The Effect of Synthetic Polymer Foams on Cellulosic Material Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Materials
2.2. Artificial Aging
2.3. Properties of PE Foams
2.3.1. Composition
2.3.2. Mechanical Properties
2.3.3. VOC Emissions Analysis
2.4. Properties of Lignocellulosic Materials
2.4.1. Colorimetry
2.4.2. UV/Vis Spectroscopy
2.4.3. pH Value of an Aqueous Extract
2.4.4. Mechanical Properties
2.4.5. Average Degree of Polymerization
3. Results
3.1. PE Foams
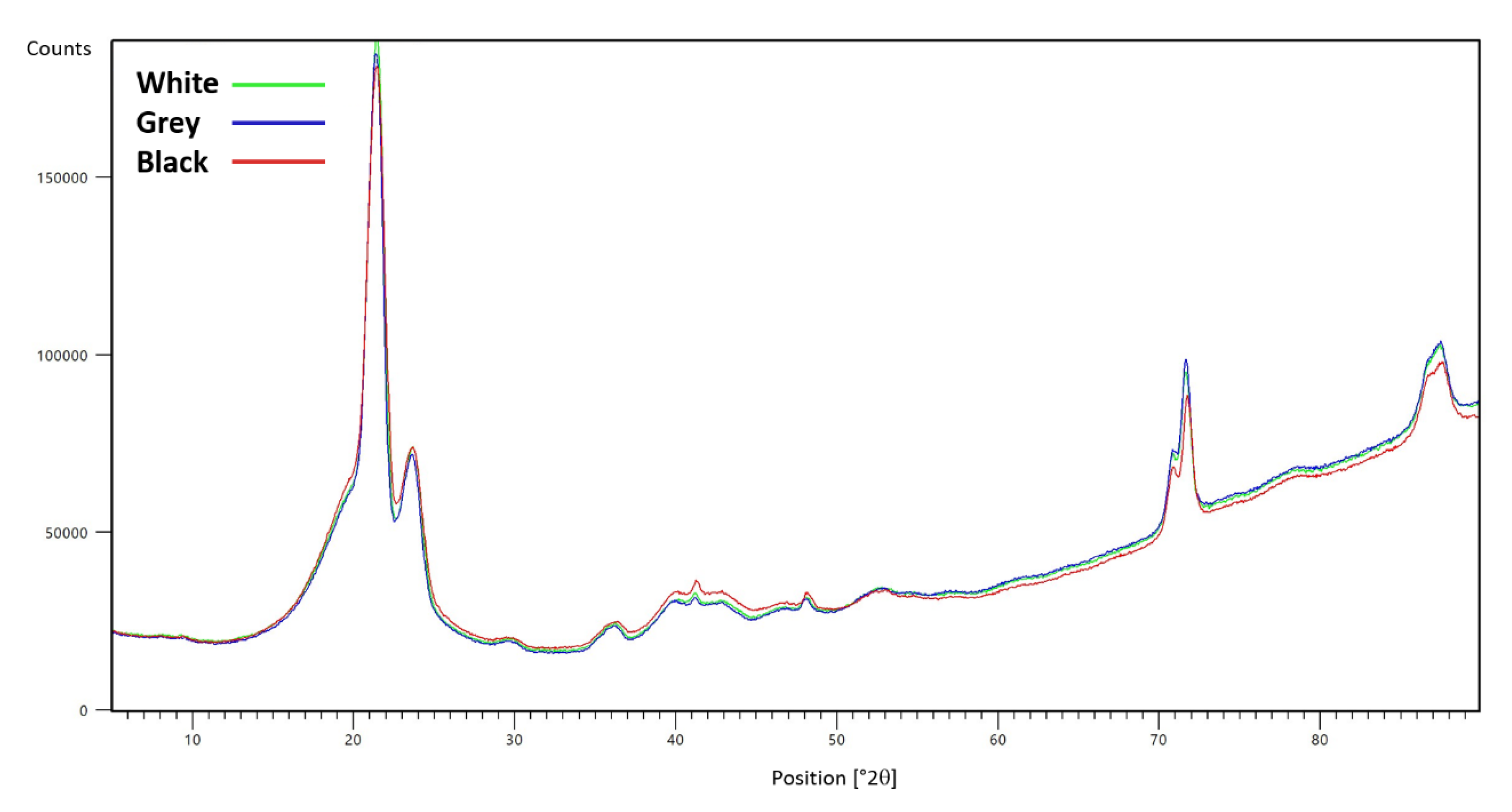
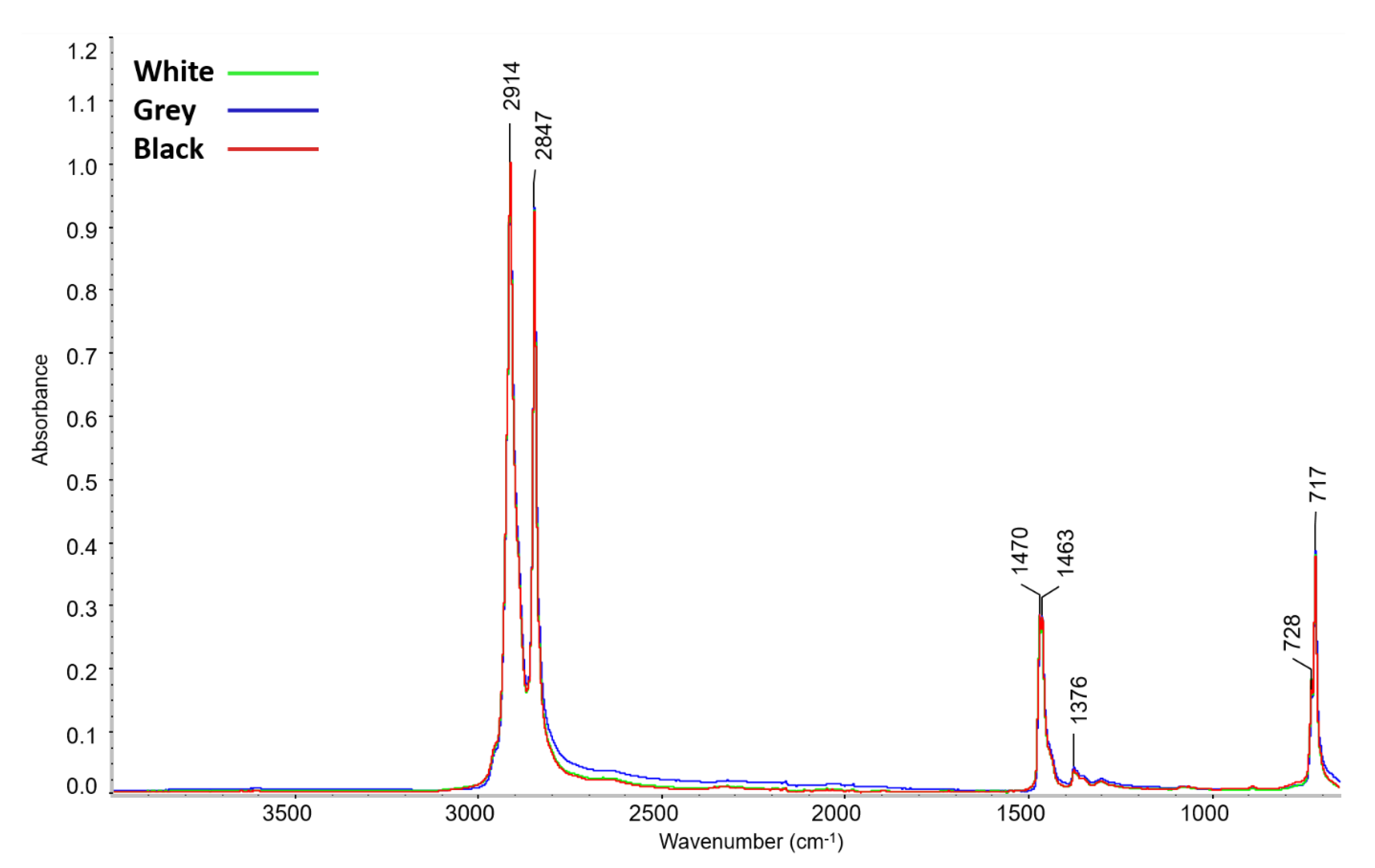
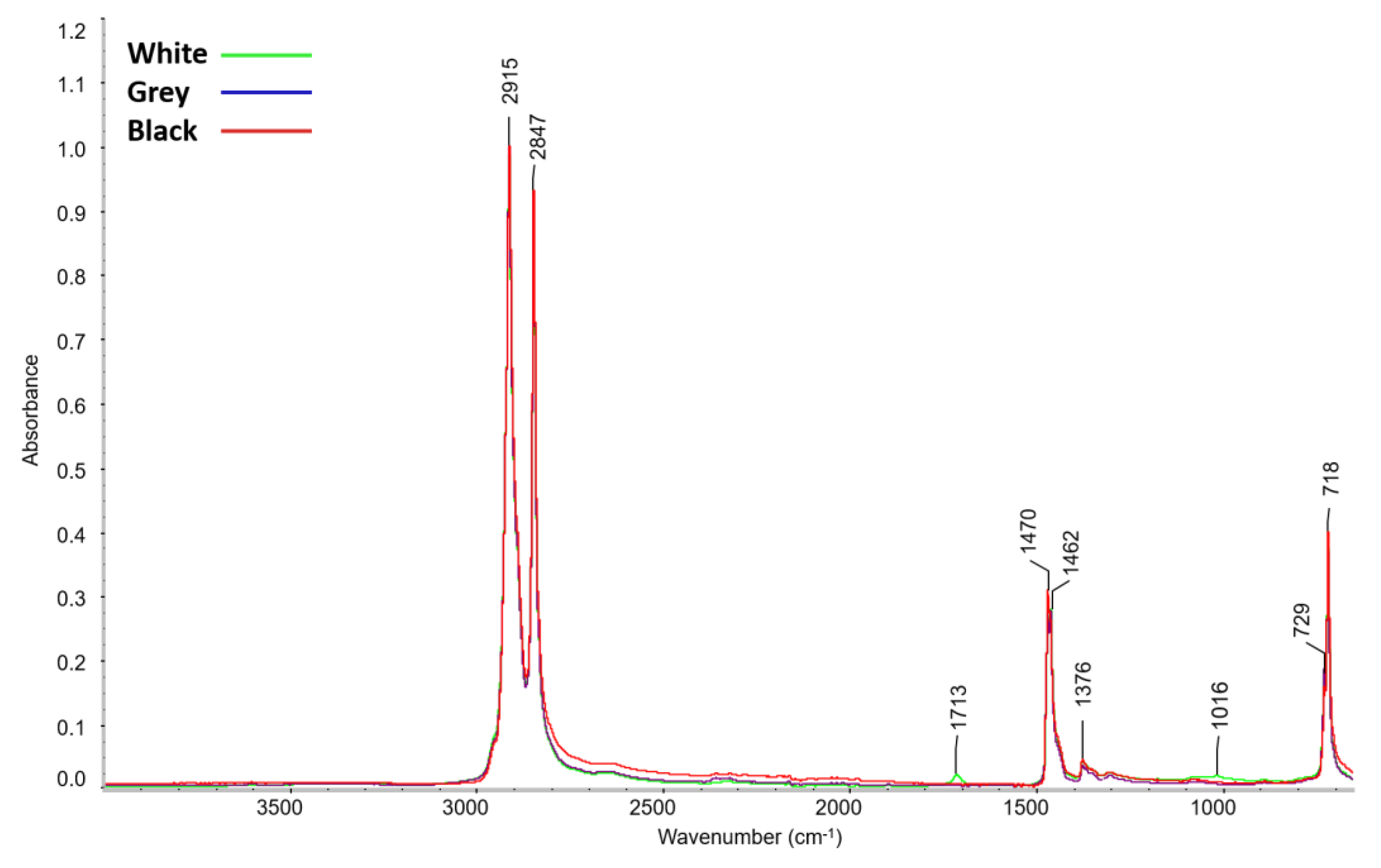
3.1.1. Composition
3.1.2. Mechanical Properties
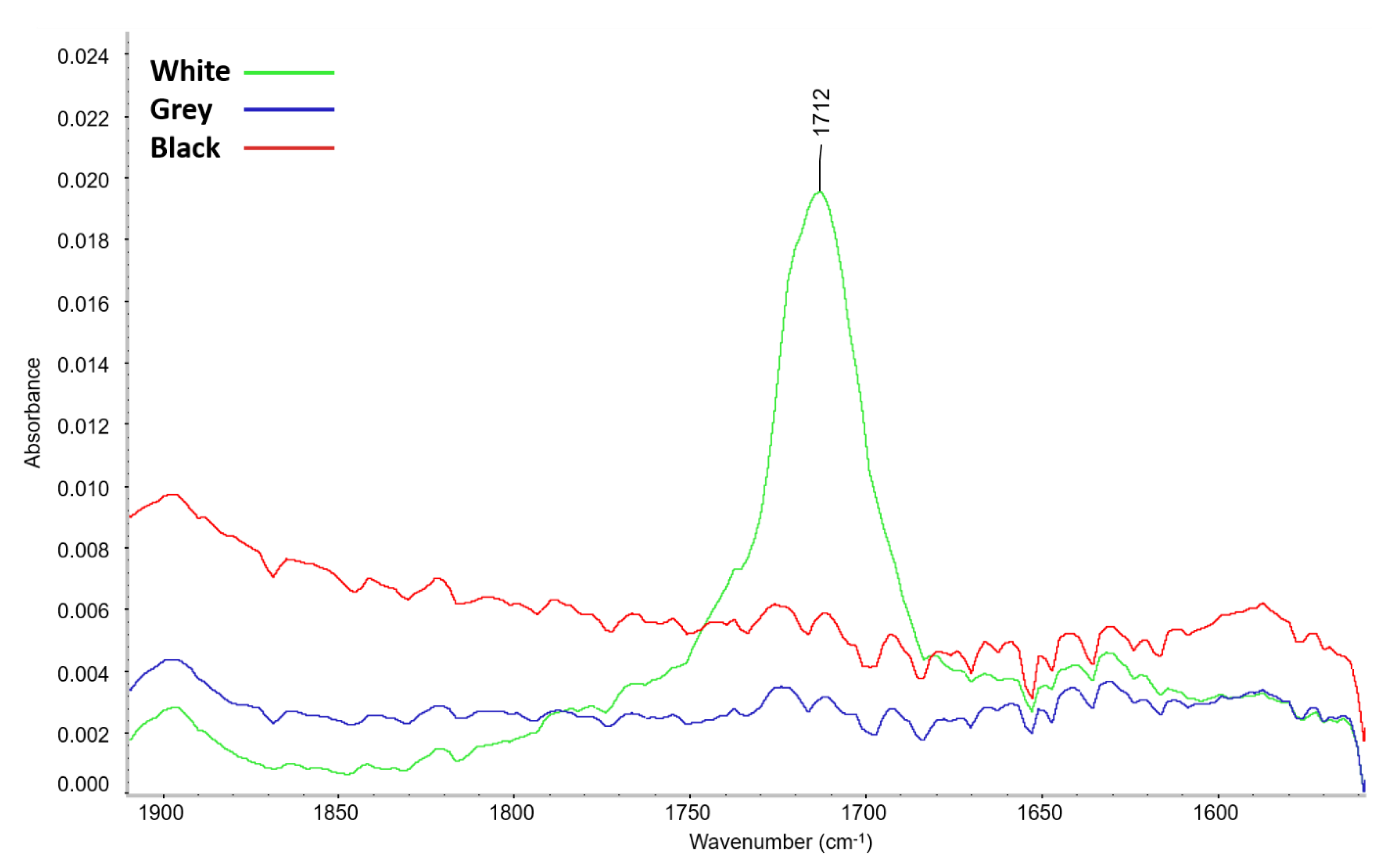
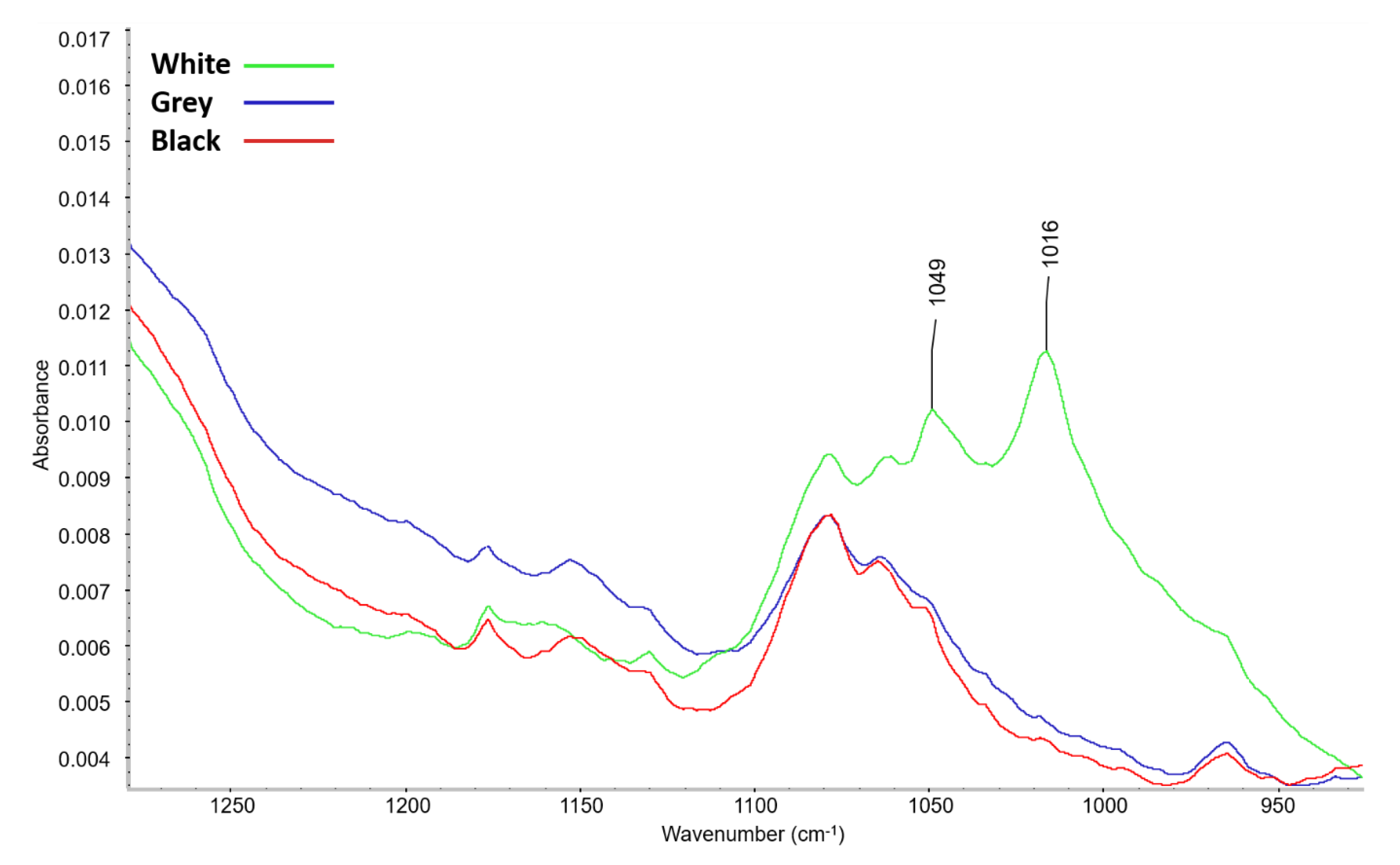
3.1.3. Volatile Organic Compounds (VOC)
3.2. Lignocellulosic Materials
3.2.1. Colorimetry
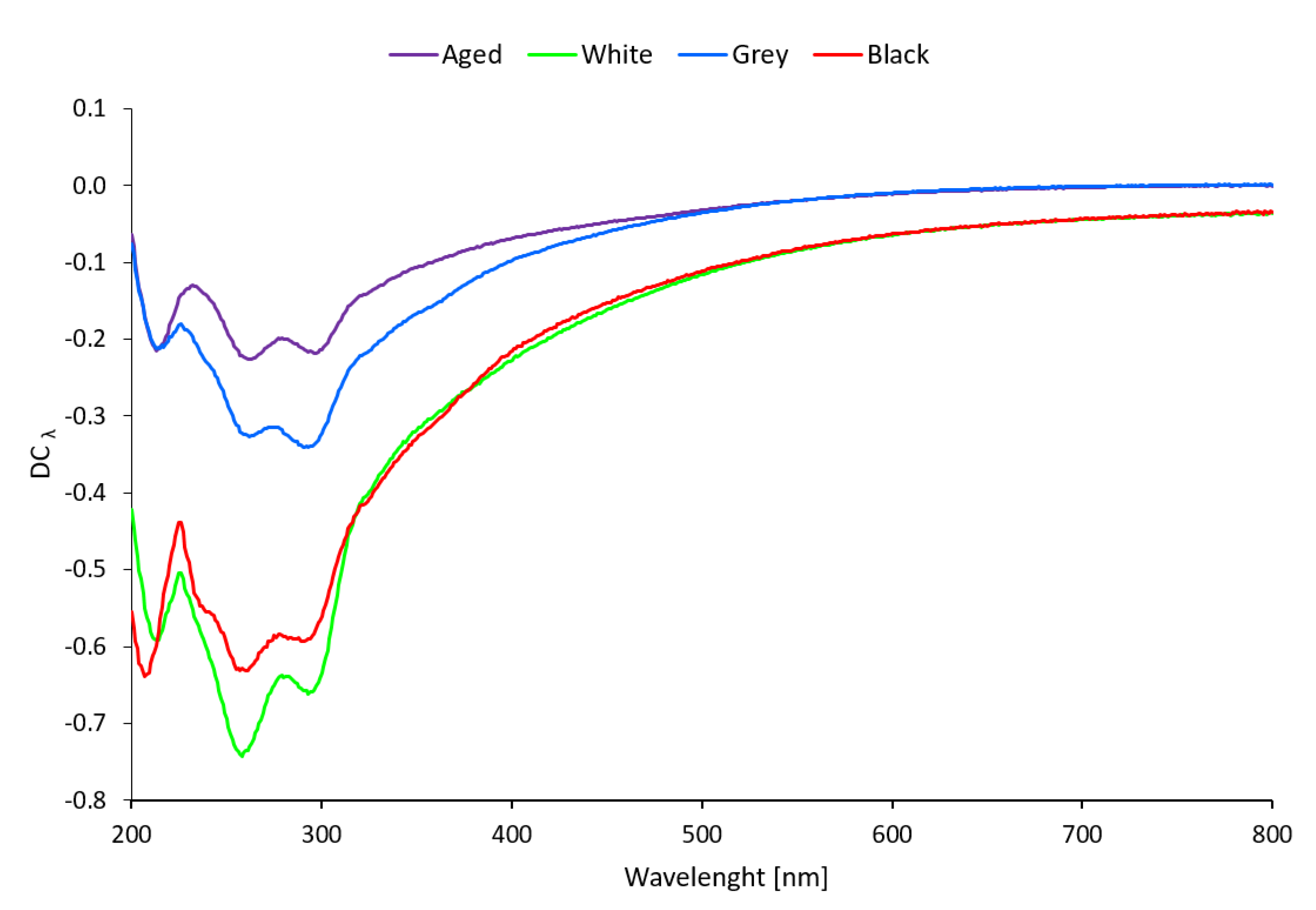
3.2.2. UV/Vis Spectroscopy
3.2.3. pH Value of an Aqueous Extract
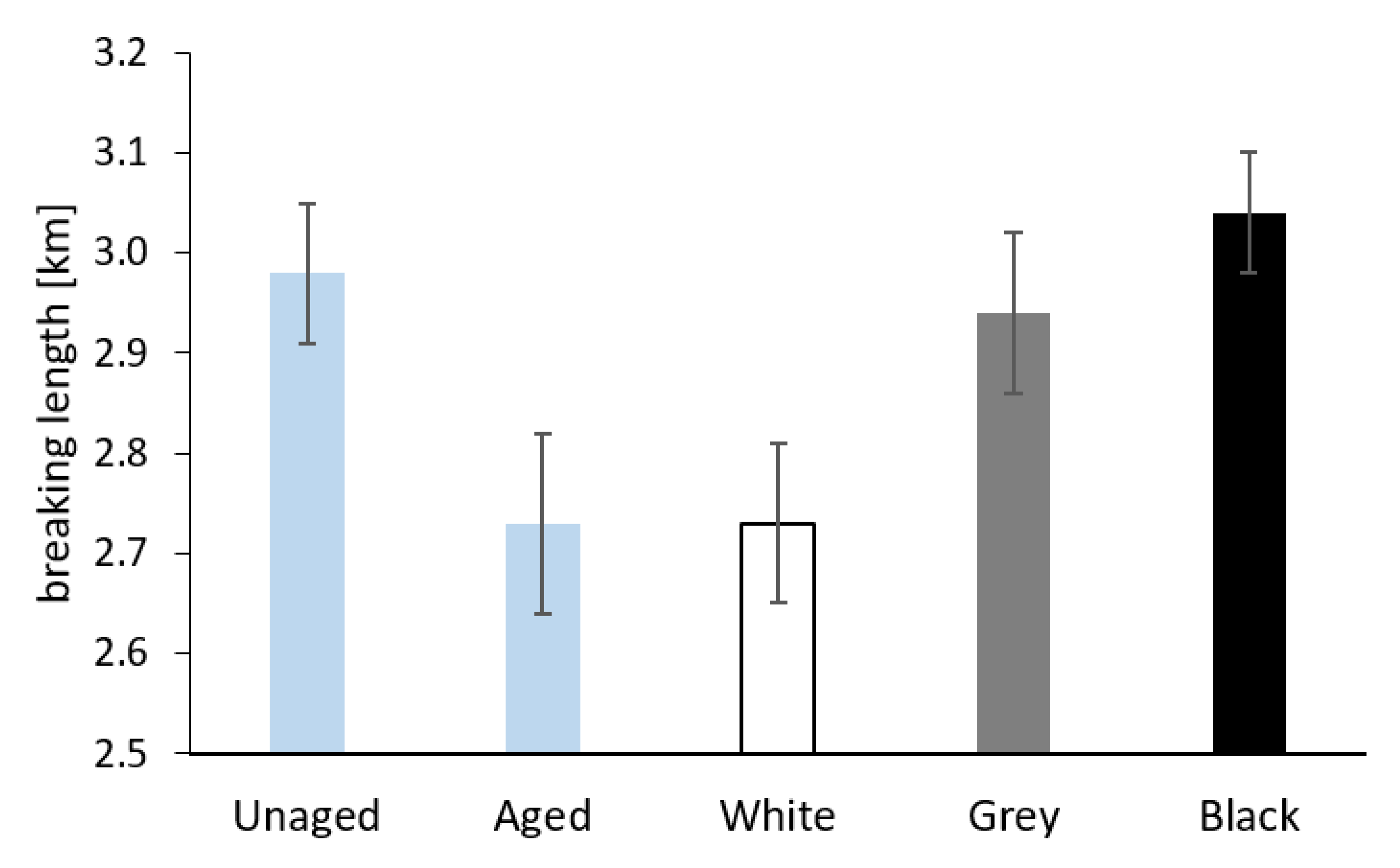
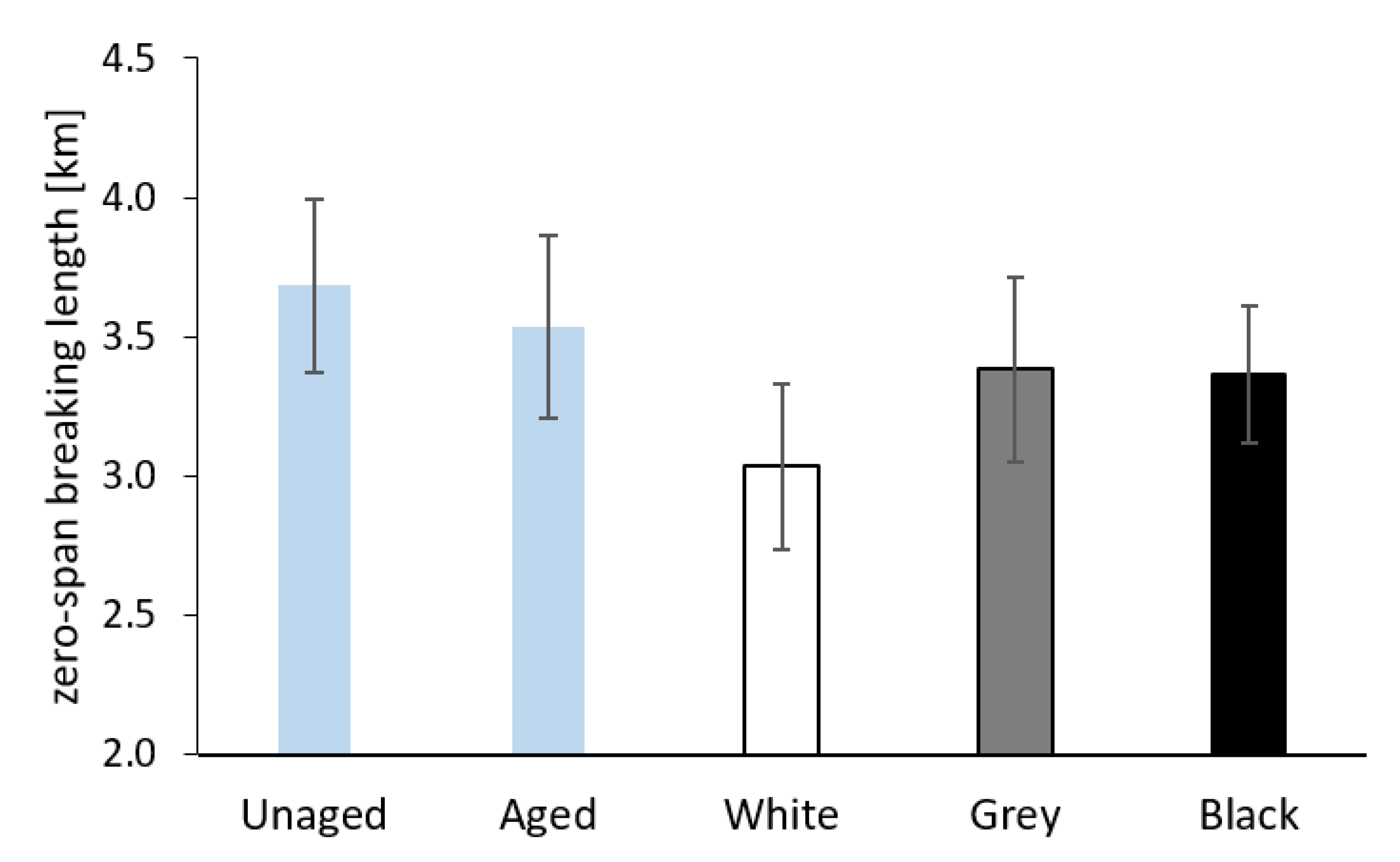
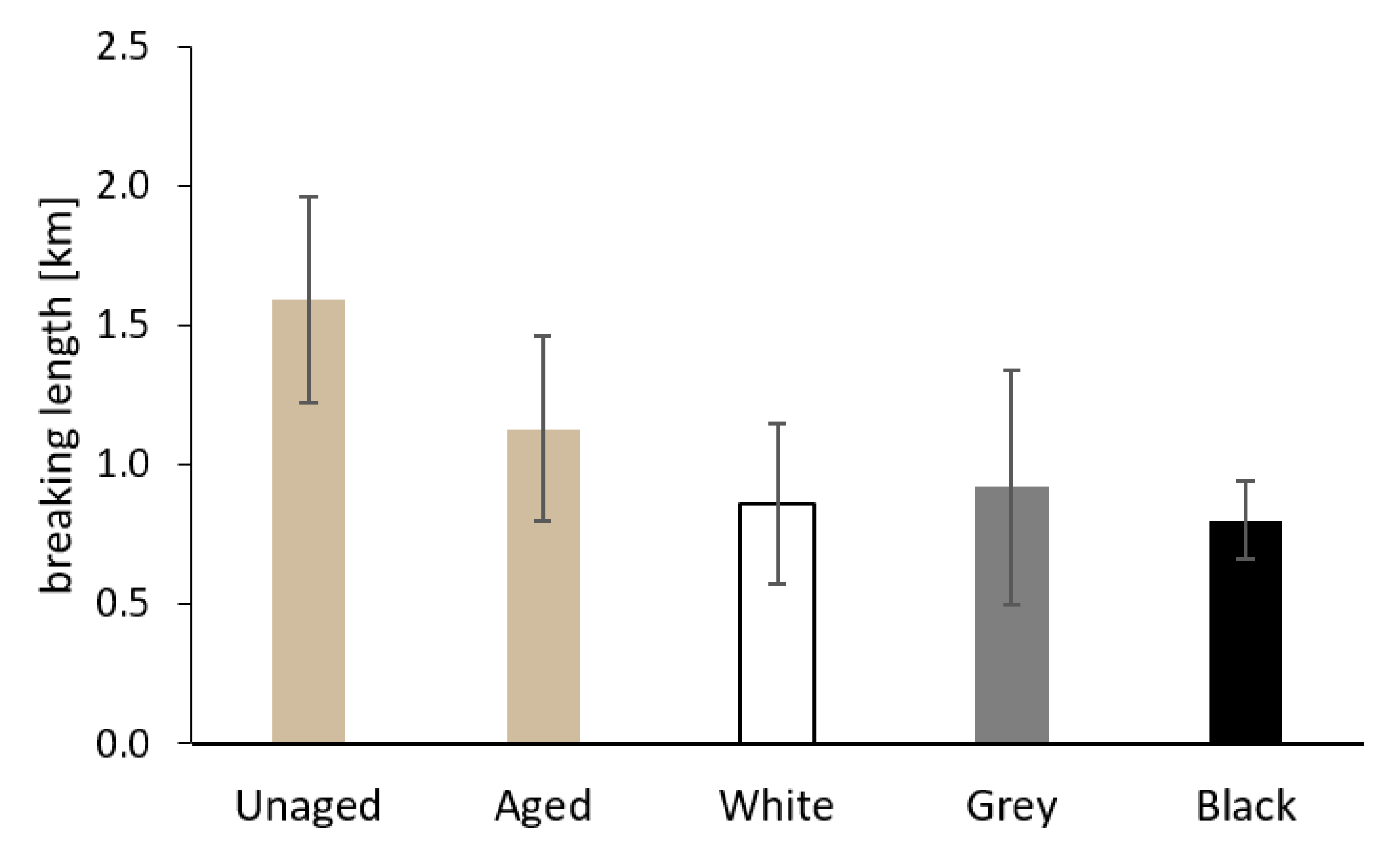
3.2.4. Mechanical Properties
3.2.5. Average Degree of Polymerization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brydson, J.A. 10—Polyethylene. In Plastics Materials, 7th ed.; Brydson, J.A., Ed.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 205–246. [Google Scholar]
- Ahmad, H.; Rodrigue, D. Crosslinked polyethylene: A review on the crosslinking techniques, manufacturing methods, applications, and recycling. Polym. Eng. Sci. 2022, 62, 2376–2401. [Google Scholar] [CrossRef]
- Sagane, N.; Harayama, H. Plastic foam—Radiation crosslinked polyethylene foam. Radiat. Phys. Chem. 1981, 18, 99–108. [Google Scholar] [CrossRef]
