12.00 | 4.80 | 1.40 | 0.10 | Balance |
| Coefficient | Value | Coefficient | Value |
|---|---|---|---|
| Coefficient | Value | Coefficient | Value |
|---|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koniorczyk, P.; Zieliński, M.; Sienkiewicz, J.; Zmywaczyk, J.; Dębski, A. Experimental Studies of Thermophysical Properties and Microstructure of X37CrMoV5-1 Hot-Work Tool Steel and Maraging 350 Steel. Materials 2023, 16, 1206. https://doi.org/10.3390/ma16031206
Koniorczyk P, Zieliński M, Sienkiewicz J, Zmywaczyk J, Dębski A. Experimental Studies of Thermophysical Properties and Microstructure of X37CrMoV5-1 Hot-Work Tool Steel and Maraging 350 Steel. Materials. 2023; 16(3):1206. https://doi.org/10.3390/ma16031206
Chicago/Turabian StyleKoniorczyk, Piotr, Mateusz Zieliński, Judyta Sienkiewicz, Janusz Zmywaczyk, and Andrzej Dębski. 2023. "Experimental Studies of Thermophysical Properties and Microstructure of X37CrMoV5-1 Hot-Work Tool Steel and Maraging 350 Steel" Materials 16, no. 3: 1206. https://doi.org/10.3390/ma16031206