- Cardoso, E.C.L.; Lugão, A.B.; Andrade, E.; Silva, L.G. Crosslinked polyethylene foams, via EB radiation. Radiat. Phys. Chem. 1998, 52, 197–200. [Google Scholar] [CrossRef]
- Weber, H.; De Grave, I.; Röhrl, E.; Altstädt, V. Foamed Plastics. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2002; pp. 1–54. [Google Scholar]
- Barsema, J.N.; Bostoen, C.L.; Jansen, R.H.S.; Mulder, M.H.V.; Nauta, W.J.; Steeman, P.A.M.; Wessling, M. Analysis of cell-Stabilizing additives in low-density polyethylene foams using low-frequency dielectric spectroscopy. Macromolecules 2003, 36, 6817–6823. [Google Scholar] [CrossRef]
- Costa, J.C.; Oliveira, M.; Machado, A.V.; Lanceros-Méndez, S.; Botelho, G. Effect of antistatic additives on mechanical and electrical properties of polyethylene foams. J. Appl. Polym. Sci. 2009, 112, 1595–1600. [Google Scholar] [CrossRef]
- Lee, S.-T. Compatibilizer system for extruded polyethylene foam and film. J. Vinyl Addit. Technol. 1996, 2, 258–262. [Google Scholar] [CrossRef]
- Mleziva, J.; Šňupárek, J. Polymery: Výroba, Struktura, Vlastnosti a Použití, 2nd ed.; Sobotáles: Praha, Czech Republic, 2000. [Google Scholar]
- Modesti, M.; Lorenzetti, A. FR Design for Foam Materials. In Fire Retardancy of Polymeric Materials, 2nd ed.; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 763–782. [Google Scholar]
- Baldursdóttir, T. Storage of the Stein Loan Collection. V & A Conserv. J. 2007, 55, 5–7. [Google Scholar]
- Konstantinidou, K. Hanging by a thread? A housing/display suggestion for single parchment leaves without the use of adhesive. J. Pap. Conserv. 2014, 15, 24–27. [Google Scholar]
- Maor, T. Packing chain mail armour: A simple solution for gaining space and maximizing support. ICON News Mag. Inst. Conserv. 2006, 5, 45–46. [Google Scholar]
- Niklas, L.E. From start to finish. ICON News Mag. Inst. Conserv. 2016, 65, 34–36. [Google Scholar]
- Doležel, B. Odolnost Plastů a Pryží, 1st ed.; Nakladatelství technické literatury: Praha, Czech Republic, 1981. [Google Scholar]
- Schnabel, W. Polymer Degradation: Principles and Practical Applications, 1st ed.; Hanser: München, Germany, 1981. [Google Scholar]
- Štěpek, J.; Zelinger, J.; Kuta, A. Technologie Zpracování a Vlastnosti Plastů, 1st ed.; Nakladatelství Technické Literatury: Praha, Czech Republic, 1989. [Google Scholar]
- Goss, B.G.S.; Barry, M.D.; Birtwhistle, D.; George, G.A. Modelling of infectious spreading in heterogeneous polymer oxidation I. Development of a stochastic model. Polym. Degrad. Stab. 2001, 74, 271–282. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.-P.; Yang, R. Infectious Behavior in Photo-oxidation of Polymers. Chin. J. Polym. Sci. 2020, 38, 248–256. [Google Scholar] [CrossRef]
- Liu, X.; Yang, R. Cross-infection in thermo-oxidation of polymers. Polym. Degrad. Stab. 2019, 161, 7–12. [Google Scholar] [CrossRef]
- Celina, M.; Clough, R.L.; Jones, G.D. Initiation of polymer degradation via transfer of infectious species. Polym. Degrad. Stab. 2006, 91, 1036–1044. [Google Scholar] [CrossRef]
- Curran, K.; Možir, A.; Underhill, M.; Gibson, L.T.; Fearn, T.; Strlič, M. Cross-infection effect of polymers of historic and heritage significance on the degradation of a cellulose reference test material. Polym. Degrad. Stab. 2014, 107, 294–306. [Google Scholar] [CrossRef]
- Pasiuk, J. Safe Plastics And Fabrics For Exhibit And Storage. Conserve O Gram 2004, 18, 1–7. [Google Scholar]
- Hakkarainen, M.; Albertsson, A.-C.; Karlsson, S. Solid phase microextraction (SPME) as an effective means to isolate degradation products in polymers. J. Environ. Polym. Degrad. 1997, 5, 67–73. [Google Scholar] [CrossRef]
- Curran, K.; Aslam, A.; Ganiaris, H.; Hodgkins, R.E.; Moon, J.; Moore, A.R.; Ramsay, L. Volatile organic compound (VOC) emissions from plastic materials used for storing and displaying heritage objects. In Proceedings of the 18th Triennial Meeting ICOM Committee for Conservation, Copenhagen, Denmark, 4–8 September 2017; Pulido & Nunes: Copenhagen, Denmark, 2017; pp. 1–8. [Google Scholar]
- Ligterink, F.; Di Pietro, G. The limited impact of acetic acid in archives and libraries. Herit. Sci. 2018, 6, 59. [Google Scholar] [CrossRef]
- Strlič, M.; Kralj Cigić, I.; Možir, A.; de Bruin, G.; Kolar, J.; Cassar, M. The effect of volatile organic compounds and hypoxia on paper degradation. Polym. Degrad. Stab. 2011, 96, 608–615. [Google Scholar] [CrossRef]
- Carter, E.C.; Ohno, Y.; Pointer, M.R.; Robertson, A.R.; Seve, R.; Schanda, J.D.; Witt, K. CIE Technical Report—Colorimetry, 3rd ed.; International Commission on Illumination: Vienna, Austria, 2004; Volume 15, pp. 17–18. [Google Scholar]
- Coleman, E.A. 21—Plastics Additives. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 489–500. [Google Scholar]
- Hodgson, S.C.; Casey, R.J.; Bigger, S.W.; Scheirs, J. Review of volatile organic compounds derived from polyethylene. Polym. Plast. Technol. Eng. 2000, 39, 845–874. [Google Scholar] [CrossRef]
- Kaszonyi, A.; Izsák, L.; Králik, M.; Jablonsky, M. Accelerated and Natural Aging of Cellulose-Based Paper: Py-GC/MS Method. Molecules 2022, 27, 2855. [Google Scholar] [CrossRef] [PubMed]
- Potthast, A.; Ahn, K.; Becker, M.; Eichinger, T.; Kostic, M.; Böhmdorfer, S.; Jeong, M.J.; Rosenau, T. Acetylation of cellulose—Another pathway of natural cellulose aging during library storage of books and papers. Carbohydr. Polym. 2022, 287, 119323. [Google Scholar] [CrossRef] [PubMed]
- Smedemark, S.H.; Ryhl-Svendsen, M.; Schieweck, A. Quantification of formic acid and acetic acid emissions from heritage collections under indoor room conditions. Part I: Laboratory and field measurements. Herit. Sci. 2020, 8, 58. [Google Scholar] [CrossRef]
- Rugg, F.M.; Smith, J.J.; Bacon, R.C. Infrared spectrophotometric studies on polyethylene. II. Oxidation. J. Polym. Sci. 1954, 13, 535–547. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Potthast, A. Improving the accuracy of estimating paper permanence for accelerated degradation in closed vials. Cellulose 2021, 28, 4053–4068. [Google Scholar] [CrossRef]
- Soares, S.; Ricardo, N.M.P.S.; Jones, S.; Heatley, F. High temperature thermal degradation of cellulose in air studied using FTIR and 1H and 13C solid-state NMR. Eur. Polym. J. 2001, 37, 737–745. [Google Scholar] [CrossRef]
- Tétreault, J.; Dupont, A.L.; Bégin, P.; Paris, S. The impact of volatile compounds released by paper on cellulose degradation in ambient hygrothermal conditions. Polym. Degrad. Stab. 2013, 98, 1827–1837. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of yellowing: Carbonyl formation during hygrothermal aging in a common amine epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef]
- Allen, S.N.; Edge, M.; Rodriguez, M.; Liauw, M.C.; Fontan, E. Aspects of the thermal oxidation, yellowing and stabilisation of ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2000, 71, 1–14. [Google Scholar] [CrossRef]
- Calvini, P.; Martinelli, G. Numerical Processing of Fourier Transform Infrared Spectra: A Powerful Tool in Paper Analysis. In Proceedings of the 9th Triennial Meeting ICOM Committee for Conservation, Dresden, Germany, 26–31 August 1990; ICOM Committee for Conservation: Dresden, Germany, 1990; pp. 453–455. [Google Scholar]
- Łojewski, T.; Miśkowiec, P.; Missori, M.; Lubańska, A.; Proniewicz, L.M.; Łojewska, J. FTIR and UV/vis as methods for evaluation of oxidative degradation of model paper: DFT approach for carbonyl vibrations. Carbohydr. Polym. 2010, 82, 370–375. [Google Scholar] [CrossRef]
- Mosini, V.; Calvini, P.F.; Mattogno, G.; Righini, G. Derivative infrared spectroscopy and electron spectroscopy for chemical analysis of ancient paper documents. Cellul. Chem. Technol. 1990, 24, 263–272. [Google Scholar]
- Alam, I.; Sood, S.; Sharma, C. Dynamic-head space GC-MS analysis of volatile odorous compounds generated from unbleached and bleached pulps and effects on strength properties during ageing. Nord. Pulp Pap. Res. J. 2021, 36, 465–474. [Google Scholar] [CrossRef]
- Tétreault, J.; Bégin, P.; Paris-Lacombe, S.; Dupont, A.L. Modelling considerations for the degradation of cellulosic paper. Cellulose 2019, 26, 2013–2033. [Google Scholar] [CrossRef]
- Jablonský, M.; Botková, M.; Hroboňová, K. Accelerated ageing of wood-containing papers: Formation of weak acids and deterioration oftensile strength. Wood Res. 2012, 57, 419–434. [Google Scholar]
- Grau-Bové, J.; Budič, B.; Cigić, I.K.; Thickett, D.; Signorello, S.; Strlič, M. The effect of particulate matter on paper degradation. Herit. Sci. 2016, 4, 2. [Google Scholar] [CrossRef]





| PE Foam | Element Content (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | S | Cl | K | Ca | Cr | Fe | Ni | Zn | |
| White | 0.043 (0.006) 1 | 0.123 (0.01) | 0.010 (0.003) | 0.093 (0.009) | 0.003 (0.002) | 0.020 (0.004) | 0.005 (0.002) | 0.003 (0.002) | 0.003 (0.002) | 0.009 (0.003) | 0.001 (0.0007) | |
| Grey | 0.003 (0.002) | 0.002 (0.001) | 0.003 (0.002) | 0.007 (0.002) | 0.001 (0.0008) | 0.002 (0.001) | 0.001 (0.0007) | |||||
| Black | 0.040 (0.006) | 0.006 (0.002) | 0.021 (0.004) | 0.034 (0.005) | 0.010 (0.003) | 0.026 (0.005) | 0.003 (0.002) | 0.011 (0.003) | 0.003 (0.002) | 0.007 (0.003) | 0.001 (0.0007) | 0.001 (0.0008) |
| PE Foam | Compression Set (%) |
|---|---|
| White | 31 ± 1 |
| Grey | 27 ± 1 |
| Black | 28 ± 1 |
| White Foam | Grey Foam | Black Foam | |||
|---|---|---|---|---|---|
| RT (Min) | Compound | RT (Min) | Compound | RT (Min) | Compound |
| 4.00 | acetaldehyde | ||||
| 4.72 | ethanol | 4.72 | ethanol | 4.73 | ethanol |
| 5.41 | acetone | 5.42 | acetone | 5.42 | acetone |
| 5.49 | propionaldehyde | ||||
| 5.64 | formic acid | ||||
| 6.17 | terc.-butanol | ||||
| 6.91 | 1-propanol | ||||
| 7.12 | trimethylsilanol | 7.13 | trimethylsilanol | 7.13 | trimethylsilanol |
| 7.67 | acetic acid | 7.34 | acetic acid | 7.29 | acetic acid |
| 7.86 | 2-butanone | ||||
| 9.81 | dimethylsilanediol | 9.81 | dimethylsilanediol | ||
| 9.49 | 1-butanol | ||||
| 10.01 | propanoic acid | 10.01 | propanoic acid | 10.01 | propanoic acid |
| 10.24 | 3-pentanone | ||||
| 10.30 | pentanal | ||||
| 11.42 | 3-methyl-2-pentanone | ||||
| 11.63 | 1-pentanol | ||||
| 11.67 | methylheptane | ||||
| 11.79 | butyric acid | 11.79 | butyric acid | 11.79 | butyric acid |
| 11.98 | 3-hexanone | ||||
| 12.06 | 2-hexanone | ||||
| 12.32 | hexamethylcyclotrisiloxane | 12.32 | hexamethylcyclotrisiloxane | 12.32 | hexamethylcyclotrisiloxane |
| 12.63 | dimethylheptane | ||||
| 12.97 | dimethylheptene | ||||
| 13.30 | valeric acid | 13.30 | valeric acid | 13.30 | valeric acid |
| 13.64 | 3-heptanone | ||||
| 13.69 | 2-heptanone | ||||
| 14.63 | hexanoic acid | 14.64 | hexanoic acid | 14.64 | hexanoic acid |
| 14.73 | iso-C10H22 | ||||
| 14.70 | gama-pentalactone | ||||
| 14.88 | 4-octanone | ||||
| 15.04 | 3-octanone | ||||
| 15.09 | 2-octanone | ||||
| 15.50 | iso-C11H24 | 15.50 | iso-C11H24 | 15.50 | iso-C11H24 |
| 15.85 | heptanoic acid | ||||
| 16.14 | 5-nonanone | ||||
| 16.35 | 2-nonanone | ||||
| 16.44 | n-undecane | 16.43 | n-undecane | ||
| 16.50 | decamethylcyclopentasiloxane | 16.50 | decamethylcyclopentasiloxane | 16.50 | decamethylcyclopentasiloxane |
| 17.28 | 5-decanone | ||||
| 17.50 | 2-decanone | ||||
| 17.56 | n-dodecane | 17.55 | n-dodecane | ||
| 18.59 | n-tridecane | ||||
| pH Value | ||
|---|---|---|
| Treatment | Whatman | Wood-Pulp Paper |
| Unaged | 6.2 | 4.2 |
| Aged | 5.3 | 4.5 |
| White | 4.7 | 4.0 |
| Grey | 5.5 | 4.0 |
| Black | 5.5 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knotek, V.; Ďurovič, M.; Kučerová, I. The Effect of Synthetic Polymer Foams on Cellulosic Material Degradation. Materials 2023, 16, 1210. https://doi.org/10.3390/ma16031210
Knotek V, Ďurovič M, Kučerová I. The Effect of Synthetic Polymer Foams on Cellulosic Material Degradation. Materials. 2023; 16(3):1210. https://doi.org/10.3390/ma16031210
Chicago/Turabian StyleKnotek, Vítězslav, Michal Ďurovič, and Irena Kučerová. 2023. "The Effect of Synthetic Polymer Foams on Cellulosic Material Degradation" Materials 16, no. 3: 1210. https://doi.org/10.3390/ma16031210
APA StyleKnotek, V., Ďurovič, M., & Kučerová, I. (2023). The Effect of Synthetic Polymer Foams on Cellulosic Material Degradation. Materials, 16(3), 1210. https://doi.org/10.3390/ma16031210







